Abstract
This review covers the literature on the chemically mediated ecology of cyanobacteria, including ultraviolet radiation protection, feeding-deterrence, allelopathy, resource competition, and signalling. To highlight the chemical and biological diversity of this group of organisms, evolutionary and chemotaxonomical studies are presented. Several technologically relevant aspects of cyanobacterial chemical ecology are also discussed.
1. Introduction
Cyanobacteria are found in almost every environment on Earth. However, historically, research on these organisms has been, for the most part, carried out by groups that work almost exclusively with either freshwater (and terrestrial) or marine strains. This is reflected in both the primary and secondary literature. A number of recent reviews have been dedicated entirely or partially to cyanobacterial chemical ecology, usually focusing on a specific type of chemo-ecological interaction or on a particular habitat type. For example, recent reviews have appeared on the chemical ecology of marine plankton1 and the benthos.2 Singh et al.3 have reviewed chemical and biological aspects of radiation protection in cyanobacteria. Allelopathic interactions, including those perpetrated by cyanobacteria, have been reviewed under slightly different perspectives.4-7 Broader-scope reviews on aquatic chemical ecological processes that have relevance for cyanobacteria were conveyed by Pohnert8 and Hay.9 Tidgewell et al.10 and Jones et al.11, 12 provide important insights into cyanobacterial natural products diversity and diversification, a key concept underlying the chemical ecology of these organisms.
Here, we review the current state of knowledge on cyanobacterial chemical ecology, regardless of the environment in which such interactions occur. The divergent treatment of freshwater and marine cyanobacteria is, of course, unavoidable and implicitly present throughout the review. Nevertheless, we find that it is useful to feature examples from both habitats (whenever possible) that result in roughly the same ecological function or purpose. We have tried to emphasize the rationale behind the discovery of the secondary metabolites covered in this review, their structural features, and, for a few more recent examples, provide some detail on their biosynthesis. We then present, in a short section, examples of evolutionary studies of selected cyanobacterial natural products followed by a discussion on the relationship between biodiversity and chemodiversity, emphasizing recent insights into the taxonomy of these organisms. A final section showcases some examples of how fundamental chemoecological findings may find application in biotechnology.
2. Ecological roles played by cyanobacterial metabolites
2.1. UV-protection
Ultra-violet radiation (UVR) is a high-energy fraction of the solar spectrum that causes detrimental effects on living systems. In particular, it can damage DNA and proteins directly,13 or indirectly through production of reactive oxygen species (ROS).14 Deleterious effects of UVR in cyanobacteria include, apart from DNA and protein damage, and production of ROS, photosynthesis inhibition, delayed cell differentiation, motility impairment, lower growth rates and decreased survivability.3 Cyanobacteria were probably the first oxygen-evolving photosynthetic organisms to have appeared on Earth, with contrasting evidence placing such an event from 2.7 to 3.5 billion years ago.15, 16 Since these photoautotrophic microorganisms are thought to be responsible for the present oxygenic atmosphere, and the ozone layer,17 their evolutionary history encompasses dramatic differences in terms of the amount of solar radiation reaching the Earth's surface. It is, therefore, perhaps unsurprising that cyanobacteria are capable of synthesizing a diverse number of metabolites that confer protection from UVR, usually termed as ‘cyanobacterial sunscreens’. Other UVR-related defence mechanisms, such as avoidance behaviour, antioxidant response (enzymatic or non-enzymatic) and cellular repair mechanisms are also known to occur among cyanobacteria.3 In the context of the present review, however, cyanobacterial secondary metabolites that confer photoprotection, especially to UVR long-term exposure, by absorbing/screening harmful UVR, deserve special attention. While cyanobacteria produce several UV-absorbing metabolites, “cyanobacterial sunscreen” is usually employed when referring to mycosporines and mycosporine-like amino acids (MAAs) or scytonemin, for which a natural role in photoprotection has been clearly established.3
2.1.1 Mycosporines and MAAs
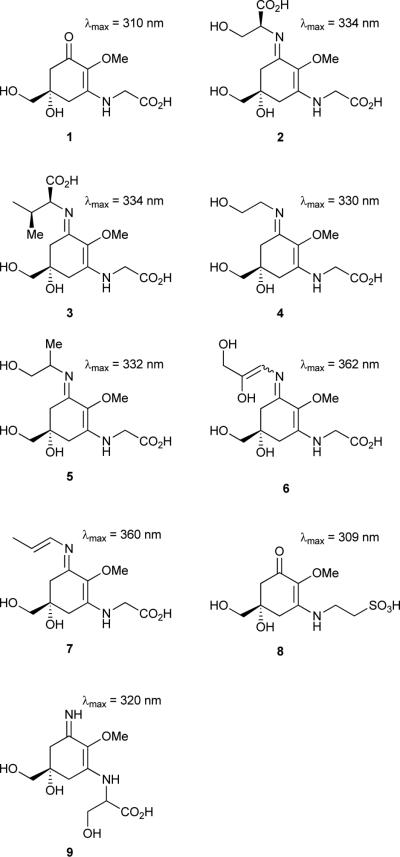
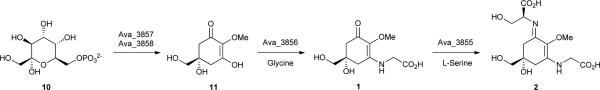
Mycosporines and MAAs are small (< 400 Da) aminocyclohexenone or aminocyclohexenimine derivatives, respectively, with λmax ranging from 310 to 362 nm. Usually the ring structure is modified by one (mycosporines) or two amino acids (MAAs), which may or may not be of proteinogenic origin. Several organisms, such as bacteria, cyanobacteria, eukaryotic phytoplankton and macroalgae are able to synthesize these compounds.18 Members from different genera of benthic and planktonic cyanobacteria, from marine and freshwater habitats are known to produce both mycosporines and MAAs,19 including mycosporine glycine (1), shinorine (2),20 porphyra-334 (3),21 asterina-330 (4), palythinol (5),20 euhalothece-362 (6)22, palythene (7),23 mycosporine-tau (8),24 and palythine serine (9)25. Sugar-linked MAAs have also been reported from cyanobacteria.26
The biosynthesis of mycosporine and MAAs was thought to proceed via the shikimate pathway intermediate 3-dehydroquinate to produce the gadusols prior to the addition of the amino acid substituents.18 However, Balskus and Walsh27 have very recently demonstrated that, at least in cyanobacteria, the starting material for mycosporine and MAAs biosynthesis is actually sedoheptulose 7-phosphate (10), a pentose-phosphate pathway intermediate. By means of genome-mining, heterologous expression, as well as feeding studies, the same study provided evidence for a four-enzyme biosynthetic pathway responsible for the production of the MAA 2 in the cyanobacterium Anabaena variabilis ATCC 29413.27 Two of the enzymes (one dehydroquinate synthase homologue, Ava_3858, and one O-methyltransferase, Ava_3857) are involved in the conversion of 10 to 4-deoxygadusol (11). Glycine and then serine are added to this intermediate, through the action of two enzymes, Ava_3856 (ATP-grasp homologue) and Ava_3855 (NRPS-like). These enzymes catalyse the installation of the amino acid substituents via imine linkages, but by distinct biochemical mechanisms.27 The genes encoding for Ava_3858 and Ava_3857 had previously been implicated in MAA biosynthesis,28 and were shown to have been horizontally transferred from cyanobacteria to dinoflagellates28, and then to metazoans.28, 29 Regarding the ecophysiological properties of this group of sunscreen molecules, they can reach high concentrations in the producing cyanobacterial cells (often above 1% of dry weight),30 and owing to their high molar absorptivities (up to 50,000 M−1 cm−1),18 cells can be effectively protected from UVR-damage. As an example, Garcia-Pichel et al.31 have estimated a sunscreen factor of 0.3 for MAAs in the cyanobacterium Gloeocapsa sp., indicating that 30% of the photons were prevented from reaching cytoplasmic targets. Other ecologically relevant findings include the increase in biosynthesis of particular MAAs by exposure to UVR,21, 24, 31, 32 and the photostability of MAA compounds under different conditions.33, 34 Interestingly, recent findings suggest that MAAs and scytonemin (12) act in a complementary fashion in the cyanobacterium Nostoc flagelliforme to provide UVR protection in both the UV-A and UV-B wavelength ranges (280-400 nm).30 The authors of the study suggest that this complementarity might be responsible for the UV-insensitivity exhibited by this cyanobacterium.30 Aside from UVR-protection, mycosporine and MAAs have also been implicated in other biological functions and considered to be “multipurpose secondary metabolites”.35 There is evidence that MAAs are involved in oxidative stress responses as antioxidants,36, 37 osmotic regulation23 and iron scavenging,22 and their synthesis has been shown to be upregulated by different abiotic stress conditions.25, 38
2.1.2 Scytonemin
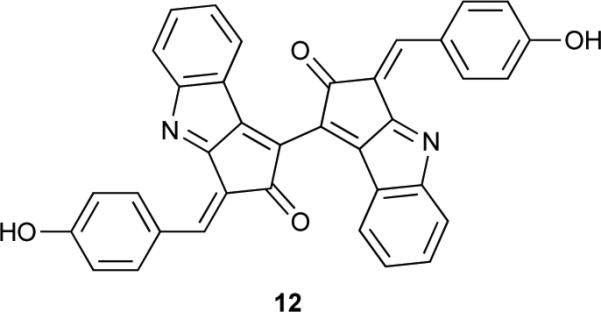
While the previously described sunscreens are found inside of cyanobacterial cells, the extracellular pigment scytonemin (12) accumulates in the mucilaginous sheath that many filamentous or unicellular cyanobacteria secrete.39 It is a dimeric alkaloid of 544 Da, composed of indolic and phenolic moieties. Its structure was determined in 1993,40 despite the existence of such a yellow-brown pigment having been reported more than 150 years ago.41 Its role as a sunscreen was established following studies in Chlorogloeopsis sp. in which the pigment was directly associated with reduced sensitivity to UVR damage and also shown to passively protect physiologically inactive (dried) cells.42 Metabolite 12 confers photoprotection to producing cells by strongly absorbing UVR in the 325-425 nm range (UVA, in vivo λmax = 370 nm), and, additionally, in the UVB and UVC spectral ranges.40 Thus, it serves as an effective measure to prevent harmful UVR from reaching cyanobacterial cells. In fact, it is estimated that approximately 90% of UVA radiation is filtered out by 12.42
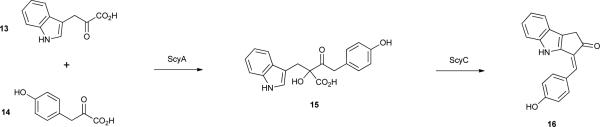
The gene cluster responsible for the biosynthesis of 12 was identified in Nostoc punctiforme ATCC 29133, following analyses of scytonemin-deficient mutants, as comprising 18 genes all transcribed in the same direction43. Some of these genes had no ascribed function, while others encoded proteins involved in amino acid biosynthesis, in particular that of tryptophan and tyrosine.43 Further studies have laid out the biochemical basis for key biosynthetic steps leading to metabolite 12.44, 45 Tryptophan serves as a building block, being converted to the corresponding pyruvic acid 13 by a leucine dehydrogenase homologue, ScyB.44 However, p-hydroxyphenylpyruvic acid (14) seems to be generated directly from prephenate (rather than tyrosine) by the prephenate dehydrogenase TyrA, also encoded in the biosynthetic pathway.44 ScyA, a homologue to thiamine diphosphate-dependent enzymes, is responsible for the acyloin coupling of both pyruvic acids, yielding 15.44 The product of one of the cluster genes with previously unknown function, ScyC, catalyses an elegant cyclization of 15 via an intramolecular reaction, followed by decarboxylation, yielding the monomer 16, a precursor which should have a propensity for oxidative dimerization to afford 12.45 Feeding studies using isotopically labeled tryptophan and tyrosine in Tolypothrix distorta46 provided in vivo evidence for both amino acids acting as substrates in the biosynthetic pathway, as well as for the decarboxylation of 13 and 14 during the biosynthesis of 12. Moreover, the data obtained suggested that enzymes involved in aromatic amino acid transformations, which are not present in the gene cluster, may also play a role in the biosynthesis of 12.46 Interestingly, two genes just outside of the initially proposed scytonemin gene cluster encode for a putative histidine kinase and a putative transcriptional regulator with a signal receiver domain.43 It has been proposed47, 48 that these configure a regulatory system controlling the transcription of the gene cluster. The 18 genes in the cluster have also been shown to be under transcriptional control from UVR, being upregulated with increased exposure to such radiation.47, 48 This is in agreement with the initial observations of the accumulation of 12 following UVR exposure.39, 42
2.2. Feeding deterrence
As primary producers, and key constituents of the phytoplankton and phytobenthos, cyanobacteria serve as food for a plethora of micro- and macro-organisms in both freshwater and marine ecosystems. As such, strategies to cope with foraging are an important theme in cyanobacterial chemical ecology. The ecotoxicity of cyanobacterial metabolites to aquatic organisms is an active field of research, and a variety of such compounds have been found to be highly toxic to potential foragers of cyanobacteria.49 Notwithstanding, assigning a natural role of defence against foraging to such compounds is not always straightforward, a classic example being the case of the microcystins. Several studies have reported the antifeedant and toxic properties of different microcystins to potential grazers.50-53 However, the finding that microcystin synthase genes evolved before the appearance of zooplankton54 has ruled out defence from foragers as the natural role for this group of metabolites, at least initially.
