Abstract
Background
Exposure of pregnant mice to corticosteroids can produce oral clefts in offspring. While data in humans are more mixed, recent reports have suggested that dermatologic steroids are associated with oral clefts.
Methods
We investigated maternal first-trimester exposure to corticosteroids (focusing on dermatologic uses) and oral clefts in offspring using two population-based studies. The Norway Cleft Study (1996–2001) is a national case-control study including 377 infants with cleft lip +/− palate (CLP), 196 infants with cleft palate only (CPO) and 763 controls. The Norwegian Mother and Child Cohort Study (MoBa, 1998–2008), is a national birth cohort including 123 infants with CLP, 61 infants with CPO and 551 controls.
Results
In the case-control study, there was the suggestion of an association of dermatological corticosteroids with both CLP (adjusted OR (aOR) = 2.3, 95% confidence interval = 0.71, 7.7) and CPO (aOR = 3.4, 0.87–13). There was no evidence of this association in the cohort data (OR for CLP = 1.2; 0.50, 2.8), OR for CPO = 1.0, 0.30–3.4), although exposure to dermatological steroids was less specifically ascertained. There were no associations with other types of corticosteroids.
Conclusion
Our data add to the suggestive but inconsistent findings for this association.
Keywords: corticosteroids, cleft lip, cleft palate, maternal exposure, pregnancy
Introduction
Corticosteroids were first recognized as a potential human teratogen in the 1950’s, when Fraser and Fainstat demonstrated that injection of cortisone in pregnant mice led to clefts in the offspring (1, 2). Corticosteroids reduce inflammation and modulate immune response, and are used to treat a range of clinical condition. Indications include asthma, autoimmune diseases, allergies, eczema, cancer, and rheumatoid arthritis. These various diseases require different modes of administration, potency, dosage, and duration of treatment, which makes epidemiologic studies challenging. Maternal use of corticosteroids during pregnancy has been associated with cleft lip and/or palate in some studies (3–9) but not all (10, 11), and the question of causation is generally regarded as unresolved. (11)
Oral corticosteroids are thought to be more of a concern than steroids applied topically because topical applications are less readily absorbed. However, a recent epidemiologic study from Denmark reported an association of dermatological corticosteroids with clefts in offspring (adjusted odds ratio = 1.5, 95% confidence interval 1.0–2.1). (10)
We explored this hypothesis in two population-based studies. One was a case-control study of facial clefts in Norway, and the other was the Norwegian national birth cohort study. We specifically addressed the question of whether mothers’ use of dermatological corticosteroids during the first trimester increased the risk of cleft lip and palate in offspring.
MATERIALS AND METHODS
Design
The Norway Cleft Study (case-control)
In Norway the treatment of all babies with cleft lip and palate is carried out in two specialized surgical centers in Oslo and Bergen. From 1996–2001 the families of all newborn infants in Norway referred for clefts surgery were invited to participate in a case-control study. Controls were randomly selected from all live births during the same time period, sampling from the Medical Birth Registry of Norway. Parents of both cases and controls were recruited within the first three months after delivery. Details on the study design have been published. (12, 13) A total of 653 infants with clefts were eligible for study, and 573 of their families (88%) agreed to participate. There were 1006 randomly selected live-born non-malformed controls eligible for study, and 763 of their families (76%) agreed to participate.
MoBa (cohort)
From 1999–2008 The Norwegian Institute of Public Health conducted a prospective population-based pregnancy cohort study (the Norwegian Mother and Child Cohort Study, or MoBa), inviting all pregnant women in Norway to participate. 39% of the expectant mothers consented, and the cohort includes 109 000 children, 91 000 mothers and 71 700 fathers. Details of study design and demographic characteristics of the cohort have been published. (14, 15) Our analysis is based on version 5 of the data files and was approved for studies on risk factors for oral clefts. Within the cohort, 123 cases with cleft lip and palate and 61 with cleft palate only were identified through the Medical Birth Registry of Norway. We randomly selected 551 mothers from the MoBa cohort to serve as controls.
Questionnaire
The Norway Cleft Study (case-control)
All mothers in the case-control study completed a self-administered questionnaire after delivery covering demographic information and a wide range of exposures during pregnancy. In particular, mothers were asked detailed questions about their use of prescribed and over-the-counter medications during the first, second and third month of pregnancy. An English translation of the questionnaire is available online. (16) Information on medications was collected for only the first three months of pregnancy, which is the period during which exposures can potentially affect the embryological fusion of the lip ( around week 4–6 of embryonic life) and palate (around week 7–10). (17) Medication was coded according to the Anatomical Therapeutic Chemical Classification System (ATC). (18) We included in our analysis all medications containing corticosteroids.
