Abstract
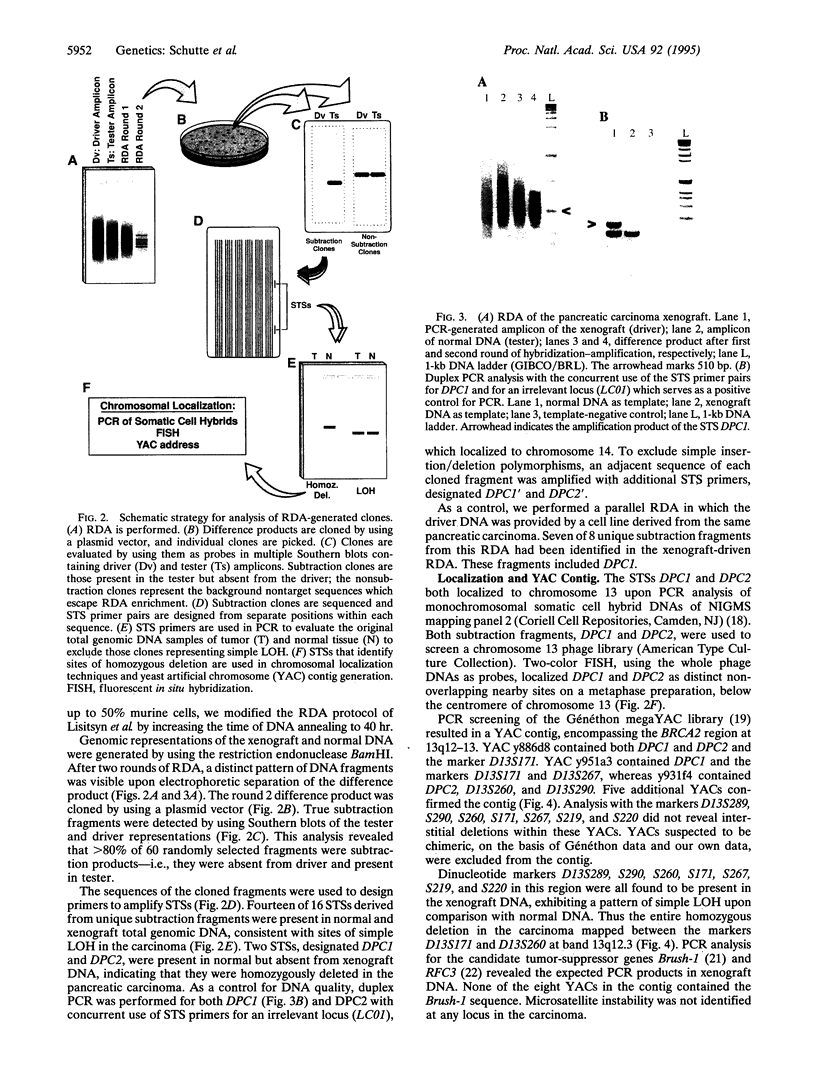
Homozygous deletions have been central to the discovery of several tumor-suppressor genes, but their finding has often been either serendipitous or the result of a directed search. A recently described technique [Lisitsyn, N., Lisitsyn, N. & Wigler, M. (1993) Science 259, 946-951] held out the potential to efficiently discover such events in an unbiased manner. Here we present the application of the representational difference analysis (RDA) to the study of cancer. We cloned two DNA fragments that identified a homozygous deletion in a human pancreatic adenocarcinoma, mapping to a 1-centimorgan region at chromosome 13q12.3 flanked by the markers D13S171 and D13S260. Interestingly, this lies within the 6-centimorgan region recently identified as the BRCA2 locus of heritable breast cancer susceptibility. This suggests that the same gene may be involved in multiple tumor types and that its function is that of a tumor suppressor rather than that of a dominant oncogene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Boring C. C., Squires T. S., Tong T. Cancer statistics, 1993. CA Cancer J Clin. 1993 Jan-Feb;43(1):7–26. doi: 10.3322/canjclin.43.1.7. [DOI] [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., da Costa L. T., Redston M. S., Schutte M., Seymour A. B., Weinstein C. L., Hruban R. H., Yeo C. J., Kern S. E. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994 Sep;8(1):27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- Chang Y., Cesarman E., Pessin M. S., Lee F., Culpepper J., Knowles D. M., Moore P. S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994 Dec 16;266(5192):1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- Cohen D., Chumakov I., Weissenbach J. A first-generation physical map of the human genome. Nature. 1993 Dec 16;366(6456):698–701. doi: 10.1038/366698a0. [DOI] [PubMed] [Google Scholar]
- Devilee P., van Vliet M., van Sloun P., Kuipers Dijkshoorn N., Hermans J., Pearson P. L., Cornelisse C. J. Allelotype of human breast carcinoma: a second major site for loss of heterozygosity is on chromosome 6q. Oncogene. 1991 Sep;6(9):1705–1711. [PubMed] [Google Scholar]
- Diaz M. O., Ziemin S., Le Beau M. M., Pitha P., Smith S. D., Chilcote R. R., Rowley J. D. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5259–5263. doi: 10.1073/pnas.85.14.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drwinga H. L., Toji L. H., Kim C. H., Greene A. E., Mulivor R. A. NIGMS human/rodent somatic cell hybrid mapping panels 1 and 2. Genomics. 1993 May;16(2):311–314. doi: 10.1006/geno.1993.1190. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Rapaport J. M., Joyce J. M., Petersen R. A. Molecular detection of deletions involving band q14 of chromosome 13 in retinoblastomas. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7391–7394. doi: 10.1073/pnas.83.19.