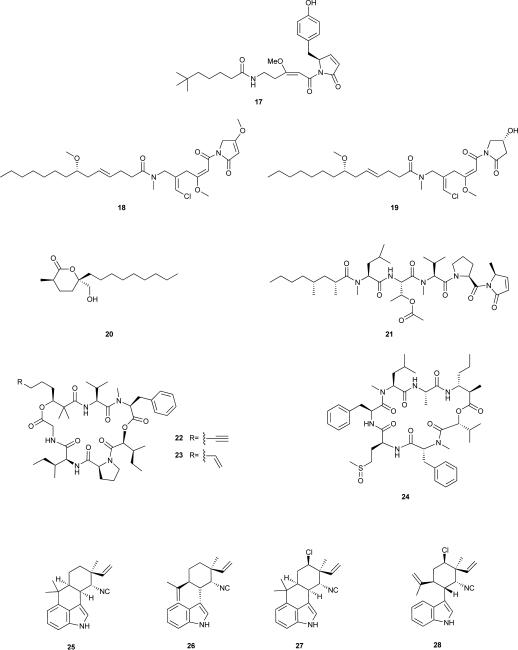
In fact, the overwhelming majority of work that has led to a hypothesized anti-predatory role for cyanobacterial natural products was conducted with tropical cyanobacteria of the genus Lyngbya. Different members of this genus display distinctly different palatabilites to predators and some actually deter feeding by natural foragers, a feature that has been frequently associated with secondary metabolite production.55, 56 A handful of Lyngbya metabolites have been isolated on the basis of such ecologically relevant activities. Ypaoamide (17) is one of such compound, isolated from a Guam L. majuscula strain.57 This cyanobacterium formed a bloom that was associated with mass mortalities of juvenile rabbitfish. Feeding deterrence toward the parrotfish Scarus schlegeli guided the isolation of 17,57 which was later found to correspond to the S-enantiomer.58 The pure metabolite, at ecologically relevant concentrations, deterred feeding by S. schlegeli, the urchin Echinometra mathaei and the rabbitfish species that motivated the study (Siganus argenteus and Siganus spinus).59 Other metabolites isolated from L. majuscula were also shown to influence the feeding behaviour of juvenile S. schlegeli and S. spinus.60 Malyngamides A (18) and B (19),61 as well as malyngolide (20),62 had low palatability to these fishes, under periodic and continuous feeding protocols.60 In fact, the low palatability of compounds 18 and 19 had previously been demonstrated experimentally with the pufferfish Canthigaster solandri and crabs from the genus Leptodius.63 The sea hare Stylocheilus longicauda was chemically deterred in its feeding activity by natural concentrations of 17, 20, and microcolin B (21),64 all produced by L. majuscula which is curiously a major part of the sea hare's diet.65 Pitipeptolides A (22) and B (23) are cyclic depsipeptides that were isolated as major constituents of another L. majuscula strain from Guam.66 Metabolite 22 was found to deter feeding by E. mathaei, Menaethius monocerus (crab), Parhyale hawaiensis and Cymadusa imbroglio (amphipods).67 A bloom-forming Lyngbya polychroa strain from Belize produces the methionine-sulfoxide containing cyclic depsipeptide carriebowmide (24).68 This metabolite was isolated from a lipophilic extract that had shown feeding deterrence activity toward a natural assemblage of Belizean coral reef fish. However, the low amount of pure 24 isolated was not enough to confirm whether this metabolite was responsible for the activity in the lipophilic extract.68
Overall, these studies emphasize, on one hand, the chemical proficiency of tropical Lyngbya species, and, on the other, the important role that limiting predation plays for these benthic cyanobacteria. Indeed, feeding deterrence may be a decisive factor allowing Lyngbya blooms to develop in tropical coastal waters.55, 67
The fact that several herbivorous insect larvae feed on cyanobacteria69 motivated a screening for cyanobacterial strains that exhibit toxicity toward grazing insects (chironomid larvae)70. This ultimately led to the identification of four hapalindoles [12-epi-hapalindole J isonitrile (25),71 12-epi-hapalindole C isonitrile (26),72 hapalindole L (27),73 and 12-epi-hapalindole E isonitrile (28)] as quite active insecticides (100% lethality at 48 h for compound 25 at 26 μM).71
A wealth of information is available concerning the toxicity of pure cyanobacterial secondary metabolites, as well as cellular extracts, toward ecologically relevant predators in freshwater, marine and brackish water environments.49, 74-76 Therefore, experimental approaches to investigate whether these substances act as feeding deterrents in their respective ecosystems are warranted.
2.3. Allelopathy
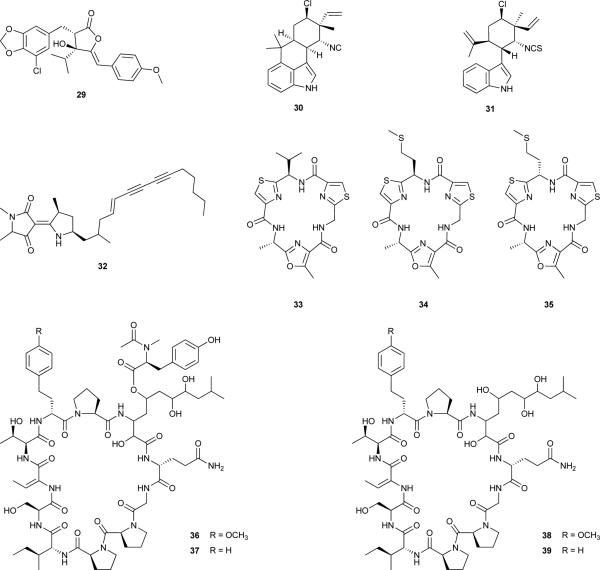
The allelopathic potential of cyanobacteria first became revealed by the work of Keating in the 1970's.77, 78 In a freshwater lake, the ability of cell-free filtrates from dominant cyanobacteria, as well as lake water, was demonstrated to induce positive or negative effects on the growth of other cyanobacteria and diatoms from the same lake. Furthermore, these studies showed that these allelopathic interactions were correlated to the succession of algal blooms in this lake. Many studies have since reported allelopathic properties of cyanobacteria, mostly in freshwater environments.4, 7, 79 Nonetheless, the majority of such evidence is drawn from co-culturing experiments, or from the exposure of target species to cell-free filtrates. Hence, information on which metabolites mediate such allelopathic interactions (allelochemicals) is scarce. The first cyanobacterial metabolite to be reported as an allelochemical was cyanobacterin (29), isolated from a freshwater Scytonema hofmanni strain.80-82 This γ-lactone contains a chlorine atom, a feature more commonly observed in marine metabolites where this element is more abundant. Cyanobacterin was isolated following laboratory observations that compounds were being released from S. hofmanni that were able to inhibit the growth of other photoautotrophs, both in liquid and solid media.80 Compound 29 was shown to target photosynthesis by specifically damaging thylakoid membranes83, 84 and inhibiting electron transport in photosystem II (PSII).83, 85 Several cyanobacteria, eukaryotic algae and angiosperms have been reported to be susceptible to this allelochemical.83-86
Based on growth inhibition of Anabaena spp. by extracellular compound(s) produced by cyanobacteria of the genus Hapalosiphon, Moore and co-workers isolated and characterized the allelochemical hapalindole A (30) from a terrestrial Hapalosiphon fontinalis.87 While several other hapalindoles were reported from this same source,73,87 the anti-cyanobacterial (and antimycotic) activities of the cyanobacterium were assigned mostly to the chlorine- and isontitrile-bearing alkaloid 30. Another member of the hapalindole family of natural products, the at-the-time known compound 12-epi-hapalindole E isonitrile (28),88 was isolated from Fischerella muscicula as a result of a screening program for allelopathic activity by cyanobacteria towards green algae and other cyanobacteria.89, 90 It was shown to have inhibitory activity towards a wide range of other organisms and cell lines.90 Further studies revealed that 28 interacts directly with the RNA polymerase, preventing RNA chain elongation in Bacillus subtilis.91 The related secondary metabolite 12-epi-hapalindole F isothiocyanate (31)72 was shown to inhibit the growth of cyanobacteria of the genera Microcystis and Synechococcus.92
Another screening effort93 tested the ability of cyanobacterial cells or cell-free filtrates to clear cyanobacterial lawns (of 30 target strains) on solid media. Strikingly, the cyanobacterium F. muscicula UTEX 1829 was found to produce an anticyanobacterial compound that was active toward all of the target strains.93 Subsequent studies led to the isolation and characterization of the allelochemical fischerellin A (32),94, 95 containing two heterocyclic rings and an enediyne moiety, and to the establishment of its function as a potent PSII inhibitor thereby affecting photosynthetic redox chemistry and O2 evolution.94-96 As in the case of 30, compound 32 is active toward cyanobacteria, green microalgae and higher plants,94-96 but does not inhibit heterotrophic bacterial growth94 nor photosynthesis in the purple bacterium Rhodospirillum rubrum.96
In the above mentioned screening study leading to the discovery of 32, the cyanobacterium Nostoc sp. 31 exhibited activity toward 29 out of the 30 target strains.93 A bioassay-guided chemical exploration of this strain allowed the isolation of the allelochemical responsible for the majority of such growth inhibitory activity, nostocyclamide (33), a cyclic peptide bearing two thiazole- and one oxazole moieties.97 A series of bioassays confirmed that 33 inhibited the growth of cyanobacteria, diatoms, and chlorophytes, but not that of the fungus Saccharomyces cerevisiae nor heterotrophic bacteria.97, 98 Interestingly, 33 was also moderately toxic to Brachionus calyciflorus. Since this freshwater rotifer feeds on cyanobacteria,99 compound 33 may actually serve more than one distinct ecological functions. The related metabolite, nostocyclamide M (34), was also present in the biomass of Nostoc sp. 31, and differs only from 33 in one amino acid residue (d-Met replacing d-Val).100 Due to its growth inhibitory activity towards cyanobacteria in agar diffusion assays, 34 was also reported to be an allelochemical.100 It is interesting to note the structural resemblance of these two metabolites with other natural products,100 in particular 34 with the epimeric substance tenuecyclamide C (35).101 Metabolite 35 was isolated from a soil Nostoc spongiaeforme strain, and displays antibacterial and toxic properties, but no clear ecological function was ascribed to this compound.101 However, it has recently been shown that 35 (and the other tenuecyclamides) are synthesized ribosomally, and not by NRPS machinery, and are thus members of the ‘cyanobactin’ group of cyanobacterial metabolites.102 It is thus likely that the nostocyclamides 33 and 34 are also of ribosomal origin, and underscores the metabolic plasticity involved in cyanobacterial chemical ecology.
More recently, a new family of compounds with allelopathic properties has been isolated from Oscillatoria sp. LEGE 05292 and named the portoamides A-D (36-39).103 This freshwater strain of Oscillatoria was identified as potentially allelopathic in a screening study during which cyanobacterial culture filtrates were used as growth media for eukaryotic microalgae.104 The extracellular products from Oscillatoria sp. LEGE 05292 inhibited the growth of Chlorella vulgaris, a ubiquitous green microalga. Moreover, it was shown that this was a cytostatic effect, as the exposed microalgae grew at normal rates when placed in regular growth medium.104 These findings motivated the bioassay-guided isolation and characterization of the portoamides, large cyclic depsipeptides containing several modified amino acids, including a β-amino acid and an N-Ac-N-Me-Tyr moiety; the latter is present in compounds 36 and 37 but not in 38 and 39.103 Compounds 36 and 37 were found to be the most abundant portoamides in the cyanobacterial biomass, while 38 and 39 were the major components in the culture medium, suggesting a specific role for the doubly modified Tyr residue in the exudation process. Furthermore, the mixture of depsipeptides 36 and 37 was found to be more active toward C. vulgaris than either a mixture of 38 and 39 or the individual pure compounds 36 or 37.103 As such, understanding the contribution of the portoamides to the allelopathic behaviour of Oscillatoria sp. LEGE 05292 requires further investigation. However, the synergistic mixture of 36 and 37 inhibited the growth of other phytoplanktonic organisms with IC50 values below 30 μg·mL−1.103 A subsequent study105 provided evidence for community wide effects of the allelochemicals produced by this cyanobacterium. A natural microbial community was exposed in a laboratory setting to a portoamides-containing fraction of the exudates from Oscillatoria sp. LEGE 05292. As a result of this exposure, the relative abundance of several bacteria (including cyanobacteria) and eukaryotes (including algae and zooplankton) was altered from a control situation. Strikingly, the Oscillatoria sp. LEGE 05292 exudates also influenced the population structure of a Microcystis sp. which dominated the community, and moreover, different Microcystis genotypes displayed distinctive sensitivities to the portoamides-containing exudates.105
The above studies highlight the role that screening approaches have had on the discovery of novel cyanobacterial allelochemicals. It can thus be expected that such experimental strategies will continue to reveal new chemical mediators of allelopathic interactions. At the same time, such molecules become tools that allow researchers to examine the ecological, biochemical and physiological aspects of allelopathy on both the producing and target organisms.