MoBa (cohort)
Mothers in the cohort study were asked to complete self-administered questionnaires at pregnancy week 15, 22 and 30. We used information from the 15-week questionnaire, which focuses on maternal health and use of medications 6 months before pregnancy and during the first 15 weeks of pregnancy. The mean time which the questionnaire was completed was 17.3 weeks (standard deviation 3.0). An English translation of the questionnaire is available online. (19) Medication was again coded according to the Anatomical Therapeutic Chemical Classification System (ATC) and all medications containing corticosteroids were included.
Case information
The Norway Cleft Study (case-control)
Information for cases on accompanying birth defects or syndromes was obtained from three sources: medical records at the hospital performing corrective surgery, the Medical Birth Registry, and the mothers’ questionnaire. Cases with no additional malformations or known syndromes were classified as “isolated clefts.”
MoBa (cohort)
Cases within the cohort were identified by linking all cohort members with the Medical Birth Registry, which includes information on all defects recorded during the newborn’s hospital stay. For oral clefts the sensitivity of the Medical Birth Registry is 94 % for CLP and 57 % for CPO.(20)
Statistical analysis
Logistic regression models were performed in STATA v. 12 to estimate the odds ratio (ORs) and 95% confidence intervals (CIs).
The Norway Cleft Study (case-control)
Steroid medications were categorized as any use, use of dermatological corticosteroids, and “other” corticosteroids. Most women who reported steroid exposure during at least one month reported exposure for more than one month. Exposure during any of the first three months of pregnancy was therefore counted as “exposed.” The outcome was total clefts and the two main cleft subtypes (cleft lip with or without cleft palate [CLP], and cleft palate only [CPO]). We adjusted for the following potential confounders: mother’s education (six categories), work status in early pregnancy (yes/no), alcohol intake (total number of drinks during first 3 months of pregnancy; none, 1–3, 4–6, 7+), smoking (none, passive only, 1–5 cigarettes/day, 6–10 cigarettes/day, 11+ cigarettes/day), folic acid supplementation (none, less than 400 ug/day, 400+ ug/day), dietary folates (quartiles with cutoffs at 171, 214 and 264 ug/day), multivitamin supplementation (yes/no), and calendar year of baby’s birth. The main analyses were performed for all cases regardless of presence of other defects; in a sensitivity analysis we restricted the outcome to isolated cases.
MoBa (cohort)
In the questionnaire mothers were asked for specific symptoms/conditions before and during pregnancy, and asked to list the medication(s) they used. The timing of exposure (spanning from 6 month prior to pregnancy to the 15th week) is reported for each condition/symptom and not for each medication. This provides difficulties in determining the timing of the exposure when the mother reports using more than one medication for a given symptom/condition. For dermatological conditions this was not a big concern as the women who reported using more than one medication, in all cases, reported using another dermatological corticosteroid. However, the majority of women who were using corticosteroids for other conditions, such as asthma and allergies, also reported using non-corticosteroid medication. Given the small numbers and uncertainty of the timing of exposure, only the dermatological corticosteroids were analyzed in this study. Exposure during the first 15 weeks of pregnancy counted as “exposed”. We adjusted for folic acid use (400 ug/day or none), smoking (none, passive only, active smoker), mother’s education (less than high school, high school or more) and alcohol consumption (none or any).
Ethical approval for the Norwegian Cleft Study was granted by the Norwegian Data Inspectorate and the Regional Medical Ethics Committee of Western Norway. The Norwegian Mother and Child Cohort Study was approved by The Regional Committee for Medical Research Ethics in South-Eastern Norway.
RESULTS
The Norway Cleft Study (case-control)
Table 1 describes demographic information for mothers of cases and controls. Overall 4.2 % (24/573) of case mothers and 2.5 % (19/763) of control mothers reported using corticosteroids during the first trimester. With regard to mode of administration, dermatological application was most frequently used by case mothers (54 %), followed by inhalation (17 %) and nasal spray (13 %). For control mothers the most frequently mode of use was inhalation (42%), nasal spray (32%) and dermatological (26%). Systemic corticosteroids were reported by only two participants, both of whom were case mothers.
Table 1.
