Abstract
Merkel cell carcinoma (MCC) is an aggressive skin cancer that is causally associated with ultraviolet light exposure and a recently discovered polyomavirus. Before 2010, MCC was staged using any of 5 unique systems in active use. In 2010, a consensus staging system for MCC was adopted worldwide and replaced these systems. This consensus system includes substages that reflect prognostic differences based on whether nodal evaluation was performed by pathologic analysis or clinical assessment alone. MCC-specific disease classification in ICD-9, to be expanded in the upcoming ICD-10, has improved the ability to track and manage this malignancy. Several biomarkers and histopathologic features have been identified that improve understanding of this cancer and may lead to future refinement of the current staging system. In 2008, the Merkel cell polyomavirus was discovered and is now thought to be a critical mechanism of transformation in at least 80% of MCCs. In patients who produce antibodies to the viral T-antigen oncoprotein, the titer increases and decreases with MCC disease burden and can be a clinically useful marker of recurrence. Diverse studies link CD8-positive T-cell function with outcomes in MCC and serve as the rational basis for ongoing trials of therapies to augment cellular immunity. This article reviews basic and translational research insights that will lead to improved staging, prognostic accuracy, and mechanism-based therapy for this often-lethal skin cancer.
Merkel cell carcinoma (MCC), or primary neuroendocrine carcinoma of the skin, is an aggressive cutaneous malignancy with 3 times the disease-specific mortality of melanoma (46% vs 15%).1 The annual incidence is approximately 1500 cases in the United States and has been increasing rapidly in recent years, likely partly because of the increasing prevalence of risk factors (eg, aging population, immunosuppression, cumulative ultraviolet light exposure) and improved detection (eg, cytokeratin 20 staining introduced in the 1990s) (Figure 1).2 MCC characteristically presents as a solitary pink or purple nodule (Figure 2) that typically has several of the features summarized in the mnemonic “AEIOU”: Asymptomatic (eg, painless, nonpruritic), Expanding rapidly, Immune suppression, Older than 50 years, and arising on Ultraviolet-exposed, fair skin.3 In 2008, the Merkel cell polyomavirus (MCPyV) was discovered and has been shown to be associated with approximately 80% of MCCs,4 thus joining 6 other viruses now known to be either direct or indirect causes of approximately 50 human malignancies.5
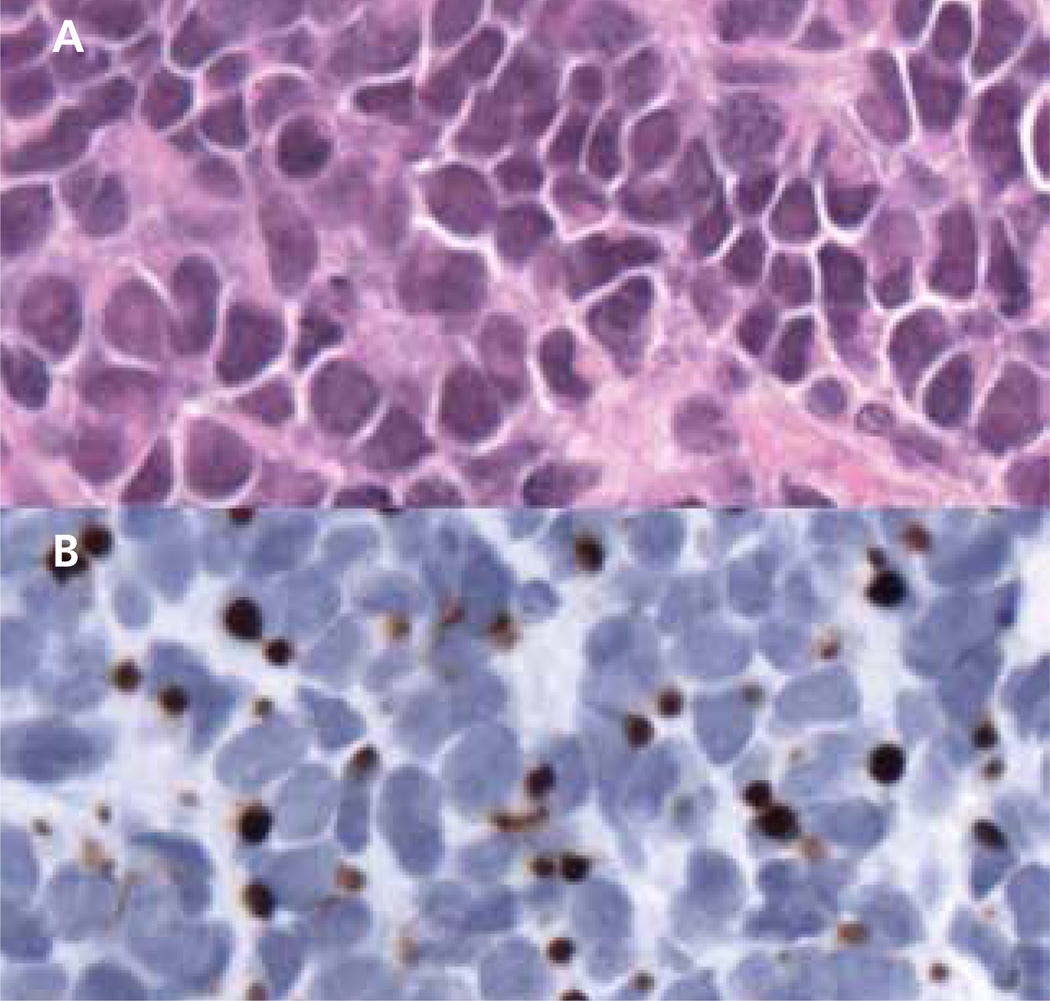
Figure 1. Light microscopy of Merkel cell carcinoma (MCC; original magnification ×400).