2.4. Resource competition
Cyanobacteria from different environments have developed various strategies by which to competitively acquire or more efficiently use available resources, which may be limiting. In certain cases, this is achieved by making use of secondary metabolites and these examples will be briefly discussed here. One of the most prominent examples of a chemically-mediated resource acquisition strategy is the production of siderophores, high-affinity ligands of metal ions, most notably iron (Fe). This element is scarce in most aquatic environments, in part due to the low solubility of Fe(III). Moreover, Fe is essential for a number of metabolic processes carried out by cyanobacteria, including photosynthesis and nitrogen fixation.106 The ability of freshwater and marine cyanobacteria to produce siderophores has been known since the 1970's,107, 108 however, only a few such compounds have been isolated and chemically characterized from this source.
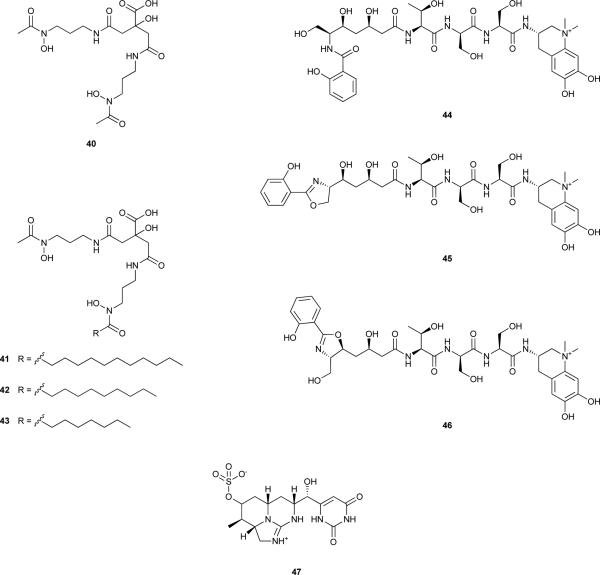
Schizokinen (40), a citrate-derived hydroxamate siderophore, first isolated from the bacterium Bacillus megaterium,109 was found to be produced and released to the culture medium by the freshwater cyanobacterium Anabaena sp. under Fe-limiting conditions.110 A regulated uptake system specific for schizokinen-bound Fe was shown to be present in Anabaena sp. ATCC 27898,111 and proof was provided that other Anabaena sp. strains were able to incorporate this ferric complex.112 Recently, an outer membrane protein, SchT, expressed by Anabaena sp. PCC 7120, was implicated in ferric 40 transport and incorporation by this cyanobacterium.113 Interestingly, this siderophore was also shown to bind copper and to considerably reduce its toxicity to Anabaena sp. PCC 7120 through lack of uptake.114 As such, the authors have suggested that this may be an additional ecological role of metabolite 40.114
Structurally related to 40, the synechobactins A (41), B (42) and C (43) were isolated from the marine cyanobacterium Synechococcus sp. PCC 7002, grown under Fe-limiting conditions.115 The fatty acid chains present in each of these hydroxamate siderophores is thought to anchor them to membranes, and may represent an evolved functionality when compared to 40. Additionally, 41 was found to be photoreactive, releasing a 46 amu fragment under fluorescent light, probably corresponding to the conversion of the citrate moiety to β-ketoglutarate with concomitant loss of two protons and CO2, but still binding Fe(III). This photoreactivity may also play a role in the Fe acquisition mechanism by Synechococcus sp. PCC 7002.115
The metabolites anachelin-H (44),116, 117 anachelin-1 (45) and anachelin-2 (46)118, 119 were isolated from two freshwater Anabaena cylindrica strains in independent research efforts, based on their Fe-binding properties116, 118 (metabolite 45 was reported in both studies, but its structure characterized only by Itou and coworkers118). These catecholate-bearing siderophores are structurally more complex than the citrate-derived hydroxamates presented above. An interesting aspect of anachelins is their hybrid biogenetic characteristics, as they feature peptide, polyketide and alkaloid fragments. It has been suggested that the natural bioactive metabolite is 44 and that 45 and 46 result as artefacts of the isolation process.120 Unfortunately, not much is known about the ecophysiological implications of any of the anachelins.
Phosphate (Pi), is also a scarce resource in many water bodies, particularly in freshwater. As photoautotrophs, cyanobacteria have an essential requirement for this inorganic nutrient. As such, cyanobacteria have developed strategies to cope with phosphorous-limiting conditions, such as intracellular polyphosphate storage, or the expression of alkaline phosphatases (APs) which hydrolyse a variety of phosphorous-containing organic compounds outside of the cellular membrane.121, 122 The activity of the APs generate Pi readily available for uptake by the AP-producing cells, but also contribute to the total dissolved Pi pool.121 Very recently, the well-studied cyanobacterial secondary metabolite cylindrospermopsin (47)123 has been associated with a new strategy to compete for Pi as perpetrated by Aphanizomenon ovalisporum.124
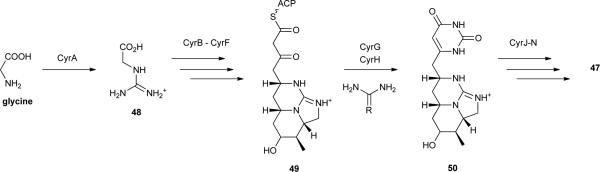
The discovery of 47, however, had no chemoecological basis. Rather, it was motivated by an outbreak of human hepatoenteritis that occurred in Queensland, Australia, in 1979, which was linked to the occurrence of Cylindrospermopsis raciborskii in a local reservoir that supplied water for domestic use.125 While this cyanobacterium was not associated with any reported toxicity at the time, the aqueous extract of a C. raciborskii strain isolated from the reservoir exhibited severe hepatotoxicity and other organ toxicity when injected intraperitoneally in mice.125 The compound responsible for the toxicity was isolated and its structure elucidated as the guanidino-containing alkaloid 47.123, 125 Due to its implications for public health and aquatic ecosystem management, this metabolite has since been extensively studied. Biosynthetic pathways responsible for the production of 47 have been reported for the cyanobacteria C. raciborskii strains AWT205126 and CS-505,127 Aphanizomenon sp. 10E6128 and Oscillatoria sp. PCC 6506.129 In spite of slight differences among the structures of the respective biosynthetic gene clusters, the biosynthesis of 47 involves NRPS/PKS and PKS enzymatic machinery, as well as several unique tailoring reactions. The starter unit for the biosynthesis of 47 is guanidinoacetate (48), derived from glycine and arginine through the action of a pathway-encoded amidinotransferase (CyrA).130 This is followed by the thiotemplated NRPS/PKS (CyrB) and PKS (CyrC to F) reactions, leading to intermediate 49. CyrG and CyrH then catalyse the formation of the uracil moiety via a urea or guanidino donor.126 Since no thioesterase or cyclization domains are present in the pathway, ring formation is believed to proceed spontaneously which also leads to the release of intermediate 50 from the enzyme.126, 131 The sulphate group present in 47 originates from the action of a pathway-encoded sulfotransferase, CyrJ. The substrate for this enzyme is 3-phosphoadenylyl sulfate, which is provided by CyrN, an adenylsulfate kinase that is also encoded in the gene cluster.126 The hydroxyl group adjacent to the uracil ring is incorporated through the action of CyrI (a 2-oxo-glutarate-dependent iron oxygenase).132 The stereoselectivities of the different orthologs of CyrI appear to be responsible for the variable proportions of 47 to its 7-epimer that are found among 47-producing strains.132
It was precisely the availability of the gene cluster for 47 that allowed the inference regarding the involvement of this alkaloid in Pi metabolism in A. ovalisporum.124 This cyanobacterium forms an annual bloom in Lake Kinneret, Israel, which is strongly correlated to AP activity in the lake water. Initially, this activity had been thought to originate from the release of APs by A. ovalisporum.133 However, subsequent observations suggested otherwise. These included lack of AP activity, a large Pi quota, and a high amount of polyphosphate bodies in A. ovalisporum filaments at the beginning of the bloom. Moreover, cyr genes were induced under Pi deprivation. These data motivated a series of laboratory- and field-based experiments that exposed the elegant mechanism by which A. ovalisporum deals with the low Pi levels. Under such conditions and when cellular reserves of this nutrient are low, this cyanobacterium enhances its production and release of 47 to the surrounding medium. Concomitantly, the transcript levels of an intracellular AP (phoD) and those of a high affinity Pi uptake system component (pstS) are upregulated. The presence of 47 in the growth medium causes an increase in extracellular AP-activity in C. reinhardtii as well as an upregulation of genes that are induced under Pi deprivation in this microalga. Similar effects were observed with other phytoplankton species. As such, Bar-Yosef et al.124 have concluded that A. ovalisporum and other cylindrospermopsin-producing organisms use this metabolite to induce a sort of “enslavement” by which susceptible phytoplankton species increase their production and release of APs. The now-available Pi is efficiently scavenged by the cylindrospermopsin-producing cyanobacterium which has readied its uptake system for this nutrient. The competitive advantage of this novel mechanism may reside in its reduced nitrogen budget and may help explain bloom-formation by cyanobacteria producing 47.112
2.5. Signalling
Bacteria communicate inter- or intra-species through chemical signals or cues that are released into the environment. Quorum-sensing (QS) systems are widespread among bacteria and constitute a mechanism by which such organisms communicate, sense their environment and coordinate their behaviours.134 Independently of being inter- or intra-specific, such systems typically comprise emitter cells, which produce a QS signal. When these signal molecules reach above a certain threshold concentration, they lead to a response in sensitive-cells.134 Different QS systems are currently known, and these have been thoroughly reviewed elsewhere from a variety of perspectives.e.g. 134-136 For the purpose of this review, we will focus on the N-acyl homoserine lactones (AHLs)-mediated signalling, an intraspecific QS-system.135 AHLs have the same general structure (51), with oxygenation possible at C3 and side chain lengths varying from C4 to C18. Usually, producing organisms synthesize more than a single AHL via LuxI or homologous proteins. These signals flow freely across cellular membranes and are recognized by cytoplasmic LuxR-type receptors, leading both to increased AHL production and to a signalling cascade that results in population-wide gene expression changes.135 AHL-mediated QS has been shown to be involved in a wide range of cellular processes (e.g. motility, bioluminescence, biofilm production, virulence, antibiotic production), in different environments.136
To date, LuxI or LuxR homologues have not been found in cyanobacteria. This, however, does not rule out AHLs production by cyanobacteria. In fact, the production of N-octanoyl homoserine lactone (52) by two Gloeothece sp. strains has been reported.137 The authors further reported that for one of the strains, the sheathless mutant Gloeothece sp. PCC 6909/1, the accumulation of 52 in the culture medium followed a pattern typical of a QS system. Initially, low accumulation of 52 was observed, despite regular cyanobacterial growth, and then there was an abrupt increase after a certain point in the culture cycle. Moreover, several proteins related to carbohydrate and amino acid metabolism were differentially expressed when this strain was treated with 52.137 This work was the first to report the finding of a QS signal in cyanobacteria. However, Marner and Moore138 had communicated the isolation of N-butyryl-l-homoserine lactone (53) in the late 1970's, a few years before AHLs were associated with QS.139
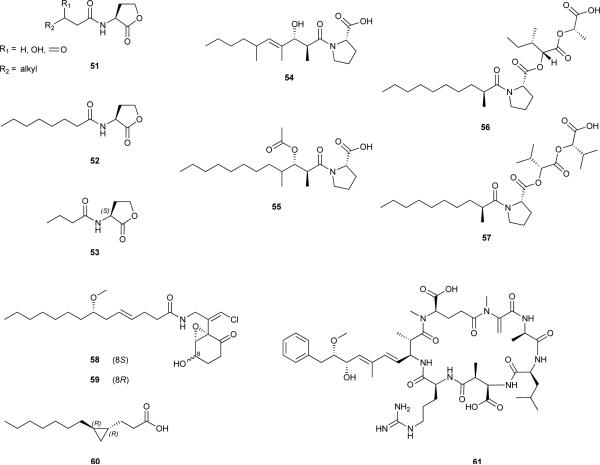
While no other studies present evidence for AHL production by cyanobacteria, given the chemoecologically dynamic nature of microbial communities, and the known roles played by AHL-mediated QS, it seems reasonable that cyanobacteria may have developed the ability to sense, or interfere with this kind of widespread QS system. This seems to be the case regarding the production of an AHL-degrading acylase by Anabaena sp. PCC 7120,140 purportedly produced as a defence mechanism against interference of AHLs with nitrogen fixation.141 Cyanobacteria produce several secondary metabolites structurally resembling AHLs and exhibiting AHL-like or QS-interfering activities. The first such metabolites to have been reported from a cyanobacterium were tumonoic acids E-H (54-57), isolated from Blennothrix cantharidosmum.142 These compounds were isolated based on their cytotoxic properties, however, given that their structures were somewhat similar to those of AHLs, the authors interrogated their activities in QS-systems. Metabolites 54-57 hampered bioluminescence production in wild-type Vibrio harveyi at micromolar concentrations.142 However, since this phenotype is regulated by different QS-systems, the mechanism of action could not be pinpointed.