Demographic Information and Characteristics of Mothers and Infants, Cases and Controls, Norway Cleft Study (Case-Control) and MoBa (Cohort). Abbreviations: cleft lip +/− palate (CLP), cleft palate only (CPO).
| Norway Cleft Study | MoBa | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cases | Controls | Cases | Controls | |||
| CLP (n = 377) | CPO (n = 196) | (n = 763) | CLP (n=123) | CPO (n=61) | (n=551) | |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Mothers | ||||||
| Age, yr | ||||||
| <20 | 8 (2) | 6 (3) | 12 (1) | 0 | 4 (7) | 5 (<1) |
| 20–29 | 199 (53) | 105 (54) | 408 (54) | 68 (55) | 19 (31) | 243 (44) |
| 30–39 | 165 (44) | 81 (41) | 328 (43) | 51 (42) | 38 (62) | 289 (52) |
| ≥40 | 5 (1) | 4 (2) | 15 (2) | 4 (3) | 0 | 14 (3) |
| Education | ||||||
| Less than high school | 93 (16) | 87 (11) | 180 (13) | 4 (3) | 2 (3) | 9 (2) |
| High school or more | 478 (83) | 675 (89) | 1153 (86) | 108 (88) | 54 (89) | 501 (91) |
| Missing/Other | 1 (<1) | 1 (<1) | 1 (<1) | 11 (9) | 5 (8) | 41 (7) |
| Smoking | ||||||
| None | 244 (43) | 414 (54) | 658 (49) | 86 (70) | 46 (75) | 436 (79) |
| Passive | 90 (16) | 106 (14) | 196 (15) | 13 (11) | 8 (13) | 37 (7) |
| Active | 239 (42) | 243 (32) | 482 (36) | 14 (11) | 4 (7) | 39 (7) |
| Missing | 0 | 0 | 0 | 10 (8) | 3 (5) | 39 (7) |
| Marital status | ||||||
| Married | 273 (48) | 405 (53) | 678 (51) | 54 (44) | 23 (38) | 245 (44) |
| Live-in | 273 (48) | 329 (43) | 602 (45) | 59 (48) | 35 (57) | 251 (46) |
| Single | 26 (4) | 28 (3) | 54 (4) | 1 (<1) | 0 | 11 (2) |
| Missing/Other | 1 (<1) | 1 (1) | 2 (<1) | 8 (7) | 3 (5) | 44 (8) |
| Alcohol beverages (first 3 months of pregnancy) | ||||||
| 0 | 360 (63) | 527 (69) | 887 (66) | 57 (93) | 101 (82) | 457 (83) |
| 1–3 | 107 (19) | 123 (16) | 230 (17) | 1 (2) | 3 (2) | 14 (3) |
| ≥4 | 110 (19) | 108 (14) | 218 (16) | 0 | 0 | 3 (<1) |
| Missing | 6 (1) | 5 (1) | 11 (1) | 3 (5) | 19 (16) | 77 (14) |
| Folic acid supplement | ||||||
| Yes | 214 (37) | 453 (59) | 667 (50) | 68 (55) | 38 (62) | 358 (65) |
| No | 359 (63) | 310 (41) | 669 (50) | 55 (45) | 23 (38) | 193 (35) |
| Folic acid supplement (highest exposed daily level); μg* | ||||||
| None | 240 (64) | 119 (61) | 453 (59) | |||
| 1–399 | 86 (23) | 46 (23) | 165 (22) | |||
| ≥400 | 51 (14) | 31 (16) | 145 (19) | |||
| Dietary folate (highest exposed daily level); μg* | ||||||
| 0–171 | 111 (31) | 62 (33) | 176 (25) | |||
| 172–214 | 88 (25) | 44 (23) | 177 (25) | |||
| 215–264 | 81 (23) | 36 (19) | 177 (25) | |||
| ≥265 | 74 (21) | 46 (25) | 174 (25) | |||
| Multivitamins* | ||||||
| Yes | 123 (33) | 71 (36) | 279 (37) | |||
| No | 254 (67) | 325 (64) | 484 (63) | |||
| Other birth defects* | 63 (17) | 78 (40) | 38 (5) | |||
| Parent with facial cleft* | 24 (6) | 12 (6) | 4 (0.5) | |||
Some characteristics were only available for the Norway Cleft Study mothers.
