Abstract
Introduction
Prisoners bear a disproportionate burden of Ukraine's volatile and transitional HIV epidemic, yet little is known in Eastern Europe about HIV testing, treatment and HIV-related risk among prisoners.
Methods
A nationally representative biobehavioural health survey linked with serological testing was conducted among soon-to-be released prisoners in 13 Ukrainian prisons from June to November 2011.
Results
Among 402 participants, 78 (19.4%) tested HIV seropositive of whom 38 (50.7%) were previously unaware of their HIV status. Independent correlates of HIV infection included drug injection (AOR=4.26; 95% CI: 2.23–8.15), female gender (AOR=2.00; 95% CI: 1.06–3.78), previous incarceration (AOR=1.99; 95% CI: 1.07–3.70) and being from Southern Ukraine (AOR=5.46; 95% CI: 2.21–13.46). Those aware of being HIV-positive reported significantly more pre-incarceration sex- and drug-related HIV risk behaviours than those who were unaware.
Conclusions
Routine rather than risk-based HIV testing and expansion of opioid substitution and antiretroviral therapy among prisoners is urgently needed to reduce HIV transmission in volatile transitional HIV epidemics.
Keywords: prisoners, substance use, HIV/AIDS, injection drug use, risk behaviours, Ukraine
Introduction
The HIV epidemics in Eastern Europe and Central Asia are among the fastest growing in the world [1]. Together, Ukraine and Russia account for 90% of all newly reported HIV cases in the region [2]. Ukraine is experiencing Europe's worst HIV epidemic [3] with 1.1% of the adult population infected [4]. As in other countries of the former Soviet Union (FSU), Ukraine's HIV epidemic started among people who inject drugs (PWIDs) [4], particularly opioids, [5] with more recent evidence of a transitional generalizing epidemic [6]. HIV prevalence among the estimated 325,000–425,000 PWIDs in Ukraine is estimated to be as high as 41.8% in certain regions [7]. Despite PWIDs accounting for 60.5% of all HIV cases in Ukraine, only 5% of PWIDs are currently receiving antiretroviral therapy (ART), yet over 25% of those not using drugs are on ART [8]. This disproportionate access to ART among PWIDs is indicative of the pervasive trend of stigmatization towards this group, resulting in poor health outcomes [9].
Ukraine has largely relied on criminal justice sanctions to address illicit drug use, favouring incarceration over medical treatment for substance use disorders (SUDs), leading to high concentrations of PWIDs within prisons [10]. Ukraine's incarceration rate (347 per 100,000) is among the world's highest, just after the United States, Russia and Cuba [11]. HIV prevalence in Ukrainian prisons is several-fold higher than in the community, resulting in health disparities among PWIDs. These poor health outcomes are compounded by low ART coverage in prisons, with only 11% of eligible HIV-positive prisoners receiving treatment [12] despite evidence that ART prevents HIV transmission among PWIDs [13, 14]. With documented high levels of drug injection and syringe sharing by HIV-positive prisoners during incarceration [15] and 7000 HIV-positive prisoners released to the community annually [16], Ukraine's criminal justice system (CJS) contributes to poor HIV-related outcomes and perpetuates HIV transmission, making it a volatile, high-risk environment. Though effective alternatives to incarceration exist, in the absence of such policies, Ukraine's CJS remains an important target for public health interventions that address the syndemic of HIV and SUDs among PWIDs by expanding screening, treatment and post-release linkage to care.
In order to better inform HIV testing, treatment and retention strategies within Ukraine's CJS, we examined the independent correlates of HIV infection and differences among those aware and unaware of being HIV-positive among a nationally representative sample of soon-to-be-released prisoners. Such findings may be useful for other countries from Eastern Europe, Central Asia and elsewhere that struggle with transitional HIV epidemics evolving from PWIDs.
Methods
Study design
Study design and procedures have been described previously [12]. In brief, a nationally representative cross-sectional health and serosurveillance survey of infectious diseases among 402 soon-to-be-released adult prisoners was conducted between May and November 2011. Eligibility criteria included: 1) ≥18 years of age; 2) <six months remaining in prison sentence; 3) able to provide informed consent; and 4) speaks Ukrainian or Russian. Participants were selected at random from the 87,717 sentenced women and men in medium-security prisons (representing 80.1% of the total prison population), using a stratified sampling strategy [17]. Of the 426 inmates randomly selected, 402 (94.4%) consented and were enrolled. An anonymous identifier linked behavioural and serological results, which was unlinked to personal identifiers unless participants were willing to release their results to prison officials to obtain care. Participants were provided with serological results, post-test counselling and a comprehensive referral packet to community-based medical and social services.