Subsequently, the L. majuscula metabolite malyngamide C143 (58) and its epimer 8-epi-malyngamide C144 (59) were shown to interfere with AHL-mediated QS in a bioreporter assay using Escherichia coli.144 A large array of malyngamides has been reported from various cyanobacteria, and therefore the role of this family of fatty acyl-containing amides in signalling is yet to be explored.
In a screening program for QS-interfering activity145, several cyanobacterial extracts were tested for their ability to impair violacein production by the reporter strain Chromobacterium violaceum CV017, a process which is mediated by short-chain AHLs. Remarkably, a large fraction of the extracts (19 out of 25) exhibited activity in the bioassay. The QS-inhibiting compound present in one of the most active extracts, a L. majuscula strain, was found to be malyngolide (20)145, a metabolite which had previously been described from this species (see Feeding Deterrence section). Further testing with Pseudomonas aeruginosa reporter strains suggested a possible detrimental effect of 20 on lasR (a luxR homologue) expression as the mechanism for QS-inhibition by this metabolite. Malyngolide was also shown to be released to the medium by L. majuscula cells.145
Lyngbyoic acid (60) is a simple cyclopropyl-containing metabolite that was isolated as an abundant constituent of a Floridan L. majuscula strain.146 When tested in E. coli QS bioassays featuring different LuxR homologues, 60 showed micromolar-level QS-inhibitory activity in three such assays. Conversely, no such activity was found with a A. tumefaciens reporter.146 Gene expression profiling in P. aeruginosa allowed inference of an important role for the cyclopropyl moiety in the QS-inhibitory activity of 60 in that the structurally related dodecanoic acid mostly elicited stimulation of QS in this bacterium.146
As a benthic and sheath-producing cyanobacterium, it is conceivable that L. majuscula utilizes secondary metabolites to condition the behaviour of the microbial communities sharing its substrate or associated with its sheath. The production of QS-interfering compounds can also be expected from other, less studied, cyanobacteria.
The natural role of the extensively studied cyanobacterial cyclic peptides microcystins, in particular that of microcystin-LR (61),147 has been a controversial topic54, 148 (see also Feeding Deterrence section). Recently, metabolite 61 has been implicated in intraspecific signalling in Microcystis sp.149 Compound 61 is not released to the surrounding medium by live Microcystis cells, but rather, upon cell lysis or as a result of grazing.150 Schatz and co-workers149 have shown that intact Microcystis sp. KLL MG cells greatly increase their production of 61 when exposed to a cell lysate from the same strain or to exogenous 61. Concomitantly, high-levels of McyB (encoded in the biosynthetic pathway for 61, see section 3.1., and involved in the production of this metabolite) accumulated in the intact cells. The authors propose that these events reflect a defence mechanism by which the Microcystis population is able to sense the lysis of nearby individuals (due, for instance, to grazing), and consequently, to increase its toxicity.149 This implies a level of biological organization close to multicellularity for Microcystis.
2.6. Other
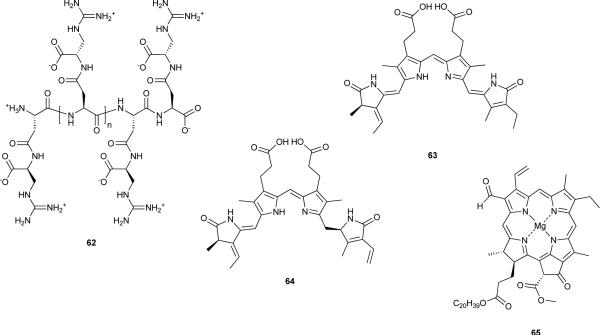
Several other ecological features of cyanobacteria reveal an interesting underlying chemistry. Cyanophycin (62)151 is a polymer composed of l-arginine and l-aspartate units that is synthesized non-ribosomally by a single protein termed cyanophycin synthetase.152 The backbone of the polymer consists of poly-l-aspartate, and the β-carboxy group of each of these residues is connected to the α-carboxy group of l-arginine via an isopeptidic bond. Cyanobacteria use 62 as a storage form of nitrogen (N).151 The polymer forms cytoplasmic “cyanophycin granules” when N is available. Under N starvation, 62 is broken down enzymatically and readily used by the cell.153 The chemical proficiency of cyanobacteria is also reflected in the way they have evolved to optimize harnessing of energy from sunlight at different wavelengths.154 Examples of this are the tetrapyrrolic chromophores of several phycobiliproteins (phycobilins), such as phycocyanobilin (63)155 or phycoerythrobilin (64),156 as well as the recently discovered chlorophyll f (65). This latter pigment was isolated from Australian stromatolites and absorbs solar radiation further to the red (λmax = 706 nm) than other chlorophylls.157
In a recent report, Zilliges et al.158 have described a potential role for microcystins (e.g. 61) in modulating protein status in producer cells. The authors compared the proteomes of microcystin-producing Microcystis aeruginosa PCC 7806 and its microcystin deficient mutant ΔmcyB under different light and oxidative stress conditions. The abundance of a number of proteins involved in photosynthesis and energy metabolism varied between the two strains. Many of these were shown to be covalently bound to microcystins, which led the authors to hypothesise a physiological regulatory role for this interaction. Further experiments supported such a hypothesis, as the mutant strain showed a higher susceptibility to high-light conditions and oxidative stress.158 These findings are particularly interesting, as the biosynthesis of microcystins has been found to be under transcriptional regulation by light.159, 160
3. Evolutionary aspects of cyanobacterial chemical ecology
The evolution of secondary metabolite structure and of the underlying biosynthetic machinery is intimately connected to the ecological history of the producing organisms. The molecular mechanisms involved in the synthesis of secondary metabolites by bacteria are among life's most diverse and rapidly evolving genetic elements, evolving over shorter time scales relative to the genes of higher organisms,161 and therefore are fundamental elements to understand the molecular evolution and chemical diversity of natural products. As one of the most ancient and chemically diverse groups of organisms on Earth, cyanobacteria present as a unique subject to study the interplay between chemistry and ecology occurring through time. In particular, the large number of NRPS or hybrid PKS-NRPS derived metabolites has provided researchers with the tools to interrogate their biosynthesis and the intriguing biochemistry that emerges from these pathways. From an evolutionary perspective, it has been possible in a few cases to integrate these genetic and biochemical findings with their putative ecological purpose. Below we highlight several such studies rather than present a comprehensive account of the evolution of biosynthetic pathways or associated enzymes.
The chemical and phylogenetic diversity among cyanobacteria also deserves attention in this review. Cyanobacterial taxonomy is currently under broad revision, and the apparent incoherence between secondary metabolite distribution and morphological diversity is an active topic of research. These subjects are of considerable interest for practical reasons, but they assume exceptional relevance to evolutionary and conservation studies.
3.1. Biosynthetic gene cluster evolution
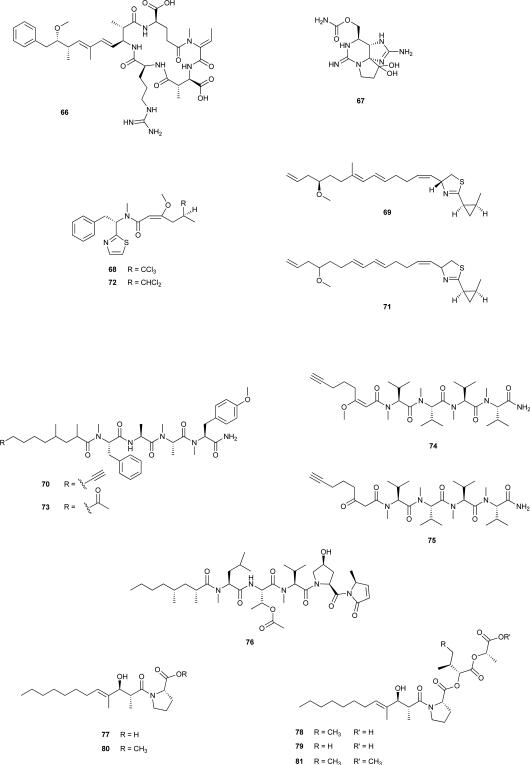
The microcystins are cyclic heptapeptides (e.g. 61) (see Section 2.4.) and are by far the most studied of the cyanobacterial toxins. They are produced by cyanobacterial strains belonging to quite distant genera, such as Microcystis, Anabaena, Anabaenopsis, Hapalosiphon, Nostoc and Planktothrix.150 The related cyclic pentapeptide nodularin (66) is produced by brackish water strains of the genus Nodularia.150 These two cyclic peptides are end products of phylogenetically related secondary metabolite pathways comprised of PKSs and NRPSs.54, 162 The biosynthesis of 61 and analogues is accomplished by a gene cluster spanning ≈55 kb composed of nine to ten genes (mcyA-J) that encode a giant enzyme complex comprising NRPSs, PKSs, and additional tailoring enzymes.163, 164 These peptide products exhibit high structural variation but all share a common cyclic structure (Adda-d-Glu-Mdha-dAla-X-d-MeAsp-Z), where X and Z are variable l-aminoacids, Adda is 3-amino-9-methoxy-2,6,8,-trimethyl-10-phenyldeca-4,6-dienoic acid, Mdha is N-methyldehydroalanine, and d-MeAsp is d-erythro-β-isoaspartic acid. Nodularin lacks the ‘X’ residue and bears an Mdhb (N-methyl-dehyrdrobutyrine) residue instead of Mdha. A variety of amino acids occupy the X and Z positions whereas all of the other positions remain conserved. This allows for the combinatorial production of an appreciable number of different molecules, with nearly 90 structural variants described to date.162 Several studies have evaluated genetic variability in the genes of the microcystin gene cluster, especially for those that are involved in the production of the various natural product isoforms,165-167 and have uncovered a high degree of recombination among the respective adenylation domains. Taken together, these studies make understandable the basis for structural variability of the microcystins, particularly in the hyper-variable residue positions. This hyper-variability likely reflects environmental adaptation and selection of different chemotypes. Interestingly, the cyanobacterial genera with microcystin-producing strains often have related strains that do not produce the toxins, and this appears to be a consequence of an absent or non-functional microcystin gene cluster.163, 168 While this distribution of Microcystis strains producing 61 and/or its analogues had first been hypothesized to be the result of horizontal gene transfer, recent phylogenetic analyses have indicated a high degree of congruence (co-evolution) between housekeeping genes and microcystin synthetase genes, which argues against the horizontal transfer hypothesis and supports an ancient origin and vertical tranmission of microcystin biosynthesis.54 One other group of cyanotoxins, saxitoxin (67) and some of its derivatives (STXs), are carbamate alkaloids that affect the neuronal impulse generation by selectively blocking the voltage-gated sodium and calcium channels, and by modifying potassium channel gating in excitable cells.169 Only a few cyanobacterial strains are know to produce STXs, and, as for the microcystins, these are from distantly related genera (Anabaena, Aphanizomenon, Cylindrospermopsis, Lyngbya, Planktothrix and Raphidiopsis).169, 170 STXs-producing isolates from these genera exhibit different STXs profiles, even within the same species.171, 172 Such varying toxin profiles appear to result from the presence or absence of genes coding for tailoring enzymes in the saxitoxin gene cluster (sxt), recently indentified in several cyanobacterial species. The biosynthetic pathway is composed of up to 26 genes whose products are involved in a complex sequence of over 30 steps.127, 173-175 Evolutionary analyses of the sxt cluster show that it has been preserved by strong stabilizing selection and relatively low recombination rate. This points toward vertical transfer and an ancient evolutionary history, dating back to the divergence of the Nostocales.176 Taking into account this ancient evolutionary origin, Murray et al.176 have proposed that the STX predecessors original targeted potassium channels, as early organisms such as bacteria had not yet evolved voltage-gated sodium channels. While the sxt cluster has been extraordinarily conserved as a whole, during evolutionary history a variety of gene losses, recombination, rearrangement and duplication events have resulted in the present diversity of STXs.176 Indeed, since the appearance of these metabolites, the aquatic environment that has harboured STX-producing cyanobacteria has undergone profound physical and biological changes. Hence, the natural role of these compounds, while not clearly established today (several roles have been put forward for 67, including homeostasis,177 chemical defence and signalling178), has certainly been of vital importance.176
The huge diversity of cyanobacteria species and related synthesized secondary metabolites provides a wealth of opportunities for genomics research. Currently, more than 60 cyanobacterial genome sequencing projects are underway and publicly available. Genome analysis is a very recent tool in cyanobacterial research but has been used quite successfully to infer important ecological, physiological and biotechnologically relevant characteristics of cyanobacteria.179 Genomic data for a few chemically prolific cyanobacteria, particularly from the orders Nostocales and Chroococcales has been made available in the past decade.12 Very recently, a genome project180 aimed precisely at gaining insight into the secondary metabolism of L. majuscula 3L, a Caribbean strain producing the lipopeptides barbamide (68),181 curacin A (69)182 and carmabin A (70)183 revealed several unexpected findings. Jones et al.180 observed that only ~293,000 nucleotides of the draft genome were attributable to secondary metabolism, a genomic portion unable to be responsible for the large number (over 200) of known Lyngbya metabolites, and suggesting that these are produced by different strains or chemotypes. In this study, the authors also found evidence for a complex gene regulatory network, which may be related to environmental adaptation, but also to microbial associations. Of note, it is conceivable that at least some of the metabolites reported from Lyngbya may in fact derive from metabolic capacities of these other bacteria.184 These first genome data for a marine Lyngbya species180 clearly calls for further genomic studies of other Lyngbya strains as these data will be invaluable to understanding the diversification of secondary metabolism in this genus. However, the chemical prolificacy of cyanobacteria identified as “Lyngbya” will be further discussed in the following section of this review (3.2.).