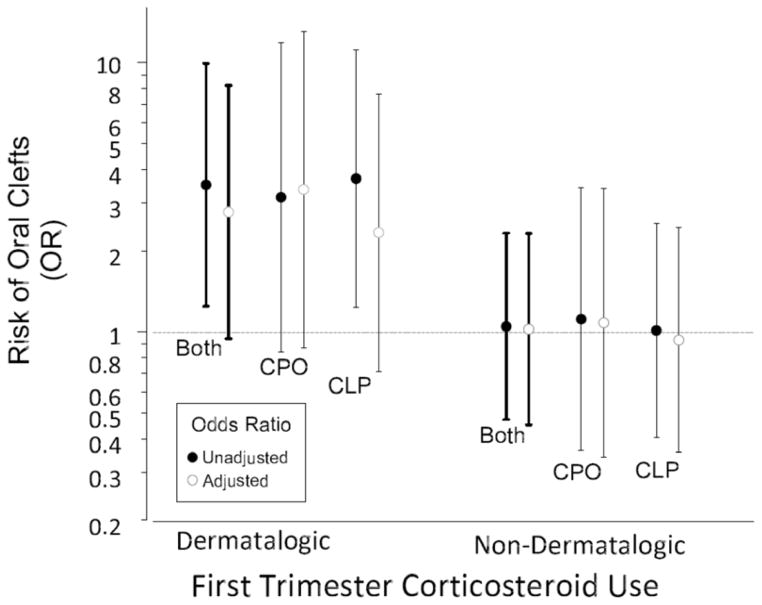
Table 2 shows crude and adjusted ORs for the association between corticosteroid use during first trimester and clefts. Mothers who reported using dermatological corticosteroids had an increased risk of a child with any cleft (crude OR = 3.5; 1.3–9.9). This risk was similar for the two subtypes of clefts (CLP, 3.7; 1.3–11; and CPO, 3.2; 0.84–12). After adjusting for potential confounders, the association was weakened for CLP although not for CPO (CLP aOR= 2.3; 0.7–7.7; and CPO, 3.4; 0.9–13). Results were weaker when restricted to isolated clefts (CLP aOR = 2.0; 0.5–7.2; CPO aOR = 2.6; 0.5–4.0). There were no associations between reported use of “other” corticosteroids during first trimester and clefts (Figure 1).
Table 2.
Corticosteroids Reported During the First Trimester of Pregnancy in the Norway Cleft Study (case-control) and MoBa (cohort). Abbreviations: cleft lip +/− palate (CLP), cleft palate only (CPO).
| Effect | N
(exposed) (CLP/CPO)/Controls |
All | OR 95% CI |
CPO | |
|---|---|---|---|---|---|
| CLP | |||||
| Norway Cleft | |||||
| Any CST | |||||
| Crude | 24 (16/8)/19 | 1.71 (0.93–3.16) | 1.73 (0.88–3.42) | 1.67 (0.72–3.87) | |
| Adjusted | 23 (15/8)/19 | 1.51 (0.80–2.86) | 1.35 (0.65–2.78) | 1.68 (0.71–3.98) | |
| Dermatological | |||||
| Crude | 13 (9/4)/5 | 3.52 (1.25–9.92) | 3.71 (1.23–11.4) | 3.16 (0.84–11.87) | |
| Adjusted | 12 (8/4)/5 | 2.79 (0.94–8.23) | 2.34 (0.71–7.66) | 3.38 (0.87–13.09) | |
| Other* | |||||
| Crude | 11 (7/4)/14 | 1.05 (0.47–2.32) | 1.01 (0.41–2.3) | 1.12 (0.36–3.42) | |
| Adjusted | 11 (7/4)/14 | 1.02 (0.45–2.32) | 0.93 (0.36–2.44) | 1.08 (0.34–3.40) | |
| Isolated cases | |||||
| Any CST | |||||
| Crude | 16 (12/4)/19 | 1.43 (0.73–2.81) | 1.48 (0.71–3.01) | 1.30 (0.44–3.90) | |
| Adjusted | 15 (11/4)/19 | 1.25 (0.61–2.55) | 1.15 (0.51–2.57) | 1.30 (0.42–4.05) | |
| Dermatological | |||||
| Crude | 9 (7/2)/5 | 3.06 (1.02–3.92) | 3.28 (1.03–10.4) | 2.48 (0.48–12.95) | |
| Adjusted | 8 (6/2)/5 | 2.27 (0.70–7.34) | 1.96 (0.54–7.17) | 2.64 (0.49–14.31) | |
| Other* | |||||
| Crude | 7 (5/2)/14 | 0.87 (0.34–2.09) | 0.82 (0.29–2.30) | 0.88 (0.20–3.90) | |
| Adjusted | 7 (5/2)/14 | 0.85 (0.33–2.18) | 0.81 (0.27–2.37) | 0.83 (0.18–3.91) | |
|
| |||||
| MoBa* | |||||
| Dermatological | |||||
| Crude | 10 (7/3)/27 | 1.10 (0.50–2.90) | 1.17 (0.50–2.75) | 1.00 (0.30–3.41) | |
| Adjusted | 9 (7/2)/27 | 0.99 (0.45–2.17) | 1.21 (0.51–2.89) | 0.60 (0.14–2.62) | |
Other use applies to non-dermatological use.
The results are presented for dermatological use only in MoBa, including all cases (isolated and non-isolated).
Figure 1.
Association of risk of delivering an infant with cleft lip and palate (CLP) and cleft palate only (CPO) among women in the Norway Cleft Study who reported using corticosteroids during the first-trimester of pregnancy.
