Abstract
Background
Acellular dermal matrices present a new alternative to supporting expanders and implants for breast reconstruction in breast cancer patients following mastectomy. However, some studies have suggested that acellular dermal matrix may increase the complication rates in certain clinical settings. DermACELL acellular dermal matrix offers advanced processing in order to attempt to decrease bio-intolerance and complications.
Methods
Ten consecutive patients that presented for breast reconstruction and were candidates for tissue expanders underwent the procedure with the use of an acellular dermal matrix. The patients underwent postoperative expansion/adjuvant cancer therapy, then tissue expander exchange for permanent silicone breast prostheses. Patients were followed through the postoperative course to assess complication outcomes. Histologic evaluation of host integration into the dermal matrix was also assessed.
Results
Of the ten patients, eight completed reconstruction while two patients failed reconstruction. The failures were related to chronic seromas and infection. Histology analysis confirms rapid integration of mesenchymal cells into the matrix compared to other acellular dermal matrices.
Conclusions
Based on our observations, DermACELL is an appropriate adjunct to reconstruction with expanders. Histological analysis of vascularization and recellularization support the ready incorporation of DermACELL into host tissue.
Level of Evidence: Level IV, therapeutic study.
Keywords: Breast, Acellular dermal matrix, Two-stage reconstruction, Tissue expanders
Introduction
Following mastectomy, tissue expander reconstruction is one of the most common methods for breast reconstruction [1]. The use of acellular dermal matrices (ADMs) has been popularized in the use of tissue expander reconstruction. This biologic matrix provides support in the lower pole of the breast and helps define the lower pole and inframammary fold. It also speeds the rate of expansion by reducing tension of the mastectomy skin and allows higher initial fill volumes. These two attributes help to decrease the overall length of the reconstruction process. Most recently, it has been suggested that ADMs help prevent capsular contracture, a common long-term problem with implants in the reconstructed breast [2–4]. Although our institutional experience has been promising, other studies have suggested mixed, although generally positive, findings concerning the use of ADMs [3, 5]. Since clinical procedures are generally standardized, this suggests that the relative ability of the various ADMs to incorporate into soft tissues may relate to the manufacturing or processing of the particular material. Incorporation confers biocompatibility, which ultimately creates symmetry within the host. Bio-incompatibility due to lack of incorporation may cause seroma, infection, and reconstructive failure [6]. The reconstructive process can be difficult enough for the patient without adding further cosmetic complications. Structural support of the breast is necessary to provide the ideal breast shape and implant position. ADMs have been recognized as being beneficial by serving as a supplemental graft in breast reconstruction cases. They can supplement muscle coverage, shape the breast and inframammary fold, and possibly reduce the number of surgeries patients need to undergo. This technique utilized in breast reconstruction brings with it many benefits [7].
Acellular dermal matrices are composed of the dermal layer and extracellular matrix of thin layers of donated skin that have had the epidermal layer removed. Donor cellular material including major histocompatibility complex (MHC) proteins are removed in a series of further treatments to theoretically minimize immunological response in ADM recipients [8]. The ability of ADMs to promote significant revascularization and cellular infiltration make them an encouraging option for an array of tissue regeneration applications, including wound healing, soft tissue reconstruction, and augmentation [9–27].
A recently developed human ADM produced by an anionic detergent and endonuclease-based decellularization process [28] is a product trade-named DermACELL® (LifeNet Health, Virginia Beach, VA, USA). Unique features of this allograft include removal of at least 97 % DNA, an indication of complete decellularization and potentially reduced immunogenicity, terminally sterilization to a Sterility Assurance Level of 10−6, and being provided at room temperature and ready to use directly out of the package.
Here, we examine the post-mastectomy outcome of a two-stage breast reconstruction using this new human acellular dermal matrix, DermACELL, in a 10 consecutive patient prospective cohort series.
Patients and methods
Case series overview
This prospective, consecutive cohort series included 10 female patients ranging in age from 33 to 59 years old who underwent mastectomies and presented to have breast reconstructions with the use of DermACELL. Of the 10 patients, eight had bilateral mastectomies and two had unilateral mastectomies, for a total of 18 breasts. One patient elected to have a prophylactic bilateral mastectomy after testing positive for the BRCA-1 gene. Two patients underwent unilateral mastectomies, one of whom had previously undergone a unilateral mastectomy and reconstruction of the contralateral breast. The remaining patients each presented with breast cancer unilaterally and elected to have a mastectomy of the affected breast, as well as prophylactic mastectomy of the contralateral breast. Of the 10 patients, two were smokers (Table 1). Two patients required neo-adjuvant chemotherapy, one required adjuvant chemotherapy, one additional patient required both neo-adjuvant and adjuvant chemotherapy, and two required radiation.
Table 1.