(A) Hematoxylin and eosin staining shows characteristic features of MCC, including densely packed cells with heterogeneity in nuclear size, nuclear molding, and sparse cytoplasm. (B) Immunohistochemistry with anti–cytokeratin 20 (CK20) is classically positive in MCC. This section highlights the typical perinuclear dot-like pattern of the stain.
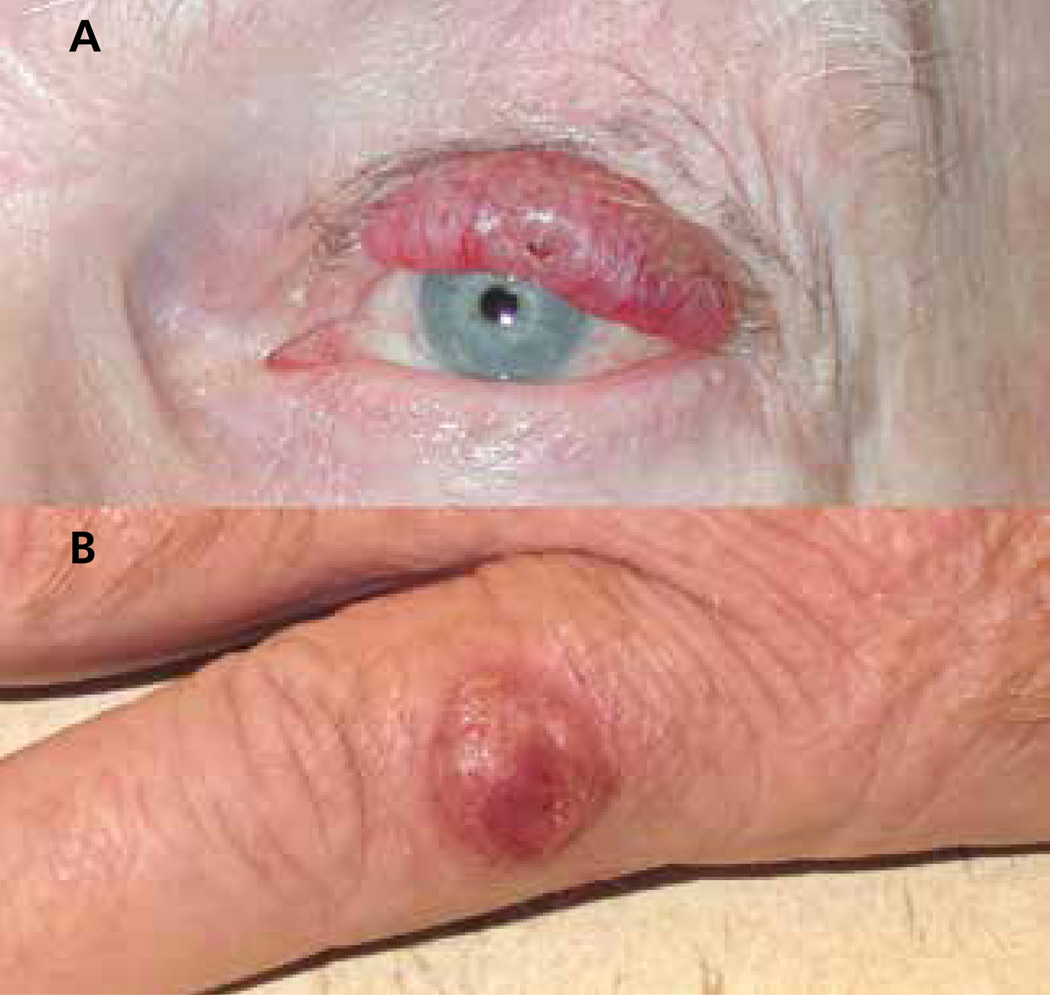
Figure 2. Two characteristic Merkel cell carcinoma (MCC) primary lesions.
(A) Primary MCC on the left upper eyelid of an 85-year-old man with chronic lymphocytic leukemia that highlights the typical red-purple color and location on sun-exposed skin. This lesion was originally presumed to be a chalazion, and was biopsied after it failed to respond to antibiotics. (B) Primary MCC on the left small finger of a 70-year-old man. Characteristically, both of these lesions were nontender and nonpruritic.