MoBa (cohort)
In the cohort study, 7 (6%) of the case mothers of children with cleft lip and palate and 3 (5 %) of the case mothers of children with cleft palate only used dermatological corticosteroids during pregnancy, compared with 27 (5%) of the control mothers. There was no association in the MoBa for any cleft type (crude OR= 1.1;0.5–2.9; adjusted OR= 1.0; 0.5–2.2), CLP (crude OR= 1.2;0.5–2.8; adjusted OR= 1.2; 0.5–2.9) nor for CPO (crude OR= 1.0;0.3–3.4; adjusted OR= 0.6; 0.1–2.6).
DISCUSSION
A recent study from Denmark reported an increase in oral clefts among mothers who had been prescribed dermatologic corticosteroids during pregnancy. (10) Our data from the Norwegian Cleft Study (a case-control study) also suggest the possibility of an association between mothers’ reported use of dermatological corticosteroids during the first trimester of pregnancy and clefts in the offspring, although the number of exposed mothers were small. This association was not present in the MoBa Study (a cohort study).
The literature
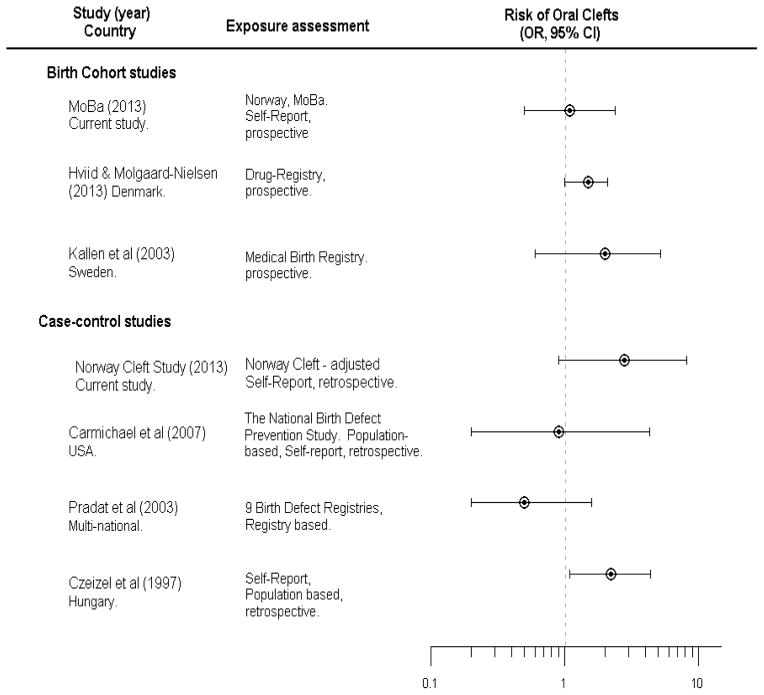
The hypothesis of an association between maternal corticosteroid use and clefts in offspring has a long history, beginning with a classic study of corticosteroids as a laboratory teratogen (the systemic cortisone dose needed to produce clefts in this study was approximately 60 mg/kg, which ranges from 15 to 150 times the recommended therapeutic dose in humans). (1, 21, 22) This hypothesis has been raised to support observed associations between stressful life-experiences to the mother during pregnancy, as stress leads to increased levels of cortisol which is a corticosteroid, and clefts in her offspring. (23, 24) Figure 2 outlines epidemiologic studies of maternal use of dermatological corticosteroids and risk of facial clefts in the offspring. Mothers’ use of oral corticosteroids has also been associated with clefts in several epidemiological studies. (3, 6, 8, 9, 25) Since only two mothers in our study (both case mothers) reported using oral corticosteroids, we were unable to evaluate this exposure.
Figure 2.
Summary of previous and current studies on dermatological corticosteroid use and risk of oral clefts.
*Estimates in the study by Hviid and Molgaard-Nielsen reflects estimates for CLP.
Studies from both Sweden (5) and Denmark (10) reported a possible association between facial clefts and dermatological corticosteroids (Figure 2). These two studies had different ascertainment of exposure. The Danish study used information from the Danish Prescription Drug Register to assess exposure. This method of defining exposure is objective but provides no information on whether women actually used the prescribed medication. In another study, only 16 % of Danish mothers who were prescribed dermatological corticosteroids actually reported using them. (26) The Swedish study used medication information from prenatal records, in which mothers had prospectively reported their medication use at week 10–12 in pregnancy, which is similar to the exposure assessment in MoBa.
Dermal absorption and placental transfer
Topical corticosteroids are generally considered to be safer than oral steroids during pregnancy (11, 27), although topical corticosteroids can be systemically absorbed. (28) The indications for topical corticosteroid use include rashes, psoriasis, dermatitis and eczema.(29) The amount of circulating corticosteroid after dermatologic application varies by the amount, frequency, and total surface area to which it is applied. Absorption also depends on the region to which it is applied (30) and the condition of the skin. (31) These factors make an estimate of exposure from topical applications extremely difficult without serum measures.