Patient overview
| Patient no. | Age (years) | Post-op chemotherapy | Radiation | Uni- or bilateral | Duration of implant prior to 2nd stage (weeks) | Expansion volume | Smoker | Implant volume |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | Adj. | No | Bilateral | 46 | 400 cc | No | 400 cc |
| 2 | 43 | No | No | Bilateral | 24 | 400 cc | No | 600 cc |
| 3 | 52 | No | No | Bilateral | 9 | 380 cc | No | 425 cc |
| 4 | 43 | Neo-adj./Adj. | Yes | Bilateral | N/A | X | Yes | X |
| 5 | 33 | No | No | Bilateral | 7 | 550 cc | No | 500 cc |
| 6 | 56 | Neo-adj. | No | Bilateral | N/A | X | Yes | X |
| 7 | 59 | No | No | Bilateral | 20 | 700 cc | No | 700 cc |
| 8 | 45 | Neo-adj. | Yes | Unilateral (R) | 41 | 600 cc | No | Flapa |
| 9 | 48 | No | No | Unilateral (R) | 43 | 220 cc | No | 175 cc |
| 10 | 33 | No | No | Bilateral | 20 | 500 cc | No | 555 cc |
aPlanned autologous reconstruction in a delayed fashion
Breast reconstruction was performed in two stages. In the first stage, DermACELL and expanders were used. Following tissue expansion, patients advanced to the second stage of the procedure of immediate reconstruction for final placement of silicone implants.
Clinical procedures
Each reconstruction included placement of Mentor CPX3 tall height contour profile implants (Mentor Worldwide LLC, Santa Barbara, CA, USA) for tissue expansion and 16 × 6 cm2 DermACELL patches, with one patient’s DermACELL patch tailored down to 12 × 6 cm2. All expanders were placed with an initial volume of 100 cc intraoperatively using sterile saline. The pectoris muscle was elevated and the DermACELL was sutured to the inferior portion of the muscle and to the inframammary fold to serve as a sling. A drain was placed in the breast pocket and in the subcutaneous pocket between the mastectomy skin and the DermACELL. Two weeks postoperative expansion began and continued between 7 and 46 weeks (average 8 weeks) until the desired volume. Drains were removed between 6 and 21 days postoperatively (average 15 days) depending on their output.
Histological analysis
Biopsy samples were taken from the patients and sent in formalin to Dominion Pathology Laboratories (Norfolk, VA, USA) for sectioning and staining. Hematoxylin and eosin (H&E) staining was undertaken to assess cellularity and general ultrastructure. Additionally, immunohistochemical staining for the endothelial cell marker CD34 (CD34) was used to assess vascularity and Verhoef-Von Gieson (VVG) staining was performed to assess elastic fibers and general ultrastructure. Histological assessments were determined by dermatopathologists Kevagn Fair, MD (Dominion Pathology) and Antoinette Hood, MD (Eastern Virginia Medical School, Norfolk, VA, USA).
Results
Patient overview and results
Eight (15 breasts) of the 10 breast reconstruction procedures were successfully completed and only minor complications were reported in two patients (Table 2). Major complications were seen in the two patients with failed breast reconstructions due to smoking and infection. Patient outcomes were as follows (Table 2): four breasts developed seromas, there were two surgical site infections, four experienced delayed healing, and three had flap necrosis. Of particular note, there was no observation of hematomas or red breast syndrome in any patient. Over half of the observed complications were limited to a single patient, limiting the complicative profile and associating patient comorbidities as the major contributor. It appears that a history of smoking caused mastectomy skin necrosis and a port-a-cath wound infection contributed to the failed reconstruction. This patient developed seromas of both breasts, a purulent right breast infection, and mastectomy flap necrosis of both breasts. This patient also had postoperative radiation and significant skin necrosis, apparently due to the radiation. She also eventually developed metastatic cancer and did not return to undergo reconstruction. The other patient who failed reconstruction developed a seroma, surgical site infection, flap necrosis, and experienced delayed healing of the left breast. These complications were most likely the result of the patient continuing to smoke throughout her postoperative recovery. The expanders were removed and the reconstruction was aborted.
Table 2.
Patient outcomes
| Patient no. | Seroma | Hematoma | Surgical site infection | Red breast | Delayed healing | Flap necrosis | Completed reconstruction | Failed reconstruction |
|---|---|---|---|---|---|---|---|---|
| 1 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 2/2 | 0/2 |
| 2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 2/2 | 0/2 |
| 3 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 2/2 | 0/2 |
| 4 | 2/2 | 0/2 | 1/2 | 0/2 | 2/2 | 2/2 | 0/2 | 2/2 |
| 5 | 1/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 2/2 | 0/2 |
| 6 | 1/2 | 0/2 | 1/2 | 0/2 | 1/2 | 1/2 | 0/2 | 2/2 |
| 7 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 2/2 | 0/2 |
| 8 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 |
| 9 | 0/1 | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | 1/1 | 0/1 |
| 10 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 2/2 | 0/2 |
| Total | 4/18 | 0/18 | 2/18 | 0/18 | 4/18 | 3/18 | 15/18 | 4/18 |
Six patients that completed reconstruction did not experience any complications. Patient 2 (Fig. 1) was one of these six patients that had a successful reconstruction with no infections, seromas, or other possible complications. She was 43 years old, had neoadjuvant chemotherapy for a left locally advanced breast cancer, and was advised to have a mastectomy. The patient also requested a contralateral prophylactic mastectomy with immediate reconstruction of both breasts. From histological analysis (Fig. 2), it is evident that the DermACELL had incorporated into the host tissue and allowed for revascularization. The tissue was infiltrated with fibroblasts and blood vessels.