Eligible sites were categorized into one of four geographic regions of the country. Approximately 100 participants were targeted from each of the four regions (north, south, west and east). Each region included two medium-security male-only prisons and one female-only prison. Pre-planned sampling was divided evenly between first time and repeat offenders, and women (20%) were oversampled. Prison facility selection criteria included being large, representative, and non-specialized; HIV/AIDS, addiction, and tuberculosis prison hospitals were not included. HIV testing is available in all prisons through voluntary counselling/testing (VCT) that primarily targets high-risk groups.
Data collection
Surveys were administered under the supervision of a trained research assistant using computer-assisted survey instruments (CASI) to ensure confidentiality. Nurses performed the phlebotomy. Serological testing was performed using the commercially available One-Step Multi-Infectious Disease Rapid Test Card (InTec Products, Inc. Xiamen, China), including testing for Hepatitis B virus surface antigen (HBsAg), antibody to Hepatitis C virus, ELISA for HIV (1&2) test and a rapid plasma reagin (RPR) test to Treponema pallidum for syphilis (RPR>1:16 was considered positive). Initially reactive HIV serology (sensitivity=99.9%; specificity=100%) was confirmed with the DetermineTM HIV-1/2 Western Blot (Abbott Laboratories, Tokyo, Japan). All HIV-seropositive participants underwent reflex CD4 T lymphocyte count assessment using FACS flow cytometry.
Definitions
All self-reported sex- and drug-risk behaviours were limited to the 30 days prior to arrest in order to minimize recall bias, with the exception of “ever injected drugs,” “ever used drugs” or “ever used opioids,” which refer to lifetime. Alcohol use and tattooing were assessed for the year before incarceration. Poverty was defined as being below the 2011 national Ukrainian poverty line income of <1776 Ukrainian Hryvnia per month (~220 USD). Pre-incarceration drug use refers to opioids, sedatives, amphetamines, hallucinogens, barbiturates or cocaine in the month before incarceration. Multiple-substance use refers to using two or more of the aforementioned substances at least once during that period.
Having moderate to severe depressive symptoms was defined for individuals who scored ≥10 on the 10-item Clinical Epidemiological Scale for Depression (CES-D) [18]; hazardous drinking of alcohol was confirmed if scores were ≥8 for men or ≥5 for women [19] using the Alcohol Use Disorders Inventory Test (AUDIT) [20]; social support was measured using a continuous standardized scale [21]; health-related quality of life (HRQoL) was measured continuously with a physical and mental health summary score using the 36-item short-form (SF-36) Medical Outcomes Survey [22].
Data analysis
Statistical analyses, using SPSS (version 19.0), involved t-test and χ 2 test for categorical and continuous variables, with significance defined as p<0.05. Bivariate and multivariate logistic regression analyses were carried out to determine the correlates of HIV seropositivity. Multivariate logistic regression assessed the independent correlates for HIV-seropositive status if bivariate associations with the dependent variable were significant at p<0.05. All variables in the final model were checked for multicollinearity, and tolerance values in the final model were high (>0.90) and all VIF values were low (≤1.10). Injection drug use (IDU) and sex with an HIV-positive partner were included over other sex and drug-risk variables because they are most directly associated with HIV risk and resulted in best goodness-of-fit. Region was included in order to account for region-specific differences. Variables that were kept in the model were tested for interactions with each other. Goodness-of-fit for the final logistic regression was measured using the Akaike Information Criterion (AIC).
Ethics statement
Institutional Review Boards at the Ukrainian Institute on Public Health Policy and Yale University approved the study. Further safety assurances were provided by the Office for Human Research Protections (OHRP) in accordance with 45 CFR 46.305(c) “Prisoner Research Certification” requirements. Participants provided written informed consent prior to study participation.
Results
Description of study participants
Table 1 provides the sample characteristics, including demographics, drug and alcohol use, and pre-incarceration HIV risk behaviours. Overall, 78 prisoners (19.4%) had confirmed HIV infection.
Table 1.