The transfer of steroids to the fetus raises further questions. In pregnant mice and rabbits, the corticosteroid betamethasone is systemically absorbed when applied to the skin, passes the placenta, and is detected in the fetus. (32) The human placenta expresses high levels of 11β-hydroxysteroid dehydrogenase type 2 that can interfere with placental transfer of corticosteroids. However this barrier is incomplete and maternal corticosteroids do cross. (33, 34) Indeed, the treatment of mothers at risk of preterm birth with intramuscular injection of corticosteroids depends on placental transfer to facilitate fetal lung maturation. (35) Since the fetus has a lower endogenous level of corticosteroids than the mother, even relatively limited transmission from the mother might be of significance at crucial stages of embryonic development. (36)
Strengths and Limitations
Our results come from two Norwegian population-based studies, one a case-control study that captured most clefts cases in 1996–2001, and the other a cohort study that was in the field from 1999–2008. While the two studies overlapped in time, we consider it unlikely that they would include more than a few of the same cases. The birth cohort had its slowest recruitment in the early years when the case-control study was in progress. Still, it is not possible to ascertain the number of overlapping cases owing to the anonymization of participants in the case-control study. Further we do not know if any of the case families were related to the control families. The two studies were not suited for a meta-analysis due to heterogeneity.
The two studies show the classic strengths and weaknesses of their respective designs. The case-control study had far more cases and thus more power. The case-control study also collected more specific information on relevant exposures during each of the first three months of pregnancy, when the structures of the face are being formed. While participation was generally high, it was less for controls than for cases (78% vs. 88%). Thus we cannot rule out bias from differential participation. Another concern is the possibility of differential reporting by cases and controls. A third issue is confounding by indication. (37, 38) It is not implausible that skin diseases and facial clefts might share certain causes. Keratinocytes play an important role in the fusion of the palatal shelves, and a dysfunction could result in defects in the skin and mucosa. (39) Mutations in interferon regulatory factor 6 (IRF6) are believed to underlie Van der Woude syndrome and popliteal pterygeum syndrome, both of which involve oral clefts and skin manifestations. (40, 41) Given that topical applications are typically for skin conditions, it would be difficult to disentangle confounding by indication in an observational study.
The cohort design in principle provides a stronger basis of inference in that exposure information is collected before the outcome is known. However, for the study of rare diseases (such as facial clefts), even very large cohorts have limited power. While the estimates of risk in the cohort study were close to the null, confidence intervals were wide and could not exclude the possibility of strong associations. Another limitation of cohort studies is the inevitable selection of volunteers when recruiting into a long-term study. Of the invited mothers-to-be in the Norwegian Mother and Child Cohort, only 39% gave their consent. Selection bias in this study has previously been described in detail. (14)
A general limitation of our analyses is our coarse grouping of corticosteroid medications, made necessary by the low frequency of exposure. Different types of corticosteroids may differ in their teratogenicity. Cortisone produces clefts in mice but not rats, while clefts in rats can be produced by betamethasone, triamcinolone, and dexamethasone. (42) Betamethasone is the steroid most frequently prescribed topically to reduce inflammation from allergy or irritation. If only one type of corticosteroid is responsible for the observed association, larger studies or more highly exposed populations would be needed to clarify this association.
In sum, the association of maternal corticosteroid exposure with oral clefts has been addressed in a range of large and relatively well-designed epidemiologic studies. The fact that a preponderance of reported associations have been positive may reflect publication bias. Still, it is not possible to exclude the possibility that corticosteroids present some underlying risk to the developing fetus. Our own attempt to clarify this question in two large studies has only added to the conflicting information. At this point, we can conclude only that a causal effect cannot be dismissed completely, and that if causal, corticosteroids could explain only a very small portion of oral clefts.