Fig. 1.
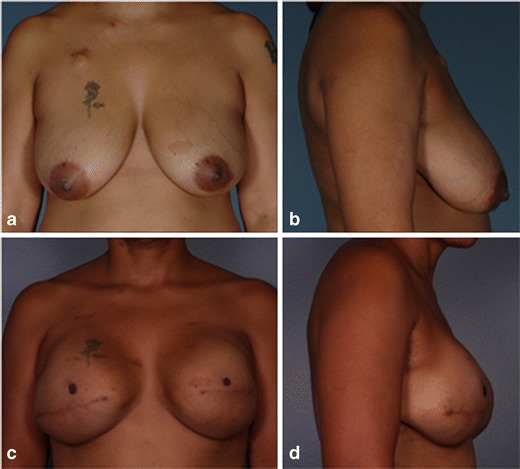
a, b Patient 2 preoperative before undergoing bilateral mastectomy with immediate reconstruction. c, d Patient 2 postoperative 7 months after placement of permanent implant
Fig. 2.
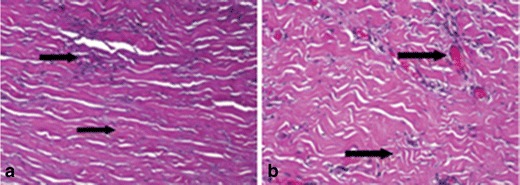
a, b H&E stain of DermACELL sample taken from patient 2’s left breast at 7 months postoperative. Viewed under ×100. The upper arrows identify blood vessels while the lower arrows identify fibroblasts
Another notable completed reconstruction was performed on patient 5 (Fig. 3). She was 33 years old, had a strong family history of breast cancer, and therefore decided to be tested for the BRCA-1 gene mutation. The patient tested positive for the mutation and elected to undergo prophylactic mastectomy of both breasts with immediate breast reconstruction. A seroma developed on the left breast between the skin and the DermACELL on day 10. A drain was placed between the skin and DermACELL, and was then removed 1 week later. The patient had expansion for about 7 weeks and then returned for tissue expander removal and placement of the permanent implants. At this time, the DermACELL was found to be incorporated into the surrounding tissues.
Fig. 3.
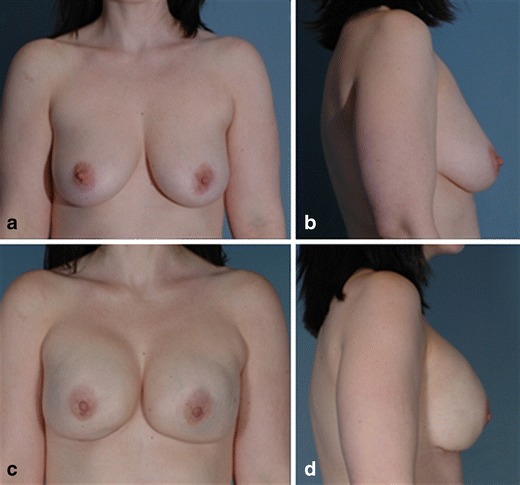
a Patient 5 preoperative before undergoing bilateral mastectomy with immediate reconstruction. b Patient 5 postoperative 1 month after exchange of tissue expander for implant
Patient 10, one of the eight patients with completed reconstruction, was a 33-year old who originally presented for breast augmentation (Fig. 4). During her preoperative screening, she was found to have left breast cancer and was counseled to undergo mastectomy. The patient agreed to have left breast mastectomy and also opted to have prophylactic right breast mastectomy followed by immediate breast reconstruction. After expansion continued to the desired volume, the tissue expander implants were removed at 20 weeks and replaced with permanent silicone breast implants. During placement of the permanent implants, it was noted that the DermACELL matrix had incorporated into the surrounding tissue and showed signs of granulation budding (Fig. 5).
Fig. 4.
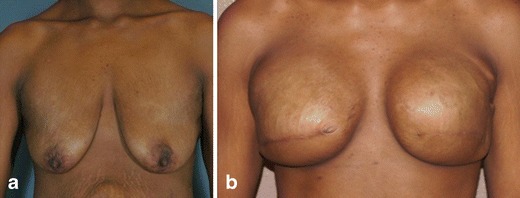
a Patient 10 preoperative before undergoing bilateral mastectomy with immediate reconstruction. b Patient 10 postoperative 7 months after mastectomy and placement of tissue expanders and DermACELL
Fig. 5.
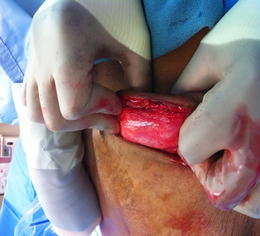
Patient 10, 5 months after expansion of the DermACELL, the matrix is incorporated into the surrounding tissue and show signs of granulation
Histological analysis
Histology analysis confirms the incorporation of the DermACELL matrix into the surrounding tissue, aiding in reconstruction. Observations for all biopsy samples included presence of fibroblasts, intact ultrastructure including elastin, and vasculature (Figs. 6, 7, and 8). Rarely, a foreign body response was observed and was noticeable at the same position as polarizable material. The foreign body response was consistent with suture material, as evidenced by the regular pattern and minimum associated inflammation. Pseudo-capsule formation was generally seen on the side of the implant facing the expander, which occurred as a benign response to the expander material. The opposite interface between the implant and the host tissue exhibited some tissue integration and minimal inflammation, which was consistent with normal healing. The implant material looked more organized with less vasculature and fewer living cells compared to host tissue. These findings are consistent for a stable material being slowly incorporated and remodeled after a few weeks to a few months after surgery when biopsy samples were taken.