History of MCC Staging Before 2010
Before 2010, 5 different staging systems were used in the management of MCC (Table 1),6–10 all of which were based on data gathered from a relatively small number of patients (70–251 cases) from a limited number of institutions (1–3). Although each adhered to the tissue-node-metastasis (TNM) system, they differed significantly with respect to several characteristics, including the overall number of stages (3 vs 4), size cutoff for the primary tumor (≤1 vs ≤2 cm), and number of involved nodes needed to constitute upstaging (any vs >2). These differences generated confusion among patients and providers, and hindered discussion of MCC management. For example, depending on the staging system being used, a 1-cm primary tumor with a single positive lymph node could be staged as II, IIA, or III, whereas a distant metastatic lesion could be III or IV. These issues led to the establishment of a single evidence-based staging system that could clarify the classification and management of MCC.
Table 1.
MCC Staging Systems
| Study (Year) | Cohort Size | Stage I | Stage II | Stage III | Stage IV |
|---|---|---|---|---|---|
| Previous Staging Systems | |||||
| Yiengpruksawan et al,6 1991 | 70 | Local | Nodal | Metastatic | --- |
| Allen et al,7 1999 | 190 | IA: Local <2 cm | Nodal | Metastatic | --- |
| IB: Local ≥2 cm | |||||
| AJCC 6th Edition,10 2002 | 0 MCCs (based on other skin cancers) |
Local ≤2 cm | Local >2 cm | Nodal or local with extradermal deep invasion | Metastatic |
| Allen et al,8 2005 | 251 | Local <2 cm | Local ≥2 cm | Nodal | Metastatic |
| Clark et al,9 2007 | 110 | Local ≤1 cm | IIA: Local ≤1 cm and ≤2 positive regional nodes IIB: Local >1 cm |
>2 positive nodes | Metastatic |
| Current Staging System | |||||
| AJCC 7th Edition,11 2010 | 5823 | IA: Local ≤2 cm, pathologic nodal evaluationa IB: Local ≤2 cm, clinical nodal evaluationb |
IIA: Local >2 cm, pathologic nodal evaluationa IIB: Local >2 cm, clinical nodal evaluationb |
IIIA: Microscopic nodalc IIIB: Macroscopic nodald |
Metastatic |
Abbreviation: MCC, Merkel cell carcinoma.
Pathologic nodal evaluation implies tissue was evaluated under a microscope for nodal metastasis.
Clinical nodal evaluation includes palpation and/or radiographic imaging (eg, CT or PET).
Microscopic nodal disease constitutes tumor cells found on pathologic evaluation only.
Macroscopic nodal disease constitutes clinically evident MCC that was either palpable or radiographically apparent.
2010 AJCC 7th Edition Consensus Staging System
In 2010, the AJCC and the Union for International Cancer Control (UICC) adopted the first consensus staging system for MCC.11 The system was based on survival analysis of 5823 MCC cases from the National Cancer Data Base, representing the largest such cohort to date.1 Analysis of this cohort led to a 4-stage system in which localized disease is distinguished by the primary tumor size (≤2 cm for stage I vs >2 cm for stage II), any nodal disease is stage III, and distant metastatic disease is stage IV (Table 1). Clinical nodal examination alone has been shown to miss upwards of a third of nodal metastases,12 and patients who were only staged with clinical nodal examination have poorer survival compared with those whose nodes were found to be negative after pathologic examination, such as with sentinel lymph node biopsy.1 Based on this significant survival difference, local disease was substaged to reflect whether the draining nodes were evaluated pathologically (IA or IIA) or only clinically via palpation or imaging (IB or IIB). For patients with nodal disease, sub-stages were created based on whether the metastasis was only detected on microscopic evaluation (IIIA), or if the nodal involvement was detectable through clinical or radiologic assessment (IIIB). Like all other cancers, MCC staging is anticipated to undergo revision as part of an 8th edition of the AJCC staging system. Recent developments in the understanding of MCC biology (discussed later) are likely to be integrated into the new staging algorithm.
ICD-9 Codes (209.3X) and Transition to ICD-10 (C4A.X)
The fact that MCC is histologically unique and far more aggressive than typical nonmelanoma skin cancers led not only to its own staging system but also to its independent codes under the International Classification of Diseases and Related Health Problems (ICD) in 2009.13 Previously grouped with cancers such as basal cell carcinoma, which is far more common, rarely lethal, and not required to be reported, MCC was frequently not tracked or managed carefully. After a request for dedicated disease codes, 7 ICD-9 codes were assigned to MCC by the Centers for Disease Control and Prevention (CDC) and became active in October 2009.13 Implementation of these codes has not only facilitated diagnosis and tracking, but also aided in obtaining insurance approval for disease-appropriate surveillance and management.
ICD-10 represents a major expansion of ICD-9, and will increase the number of codes by roughly 10-fold across all diseases. With respect to MCC diagnosis, the increasing number of codes affords greater specificity for anatomic site, including unique codes for laterality (Table 2). Despite a delay in implementation, health care systems in the United States are expected to continue the transition to ICD-10.
Table 2.