Acknowledgments
This work was funded by the Western Norwegian Health Authorities. The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, US National Institute of Environmental Health Sciences (grants Z01 ES049027-11 and Z01 ES040007), and the Research Council of Norway (grant 166026/V50). Special thanks to Quaker Harmon and Donna Baird for useful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fraser FC, Fainstat TD. Production of congenital defects in the offspring of pregnant mice treated with cortisone: Progress report. Pediatrics 1951. 1951 Oct 1;8(4):527–33. [PubMed] [Google Scholar]
- 2.Walker BE, Fraser FC. The Embryology of Cortisone-induced Cleft Palate. Journal of Embryology and Experimental Morphology 1957. 1957 Jun;5(2):201–9. [Google Scholar]
- 3.Carmichael SL, Shaw GM. Maternal corticosteroid use and risk of selected congenital anomalies. Am J Med Genet. 1999;86(3):242–4. doi: 10.1002/(sici)1096-8628(19990917)86:3<242::aid-ajmg9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Carmichael SL, Shaw GM, Ma C, Werler MM, Rasmussen SA, Lammer EJ. Maternal corticosteroid use and orofacial clefts. Am J Obstet Gynecol. 2007;197(6):585, e1–e7. doi: 10.1016/j.ajog.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 5.Källén B. Maternal drug use and infant cleft lip/palate with special reference to corticoids. The Cleft Palate-Craniofacial Journal 2003. 2003 Nov 01;40(6):624–8. doi: 10.1597/02-077. [DOI] [PubMed] [Google Scholar]
- 6.Edwards MJ, Agho K, Attia J, Diaz P, Hayes T, Illingworth A, et al. Case-control study of cleft lip or palate after maternal use of topical corticosteroids during pregnancy. American Journal of Medical Genetics Part A. 2003;120A(4):459–63. doi: 10.1002/ajmg.a.20130. [DOI] [PubMed] [Google Scholar]
- 7.Czeizel AE, Hirschberg J. Orofacial clefts in Hungary. Epidemiological and genetic data, primary prevention. Folia Phoniatr Logop. 1997;49(3–4):111–6. doi: 10.1159/000266446. Epub 1997/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 8.Pradat P, Robert-Gnansia E, Di Tanna GL, Rosano A, Lisi A, Mastroiacovo P. First trimester exposure to corticosteroids and oral clefts. Birth Defects Research Part A: Clinical and Molecular Teratology. 2003;67(12):968–70. doi: 10.1002/bdra.10134. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Pinilla E, Luisa Martínez-Frías M. Corticosteroids during pregnancy and oral clefts: A case-control study. Teratology. 1998;58(1):2–5. doi: 10.1002/(SICI)1096-9926(199807)58:1<2::AID-TERA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Hviid A, Mølgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. Canadian Medical Association Journal 2011. 2011 Apr 19;183(7):796–804. doi: 10.1503/cmaj.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi C-C, Wang S-H, Kirtschig G, Wojnarowska F. Systematic review of the safety of topical corticosteroids in pregnancy. Journal of the American Academy of Dermatology. 2010;62(4):694–705. doi: 10.1016/j.jaad.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox AJ, Lie RT, Solvoll K, Taylor J, McConnaughey DR, Abyholm F, et al. Folic acid supplements and risk of facial clefts: national population based case-control study. British Medical Journal. 2007 Mar 3;334(7591):464–7. doi: 10.1136/bmj.39079.618287.0B. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lie RT, Wilcox AJ, Taylor J, Gjessing HK, Saugstad OD, Aabyholm F, et al. Maternal smoking and oral clefts - The role of detoxification pathway genes. Epidemiology. 2008 Jul;19(4):606–15. doi: 10.1097/EDE.0b013e3181690731. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009 Nov;23(6):597–608. doi: 10.1111/j.1365-3016.2009.01062.x. Epub 2009/10/21. eng. [DOI] [PubMed] [Google Scholar]
- 15.Magnus P, Irgens LM, Haug K, Nystad W, Skjærven R, Stoltenberg C, et al. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa) International Journal of Epidemiology 2006. 2006 Oct 1;35(5):1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 16.NIEHS. Available at: http://www.niehs.nih.gov/research/atniehs/labs/epi/studies/ncl/question/index.cfm.
- 17.Sperber GH. Formation of the Primary Palate and Palatogenesis: Closure of the Secondary Palate. 2002. pp. 5–24. [Google Scholar]
- 18.WHO. ATC system. http://www.whocc.no/atc_ddd_index/
- 19.The Norwegian Institute of Public Health. Available at: http://www.fhi.no/dokumenter/1f32a49514.pdf.
- 20.Kubon C, Sivertsen Å, Andreas Vindenes H, Åbyholm F, Wilcox A, Terje Lie R. Completeness of registration of oral clefts in a medical birth registry: a population-based study. Acta Obstetricia et Gynecologica Scandinavica. 2007;86(12):1453–7. doi: 10.1080/08037050701645090. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka S, Mizorogi T, Nishijima K, Kuwahara S, Tsujio M, Aoyama H, et al. Body and major organ weights of A/J-Chr 11(SM) consomic mice. Exp Anim. 2009 Jul;58(4):357–61. doi: 10.1538/expanim.58.357. Epub 2009/08/06. eng. [DOI] [PubMed] [Google Scholar]
- 22.PDR.net. http://www.pdr.net/drug-summary/prednisolone-tablets?druglabelid=2138&id=900.