Fig. 6.
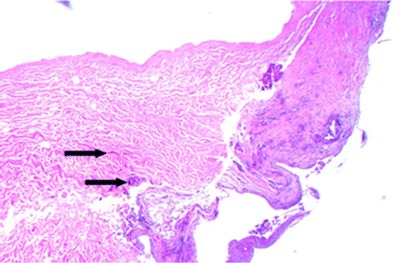
Hematoxylin and eosin staining of biopsy from patient #1 following 16 weeks in situ placement of acellular dermal matrix. Note the intact ultrastructure and also evidence of cellular in-growth as apparent fibroblasts (arrows) at ×10 magnification
Fig. 7.
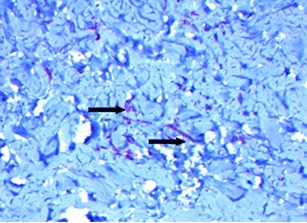
CD34 staining of biopsy from patient #1 following 16 weeks in situ placement of acellular dermal matrix. Evidence of robust vascularization is noted by reddish-brown stains apparently associated with blood vessels (arrows) at ×10 magnification
Fig. 8.
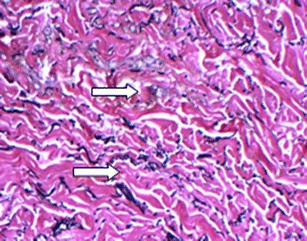
Verhoef-VanGeisen staining of biopsy from patient #1 following 16 weeks in situ placement of acellular dermal matrix. Note abundance of elastin (arrows) in this ×10 magnification
Discussion
Overall, excellent results were noted with DermACELL in two-stage breast reconstruction. This consecutive cohort series did not exclude challenging patients. Most complications were isolated to two compromised patients. The most complications were observed in the patient who was an occasional smoker. This was not unexpected as Anthony et al. [29] noted a trend towards significance in their study on the incidence of complications in breast reconstruction using acellular dermal matrices. Padubidri et al. [30] demonstrated a significant complication rate for smokers in their retrospective study of hundreds of breast reconstruction patients. Other studies have also noted an increased incidence of complications of patients who smoke after post-mastectomy breast reconstructions [31, 32].
In addition to the immediate advantages of providing structural support, the ultimate goal of using ADM’s is to enable integration of the matrix into the host tissue. This integration can be quantified by examining the revascularization and recellularization of the ADM. The two blinded dermatopathologists, who assessed DermACELL samples, identified numbers of blood vessels and fibroblasts that support incorporation into the host cell.
A similar case series [33] was recently published where DermACELL was used for two-stage breast reconstructions in 10 breast cancer patients. After excluding one patient for tobacco use, the author found comparable results with 14 out of 17 breasts successfully reconstructed to the second stage implant sequence. There were few complications reported, including the noteworthy absence of red breast syndrome in all patients, and most postoperative complications occurred in a patient who relapsed into tobacco use. Histological analysis of biopsy samples taken of the ADM from eight patients indicated incorporation with minimal inflammation and normal healing, consistent with our findings.
Another study [34] using in vivo rat models compared several different ADM’s, including DermACELL. The results of the study of Capito et al. support our findings. At day 7 postoperative, the DermACELL matrices had nearly twice the number of blood vessels as the next highest ADM. In regards to recellularization, it was found that DermACELL had the statistically significant highest amount of cell density at days 7, 14, and 21 postoperative as well as the greatest amount of cell infiltration.
The success reported here with DermACELL may be due to the processing and sterility of the material. The process involves the use of a solution of non-denaturing anionic detergent (N-Laurol sarcosinate), recombinant endonuclease (Benzonase® (Merck KGaA, Darmstadt, Germany), and antibiotics (Polymixin B, Vancomycin, and Lincomycin), resulting in a material that exhibits at least 97 % nucleic acid removal, while maintaining biomechanical strength. DermACELL allografts are preserved in glycerol, stored at room temperature, and are ready to use without needing to be rehydrated [35]. The allografts are terminally sterilized at ultra-low temperatures to achieve a 10−6 sterility assurance level [35, 36]. The temperature of irradiation has proved to be important because sterilizing grafts at very low temperatures minimizes free radicals and eventual tissue damage [37, 38]. In addition to the temperature at which irradiation occurs, the solutions used to clean the human tissue can determine the quality of the allograft. When harsh chemicals such as acetone, sodium hydroxide, and hydrogen peroxide are used, they can greatly damage the soft tissue [39]. Many factors are involved in the processing of a human tissue allograft with a sterility assurance level of 10−6. An allograft with a sterility assurance level of 10−6 is equivalent to the level of sterility provided with implantable medical devices, and the use of such sterile allografts could result in a lower rate of infection than when using autografts obtained from a second procedural site [40, 41]. By using low doses of gamma irradiation at very low temperatures, this process is able to ensure a high degree of safety without compromising biological and biomechanical properties [42–46]. The decellularization process used to prepare DermACELL is designed to remove cellular elements while retaining matrix structure, theoretically yielding a material with reduced antigenicity, increased biocompatibility, and subsequently reduced complications.