Selected characteristics of study participants (N=402)
| Characteristics | N=402 (%) |
|---|---|
| Age | |
| Mean, years (range) | 31.9 (18–58) |
| ≤30 years | 212 (52.7) |
| >30 years | 190 (47.3) |
| Sex | |
| Male | 321 (79.9) |
| Female | 81 (20.1) |
| High school graduate | |
| Yes | 305 (75.9) |
| No | 97 (24.1) |
| Ethnicity | |
| Ukrainian or Russian | 381 (95.0) |
| Other | 21 (5.2) |
| Criminal justice history | |
| Mean number of lifetime arrests (SD) | 5.4 (7.0) |
| Mean number of previous incarcerations (range) | 2.2 (0–11) |
| Mean current incarceration duration, years (SD) | 2.6 (1.9) |
| Mean time before community release, months (SD) | 2.1 (1.7) |
| In a relationship | |
| Yes | 105 (26.1) |
| No | 297 (73.9) |
| Below national poverty line | |
| Yes | 243 (60.4) |
| No | 159 (39.6) |
| Hazardous drinking | |
| Yes | 229 (57.0) |
| No | 167 (41.5) |
| Substance use 30 days before incarceration | |
| Any substance use | 171 (42.5) |
| Multiple-substance use | 127 (31.6) |
| Opioid use | 138 (34.3) |
| Amphetamine use | 85 (21.1) |
| Sedatives use | 72 (17.9) |
| Ever injected drugs, lifetime | |
| Yes | 193 (48.7) |
| No | 209 (51.3) |
| Re-used syringe, container or needle (N=144) | |
| Yes | 104 (25.9) |
| No | 40 (10.0) |
| Major depressive disorder | |
| Moderate to severe depressive symptoms | 158 (40.3) |
| Social support | |
| Mean social support score (SD) | 2.99 (1.1) |
| Health-related quality of life | |
| Mean Physical Composite Score (SD) | 47.3 (5.8) |
| Mean Mental Composite Score (SD) | 38.4 (8.9) |
| HIV seropositive | 78 (19.4) |
| HIV outcomes (N=78) | |
| Mean CD4 count, cells/mL (range) | 355.1 (5–1239) |
| CD4>350 | 34 (43.6) |
| CD4≤350 | 44 (56.4) |
| Ever prescribed antiretroviral therapy | 8 (10.3) |
| Currently prescribed antiretroviral therapy | 5 (6.4) |
| Hepatitis C antibody | |
| Negative | 161 (40.0) |
| Positive | 241 (60.0) |
SD=standard deviation.
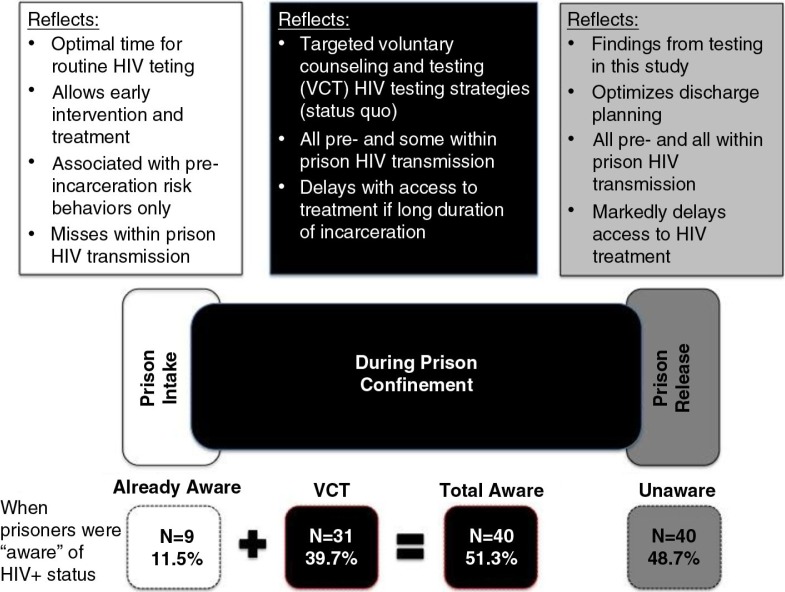
The findings, illustrating when prisoners became aware of their HIV status, are depicted in Figure 1, which provides insights into the attributes of each testing strategy.
Figure 1.
Analytic strategy when prisoners learn about their HIV-seropositive status.