- 23.Carmichael SL, Shaw GM, Yang W, Abrams B, Lammer EJ. Maternal stressful life events and risks of birth defects. Epidemiology. 2007 May;18(3):356–61. doi: 10.1097/01.ede.0000259986.85239.87. Epub 2007/04/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingstrup KG, Liang H, Olsen J, Nohr EA, Bech BH, Wu CS, et al. Maternal bereavement in the antenatal period and oral cleft in the offspring. Hum Reprod. 2013 Jan 4; doi: 10.1093/humrep/des434. Epub 2013/01/08. Eng. [DOI] [PubMed] [Google Scholar]
- 25.Carmichael SL, Shaw GM, Ma C, Werler MM, Rasmussen SA, Lammer EJ. Maternal corticosteroid use and orofacial clefts. Am J Obstet Gynecol. 2007 Dec;197(6):585, e1–7. doi: 10.1016/j.ajog.2007.05.046. discussion 683–4, e1–7. Epub 2007/12/07. eng. [DOI] [PubMed] [Google Scholar]
- 26.Olesen C, Søndergaard C, Thrane N, Lauge Nielsen G, de Jong-van den Berg L, Olsen J, et al. Do pregnant women report use of dispensed medications? Epidemiology. 2001;12(5):497–501. doi: 10.1097/00001648-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Lam J, Polifka JE, Dohil MA. Safety of dermatologic drugs used in pregnant patients with psoriasis and other inflammatory skin diseases. Journal of the American Academy of Dermatology. 2008;59(2):295–315. doi: 10.1016/j.jaad.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. Journal of the American Academy of Dermatology. 2006;54(1):1–15. doi: 10.1016/j.jaad.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Habif TP. Clinical dermatology: a color guide to diagnosis and therapy. 2. St. Louis: Mosby; 1990. [Google Scholar]
- 30.Feldmann RJ, Maibach HI. Regional Variation in Percutaneous Penetration of 14C Cortisol in Man1. The Journal of Investigative Dermatology. 1967;48(2):181–3. doi: 10.1038/jid.1967.29. [DOI] [PubMed] [Google Scholar]
- 31.Turpeinen M, Lehtokoski-Lehtiniemi E, Leisti S, Salo OP. Percutaneous absorption of hydrocortisone during and after the acute phase of dermatitis in children. Pediatric Dermatology. 1988;5(4):276–9. doi: 10.1111/j.1525-1470.1988.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 32.Yamada H, Nakano M, Ichihashi T. Fetal concentration after topical application of betamethasone 17,21-dipropionate (S-3440) ointment and teratogenesis in mice and rabbits. Pharmacometrics. 1981;21(4):645–55. [Google Scholar]
- 33.Seckl Prenatal glucocorticoids and long-term programming. European Journal of Endocrinology 2004. 2004 Nov 1;151(Suppl 3):U49–U62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- 34.Benediktsson R, Calder AA, Edwards CRW, Seckl JR. Placental 11β-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clinical Endocrinology. 1997;46(2):161–6. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- 35.Crowley P. Prophylactic corticosteroids for preterm birth (Withdrawn Paper. 2006, art no. CD000065) Cochrane Db Syst Rev. 2006;(3) doi: 10.1002/14651858.CD000065.pub2. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. The Lancet. 1998;352(9129):707–8. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- 37.Walker AM. Confounding by indication. Epidemiology. 1996 Jul;7(4):335–6. Epub 1996/07/01. eng. [PubMed] [Google Scholar]
- 38.Wilkins I, Loy G, Rogers D, Chor J, To M-L, Velez B. Maternal corticosteroid use and orofacial clefts: A study by Carmichael et al. Am J Obstet Gynecol. 2007;197(6):683–4. doi: 10.1016/j.ajog.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 39.Ingraham CR, Kinoshita A, Kondo S, Yang B, Sajan S, Trout KJ, et al. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6) Nat Genet. 2006;38(11):1335–40. doi: 10.1083/ng1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson RJ, Dixon J, Malhotra S, Hardman MJ, Knowles L, Boot-Handford RP, et al. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat Genet. 2006;38(11):1329–34. doi: 10.1038/ng1894. [DOI] [PubMed] [Google Scholar]
- 41.Zucchero TM, Cooper ME, Maher BS, Daack-Hirsch S, Nepomuceno B, Ribeiro L, et al. Interferon Regulatory Factor 6 (IRF6) Gene Variants and the Risk of Isolated Cleft Lip or Palate. New England Journal of Medicine. 2004;351(8):769–80. doi: 10.1056/NEJMoa032909. [DOI] [PubMed] [Google Scholar]
- 42.Walker BE. Induction of cleft palate in rats with antiinflammatory drugs. Teratology. 1971;4(1):39–42. doi: 10.1002/tera.1420040107. [DOI] [PubMed] [Google Scholar]