AlloDerm® (LifeCell Corporation, Bridgewater, NJ, USA), a decellularized human dermal matrix, is aseptically processed and freeze-dried. It is treated with a buffered salt solution to separate and eliminate the epidermis, then washed with a series of mild non-denaturing detergent solutions to solubilize and eliminate all cells [47]. AlloDerm undergoes a sterilization process that includes electron beam irradiation, which provides for a sterility assurance level of 10−3. FlexHD® (Musculoskeletal Transplant Foundation, Edison, New Jersey, USA) is human allograft skin that is minimally processed to remove epidermal and dermal cells, a process that maintains the integrity of the matrix. It is packaged in an ethanol solution and must be soaked in a sterile solution before implantation. The allograft tissue complies with the requirements of United States Pharmacopeia (USP) <71> Sterility tests but is not terminally sterilized, and thus claims no level of sterility assurance [48].
While all of these products are acellular dermal matrices, there are many differences between the three, including the degree of sterilization. DermACELL maintains a sterility assurance level of 10−6, which assures that no more than one in a million implants could potentially be infected with microorganisms. In comparison, AlloDerm is only aseptic with a sterility assurance level of 10−3 and, in theory, about one in one thousand implants could potentially be infected. FlexHD is not terminally sterilized, but meets the requirements of USP <71> Sterility tests.
The same sterility standards are not followed by all organizations that supply bone and tissue allografts. Since there is no standard definition of sterile, there can be clinical concern and confusion regarding infection [49]. Contaminated allografts can cause significant complications or even death in patients who receive the infected tissue [50]. In addition to compromising the health of patients, surgical site infections due to contaminated allografts can place a notable financial burden on patients, hospitals, and health care providers. As determined by the Centers for Medicare and Medicaid Services (CMS), surgical site infections are considered to be preventable complications and hospitals will not be reimbursed for the care of patients suffering from such complications [51]. In 2005, a study performed in Pennsylvania reported that the average charge for patients that developed a hospital infection ($173,206) was about four times as high as the charge for patients with the same diagnosis who did not contract an infection ($44,367) [52]. Therefore, allograft-associated infections place additional financial burden on patients and have a significant economic impact on hospitals. Rigorous screening, in combination with stringent aseptic tissue processing followed by terminal sterilization, can help eliminate allograft-associated infections [48].
Decellularization of human allograft tissue performed during tissue processing results in a material devoid of immunogenic components, theoretically allowing for improved graft biocompatibility, incorporation, and healing. A key measure of the effectiveness of decellularization is removal of DNA. The decellularization process used in the treatment of DermACELL results in at least a 97 % reduction in the DNA content of the tissue, reduced, in one analysis, to approximately 15.97 ng/mg dry weight. The residual DNA value for AlloDerm is much higher at 272.8 ng/mg dry weight [36].
The presence of erythema of mastectomy skin flaps following matrix implant, also known as “red breast syndrome,” has been reported when using either human or xenograft matrices [20, 53, 54]. While not fully understood, it is suggested that this may be an inflammatory response to the implanted matrix or preservatives [20, 53]. It is of special interest to note that there were no cases of red breast syndrome in this case series using DermACELL. While no generalizable conclusions can be reached from this small patient population, the processing of DermACELL ADM resulting in significant reduction in DNA and cellular content may result in the absence of erythema.
To compare the effectiveness of different acellular dermal matrices, histological analysis was performed on biopsies taken as part of this cohort and from additional two-stage breast reconstruction patients, also treated by this study author. One patient had received AlloDerm, while FlexHD was used in the second patient. At 6 weeks postoperative, DermACELL tissue was infiltrated with fibroblasts and blood vessels (Fig. 9). The revascularization of the DermACELL tissue indicates that incorporation has occurred. In comparison, the AlloDerm tissue biopsy (Fig. 10) did not have nearly as many blood vessels and fibroblasts as the DermACELL tissue. A postoperative biopsy of FlexHD also showed a lack of revascularization (Fig. 11) in our study at the same time point. Further studies are needed for direct comparisons between DermACELL and other ADMs.
Fig. 9.
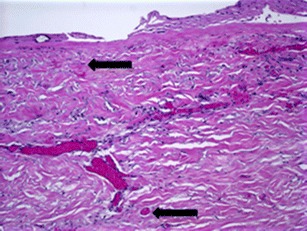
Six-week postoperative biopsy for DermACELL. The upper arrow identifies a fibroblast and the lower arrow identifies a blood vessel
Fig. 10.
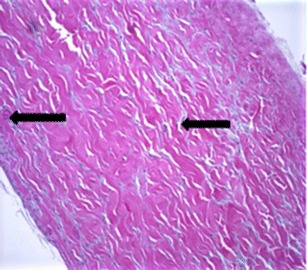
Postoperative biopsy for AlloDerm. The arrow on the left identifies a blood vessel and the arrow on the right identifies a fibroblast
Fig. 11.