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Park S. H., Bogenmann E., Cheng J. C., Yandell D. W., Kaye F. J., Minna J. D., Dryja T. P., Weinberg R. A. Frequent inactivation of the retinoblastoma anti-oncogene is restricted to a subset of human tumor cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2775–2779. doi: 10.1073/pnas.87.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Redston M., Seymour A. B., Caldas C., Powell S. M., Kornacki S., Kinzler K. W. Molecular genetic profiles of colitis-associated neoplasms. Gastroenterology. 1994 Aug;107(2):420–428. doi: 10.1016/0016-5085(94)90167-8. [DOI] [PubMed] [Google Scholar]
- Knudson A. G. Antioncogenes and human cancer. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10914–10921. doi: 10.1073/pnas.90.23.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M., Monaco A. P., Middlesworth W., Ochs H. D., Latt S. A. Specific cloning of DNA fragments absent from the DNA of a male patient with an X chromosome deletion. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4778–4782. doi: 10.1073/pnas.82.14.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Schork N. J. Genetic dissection of complex traits. Science. 1994 Sep 30;265(5181):2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- Lisitsyn N. A., Lisitsina N. M., Dalbagni G., Barker P., Sanchez C. A., Gnarra J., Linehan W. M., Reid B. J., Wigler M. H. Comparative genomic analysis of tumors: detection of DNA losses and amplification. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):151–155. doi: 10.1073/pnas.92.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisitsyn N. A., Segre J. A., Kusumi K., Lisitsyn N. M., Nadeau J. H., Frankel W. N., Wigler M. H., Lander E. S. Direct isolation of polymorphic markers linked to a trait by genetically directed representational difference analysis. Nat Genet. 1994 Jan;6(1):57–63. doi: 10.1038/ng0194-57. [DOI] [PubMed] [Google Scholar]
- Lisitsyn N., Lisitsyn N., Wigler M. Cloning the differences between two complex genomes. Science. 1993 Feb 12;259(5097):946–951. doi: 10.1126/science.8438152. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Fitzsimmons M. L., Smyrk T. C., Lanspa S. J., Watson P., McClellan J., Lynch J. F. Familial pancreatic cancer: clinicopathologic study of 18 nuclear families. Am J Gastroenterol. 1990 Jan;85(1):54–60. [PubMed] [Google Scholar]
- McQueen H. A., Wyllie A. H., Piris J., Foster E., Bird C. C. Stability of critical genetic lesions in human colorectal carcinoma xenografts. Br J Cancer. 1991 Jan;63(1):94–96. doi: 10.1038/bjc.1991.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan L. M., Eng C., Healey C. S., Clayton D., Kwok J. B., Gardner E., Ponder M. A., Frilling A., Jackson C. E., Lehnert H. Specific mutations of the RET proto-oncogene are related to disease phenotype in MEN 2A and FMTC. Nat Genet. 1994 Jan;6(1):70–74. doi: 10.1038/ng0194-70. [DOI] [PubMed] [Google Scholar]