ICD-9 and ICD-10 Codes for Merkel Cell Carcinoma
| ICD-9 Code | Description | ICD-10 Code | Further specification |
|---|---|---|---|
| 209.3 | MCC | C4A | --- |
| 209.31 | MCC of the face | C4A.0 | Lip |
| C4A.1 | Eyelid (including canthus) | ||
| C4A.10 | Eyelid, unspecified | ||
| C4A.11 | Eyelid, right | ||
| C4A.12 | Eyelid, left | ||
| C4A.2 | Ear (and external auricular canal) | ||
| C4A.20 | Ear, unspecified | ||
| C4A.21 | Ear, right | ||
| C4A.22 | Ear, left | ||
| C4A.3 | Other and unspecified parts of face Face, unspecified |
||
| C4A.30 | Nose | ||
| C4A.31 | Face, other part | ||
| C4A.39 | |||
| 209.32 | MCC of the scalp and neck | C4A.4 | --- |
| 209.33 | MCC of the upper limb | C4A.6 | Upper limb (including shoulder) |
| C4A.60 | Upper limb, unspecified | ||
| C4A.61 | Upper limb, right | ||
| C4A.62 | Upper limb, left | ||
| 209.34 | MCC of the lower limb | C4A.7 | Lower limb (including hip) |
| C4A.70 | Lower limb, unspecified | ||
| C4A.71 | Lower limb, right | ||
| C4A.72 | Lower limb, left | ||
| 209.35 | MCC of the trunk | C4A.5 | --- |
| C4A.51 | Anal or perianal skin | ||
| C4A.52 | Skin of breast | ||
| C4A.59 | Trunk, other part | ||
| 209.36 | MCC of the other sites | C4A.8 | Overlapping sites (eg, junction of neck and trunk, junction of abdomen and lower limb) |
| C4A.9 | unspecified site | ||
| 209.75 | Secondary MCC (refers to MCC presenting in nodal or visceral sites without a known primary) | C7B.1 | --- |
| V10.91 | Personal history of malignant neuroendocrine tumor | Z85.821 | History of MCC of the skin |
Abbreviation: MCC, Merkel cell carcinoma.
Etiology
Ultraviolet Radiation
Several lines of evidence suggest a strong link between ultraviolet (UV) light exposure and the development of MCC. These include the predilection of MCC for sun-exposed skin, its low incidence among dark-skinned people, and the predominance of UV-induced genetic mutations (eg, C → T and CC → TT nucleotide transitions) similar to those found in other cutaneous malignancies (eg, squamous cell carcinoma).14,15 In addition to UV being a direct carcinogen, UV-mediated immune suppression may facilitate the tumor’s evasion of the immune system.16 Lastly, it has been suggested that UV plays a role in the integration of MCPyV into the host genome and the induction of truncation mutations in the virus thought to be necessary for oncogenesis.5
Immune Suppression
Although most patients with MCC have no known immune dysfunction, approximately 8% have some form of profound, chronic immune suppression.3 This is a significant overrepresentation compared with the general population, and suggests an important role for the immune system as a gatekeeper of carcinogenesis for MCC. Immunosuppression in the setting of MCC is most commonly caused by comorbid hematologic malignancy (eg, chronic lymphocytic leukemia [CLL]),17 HIV/AIDS,18 and immunosuppressive medications in the setting of advanced autoimmune disease19 or solid organ transplantation.20
Merkel Cell Polyomavirus
The observation in 2002 that HIV was associated with a 13.4-fold increase in the risk of MCC led to the search for an infectious origin.18 In 2008, Feng et al4 discovered MCPyV, the seventh virus now thought to be either a direct or indirect cause of human malignancy.5 MCPyV is currently thought to be associated with approximately 80% of MCCs, although a recent study has suggested this may be an underestimate.21 Asymptomatic infection with MCPyV is highly prevalent in the general population (reportedly as high as 80%–90%),22 and is likely transmitted at an early age.23 Despite its ubiquity, only a small fraction of individuals infected with MCPyV develop MCC.
MCPyV encodes 2 main oncoproteins: large tumor antigen (LT) and small tumor antigen (sT). Both have been shown to be constitutively expressed in MCC, and LT is capable of binding and inactivating the retinoblastoma tumor suppressor protein as one key mechanism of tumorigenesis.24 In vitro studies of MCC cell lines have shown knockdown of these oncoproteins to be sufficient to arrest growth and/or induce cell death.25 These studies suggest that MCPyV-positive MCCs require expression of oncoproteins for survival. Taken together, these findings have led to the current thinking that MCPyV is a major mechanism of transformation in MCC.
Prognosis
Even within a given stage, the clinical course for patients with MCC is often highly variable. For this reason, several groups have attempted to identify clinical and histopathologic biomarkers that can predict outcome independent of stage (Table 3).
Table 3.