3.2. Morpohological, ecological and chemical diversity
As one of the most ancient organisms on the planet, cyanobacteria have, over the last three billion years, adapted to almost all conceivable habitats and are among the most abundant and geographically widespread group of prokaryotes known.185, 186 A consequence of these adaptations over this long evolutionary period is a rather extensive degree of biological and chemical diversity. Unfortunately, the taxonomic systems used to traditionally classify cyanobacteria have not recognized the true extent of this biodiversity. Moreover, the taxonomic distribution of secondary metabolites in cyanobacteria has been described as highly uneven with certain taxa greatly overrepresented in their chemical richness.10 For example, the cyanobacterial genus Lyngbya has been attributed with a total of 326 secondary metabolites or over 60% of all marine cyanobacterial metabolites reported to date.187 However, a major reason for this skewed taxonomic perception is that the taxonomy of cyanobacteria has been oversimplified and this has resulted in lumping together organisms with very different evolutionary histories.
Historically, the taxonomic classification of cyanobacteria has been based primarily upon morphological characterizations.188 Moreover, the first comprehensive classification system was constructed based on morphology of European field-collected specimens, and this then formed the basis of relatively modern cyanobacterial systematics.189 However, many of the traditional phenotypic traits used for taxonomic characterization have been shown to be variable and influenced by environmental factors.190 More recently, cyanobacteria have largely been accepted to represent a bacterial phylum, and a more comprehensive Bacteriological Code of systematics that incorporates biochemical, genetic, and physiological data has consequently been proposed.191-193 More importantly, both the Bacteriological Code and the traditional Botanical Code have begun incorporating the evolutionary histories and phylogenetic relationships as a fundamental framework to establish biologically informative classification systems.194-196 Therefore, to uncover and understand true cyanobacterial biodiversity, new specimens must be placed in a phylogenetic perspective with the type-strains that were used to establish our current taxonomic system.
In regards to the chemically prolific organism Lyngbya, this genus has been shown to be a polyphyletic group composed of multiple morphologically similar but phylogenetically distant groups.197, 198 Moreover, natural products attributed to Lyngbya have been isolated from the various unrelated evolutionary lineages and the polyphyly of this group has consequently been shown to be a major reason for some of the chemical prolificacy of Lyngbya.199
In attempts to better understand biological diversity of chemically rich cyanobacteria, one of the tropical marine Lyngbya lineages was recently described as the new genus Moorea gen. nov. by polyphasic approaches.200 Several important Lyngbya-attributed natural products, such as curacins A (69) and D (71),201 barbamide (68), dechlorobarbamide (72),202 carmabins A (70) and B (73),183 dragonamides C (74) and D (75)203 have been shown to be produced by Moorea.197, 199 At the same time, other Lyngbya-attributed natural products were shown to be produced by other yet undescribed cyanobacteria with morphological similarities to Lyngbya. For example, microcolins A (76) and B (21),64 tumonoic acids A-C (77-79), and methyl tumonates A (80) and B (81)204 have all been re-isolated from specimens most closely related to the genus Trichodesmium,197, 199 but which, in all likelihood, need to be described as novel generic entities.205 While further studies will need to determine how many Lyngbya-attributed natural products are actually being produced by Lyngbya, it is interesting to note that to date, no secondary metabolites have been shown to be produced by Lyngbya reference-strain PCC 7419 or related specimens.
In fact, most natural product-rich cyanobacterial groups appear to be polyphyletic, which suggests a great extent of novel biodiversity among these prokaryotes. Moreover, the current lack of description of these groups has skewed our taxonomic understanding of the distribution and evolution of bioactive secondary metabolites in cyanobacteria.
4. Making use of chemoecological interactions
The potential applications of cyanobacterial metabolites, in particular for biotechnological purposes, are based on a substantial body of research which has been previously reviewed.206, 207 In this section, we emphasize the contribution of chemical ecology to helping to realize the biotechnological potential of these organisms. This contribution is most evident when the natural role played by a particular metabolite is closely related to the proposed technological application. For example, compounds that are discovered based on their ecologically-relevant antibacterial or antifungal properties, may find direct use in the clinic, should they act on features shared by clinically relevant targets. However, studies describing antibacterial or antifungal activity from cyanobacterial extracts or purified metabolites have most often employed clinical isolates or other biotechnologically important bacteria or fungi as test strains.208 It is, therefore, difficult to ascertain whether such biotechnologically-relevant antimicrobial agents have a similar function in the natural setting. A large fraction of the reported biologically active cyanobacterial metabolites have been isolated precisely on the basis of application-related activities (e.g. cancer cell cytotoxicity,209 enzyme inhibition,210 ion channel activation211). Consequently, the natural function for the majority of these compounds is not known, and therefore, they are not covered in the present review.
Naturally occurring cyanobacterial sunscreens may find a homologous application in human photoprotection. In fact, the incorporation of particular mycosporines/MAAs or scytonemin in cosmetic sunscreens has been proposed,206 and evidence supports the photoprotective role of compounds 1, 2 and 3 in human fibroblasts.212 A formulation containing a mixture of MAAs 2 and 3 is currently commercialized under the brand name Helioguard 365®. Additionally, the antioxidant properties (see Section 2.1.) of mycosporines and MAAs may increase the attractiveness of these metabolites as components of cosmetic formulations.
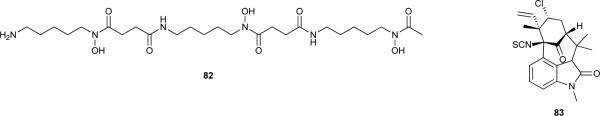
A recent study213 revealed that siderophores may be used to bring into culture previously uncultured microorganisms. Of special relevance, the authors have demonstrated that the presence of siderophores produced by microorganisms isolated from the same environment are needed in the culture of other isolates.213 Because the overwhelming majority of marine bacteria have not yet been cultured,214 it is expected that an immense biological and chemical diversity will arise from the successful culture of these microorganisms.215 The siderophore-based strategy presented by D’Onofrio et al.213 to reach untapped biological diversity is therefore promising, and, in that context, cyanobacterial siderophores (known or not) may assume an important role in future drug discovery efforts. These iron chelators may also hold direct pharmaceutical potential, for example, in the treatment of Fe-related pathologies. The bacterial siderophore desferrioxamine B (82) is widely used to treat Fe overload occurring as a result of diverse aetiologies.216 Chemical synthesis efforts may also benefit from chemoecological information. The metal-binding properties of siderophore 44 were exploited to anchor poly(ethylene glycol) (PEG) to a metal oxide surface, resulting in a stable, fouling-resistant coating.217
Insecticidal activity has not been cited often as the natural role of known cyanobacterial secondary metabolites. However, the toxicity of several hapalindoles towards grazing insects (see section 2.2), together with the insecticidal activity of N-methylwelwitindolinone C isothiocyanate (83) to blow-fly larvae72 suggest that this kind of interaction may be a common and useful feature of cyanobacteria. As such, cyanobacterial secondary metabolites may find more broad application as insect repellents and control agents of disease vectors in the future. Analogously, novel algal bloom mitigation or antifouling agents and herbicides may draw inspiration from cyanobacterial allelochemicals (see section 2.3.), as recently reviewed.79, 218
One particularly promising application of cyanobacterial natural products concerns QS-inhibition. Since many bacteria coordinate virulence and biofilm-forming phenotypes using QS,219 accessing the chemotypes that cyanobacteria have evolved to interfere with this process may help develop effective treatments for QS-associated pathologies. Alternatively, some cyanobacterial metabolites may be useful tool compounds for defining the role of QS in human disease. For example, the QS-inhibiting fatty acid 60 elicits a response in P. aeruginosa which is similar to the phenotype this bacterium exhibits in cystic fibrosis patients.146 The authors argue that fatty acid-related signalling could be involved in modulating the changes in genetic composition that occur during chronic infections of P. aeruginosa in these patients, and, as such, 60 may provide useful as a research tool.146
5. Conclusions
Cyanobacteria continue to constitute an extremely productive source of novel secondary metabolites. The underlying chemical ecology is gradually being harnessed, and the field continues to grow into a truly interdisciplinary area of research.
It becomes clear, from this review and others, that screening studies and field observations of cyanobacteria have been the most productive approach for uncovering and characterizing chemoecological interactions. In particular, field studies followed by laboratory investigations (both biological and chemical) have allowed a clear identification of the natural roles of several cyanobacterial metabolites.
However, emerging methodologies in genomics and analytical chemistry will certainly strongly impact this field in coming years. The steeply-decreasing price of DNA sequencing is expected to modify the paradigm of natural product discovery from marine microorganisms. Genes from environmental samples will be used to predict interesting chemical scaffolds with subsequent functional expression in heterologous hosts, even for gene clusters that are silent in the originating wild type organism.220 While this may be more difficult to accomplish for cyanobacteria than for other microorganisms,12 we can still expect this to become a mainstream approach in the future. As a result, large chemical and biological diversity datasets are expected to be available, which in turn will allow for better chemotaxonomic trends to be determined. Further, these data will enable a better understanding of the geographical patterns of secondary metabolite distribution, and this can easily be linked with quantitative data from the field and to the ecology of the producing cyanobacteria.
Mass spectrometric (MS) imaging methodologies221 will certainly have an increasingly important role in chemoecological studies, as hinted by pioneering work from the past few years.222, 223 These techniques should facilitate the study of metabolite distribution at the organism level as well as give insight into information exchange between microorganisms or even some macro-organisms, and thus become instrumental for the discipline of chemical ecology. Furthermore, when combined with chemotaxonomy, MS imaging techniques will allow scrutinizing the biological origin of a natural product of interest184 and allow development of a better chemical picture of the ecological relationships between cyanobacteria, various eukaryotic hosts, and heterotrophic bacteria.
Supplementary Material
Structure 1.
Structure 2.
Structure 3.
Structure 4.
Structure 5.
Structure 6.
Structure 7.
Structure 8.
Structure 9.
Structure 10.
Structure 11.
Structure 12.
Acknowledgments
PNL acknowledges a postdoctoral fellowship (SFRH/BPD/70233/2010) from Fundação para a Ciência e a Tecnologia (FCT); AA was funded in part by the PTDC/AAC-AMB/104983/2008 (FCOMP-01-0124-FEDER-008610) and PTDC/AAC-CLI/116122/2009 (FCOMP-01-0124-FEDER-014029) projects from FCT. VV acknowledges financial support from Interreg Programs, STC-CP2008-1-555612 Atlantox, 2009-1/117 Pharmatlantic. Work in the Gerwick laboratory is supported by NIH grants CA100851, TW006634, CA108874, and NS053398.