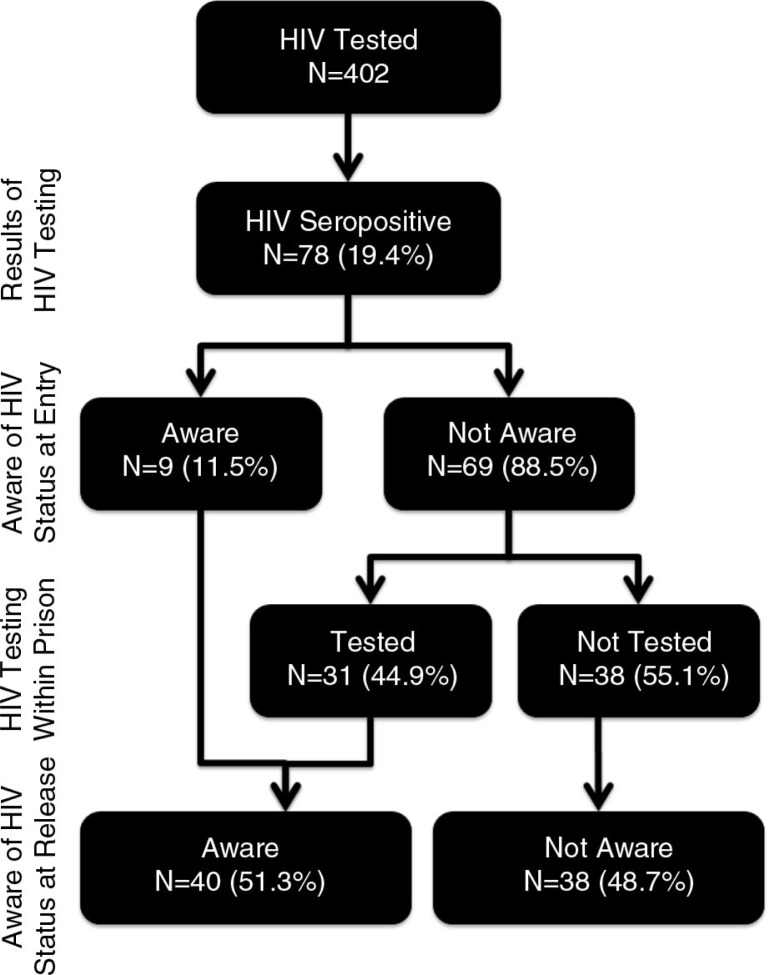
Figure 2 depicts knowledge about HIV status, based on pre-specified HIV testing periods (prison entry, during incarceration and at prison release); testing at prison release was testing that occurred as a result of this study. Prison intake reflects a time when “routine” testing is most optimized [23–25]. Nearly all HIV-seropositives were unaware of their status at intake (N=69; 88.5%). During imprisonment, 31 of those initially unaware were tested and informed about their status, leaving 38 (48.7%) not knowing their status were it not for this study. Despite 45 HIV-seropositives reporting previous HIV testing, eight of them (17.8%) believed themselves to be HIV-uninfected (of which three had tested negative during the current incarceration) and another eight never received test results. Seven of the eight who had never received their results had undergone testing during their current incarceration.
Figure 2.
When participants became aware of their HIV status.
Correlates of HIV infection
Correlates of HIV status are presented in Table 2. After controlling for variables significant in the bivariate analysis, multivariable logistic regression analysis showed that having ever injected drugs was the single most important correlate of HIV infection, portending more than a four-fold increased association (AOR: 4.26; 95% CI: 2.23–8.15). Other independent correlates of HIV infection included being female (AOR: 2.00; 95% CI: 1.06–3.78), in Southern Ukraine (AOR: 5.46; 95% CI: 2.21–13.46) and being a recidivist offender (AOR: 1.99; 95% CI: 1.07–3.70).
Table 2.