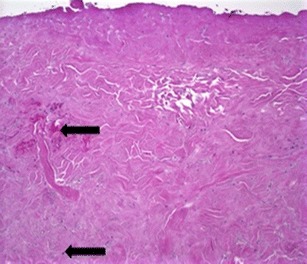
Postoperative biopsy for FlexHD. The upper arrow identifies a hematoma and the lower arrow identifies a fibroblast
It is important for successful breast reconstructions utilizing acellular dermal matrices to facilitate the incorporation of the host tissue into the allograft material. Acellular dermal matrices are able to support larger and faster tissue expansion, reduce capsular contracture, and decrease the rate of revision [55–58]. The ability of DermACELL to incorporate into host tissue with good revascularization and recellularization could provide for quicker breast reconstructions with fewer complications.
Conclusion
DermACELL appears to be an appropriate adjunct to reconstruction with expanders. Overall, patients that experienced the most complications postoperatively were those with unhealthy lifestyle behaviors, most notably smoking. The results of our case series suggest that DermACELL is at least comparable to other ADMs, within the limitations of the small cohort size. DermACELL’s advanced processing may also reduce complications that lead to reconstruction failure. Histologic analysis reveals early integration at 6 weeks post-implantation.
Acknowledgments
Conflict of Interest
None
Ethical standards
All patients gave informed consent for inclusion in this study. This study meets the 1964 Declaration of Helsinki in protecting human subjects.
References
- 1.American Society of Plastic Surgery (2011) Reconstructive plastic surgery statistics. Available at: www.plasticsurgery.org. Accessed 9 Sept 2013
- 2.Macadam SA, Lennox PA. Acellular dermal matrices: use in reconstructive and aesthetic breast surgery. Can J Plast Surg. 2012;20:75–89. doi: 10.1177/229255031202000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho G, Nguyen TJ, Shahabi A, Hwang BH, Chan LS, Wong AK. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg. 2012;68:346–356. doi: 10.1097/SAP.0b013e31823f3cd9. [DOI] [PubMed] [Google Scholar]
- 4.Breuing KH, Colwell AS. Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg. 2007;59:250–255. doi: 10.1097/SAP.0b013e31802f8426. [DOI] [PubMed] [Google Scholar]
- 5.Chun YS, Verma K, Rosen H, Lipsitz S, Morris D, Kenney P, Eriksson E. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125:429–436. doi: 10.1097/PRS.0b013e3181c82d90. [DOI] [PubMed] [Google Scholar]
- 6.Becker S, Saint-Cyr M, Wong C, Dauwe P, Nagarkar P, Thornton J, Peng Y. AlloDerm versus DermaMatrix in immediate expander-based breast reconstruction: a preliminary comparison of complication profiles and material compliance. Plast Reconstr Surg. 2008;123:1–6. doi: 10.1097/PRS.0b013e3181904bff. [DOI] [PubMed] [Google Scholar]
- 7.Salzberg A. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm) Ann Plast Surg. 2006;57:1–5. doi: 10.1097/01.sap.0000214873.13102.9f. [DOI] [PubMed] [Google Scholar]
- 8.Wainwright DJ, Bury SB. Acellular dermal matrix in the management of the burn patient. Aesthet Surg J. 2011;31:13s–23s. doi: 10.1177/1090820X11418202. [DOI] [PubMed] [Google Scholar]
- 9.Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19:104–109. doi: 10.1016/j.jse.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Snyder S, Bone J. Technique for arthroscopic replacement of severely damaged rotator cuff using “GraftJacket” allograft. Oper Tech Sports Med. 2007;15:86–94. doi: 10.1053/j.otsm.2006.07.006. [DOI] [Google Scholar]
- 11.Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24:20–24. doi: 10.1016/j.arthro.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Burkhead WZ, Schiffern SC, Krishnan SG. Use of GraftJacket as an augmentation for massive rotator cuff tears. Semin Arthroplast. 2007;18:11–18. doi: 10.1053/j.sart.2006.11.017. [DOI] [Google Scholar]
- 13.Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a Graft-Jacket allograft: technique and preliminary results. Arthroscopy. 2008;24:403–409. doi: 10.1016/j.arthro.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 14.Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular human dermal allograft matrix. Int J Shoulder Surg. 2007;1:7–15. doi: 10.4103/0973-6042.30673. [DOI] [Google Scholar]
- 15.Wilkins R. Acellular dermal graft augmentation in quadriceps tendon rupture repair. Curr Orthop Pract. 2010;21:315–319. doi: 10.1097/BCO.0b013e3181c7ea2e. [DOI] [Google Scholar]