References
- 1.Sieg RD, Poulson-Ellestad KL, Kubanek J. Nat. Prod. Rep. 2011;28:388–399. doi: 10.1039/c0np00051e. [DOI] [PubMed] [Google Scholar]
- 2.Paul VJ, Ritson-Williams R, Sharp K. Nat. Prod. Rep. 2011;28:345–387. doi: 10.1039/c0np00040j. [DOI] [PubMed] [Google Scholar]
- 3.Singh SP, Hader DP, Sinha RP. Ageing Res. Rev. 2010;9:79–90. doi: 10.1016/j.arr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Leflaive J, Ten-Hage L. Freshwat. Biol. 2007;52:199–214. [Google Scholar]
- 5.Camacho FA. In: Algal Chemical Ecology. Amsler CD, editor. Springer-Verlag; Berlin: 2008. pp. 105–119. [Google Scholar]
- 6.Gantar M, Berry JP, Thomas S, Wang M, Perez R, Rein KS. FEMS Microbiol. Ecol. 2008;64:55–64. doi: 10.1111/j.1574-6941.2008.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leão PN, Vasconcelos MTSD, Vasconcelos VM. Crit. Rev. Microbiol. 2009;35:271–282. doi: 10.3109/10408410902823705. [DOI] [PubMed] [Google Scholar]
- 8.Pohnert G, Steinke M, Tollrian R. Trends Ecol. Evol. 2007;22:198–204. doi: 10.1016/j.tree.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Hay ME. Annu. Rev. Mar. Sci. 2009;1:193–212. doi: 10.1146/annurev.marine.010908.163708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tidgewell K, Clark BR, Gerwick WH. In: Comprehensive Natural Products II Chemistry and Biology. Mander L, Lui H-W, editors. Vol. 2. Elsevier; Oxford: 2010. pp. 141–188. [Google Scholar]
- 11.Jones AC, Gu LC, Sorrels CM, Sherman DH, Gerwick WH. Curr. Opin. Chem. Biol. 2009;13:216–223. doi: 10.1016/j.cbpa.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones AC, Monroe EA, Eisman EB, Gerwick L, Sherman DH, Gerwick WH. Nat. Prod. Rep. 2010;27:1048–1065. doi: 10.1039/c000535e. [DOI] [PubMed] [Google Scholar]
- 13.Hader DP, Kumar HD, Smith RC, Worrest RC. Photoch. Photobio. Sci. 2007;6:267–285. doi: 10.1039/b700020k. [DOI] [PubMed] [Google Scholar]
- 14.Hargreaves A, Taiwo FA, Duggan O, Kirk SH, Ahmad SI. J. Photoch. Photobio. B. 2007;89:110–116. doi: 10.1016/j.jphotobiol.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Brocks JJ, Logan GA, Buick R, Summons RE. Science. 1999;285:1033–1036. doi: 10.1126/science.285.5430.1033. [DOI] [PubMed] [Google Scholar]
- 16.Hoashi M, Bevacqua DC, Otake T, Watanabe Y, Hickman AH, Utsunomiya S, Ohmoto H. Nat. Geosci. 2009;2:301–306. [Google Scholar]
- 17.Cavalier-Smith T. Phil. Trans. R. Soc. B. 2006;361:969–1006. doi: 10.1098/rstb.2006.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shick JM, Dunlap WC. Annu. Rev. Physiol. 2002;64:223–262. doi: 10.1146/annurev.physiol.64.081501.155802. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Pichel F, Castenholz RW. Appl. Environ. Microbiol. 1993;59:163–169. doi: 10.1128/aem.59.1.163-169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sommaruga R, Garcia-Pichel F. Arch. Hydrobiol. 1999;144:255–269. [Google Scholar]
- 21.Sinha RP, Ambasht NK, Sinha JP, Klisch M, Hader DP. J. Photoch. Photobio. B. 2003;71:51–58. doi: 10.1016/j.jphotobiol.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Volkmann M, Gorbushina AA, Kedar L, Oren A. FEMS Microbiol. Lett. 2006;258:50–54. doi: 10.1111/j.1574-6968.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 23.Oren A. Geomicrobiology Journal. 1997;14:231–240. [Google Scholar]
- 24.Zhang L, Li L, Wu Q. J. Photoch. Photobio. B. 2007;86:240–245. doi: 10.1016/j.jphotobiol.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Singh SP, Klisch M, Sinha RP, Hader DP. Photochem. Photobiol. 2010;86:862–870. doi: 10.1111/j.1751-1097.2010.00736.x. [DOI] [PubMed] [Google Scholar]
- 26.Bohm GA, Pfleiderer W, Boger P, Scherer S. J. Biol. Chem. 1995;270:8536–8539. doi: 10.1074/jbc.270.15.8536. [DOI] [PubMed] [Google Scholar]
- 27.Balskus EP, Walsh CT. Science. 2010;329:1653–1656. doi: 10.1126/science.1193637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh SP, Klisch M, Sinha RP, Hader DP. Genomics. 2010;95:120–128. doi: 10.1016/j.ygeno.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Starcevic A, Akthar S, Dunlap WC, Shick JM, Hranueli D, Cullum J, Long PF. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2533–2537. doi: 10.1073/pnas.0707388105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferroni L, Klisch M, Pancaldi S, Hader DP. Mar. Drugs. 2010;8:106–121. doi: 10.3390/md8010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Pichel F, Wingard CE, Castenholz RW. Appl. Environ. Microbiol. 1993;59:170–176. doi: 10.1128/aem.59.1.170-176.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinha RP, Ambasht NK, Sinha JP, Hader DP. Photoch. Photobio. Sci. 2003;2:171–176. doi: 10.1039/b204167g. [DOI] [PubMed] [Google Scholar]
- 33.Conde FR, Churio MS, Previtali CM. J. Photoch. Photobio. B. 2000;56:139–144. doi: 10.1016/s1011-1344(00)00066-x. [DOI] [PubMed] [Google Scholar]
- 34.Whitehead K, Hedges JI. J. Photoch. Photobio. B. 2005;80:115–121. doi: 10.1016/j.jphotobiol.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Oren A, Gunde-Cimerman N. FEMS Microbiol. Lett. 2007;269:1–10. doi: 10.1111/j.1574-6968.2007.00650.x. [DOI] [PubMed] [Google Scholar]
- 36.Dunlap WC, Yamamoto Y. Comparative Biochemistry and Physiology -B Biochemistry and Molecular Biology. 1995;112:105–114. doi: 10.1016/0305-0491(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 37.Tao C, Sugawara T, Maeda S, Wang X, Hirata T. Fisheries Sci. 2008;74:1166–1172. [Google Scholar]
- 38.Singh SP, Klisch M, Sinha RP, Hader DP. Photochem. Photobiol. 2008;84:1500–1505. doi: 10.1111/j.1751-1097.2008.00376.x. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Pichel F, Castenholz RW. J. Phycol. 1991;27:395–409. [Google Scholar]
- 40.Proteau PJ, Gerwick WH, Garcia-Pichel F, Castenholz R. Experientia. 1993;49:825–829. doi: 10.1007/BF01923559. [DOI] [PubMed] [Google Scholar]
- 41.Nägeli C. Neue Denkschr. allg. schweiz. Ges. ges. Naturw. 1849;10:1–139. [Google Scholar]
- 42.Garcia-Pichel F, Sherry ND, Castenholz RW. Photochem. Photobiol. 1992;56:17–23. doi: 10.1111/j.1751-1097.1992.tb09596.x. [DOI] [PubMed] [Google Scholar]
- 43.Soule T, Stout V, Swingley WD, Meeks JC, Garcia-Pichel F. J. Bacteriol. 2007;189:4465–4472. doi: 10.1128/JB.01816-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balskus EP, Walsh CT. J. Am. Chem. Soc. 2008;130:15260–15261. doi: 10.1021/ja807192u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balskus EP, Walsh CT. J. Am. Chem. Soc. 2009;131:14648–14649. doi: 10.1021/ja906752u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones CS, Esquenazi E, Dorrestein PC, Gerwick WH. Bioorg. Med. Chem. 2011 [Google Scholar]
- 47.Soule T, Garcia-Pichel F, Stout V. J. Bacteriol. 2009;191:4639–4646. doi: 10.1128/JB.00134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorrels CM, Proteau PJ, Gerwick WH. Appl. Environ. Microbiol. 2009;75:4861–4869. doi: 10.1128/AEM.02508-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Landsberg JH. Rev. Fish. Sci. 2002;10:113–390. [Google Scholar]
- 50.Kurmayer R, Jüttner F. J. Plankton Res. 1999;21:659–683. [Google Scholar]
- 51.Shaw BA, Andersen RJ, Harrison PJ. Mar. Biol. 1997;128:273–280. [Google Scholar]
- 52.Blom JF, Robinson JA, Jüttner F. Toxicon. 2001;39:1923–1932. doi: 10.1016/s0041-0101(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 53.Blom JF, Baumann HI, Codd GA, Jüttner F. Arch. Hydrobiol. 2006;167:547–559. [Google Scholar]
- 54.Rantala A, Fewer DP, Hisbergues M, Rouhiainen L, Vaitomaa J, Borner T, Sivonen K. Proc. Natl. Acad. Sci. U. S. A. 2004;101:568–573. doi: 10.1073/pnas.0304489101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagle DG, Paul VJ. J. Phycol. 1999;35:1412–1421. [Google Scholar]
- 56.Capper A, Cruz-Rivera E, Paul VJ, Tibbetts IR. Hydrobiologia. 2006;553:319–326. [Google Scholar]
- 57.Nagle DG, Paul VJ, Roberts MA. Tetrahedron Lett. 1996;37:6263–6266. [Google Scholar]
- 58.Chen J, Huang PQ, Queneau Y. J. Org. Chem. 2009;74:7457–7463. doi: 10.1021/jo901557h. [DOI] [PubMed] [Google Scholar]
- 59.Nagle DG, Paul VJ. J. Exp. Mar. Biol. Ecol. 1998;225:29–38. [Google Scholar]
- 60.Thacker RW, Nagle DG, Paul VJ. Mar. Ecol. Prog. Ser. 1997;147:21–29. [Google Scholar]
- 61.Cardellina JH, II, Dalietos D, Marner FJ, Mynderse JS, Moore RE. Phytochemistry. 1978;17:2091–2095. [Google Scholar]
- 62.Cardellina JH, II, Moore RE, Arnold EV, Clardy J. J. Org. Chem. 1979;44:4039–4042. [Google Scholar]
- 63.Pennings SC, Weiss AM, Paul VJ. Mar. Biol. 1996;126:735–743. [Google Scholar]
- 64.Koehn FE, Longley RE, Reed JK. J. Nat. Prod. 1992;55:613–619. doi: 10.1021/np50083a009. [DOI] [PubMed] [Google Scholar]
- 65.Nagle DG, Camacho FT, Paul VJ. Mar. Biol. 1998;132:267–273. [Google Scholar]
- 66.Luesch H, Pangilinan R, Yoshida WY, Moore RE, Paul VJ. J. Nat. Prod. 2001;64:304–307. doi: 10.1021/np000456u. [DOI] [PubMed] [Google Scholar]
- 67.Cruz-Rivera E, Paul V. J. Chem. Ecol. 2007;33:213–217. doi: 10.1007/s10886-006-9212-y. [DOI] [PubMed] [Google Scholar]
- 68.Gunasekera SP, Ritson-Williams R, Paul VJ. J. Nat. Prod. 2008;71:2060–2063. doi: 10.1021/np800453t. [DOI] [PubMed] [Google Scholar]
- 69.Robles CD, Cubit J. Ecology. 1981;62:1536–1547. [Google Scholar]
- 70.Becher PG, Jüttner F. Environ. Toxicol. 2005;20:363–372. doi: 10.1002/tox.20113. [DOI] [PubMed] [Google Scholar]
- 71.Becher PG, Keller S, Jung G, Süssmuth RD, Jüttner F. Phytochemistry. 2007;68:2493–2497. doi: 10.1016/j.phytochem.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 72.Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. J. Am. Chem. Soc. 1994;116:9935–9942. [Google Scholar]
- 73.Moore RE, Cheuk C, Yang XQG, Patterson GML, Bonjouklian R, Smitka TA, Mynderse JS, Foster RS, Jones ND, Swartzendruber JK, Deeter JB. J. Org. Chem. 1987;52:1036–1043. [Google Scholar]
- 74.Martins R, Fernandez N, Beiras R, Vasconcelos V. Toxicon. 2007;50:791–799. doi: 10.1016/j.toxicon.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 75.Lecoz N, Malécot M, Quiblier C, Puiseux-Dao S, Bernard C, Crespeau F, Edery M. Toxicon. 2008;51:262–269. doi: 10.1016/j.toxicon.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 76.Lopes VR, Fernandez N, Martins RF, Vasconcelos V. Mar. Drugs. 2010;8:471–482. doi: 10.3390/md803471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keating KI. Science. 1977;196:885–887. doi: 10.1126/science.196.4292.885. [DOI] [PubMed] [Google Scholar]
- 78.Keating KI. Science. 1978;199:971–973. doi: 10.1126/science.199.4332.971. [DOI] [PubMed] [Google Scholar]