MCC Prognostic Factors
| Prognostic Factor | Description | Potential Impact on Prognosis |
|---|---|---|
| MCPyV VP1 serology | Antibodies against MCPyV capsid protein VP1; not specific to MCC | High titer is positive |
| MCPyV oncoprotein serology | Antibodies against MCPyV small and large T antigens; specific to MCC | Titer tracks with disease burden |
| LV I | Tumor cells found within lymphatic and/or blood vessels | Negative |
| Impaired immune function | Profoundly deficient cellular immunity, usually secondary to hematologic malignancy, HIV/AIDS, or medication | Negative |
| TILs | Lymphocytes found between tumor cells (stromal invasion does not count) | Positive |
| Unknown primary | Extracutaneous MCC without a primary tumor | Positive |
| p63 expression | Multifunctional transcription factor | Negative/unclear |
Abbreviations: LVI, lymphovascular invasion; MCC, Merkel cell carcinoma; MCPyV, Merkel cell polyomavirus; TILs, tumor-infiltrating lymphocytes.
Antibodies to MCPyV Capsid Protein and T-Antigen Oncoprotein
Approximately 90% of patients with MCC and 60% of healthy people produce antibodies to the MCPyV capsid protein VP1.26 Titers of these antibodies are stably elevated over time, suggesting that this is likely a marker of prior MCPyV exposure rather than of disease status.27 A study in a cohort of French patients found that higher VP1 titers correlate with significantly improved disease-free survival,28 suggesting that VP1 antibodies may be a measure of immune response to MCC.
In contrast to VP1, antibodies against the viral T-antigen oncoprotein are present in less than 1% of healthy population controls versus approximately half of patients with MCC,27 making these antibodies specific to MCC. Among 20 evaluable patients with MCC, T-antigen antibody titers declined in 10 of 10 patients who remained disease-free after treatment, and increased in 8 of 10 patients who eventually experienced disease progression. In 3 of the patients experiencing disease progression, the increase in T-antigen titer preceded clinical detection of the recurrence.27 These results suggest that serology against MCPyV T antigens may be a useful means of monitoring disease burden in MCC, and this test is clinically available as of 2014 (www.merkelcell.org/sero). A larger validation study has been completed (manuscript in preparation) and will help more accurately determine the role of this biomarker.
Lymphovascular Invasion
Lymphovascular invasion (LVI) by tumor cells may be a precursor event to metastasis and has repeatedly been shown to be independently associated with poorer prognosis in MCC.29–31 In one cohort, the absence of LVI was associated with less than 1% MCC-specific mortality.31 Although typically correlated with larger tumor size, LVI has been demonstrated in MCCs as small as 0.3 cm.32 The ability of MCC to spread very early may account for the unusual aggressiveness of this malignancy. The strong association of LVI with poor prognosis in several cohorts may lead to its incorporation into the next staging algorithm.
Impaired Immune Function
Not only are profoundly immunosuppressed patients at a higher risk of developing MCC, they are also more likely to die from MCC than patients with intact immune function. In an analysis of a large cohort from the SEER database, Brewer et al17 found that patients with MCC and CLL were nearly 4 times as likely to die from MCC as those without. This finding may reflect a direct negative impact of CLL on cellular immunity. However, because SEER does not capture prior treatment history, the potential effect of T-cell–sup-pressive therapies used to manage CLL could not be excluded. Analysis of a distinct cohort that captured multiple forms of immunosuppression (eg, CLL and other hematologic malignancies, HIV/AIDS, long-term immunosuppressive medications, solid organ transplant) also found that chronic immune suppression was associated with increased MCC-specific mortality.33 In line with this theme, a recent study showed that patients with absolute lymphocyte counts lower than 1100/mm3 at the time of initial management had significantly reduced overall survival in multivariate analysis.34 These observations suggest that immunosuppressed patients with MCC should be monitored particularly closely and that medications impeding T-cell function should be avoided whenever possible in the management of MCC.
Tumor-Infiltrating Lymphocytes
Multivariate models in several cohorts have shown that tumor-infiltrating lymphocytes (TILs), specifically CD8-positive T cells, are independently associated with improved survival in MCC.35,36 Sihto et al36 also found that MCPyV-positive MCCs had significantly greater lymphocyte infiltration than MCPyV-negative MCCs. Interestingly, among MCPyV-negative tumors, those with high T-cell infiltration also enjoyed a prognostic benefit.36 These findings suggest that MCPyV-positive MCCs are generally more immunogenic, but that immunogenic tumors, irrespective of viral status, are linked to better outcomes.
Despite evidence that infiltrating T cells are associated with improved survival, evidence also shows that MCPyV-specific T cells are often dysfunctional. A recent study showed that although MCPyV-specific T cells were commonly present in the blood of patients with MCC, they persistently expressed high levels of 2 markers that indicate they were “exhausted” and not optimally functional: programmed death 1 (PD-1) and T-cell immunoglobulin and mucin domain (Tim-3).37 Using immunohistochemistry, Lipson et al38 found high levels of the complementary receptor to PD-1, programmed death ligand 1 (PD-L1), expressed on both TILs and MCPyV-positive MCC tumor cells. These findings suggest that although TILs may be in the right place at the right time, their capacity for tumor destruction may be limited. Therapeutic trials aimed at reversing these exhaustion phenotypes are currently underway (discussed later).