- Okumura K., Nogami M., Taguchi H., Dean F. B., Chen M., Pan Z. Q., Hurwitz J., Shiratori A., Murakami Y., Ozawa K. Assignment of the 36.5-kDa (RFC5), 37-kDa (RFC4), 38-kDa (RFC3), and 40-kDa (RFC2) subunit genes of human replication factor C to chromosome bands 12q24.2-q24.3, 3q27, 13q12.3-q13, and 7q11.23. Genomics. 1995 Jan 1;25(1):274–278. doi: 10.1016/0888-7543(95)80135-9. [DOI] [PubMed] [Google Scholar]
- Redston M. S., Caldas C., Seymour A. B., Hruban R. H., da Costa L., Yeo C. J., Kern S. E. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res. 1994 Jun 1;54(11):3025–3033. [PubMed] [Google Scholar]
- Sato T., Tanigami A., Yamakawa K., Akiyama F., Kasumi F., Sakamoto G., Nakamura Y. Allelotype of breast cancer: cumulative allele losses promote tumor progression in primary breast cancer. Cancer Res. 1990 Nov 15;50(22):7184–7189. [PubMed] [Google Scholar]
- Schott D. R., Chang J. N., Deng G., Kurisu W., Kuo W. L., Gray J., Smith H. S. A candidate tumor suppressor gene in human breast cancers. Cancer Res. 1994 Mar 15;54(6):1393–1396. [PubMed] [Google Scholar]
- Seymour A. B., Hruban R. H., Redston M., Caldas C., Powell S. M., Kinzler K. W., Yeo C. J., Kern S. E. Allelotype of pancreatic adenocarcinoma. Cancer Res. 1994 May 15;54(10):2761–2764. [PubMed] [Google Scholar]
- Thorlacius S., Jonasdottir O., Eyfjord J. E. Loss of heterozygosity at selective sites on chromosomes 13 and 17 in human breast carcinoma. Anticancer Res. 1991 Jul-Aug;11(4):1501–1507. [PubMed] [Google Scholar]
- Toguchida J., Ishizaki K., Sasaki M. S., Nakamura Y., Ikenaga M., Kato M., Sugimot M., Kotoura Y., Yamamuro T. Preferential mutation of paternally derived RB gene as the initial event in sporadic osteosarcoma. Nature. 1989 Mar 9;338(6211):156–158. doi: 10.1038/338156a0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- Wadayama B., Toguchida J., Shimizu T., Ishizaki K., Sasaki M. S., Kotoura Y., Yamamuro T. Mutation spectrum of the retinoblastoma gene in osteosarcomas. Cancer Res. 1994 Jun 1;54(11):3042–3048. [PubMed] [Google Scholar]
- Wieland I., Bolger G., Asouline G., Wigler M. A method for difference cloning: gene amplification following subtractive hybridization. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2720–2724. doi: 10.1073/pnas.87.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland I., Böhm M., Bogatz S. Isolation of DNA sequences deleted in lung cancer by genomic difference cloning. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9705–9709. doi: 10.1073/pnas.89.20.9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooster R., Neuhausen S. L., Mangion J., Quirk Y., Ford D., Collins N., Nguyen K., Seal S., Tran T., Averill D. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994 Sep 30;265(5181):2088–2090. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]
- Yoo G. H., Xu H. J., Brennan J. A., Westra W., Hruban R. H., Koch W., Benedict W. F., Sidransky D. Infrequent inactivation of the retinoblastoma gene despite frequent loss of chromosome 13q in head and neck squamous cell carcinoma. Cancer Res. 1994 Sep 1;54(17):4603–4606. [PubMed] [Google Scholar]