- 79.Berry JP, Gantar M, Perez MH, Berry G, Noriega FG. Mar. Drugs. 2008;6:117–146. doi: 10.3390/md20080007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mason CP, Edwards KR, Carlson RE, Pignatello J, Gleason FK, Wood JM. Science. 1982;213:400–402. doi: 10.1126/science.6800032. [DOI] [PubMed] [Google Scholar]
- 81.Pignatello JJ, Porwoll J, Carlson RE, Xavier A, Gleason FK, Wood JM. J. Org. Chem. 1983;48:4035–4038. [Google Scholar]
- 82.Jong TT, Williard PG, Porwoll JP. J. Org. Chem. 1984;49:735–736. [Google Scholar]
- 83.Gleason FK, Paulson JL. Arch. Microbiol. 1984;138:273–277. [Google Scholar]
- 84.Gleason FK. FEMS Microbiol. Lett. 1990;68:77–81. [Google Scholar]
- 85.Gleason FK, Case DE. Plant Physiol. 1986;80:834–837. doi: 10.1104/pp.80.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gleason FK, Baxa CA. FEMS Microbiol. Lett. 1986;33:85–88. [Google Scholar]
- 87.Moore RE, Cheuk C, Patterson GML. J. Am. Chem. Soc. 1984;106:6456–6457. [Google Scholar]
- 88.Schwartz RE, Hirsch CF, Springer JP, Pettibone DJ, Zink DL. J. Org. Chem. 1987;52:3704–3706. [Google Scholar]
- 89.Schlegel I, Doan NT, Chazal N. d., Smith GD. J. Appl. Phycol. 1999;10:471–479. [Google Scholar]
- 90.Doan NT, Rickards RW, Rothschild JM, Smith GD. J. Appl. Phycol. 2000;12:409–416. [Google Scholar]
- 91.Doan NT, Stewart PR, Smith GD. FEMS Microbiol. Lett. 2001;196:135–139. doi: 10.1111/j.1574-6968.2001.tb10554.x. [DOI] [PubMed] [Google Scholar]
- 92.Etchegaray A, Rabello E, Dieckmann R, Moon DH, Fiore MF, Döhren H. v., Tsai SM, Neilan BA. J. Appl. Phycol. 2004;16:237–243. [Google Scholar]
- 93.Flores E, Wolk CP. Arch. Microbiol. 1986;145:215–219. doi: 10.1007/BF00443648. [DOI] [PubMed] [Google Scholar]
- 94.Gross EM, Wolk CP, Jüttner F. J. Phycol. 1991;27:686–692. [Google Scholar]
- 95.Hagmann L, Jüttner F. Tetrahedron Lett. 1996;37:6539–6542. [Google Scholar]
- 96.Srivastava A, Jüttner F, Strasser RJ. Biochim. Biophys. Acta. 1998;1364:326–336. doi: 10.1016/s0005-2728(98)00014-0. [DOI] [PubMed] [Google Scholar]
- 97.Todorova AK, Jüttner F. J. Org. Chem. 1995;60:7891–7895. [Google Scholar]
- 98.Todorova A, Jüttner F. Phycologia. 1996;35:183–188. [Google Scholar]
- 99.Starkweather PL, Kellar PE. Hydrobiologia. 1983;104:373–377. [Google Scholar]
- 100.Jüttner F, Todorova AK, Walch N, Philipsborn W. v. Phytochemistry. 2001;57:613–619. doi: 10.1016/s0031-9422(00)00470-2. [DOI] [PubMed] [Google Scholar]
- 101.Banker R, Carmeli S. J. Nat. Prod. 1998;61:1248–1251. doi: 10.1021/np980138j. [DOI] [PubMed] [Google Scholar]
- 102.Donia MS, Ravel J, Schmidt EW. Nat. Chem. Biol. 2008;4:341–343. doi: 10.1038/nchembio.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leão PN, Pereira AR, Liu W-T, Ng J, Pevzner PA, Dorrestein PC, König GM, Vasconcelos VM, Gerwick WH. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11183–11188. doi: 10.1073/pnas.0914343107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leão PN, Vasconcelos MTSD, Vasconcelos VM. Eur. J. Phycol. 2009;44:347–355. [Google Scholar]
- 105.Leão PN, Ramos V, Vale M, Machado JP, Vasconcelos VM. Microb. Ecol. 2011 doi: 10.1007/s00248-011-9939-z. [DOI] [PubMed] [Google Scholar]
- 106.Boyer GL, Gilliam AH, Trick CG. In: The cyanobacteria. Fay P, Van Baalen C, editors. Elsevier; Amsterdam: 1987. pp. 415–436. [Google Scholar]
- 107.Murphy TP, Lean DRS, Nalewajko C. Science. 1976;192:900–902. doi: 10.1126/science.818707. [DOI] [PubMed] [Google Scholar]
- 108.Armstrong JE, Van Baalen C. J. Gen. Microbiol. 1979;111:253–262. [Google Scholar]
- 109.Mullis KB, Pollack JR, Neilands JB. Biochemistry. 1971;10:4894–4897. doi: 10.1021/bi00802a010. [DOI] [PubMed] [Google Scholar]
- 110.Simpson FB, Neilands JB. J. Phycol. 1976;12:44–48. [Google Scholar]
- 111.Lammers PJ, Sanders-Loehr J. J. Bacteriol. 1982;151:288–294. doi: 10.1128/jb.151.1.288-294.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goldman SJ, Lammers PJ, Berman MS, Sanders Loehr J. J. Bacteriol. 1983;156:1144–1150. doi: 10.1128/jb.156.3.1144-1150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nicolaisen K, Moslavac S, Samborski A, Valdebenito M, Hantke K, Maldener I, Muro-Pastor AM, Flores E, Schleiff E. J. Bacteriol. 2008;190:7500–7507. doi: 10.1128/JB.01062-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clarke SE, Stuart J, Sanders-Loehr J. Appl. Environ. Microbiol. 1987;53:917–922. doi: 10.1128/aem.53.5.917-922.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ito Y, Butler A. Limnol. Oceanogr. 2005;50:1918–1923. [Google Scholar]
- 116.Beiderbeck H, Taraz K, Budzikiewicz H, Walsby AE. Z. Naturforsch. C. 2000;55:681–687. doi: 10.1515/znc-2000-9-1002. [DOI] [PubMed] [Google Scholar]
- 117.Gademann K, Bethuel Y. Organic Letters. 2004;6:4707–4710. doi: 10.1021/ol048068x. [DOI] [PubMed] [Google Scholar]
- 118.Itou Y, Okada S, Murakami M. Tetrahedron. 2001;57:9093–9099. [Google Scholar]
- 119.Ito Y, Ishida K, Okada S, Murakami M. Tetrahedron. 2004;60:9075–9080. [Google Scholar]
- 120.Gademann K, Portmann C. Curr. Org. Chem. 2008;12:326–341. [Google Scholar]
- 121.Hernández I, Niell FX, Whitton BA. J. Appl. Phycol. 2002;14:475–487. [Google Scholar]
- 122.Mateo P, Douterelo I, Berrendero E, Perona E. J. Phycol. 2006;42:61–66. [Google Scholar]
- 123.Ohtani I, Moore RE, Runnegar MTC. J. Am. Chem. Soc. 1992;114:7941–7942. [Google Scholar]
- 124.Bar-Yosef Y, Sukenik A, Hadas O, Viner-Mozzini Y, Kaplan A. Curr. Biol. 2010;20:1557–1561. doi: 10.1016/j.cub.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 125.Hawkins PR, Runnegar MTC, Jackson ARB, Falconer IR. Appl. Environ. Microbiol. 1985;50:1292–1295. doi: 10.1128/aem.50.5.1292-1295.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mihali TK, Kellmann R, Muenchhoff J, Barrow KD, Neilan BA. Appl. Environ. Microbiol. 2008;74:716–722. doi: 10.1128/AEM.01988-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stucken K, John U, Cembella A, Murillo AA, Soto-Liebe K, Fuentes-Valdés JJ, Friedel M, Plominsky AM, Vásquez M, Glöckner G. PLoS ONE. 2010:5. doi: 10.1371/journal.pone.0009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stüken A, Jakobsen KS. Microbiology. 2010;156:2438–2451. doi: 10.1099/mic.0.036988-0. [DOI] [PubMed] [Google Scholar]
- 129.Méjean A, Mazmouz R, Mann S, Calteau A, Médigue C, Ploux O. J. Bacteriol. 2010;192:5264–5265. doi: 10.1128/JB.00704-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Muenchhoff J, Siddiqui KS, Poljak A, Raftery MJ, Barrow KD, Neilan BA. FEBS Journal. 2010;277:3844–3860. doi: 10.1111/j.1742-4658.2010.07788.x. [DOI] [PubMed] [Google Scholar]
- 131.Burgoyne DL, Hemscheidt TK, Moore RE, Runnegar MTC. J. Org. Chem. 2000;65:152–156. doi: 10.1021/jo991257m. [DOI] [PubMed] [Google Scholar]
- 132.Mazmouz R, Chapuis-Hugon F, Pichon V, Méjean A, Ploux O. ChemBioChem. 2011;12:858–862. doi: 10.1002/cbic.201000726. [DOI] [PubMed] [Google Scholar]
- 133.Hadas O, Pinkas R, Malinsky-Rushansky N, Shalev-Alon G, Delphine E, Berner T, Sukenik A, Kaplan A. Eur. J. Phycol. 2002;37:259–267. [Google Scholar]
- 134.Keller L, Surette MG. Nat. Rev. Microbiol. 2006;4:249–258. doi: 10.1038/nrmicro1383. [DOI] [PubMed] [Google Scholar]
- 135.Waters CM, Bassler BL. Annu. Rev. Cell Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 136.Williams P, Winzer K, Chan WC, Camara M. Phil. Trans. R. Soc. B. 2007;362:1119–1134. doi: 10.1098/rstb.2007.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sharif DI, Gallon J, Smith CJ, Dudley E. ISME J. 2008;2:1171–1182. doi: 10.1038/ismej.2008.68. [DOI] [PubMed] [Google Scholar]
- 138.Marner FJ, Moore RE. Phytochemistry. 1978;17:553–554. [Google Scholar]
- 139.Eberhard A, Burlingame AL, Eberhard C, Kenyon GL, Nealson KH, Oppenheimer NJ. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 140.Romero M, Diggle SP, Heeb S, Cámara M, Otero A. FEMS Microbiol. Lett. 2008;280:73–80. doi: 10.1111/j.1574-6968.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- 141.Romero M, Muro-Pastor AM, Otero A. FEMS Microbiol. Lett. 2011;315:101–108. doi: 10.1111/j.1574-6968.2010.02175.x. [DOI] [PubMed] [Google Scholar]
- 142.Clark BR, Engene N, Teasdale ME, Rowley DC, Matainaho T, Valeriote FA, Gerwick WH. J. Nat. Prod. 2008;71:1530–1537. doi: 10.1021/np800088a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ainslie RD, Barchi JJ, Jr, Kuniyoshi M, Moore RE, Mynderse JS. J. Org. Chem. 1985;50:2859–2862. [Google Scholar]
- 144.Kwan JC, Teplitski M, Gunasekera SP, Paul VJ, Luesch H. J. Nat. Prod. 2010;73:463–466. doi: 10.1021/np900614n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dobretsov S, Teplitski M, Alagely A, Gunasekera SP, Paul VJ. Environ. Microbiol. Rep. 2010;2:739–744. doi: 10.1111/j.1758-2229.2010.00169.x. [DOI] [PubMed] [Google Scholar]
- 146.Kwan JC, Meickle T, Ladwa D, Teplitski M, Paul V, Luesch H. Mol. Biosyst. 2011;7:1205–1216. doi: 10.1039/c0mb00180e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Botes DP, Wessels PL, Kruger H, Runnegar MTC, Santikarn S, Smith RJ, Barna JCJ, Williams DH. J. Chem. Soc. Perk. T. 1985;1:2747–2748. [Google Scholar]
- 148.Babica P, Blaha L, Marsalek B. J. Phycol. 2006;42:9–20. [Google Scholar]
- 149.Schatz D, Keren Y, Vardi A, Sukenik A, Carmeli S, Borner T, Dittmann E, Kaplan A. Environ. Microbiol. 2007;9:965–970. doi: 10.1111/j.1462-2920.2006.01218.x. [DOI] [PubMed] [Google Scholar]
- 150.Sivonen K, Jones G. In: Toxic cyanobacteria in water: A guide to their public health consequences, monitoring and management. Chorus I, Bartram J, editors. E&FN Spon; London: 1999. pp. 26–68. [Google Scholar]
- 151.Simon RD. Proc. Natl. Acad. Sci. U. S. A. 1971;68:265–267. doi: 10.1073/pnas.68.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ziegler K, Diener A, Herpin C, Richter R, Deutzmann R, Lockau W. Eur. J. Biochem. 1998;254:154–159. doi: 10.1046/j.1432-1327.1998.2540154.x. [DOI] [PubMed] [Google Scholar]
- 153.Allen MM, Hutchison F. Arch. Microbiol. 1980;128:1–7. [Google Scholar]
- 154.Kehoe DM. Proc. Natl. Acad. Sci. U. S. A. 2010;107:9029–9030. doi: 10.1073/pnas.1004510107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cole WJ, Chapman DJ, Siegelman HW. J. Am. Chem. Soc. 1967;89:3643–3645. [Google Scholar]
- 156.Chapman DJ, Cole WJ, Siegelman HW. J. Am. Chem. Soc. 1967;89:5976–5977. [Google Scholar]
- 157.Chen M, Schliep M, Willows RD, Cai ZL, Neilan BA, Scheer H. Science. 2010;329:1318–1319. doi: 10.1126/science.1191127. [DOI] [PubMed] [Google Scholar]