Unknown Primary
Although most MCCs arise in the skin, estimates show that as many as 19% of all MCCs39 and 40% of stage IIIB MCCs40 present with no known primary. Several studies have shown that patients with MCC with no known primary have significantly improved survival.40–42 A 2-year survival rate of 76.9% has been reported for patients with stage IIIB MCC without a known primary versus 36.4% for those with a primary. 43 The absence of a primary lesion in the setting of nodal disease may reflect the ability of cell-mediated immunity to eliminate the primary tumor. If that is true, the impressive prognostic benefit associated with no known primary may be a function of cell-mediated containment of residual disease. Given the strength of this association across several cohorts, incorporation of the presence or absence of a primary tumor as a prognostic factor may be considered in the next MCC staging algorithm.
p63 Expression
p63 is a multifunctional transcription factor necessary for normal epithelial development.44 p63 expression has also been implicated as a prognostic factor in several cancers, including breast cancer, squamous cell carcinoma of the head and neck, and bladder carcinoma.44 Several studies have suggested that p63 expression is an indicator of poor prognosis in MCC.45–47 In a univariate analysis of localized MCCs, Asioli et al45 reported a 20% 5-year survival rate for 21 patients with MCC with p63-positive tumors compared with 100% for 19 patients with p63-negative MCC (hazard ratio [HR], 7.26; P<.001). In an independent and larger cohort, Stetsenko et al47 also found that p63 expression was significantly correlated with decreased survival, but the magnitude of the association was smaller (HR, 2.05; P=.02). In multivariate subgroup analysis, p63 expression did not significantly predict mortality within a given stage.47 Currently, the additional prognostic value p63 expression adds to MCC staging or clinical management is unclear.
Toward Immune Therapy for MCC
Appropriate therapeutic options and an algorithm for MCC management are now included in the NCCN Clinical Practice Guidelines in Oncology for Merkel Cell Carcinoma (to view the most recent version of these guidelines, visit NCCN.org).48 Although localized MCC is effectively controlled with surgery and radiation, chemotherapy for advanced disease carries significant morbidity and rarely offers durable benefit. This article focuses on emerging therapeutic approaches designed to activate tumor-specific T cells and augment cellular immunity in an effort to prevent or control advanced disease. A more comprehensive assessment of established and experimental therapies for advanced MCC was recently published by Miller et al.49
Binding of PD-1 by its ligand PD-L1 induces T-cell exhaustion, thereby inactivating T cells that have been chronically presented with antigens they have failed to clear. Targeted inhibitors of PD-1 and PD-L1 have been shown in early-phase trials to durably shrink a variety of advanced cancers, with particularly promising results in cases with high expression of PD-L1 in the tumor.50,51 The observations that patients with MCC often have MCPyV-specific T cells expressing PD-1,37 and that high tumoral PD-L1 expression is often seen in MCPyV-positive MCCs,38 provide a strong rationale for a trial of PD-1 pathway blockade in patients with advanced MCC.
Monoclonal antibodies targeting 4-1BB (CD137, a costimulatory receptor found on activated T cells) have been shown to increase leukocyte proliferation and reduce tumor growth in preclinical studies.52 Because MCPyV-specific T cells have increased expression of CD137,37 MCC is an attractive target for 4-1BB agonists. A multicenter phase I trial for patients with MCC is currently underway (Clinical Trials.gov identifier: NCT01307267).
Adoptive T-cell therapy is the process of extracting T cells from patients, selecting for and expanding those of the desired antigen specificity, and then reinfusing them into the patient. The strong links between T-cell function and survival,17,33,35,36 and the ongoing expression of viral oncoproteins,24 make adoptive T-cell therapy an attractive modality for treating MCPyV-positive MCCs. In an ongoing phase I/II trial, MCPyV-specific T cells are being used to treat patients with metastatic MCC (Clinical Trials.gov identifier: NCT01758458).
Conclusions
Before 2008, little was known regarding the precise cause of MCC, no MCC-specific diagnostic codes existed, MCC was staged along with comparatively benign skin cancers such as basal cell carcinoma, and virtually no active clinical trials were underway. Currently, 9 clinical trials are specifically recruiting patients with MCC, with several others pending (see ClinicalTrials.gov). According to PubMed, between 2000 and 2008, an average of 80 papers were published per year relating to MCC. In the years since, that number has more than doubled. This increase in clinical interest and scientific attention is no doubt partly because of the discovery of MCPyV and subsequent studies establishing the important role of the immune system in the genesis and prognosis of MCC. Although in their early stages, trials of mechanism-based therapies aimed at activating antitumor T cells have already shown promise in several malignancies, and may be particularly effective for this virus-associated cancer.
Acknowledgments
This work was funded by NCATS Grant TL1 TR000422, NIH K24-CA139052, NIH R01-CA162522, the David & Rosalind Bloom Endowment for MCC Research, the Michael Piepkorn Endowment, and the UW MCC Patient Gift Fund. Dr. Nghiem has disclosed that he is a consultant for EMD Serono, Inc.
Footnotes
Dr. Moshiri has disclosed that he has no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors.