- 16.Lee D. Achilles tendon repair with acellular tissue graft augmentation in neglected ruptures. J Foot Ankle Surg. 2007;46:451–455. doi: 10.1053/j.jfas.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Lee D. A preliminary study on the effects of acellular tissue graft augmentation in acute Achilles tendon ruptures. J Foot Ankle Surg. 2008;47:8–12. doi: 10.1053/j.jfas.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Barber F, Herbert M, Coons D. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22:534–538. doi: 10.1016/j.arthro.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Sbitany H, Sandeen S, Amalfi A, Davenport M, Langstein H. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg. 2009;124:1735–1740. doi: 10.1097/PRS.0b013e3181bf803d. [DOI] [PubMed] [Google Scholar]
- 20.Nahabedian M. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg. 2009;124:1743–1753. doi: 10.1097/PRS.0b013e3181bf8087. [DOI] [PubMed] [Google Scholar]
- 21.Kapfer S, Keshen T. The use of human acellular dermis in the operative management of giant omphalocele. J Pediatr Surg. 2006;41:216–220. doi: 10.1016/j.jpedsurg.2005.10.093. [DOI] [PubMed] [Google Scholar]
- 22.Albo D, Awad S, Berger D, Bellows C. Decellularized human cadaveric dermis provides a safe alternative for primary inguinal hernia repair in contaminated surgical fields. Am J Surg. 2006;192:12–17. doi: 10.1016/j.amjsurg.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 23.Candage R, Jones K, Luchette F, Sinacore JM, Vandevender D, Reed RL., 2nd Use of human acellular dermal matrix for hernia repair: friend or foe? Surgery. 2008;144:703–711. doi: 10.1016/j.surg.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell C, Clma R. A novel technique for the repair of urostomal hernias using human acellular dermal matrix. Urology. 2011;77:746–750. doi: 10.1016/j.urology.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Winters C, Brigido S, Liden B, Simmons M, Hartman JF, Wright ML. A multicenter study involving the use of a human acellular dermal regenerative tissue matrix for the treatment of diabetic lower extremity wounds. Adv Skin Wound Care. 2008;21:375–381. doi: 10.1097/01.ASW.0000323532.98003.26. [DOI] [PubMed] [Google Scholar]
- 26.Randall K, Booth B, Miller A, Russel C, Laughlin R. Use of an acellular regenerative tissue matrix in combination with vacuum-assisted closure therapy for treatment of a diabetic foot wound. J Foot Ankle Surg. 2008;47:430–433. doi: 10.1053/j.jfas.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Brigido SA, Boc SF, Lopez RC. Effective management of major lower extremity wounds using an acellular regenerative tissue. Orthopedics. 2004;27:s145–s149. doi: 10.3928/0147-7447-20040102-14. [DOI] [PubMed] [Google Scholar]
- 28.US Patents 7,338,757; 6,743,574; 6,734,018
- 29.Antony A, McCarthy C, Cordeiro P, Mehrara BJ, Pusic AL, Teo EH, Arriaga AF, Disa JJ. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg. 2010;125:1606–1614. doi: 10.1097/PRS.0b013e3181d4fb2a. [DOI] [PubMed] [Google Scholar]
- 30.Padubidri AN, Yetman R, Browne E, Lucas A, Papay F, Larive B, Zins J. Complications of postmastectomy breast reconstructions in smokers, ex-smokers, and nonsmokers. Plast Reconstr Surg. 2001;107:342–349. doi: 10.1097/00006534-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Goodwin SJ, McCarthy CM, Pusic AL, Bui D, Howard M, Disa JJ, Cordeiro PG, Mehrara BJ. Complications in smokers after postmastectomy tissue expander/implant breast reconstruction. Ann Plast Surg. 2005;55:16–19. doi: 10.1097/01.sap.0000168282.81348.b3. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy CM, Mehrara BJ, Riedel E, Davidge K, Hinson A, Disa JJ, Cordeiro PG, Pusic AL. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121:1886–1892. doi: 10.1097/PRS.0b013e31817151c4. [DOI] [PubMed] [Google Scholar]
- 33.Vashi C. Clinical outcomes for breast cancer patients undergoing mastectomy and reconstruction with use of DermACELL, a sterile, room temperature acellular dermal matrix. Plast Surg Int. 2014;2014:1–7. doi: 10.1155/2014/704323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capito AE, Tholpady SS, Agrawal H, Drake D, Katz A. Evaluation of host tissue integration, revascularization, and cellular infiltration within various dermal substrates. Ann Plast Surg. 2012;68:495–500. doi: 10.1097/SAP.0b013e31823b6b01. [DOI] [PubMed] [Google Scholar]
- 35.Chen SG, Tzeng YS, Wang CH. Treatment of severe burn with DermACELL, an acellular dermal matrix. Int J Burns Trauma. 2012;2:105–109. [PMC free article] [PubMed] [Google Scholar]
- 36.Vangsness CT, Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31:474–481. doi: 10.1177/03635465030310032701. [DOI] [PubMed] [Google Scholar]
- 37.Anderson MJ, Keyak JH, Skinner HB. Compressive mechanical properties of human cancellous bone after gamma irradiation. J Bone Joint Surg Am. 1992;74:747–752. [PubMed] [Google Scholar]
- 38.Hamer AJ, Stockley I, Elson RA. Changes in allograft bone irradiated at different temperatures. J Bone Joint Surg (Br) 1999;81:342–344. doi: 10.1302/0301-620X.81B2.9083. [DOI] [PubMed] [Google Scholar]