- 158.Zilliges Y, Kehr JC, Meissner S, Ishida K, Mikkat S, Hagemann M, Kaplan A, Börner T, Dittmann E. PLoS ONE. 2011:6. doi: 10.1371/journal.pone.0017615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kaebernick M, Neilan BA, Börner T, Dittmann E. Appl. Environ. Microbiol. 2000;66:3387–3392. doi: 10.1128/aem.66.8.3387-3392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kaebernick M, Dittmann E, Börner T, Neilan BA. Appl. Environ. Microbiol. 2002;68:449–455. doi: 10.1128/aem.68.2.449-455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fischbach MA, Walsh CT, Clardy J. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4601–4608. doi: 10.1073/pnas.0709132105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Welker M, von Dohren H. FEMS Microbiol. Rev. 2006;30:530–563. doi: 10.1111/j.1574-6976.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- 163.Tillett D, Dittmann E, Erhard M, von Dohren H, Borner T, Neilan BA. Chem. Biol. 2000;7:753–764. doi: 10.1016/s1074-5521(00)00021-1. [DOI] [PubMed] [Google Scholar]
- 164.Christiansen G, Fastner J, Erhard M, Borner T, Dittmann E. J. Bacteriol. 2003;185:564–572. doi: 10.1128/JB.185.2.564-572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kurmayer R, Gumpenberger M. Mol. Ecol. 2006;15:3849–3861. doi: 10.1111/j.1365-294X.2006.03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fewer DP, Rouhiainen L, Jokela J, Wahlsten M, Laakso K, Wang H, Sivonen K. BMC Evol. Biol. 2007:7. doi: 10.1186/1471-2148-7-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tooming-Klunderud A, Fewer DP, Rohrlack T, Jokela J, Rouhiainen L, Sivonen K, Kristensen T, Jakobsen KS. BMC Evol. Biol. 2008:8. doi: 10.1186/1471-2148-8-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mikalsen B, Boison G, Skulberg OM, Fastner J, Davies W, Gabrielsen TM, Rudi K, Jakobsen KS. J. Bacteriol. 2003;185:2774–2785. doi: 10.1128/JB.185.9.2774-2785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Llewellyn LE. Nat. Prod. Rep. 2006;23:200–222. doi: 10.1039/b501296c. [DOI] [PubMed] [Google Scholar]
- 170.Yunes JS, De La Rocha S, Giroldo D, da Silveira SB, Comin R, Bicho M. d. S., Melcher SS, Sant'anna CL, Henriques Vieira AA. J. Phycol. 2009;45:585–591. doi: 10.1111/j.1529-8817.2009.00673.x. [DOI] [PubMed] [Google Scholar]
- 171.Beltran EC, Neilan BA. Appl. Environ. Microbiol. 2000;66:4468–4474. doi: 10.1128/aem.66.10.4468-4474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Llewellyn LE, Negri AP, Doyle J, Baker PD, Beltran EC, Neilan BA. Environ. Sci. Technol. 2001;35:1445–1451. doi: 10.1021/es001575z. [DOI] [PubMed] [Google Scholar]
- 173.Kellmann R, Mihali TK, Jeon YJ, Pickford R, Pomati F, Neilan BA. Appl. Environ. Microbiol. 2008;74:4044–4053. doi: 10.1128/AEM.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mihali TK, Kellmann R, Neilan BA. BMC Biochem. 2009;10:8. doi: 10.1186/1471-2091-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Moustafa A, Loram JE, Hackett JD, Anderson DM, Plumley FG, Bhattacharya D. PLoS ONE. 2009:4. doi: 10.1371/journal.pone.0005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Murray SA, Mihali TK, Neilan BA. Mol. Biol. Evol. 2011;28:1173–1182. doi: 10.1093/molbev/msq295. [DOI] [PubMed] [Google Scholar]
- 177.Pomati F, Rossetti C, Manarolla G, Burns BP, Neilan BA. Microbiology-Sgm. 2004;150:455–461. doi: 10.1099/mic.0.26350-0. [DOI] [PubMed] [Google Scholar]
- 178.Zimmer RK, Ferrer RP. Biol. Bull. 2007;213:208–225. doi: 10.2307/25066641. [DOI] [PubMed] [Google Scholar]
- 179.Hess WR. Curr. Opin. Microbiol. 2011 doi:10.1016/j.mib.2011.07.024. [Google Scholar]
- 180.Jones AC, Monroe EA, Podell S, Hess WR, Klages S, Esquenazi E, Niessen S, Hoover H, Rothmann M, Lasken RS, Yates Iii JR, Reinhardt R, Kube M, Burkart MD, Allen EE, Dorrestein PC, Gerwick WH, Gerwick L. Proc. Natl. Acad. Sci. U. S. A. 2011;108:8815–8820. doi: 10.1073/pnas.1101137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Orjala J, Gerwick WH. J. Nat. Prod. 1996;59:427–430. doi: 10.1021/np960085a. [DOI] [PubMed] [Google Scholar]
- 182.Gerwick WH, Proteau PJ, Nagle DG, Hamel E, Blokhin A, Slate DL. J. Org. Chem. 1994;59:1243–1245. [Google Scholar]
- 183.Hooper GJ, Orjala J, Schatzman RC, Gerwick WH. J. Nat. Prod. 1998;61:529–533. doi: 10.1021/np970443p. [DOI] [PubMed] [Google Scholar]
- 184.Simmons TL, Coates RC, Clark BR, Engene N, Gonzalez D, Esquenazi E, Dorrestein PC, Gerwick WH. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4587–4594. doi: 10.1073/pnas.0709851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Whitton BA, Potts M. The ecology of Cyanobacteria: their diversity in time and space. Kluwer Academic Publishers; Dordrecht: 2000. [Google Scholar]
- 186.Stanley SM. Earth system history. 2nd edn. W.H. Freeman; New York: 2004. p. 263. [Google Scholar]
- 187.MarinLit, a marine literature database. Department of Chemistry, University of Canterbury; 2011. http://www.chem.canterbury.ac.nz/marinlit/marinlit.shtml. [Google Scholar]
- 188.Anagnostidis K, Komarek J. Arch. Hydrobiol. Suppl. 1988;80:327–472. [Google Scholar]
- 189.Geitler L. In: Kryptogamen-Flora von Deutschland, Österreich und der Schweiz. Rabenhorst L, editor. XIV. Akademische Verlag; Leipzig: 1932. pp. 1027–1068. [Google Scholar]
- 190.Sumina EL. Microbiology. 2006;75:459–464. [Google Scholar]
- 191.Stanier RY, Sistrom WR, Hansen TA, Whitton BA, Castenholz RW, Pfennig N, Gorlenko VN, Kondratieva EN, Eimhjellen KE, Whittenbury R, Gherna RL, Truper HG. Int. J. Syst. Bacteriol. 1978;28:335–336. [Google Scholar]
- 192.Rippka R, Deruelles J, Waterbury JB. J. Gen. Microbiol. 1979;111:1–61. [Google Scholar]
- 193.Oren A. Int. J. Syst. Evol. Microbiol. 2004;54:1895–1902. doi: 10.1099/ijs.0.03008-0. [DOI] [PubMed] [Google Scholar]
- 194.Castenholz RW. In: Bergey's Manual of Systematic Bacteriology. Boone DR, Castenholz RW, editors. Springer; New York: 2001. pp. 473–553. [Google Scholar]
- 195.Komárek J, Anagnostidis K. Cyanoprokaryota, Part 2: Oscillatoriales. Spektrum Akademischer Verlag; Heidelberg: 2005. [Google Scholar]
- 196.Komárek J. Algae. 2006;21:349–375. [Google Scholar]
- 197.Sharp K, Arthur KE, Gu L, Ross C, Harrison G, Gunasekera SP, Meickle T, Matthew S, Luesch H, Thacker RW, Sherman DH, Paul VJ. Appl. Environ. Microbiol. 2009;75:2879–2888. doi: 10.1128/AEM.02656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Engene N, Cameron Coates R, Gerwick WH. J. Phycol. 2010;46:591–601. [Google Scholar]
- 199.Engene N, Choi H, Esquenazi E, Rottacker EC, Ellisman MH, Dorrestein PC, Gerwick WH. Environ. Microbiol. 2011;13:1601–1610. doi: 10.1111/j.1462-2920.2011.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Engene N, Rottacker EC, Kaštovský J, Byrum T, Choi H, Ellisman MH, Komárek J, Gerwick WH. Int. J. Syst. Evol. Microbiol. 2011 doi: 10.1099/ijs.0.033761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Márquez B, Verdier-Pinard P, Hamel E, Gerwick WH. Phytochemistry. 1998;49:2387–2389. doi: 10.1016/s0031-9422(98)00365-3. [DOI] [PubMed] [Google Scholar]
- 202.Sitachitta N, Márquez BL, Thomas Williamson R, Rossi J, Ann Roberts M, Gerwick WH, Nguyen VA, Willis CL. Tetrahedron. 2000;56:9103–9113. [Google Scholar]
- 203.Gunasekera SP, Ross C, Paul VJ, Matthew S, Luesch H. J. Nat. Prod. 2008;71:887–890. doi: 10.1021/np0706769. [DOI] [PubMed] [Google Scholar]
- 204.Harrigan GG, Luesch H, Yoshida WY, Moore RE, Nagle DG, Biggs J, Park PU, Paul VJ. J. Nat. Prod. 1999;62:464–467. doi: 10.1021/np980460u. [DOI] [PubMed] [Google Scholar]
- 205.Engene N, Choi H, Esquenazi E, Byrum T, Villa FA, Cao Z, Murray TF, Dorrestein PC, Gerwick L, Gerwick WH. J. Nat. Prod. 2011;74:1737–1743. doi: 10.1021/np200236c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rastogi RP, Sinha RP. Biotechnology Advances. 2009;27:521–539. doi: 10.1016/j.biotechadv.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 207.Nunnery JK, Mevers E, Gerwick WH. Curr. Opin. Biotechnol. 2010;21:787–793. doi: 10.1016/j.copbio.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mo S, Krunic A, Chlipala G, Orjala J. J. Nat. Prod. 2009;72:894–899. doi: 10.1021/np800751j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Simmons TL, Nogle LM, Media J, Valeriote FA, Mooberry SL, Gerwick WH. J. Nat. Prod. 2009;72:1011–1016. doi: 10.1021/np9001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zafrir-Ilan E, Carmeli S. Tetrahedron. 2010;66:9194–9202. [Google Scholar]
- 211.Pereira A, Cao Z, Murray TF, Gerwick WH. Chem. Biol. 2009;16:893–906. doi: 10.1016/j.chembiol.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Oyamada C, Kaneniwa M, Ebitani K, Murata M, Ishihara K. Mar. Biotechnol. 2008;10:141–150. doi: 10.1007/s10126-007-9043-z. [DOI] [PubMed] [Google Scholar]
- 213.D'Onofrio A, Crawford JM, Stewart EJ, Witt K, Gavrish E, Epstein S, Clardy J, Lewis K. Chem. Biol. 2010;17:254–264. doi: 10.1016/j.chembiol.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Amann RI, Ludwig W, Schleifer KH. Microbiol. Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Clardy J, Walsh C. Nature. 2004;432:829–837. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- 216.Muñoz M, García-Erce JA, Remacha ÁF. J. Clin. Pathol. 2011;64:287–296. doi: 10.1136/jcp.2010.086991. [DOI] [PubMed] [Google Scholar]
- 217.Zürcher S, Wäckerlin D, Bethuel Y, Malisova B, Textor M, Tosatti S, Gademann K. J. Am. Chem. Soc. 2006;128:1064–1065. doi: 10.1021/ja056256s. [DOI] [PubMed] [Google Scholar]
- 218.Dahms HU, Ying X, Pfeiffer C. Biofouling. 2006;22:317–327. doi: 10.1080/08927010600967261. [DOI] [PubMed] [Google Scholar]
- 219.Dickschat JS. Nat. Prod. Rep. 2010;27:343–369. doi: 10.1039/b804469b. [DOI] [PubMed] [Google Scholar]
- 220.Hertweck C. Nat. Chem. Biol. 2009;5:450–452. doi: 10.1038/nchembio0709-450. [DOI] [PubMed] [Google Scholar]
- 221.Watrous JD, Dorrestein PC. Nat. Rev. Microbiol. 2011;9:683–694. doi: 10.1038/nrmicro2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lane AL, Nyadong L, Galhena AS, Shearer TL, Stout EP, Parry RM, Kwasnik M, Wang MD, Hay ME, Fernandez FM, Kubanek J. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7314–7319. doi: 10.1073/pnas.0812020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Esquenazi E, Jones AC, Byrum T, Dorrestein PC, Gerwick WH. Proc. Natl. Acad. Sci. U. S. A. 2011;108:5226–5231. doi: 10.1073/pnas.1012813108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.