References
- 1.Lemos BD, Storer BE, Iyer JG, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63:751–761. doi: 10.1016/j.jaad.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemos B, Nghiem P. Merkel cell carcinoma: more deaths but still no pathway to blame. J Invest Dermatol. 2007;127:2100–2103. doi: 10.1038/sj.jid.5700925. [DOI] [PubMed] [Google Scholar]
- 3.Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375–381. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10:878–889. doi: 10.1038/nrc2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yiengpruksawan A, Coit DG, Thaler HT, Merkel cell carcinoma, et al. Prognosis and management. Arch Surg. 1991;126:1514–1519. doi: 10.1001/archsurg.1991.01410360088014. [DOI] [PubMed] [Google Scholar]
- 7.Allen PJ, Zhang ZF, Coit DG. Surgical management of Merkel cell carcinoma. Ann Surg. 1999;229:97–105. doi: 10.1097/00000658-199901000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300–2309. doi: 10.1200/JCO.2005.02.329. [DOI] [PubMed] [Google Scholar]
- 9.Clark JR, Veness MJ, Gilbert R, et al. Merkel cell carcinoma of the head and neck: is adjuvant radiotherapy necessary? Head Neck. 2007;29:249–257. doi: 10.1002/hed.20510. [DOI] [PubMed] [Google Scholar]
- 10.Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging manual. 6th ed. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 11.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 12.Gupta SG, Wang LC, Penas PF, et al. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: rhe Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685–690. doi: 10.1001/archderm.142.6.685. [DOI] [PubMed] [Google Scholar]
- 13.Iyer JG, Koba S, Nghiem P. Toward better management of merkel cell carcinoma using a consensus staging system, new diagnostic codes and a recently discovered virus. Actas Dermosifliogr. 2009;100(Suppl 2):49–54. doi: 10.1016/s0001-7310(09)73378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popp S, Waltering S, Herbst C, et al. UV-B-type mutations and chromosomal imbalances indicate common pathways for the development of Merkel and skin squamous cell carcinomas. Int J Cancer. 2002;99:352–360. doi: 10.1002/ijc.10321. [DOI] [PubMed] [Google Scholar]
- 15.Boukamp P. UV-induced skin cancer: similarities—variations. J Dtsch Dermatol Ges. 2005;3:493–503. doi: 10.1111/j.1610-0387.2005.05037.x. [DOI] [PubMed] [Google Scholar]
- 16.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 17.Brewer JD, Shanafelt TD, Otley CC, et al. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignant melanoma and Merkel cell carcinoma in a SEER population-based study. J Clin Oncol. 2012;30:843–849. doi: 10.1200/JCO.2011.34.9605. [DOI] [PubMed] [Google Scholar]
- 18.Engels EA, Frisch M, Goedert JJ, et al. Merkel cell carcinoma and HIV infection. Lancet. 2002;359:497–498. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]
- 19.Hemminki K, Liu X, Ji J, et al. Kaposi sarcoma and Merkel cell carcinoma after autoimmune disease. Int J Cancer. 2012;131:E326–E328. doi: 10.1002/ijc.27376. [DOI] [PubMed] [Google Scholar]
- 20.Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717–1721. doi: 10.1097/00007890-199912150-00015. [DOI] [PubMed] [Google Scholar]
- 21.Rodig SJ, Cheng J, Wardzala J, et al. Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J Clin Invest. 2012;122:4645–4653. doi: 10.1172/JCI64116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicol JT, Robinot R, Carpentier A, et al. Age-specific seroprevalences of merkel cell polyomavirus, human polyomaviruses 6, 7, and 9, and trichodysplasia spinulosa-associated polyomavirus. Clin Vaccine Immunol. 2013;20:363–368. doi: 10.1128/CVI.00438-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martel-Jantin C, Pedergnana V, Nicol JT, et al. Merkel cell polyomavirus infection occurs during early childhood and is transmitted between siblings. J Clin Virol. 2013;58:288–291. doi: 10.1016/j.jcv.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 24.DeCaprio JA, Garcea RL. A cornucopia of human polyomaviruses. Nat Rev Microbiol. 2013;11:264–276. doi: 10.1038/nrmicro2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houben R, Shuda M, Weinkam R, et al. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010;84:7064–7072. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter JJ, Paulson KG, Wipf GC, et al. Association of Merkel cell polyomavirus-specific antibodies with Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:1510–1522. doi: 10.1093/jnci/djp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in merkel cell carcinoma patients. Cancer Res. 2010;70:8388–8397. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Touze A, Le Bidre E, Laude H, et al. High levels of antibodies against merkel cell polyomavirus identify a subset of patients with merkel cell carcinoma with better clinical outcome. J Clin Oncol. 2011;29:1612–1619. doi: 10.1200/JCO.2010.31.1704. [DOI] [PubMed] [Google Scholar]