- 39.Samsell BJ, Moore MA. Use of controlled low dose gamma irradiation to sterilize allograft tendons for ACL reconstruction: biomechanical and clinical perspective. Cell Tissue Bank. 2012;13:217–223. doi: 10.1007/s10561-011-9251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bryans T, Darnell K, Hitchins V, Lappalainen S, Liu D, Masefield J (2010) Sterility assurance levels for terminal sterilization of new/emerging healthcare products. Paper presented at: US Centers for Disease Control and Prevention (CDC) Decennial Meeting; March 18-22, 2010; Atlanta, GA
- 41.Katz L, Battaglia T, Patino P, Reichmann W, Hunter DJ, Richmond JC. A retrospective comparison of the incidence of bacterial infection following anterior cruciate ligament reconstruction with autograft versus allograft. Arthroscopy. 2008;24:1330–1335. doi: 10.1016/j.arthro.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Balsly C, Cotter A, Williams L, Gaskins BD, Moore MA, Wolfinbarger L. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9:289–298. doi: 10.1007/s10561-008-9069-0. [DOI] [PubMed] [Google Scholar]
- 43.McGilvray KC, Santoni BG, Turner AS, Bogdansky S, Wheeler DL, Puttlitz CM. Effects of (60)Co gamma irradiation dose on the initial structural biomechanical properties of ovine bone-patellar tendon-bone allografts. Cell Tissue Bank. 2011;12:89–98. doi: 10.1007/s10561-010-9170-z. [DOI] [PubMed] [Google Scholar]
- 44.Greaves L, Hecker A, Brown C. The effect of donor age and low-dose gamma irradiation on the initial biomechanical properties of human tibialis tendon allografts. Am J Sports Med. 2008;36:1358–1366. doi: 10.1177/0363546508314394. [DOI] [PubMed] [Google Scholar]
- 45.Goertzen M, Clahsen H, Burrig K, Schulitz K. Sterilization of canine anterior cruciate allografts by gamma irradiation in argon. J Bone Joint Surg (Br) 1995;77:205–212. [PubMed] [Google Scholar]
- 46.Rihn JA, Irrgang JJ, Chhabra A, Fu F, Harner C. Does irradiation affect the clinical outcome of patellar tendon allograft ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2006;14:885–896. doi: 10.1007/s00167-006-0036-7. [DOI] [PubMed] [Google Scholar]
- 47.LifeCell Corporation (2011) AlloDerm instructions for use. Available at: http://www.lifecell.com/fileadmin/media/files/downloads/Alloderm_IFU_D.pdf. Accessed 11 Jul 2013
- 48.Musculoskeletal Transplant Foundation (2012) Read before using, FlexHD and FlexHD Diamond Acellular Dermis. Available at: http://www.mtf.org/documents/PI_-31_Rev_12_.pdf. Accessed 11 Jul 2013
- 49.Micarelli A, Moore M, Peycelon F. Variations in allograft sterility levels: understanding the clinical and economic implication. Virginia Beach: LifeNet Health; 2008. [Google Scholar]
- 50.Kainer MA, Linden JV, Whaley DN, Holmes HT, Jarvis WR, Jernigan DB, Archibald LK. Clostridium infections associated with musculoskeletal-tissue allografts. N Engl J Med. 2004;350:2564–2571. doi: 10.1056/NEJMoa023222. [DOI] [PubMed] [Google Scholar]
- 51.Rosenthal M. Nonpayment for performance? Medicare’s new reimbursement rule. N Engl J Med. 2007;357:1573–1575. doi: 10.1056/NEJMp078184. [DOI] [PubMed] [Google Scholar]
- 52.Peng MM, Kurt S, Johannes RS. Adverse outcomes from hospital-acquired infection in Pennsylvania cannot be attributed to increased risk on admission. Am J Med Qual. 2006;21:17s–28s. doi: 10.1177/1062860606294632. [DOI] [PubMed] [Google Scholar]
- 53.Nahabedian M. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation (Letter) Plast Reconstr Surg. 2010;126:1120–1121. doi: 10.1097/PRS.0b013e3181e3b7bb. [DOI] [PubMed] [Google Scholar]
- 54.Newman MI, Hanabergh E, Samson MC. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation (Letter) Plast Reconstr Surg. 2010;126:1120. doi: 10.1097/PRS.0b013e3181e60652. [DOI] [PubMed] [Google Scholar]
- 55.Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg. 2007;120:373–381. doi: 10.1097/01.prs.0000267340.31742.1. [DOI] [PubMed] [Google Scholar]
- 56.Bindingnavele V, Gaon M, Ota KS, Kulber D, Lee D. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg. 2007;60:1214–1218. doi: 10.1016/j.bjps.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 57.Namnoum JD. Expander/implant reconstruction with AlloDerm: recent experience. Plast Reconstr Surg. 2009;124:387–394. doi: 10.1097/PRS.0b013e3181aee95b. [DOI] [PubMed] [Google Scholar]
- 58.Topol BM, Dalton EF, Ponn T, Campbell C. Immediate single-stage breast reconstruction using implants and human acellular dermal tissue matrix with adjustment of the lower pole of the breast to reduce unwanted lift. Ann Plast Surg. 2008;6:494–499. doi: 10.1097/SAP.0b013e31816d82d9. [DOI] [PubMed] [Google Scholar]