- 29.Andea AA, Coit DG, Amin B, Busam KJ. Merkel cell carcinoma: histologic features and prognosis. Cancer. 2008;113:2549–2558. doi: 10.1002/cncr.23874. [DOI] [PubMed] [Google Scholar]
- 30.Ng L, Beer TW, Murray K. Vascular density has prognostic value in Merkel cell carcinoma. Am J Dermatopathol. 2008;30:442–445. doi: 10.1097/DAD.0b013e318172364d. [DOI] [PubMed] [Google Scholar]
- 31.Fields RC, Busam KJ, Chou JF, et al. Five hundred patients with Merkel cell carcinoma evaluated at a single institution. Ann Surg. 2011;254:465–473. doi: 10.1097/SLA.0b013e31822c5fc1. [DOI] [PubMed] [Google Scholar]
- 32.Kukko HM, Koljonen VS, Tukiainen EJ, et al. Vascular invasion is an early event in pathogenesis of Merkel cell carcinoma. Mod Pathol. 2010;23:1151–1156. doi: 10.1038/modpathol.2010.100. [DOI] [PubMed] [Google Scholar]
- 33.Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642–646. doi: 10.1038/jid.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson ME, Zhu F, Li T, et al. Absolute lymphocyte count: a potential prognostic factor for Merkel cell carcinoma. J Am Acad Dermatol. 2014;70:1028–1035. doi: 10.1016/j.jaad.2014.01.890. [DOI] [PubMed] [Google Scholar]
- 35.Paulson KG, Iyer JG, Tegeder AR, et al. Transcriptome-wide studies of merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J Clin Oncol. 2011;29:1539–1546. doi: 10.1200/JCO.2010.30.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sihto H, Bohling T, Kavola H, et al. Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: a population-based study. Clin Cancer Res. 2012;18:2872–2881. doi: 10.1158/1078-0432.CCR-11-3020. [DOI] [PubMed] [Google Scholar]
- 37.Afanasiev OK, Yelistratova L, Miller N, et al. Merkel polyomavirus-specific T cells fluctuate with Merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin Cancer Res. 2013;19:5351–5360. doi: 10.1158/1078-0432.CCR-13-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipson EJ, Vincent JG, Loyo M, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus, and overall survival. Cancer Immunology Research. 2013;1:54–63. doi: 10.1158/2326-6066.CIR-13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veness MJ, Perera L, McCourt J, et al. Merkel cell carcinoma: improved outcome with adjuvant radiotherapy. ANZ J Surg. 2005;75:275–281. doi: 10.1111/j.1445-2197.2005.03353.x. [DOI] [PubMed] [Google Scholar]
- 40.Foote M, Veness M, Zarate D, Poulsen M. Merkel cell carcinoma: the prognostic implications of an occult primary in stage IIIB (nodal) disease. J Am Acad Dermatol. 2012;67:395–399. doi: 10.1016/j.jaad.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Deneve JL, Messina JL, Marzban SS, et al. Merkel cell carcinoma of unknown primary origin. Ann Surg Oncol. 2012;19:2360–2366. doi: 10.1245/s10434-011-2213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen KT, Papavasiliou P, Edwards K, et al. A better prognosis for Merkel cell carcinoma of unknown primary origin. Am J Surg. 2013;206:752–757. doi: 10.1016/j.amjsurg.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Tarantola TI, Vallow LA, Halyard MY, et al. Unknown primary Merkel cell carcinoma: 23 new cases and a review. J Am Acad Dermatol. 2013;68:433–440. doi: 10.1016/j.jaad.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 44.Yao JY, Chen JK. Roles of p63 in epidermal development and tumorigenesis. Biomed J. 2012;35:457–463. doi: 10.4103/2319-4170.104410. [DOI] [PubMed] [Google Scholar]
- 45.Asioli S, Righi A, de Biase D, et al. Expression of p63 is the sole independent marker of aggressiveness in localised (stage I-II) Merkel cell carcinomas. Mod Pathol. 2011;24:1451–1461. doi: 10.1038/modpathol.2011.100. [DOI] [PubMed] [Google Scholar]
- 46.Hall BJ, Pincus LB, Yu SS, et al. Immunohistochemical prognostication of Merkel cell carcinoma: p63 expression but not polyomavirus status correlates with outcome. J Cutan Pathol. 2012;39:911–917. doi: 10.1111/j.1600-0560.2012.01964.x. [DOI] [PubMed] [Google Scholar]
- 47.Stetsenko GY, Malekirad J, Paulson KG, et al. p63 Expression in Merkel cell carcinoma predicts poorer survival yet may have limited clinical utility. Am J Clin Pathol. 2013;140:838–844. doi: 10.1309/AJCPE4PK6CTBNQJY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bichakjian CK, Olencki T, Alam M, et al. [Accessed July 30, 2014];NCCN Clinical Practice Guidelines in Oncology: Merkel Cell Carcinoma. Version 1. 2014 doi: 10.6004/jnccn.2018.0055. Available at: NCCN.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller NJ, Bhatia S, Parvathaneni U, et al. Emerging and mechanism-based therapies for recurrent or metastatic Merkel cell carcinoma. Curr Treat Options Oncol. 2013;14:249–263. doi: 10.1007/s11864-013-0225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fisher TS, Kamperschroer C, Oliphant T, et al. Targeting of 4-1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Cancer Immunol Immunother. 2012;61:1721–1733. doi: 10.1007/s00262-012-1237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]