Correlates of HIV infection among soon-to-be-released Ukrainian prisoners (N=402)
| Covariate | Bivariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Unadjusted odds ratio | 95% C.I. | p | Adjusted odds ratio | 95% C.I. | p | |
| Female | 1.92 | 1.09–3.37 | 0.020* | 2.00 | 1.06–3.78 | 0.032* |
| Male | Ref. | – | – | Ref. | – | – |
| Recidivist | 2.03 | 1.22–3.38 | 0.006* | 1.99 | 1.07–3.70 | 0.030* |
| First time offender | Ref. | – | – | Ref. | – | – |
| Region | ||||||
| East | Ref. | – | – | Ref. | – | – |
| West | 1.89 | 0.79–4.55 | 0.155 | 1.47 | 0.56–3.88 | 0.432 |
| North | 3.44 | 1.52–7.79 | 0.003* | 2.36 | 1.00–5.67 | 0.055 |
| South | 4.13 | 1.83–9.32 | 0.001* | 5.46 | 2.21–13.46 | <0.001* |
| Completed high school | 1.57 | 0.84–2.96 | 0.158 | – | – | – |
| Income below poverty | 1.07 | 0.64–1.76 | 0.806 | – | – | – |
| Major depression | 1.93 | 1.16–3.20 | 0.011* | 1.31 | 0.72–2.39 | 0.385 |
| Hazardous drinking | 0.70 | 0.42–1.15 | 0.156 | – | – | – |
| Syphilis | 1.04 | 0.46–2.36 | 0.920 | – | – | – |
| Told by a doctor they had a sexually transmitted infection | 1.95 | 1.06–3.56 | 0.031* | 1.88 | 0.97–3.67 | 0.062 |
| Has partner | 1.17 | 0.67–2.03 | 0.587 | – | – | – |
| Use in 30 days before incarceration | ||||||
| Injected drugs | 4.07 | 2.43–6.84 | <0.001* | – | – | – |
| Any substance use | 3.45 | 2.04–5.81 | <0.001* | – | – | – |
| Multiple-substance use | 2.87 | 1.73–4.76 | <0.001* | – | – | – |
| Amphetamine user | 1.77 | 1.01–3.09 | 0.046* | – | – | – |
| Opioid user | 3.88 | 2.32–6.49 | <0.001* | – | – | – |
| Sedatives user | 2.56 | 1.45–4.52 | 0.001* | – | – | – |
| Lifetime use | ||||||
| Injected drugs | 3.34 | 1.94–5.73 | <0.001* | 4.26 | 2.23–8.15 | <0.001* |
| Any illicit drug | 2.52 | 1.48–4.26 | 0.001* | – | – | – |
| Re-used syringe, container or needle | 1.52 | 0.68–3.38 | 0.307 | – | – | – |
| Sex without condom with at least one person | 0.88 | 0.53–1.47 | 0.634 | – | – | – |
| Sex without a condom with a HIV+ person | 2.91 | 1.31–6.43 | 0.008* | 1.71 | 0.72–4.09 | 0.225 |
| Money or drugs in exchange for sex | 0.46 | 0.06–3.64 | 0.458 | – | – | – |
| Sex under influence of alcohol | 0.33 | 0.17–0.64 | 0.001* | – | – | – |
| Sex under influence of drugs | 2.09 | 1.10–3.96 | 0.025* | – | – | – |
| Tattoo from non-professional | 0.59 | 0.30–1.15 | 0.119 | – | – | – |
| Akaike Information Criterion (AIC) | 198.49 | |||||
Denotes a significant difference, defined as p≤0.05.
Correlates of being aware of being HIV-positive
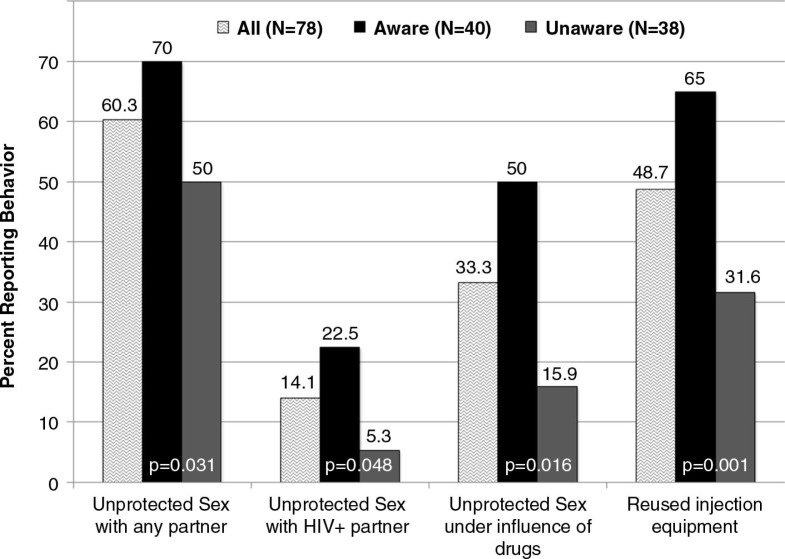
Table 3 compares pre-incarceration substance use and HIV risk behaviours among all HIV-seropositive respondents, stratified by whether they knew their HIV-positive status upon entry into prison. Compared to those being unaware, the nine respondents who knew they were HIV-positive upon entry reported significantly higher drug use and sexual risk behaviours in the six months before incarceration; six of these nine “aware” respondents also reported sharing injection equipment, sex without a condom and sex under the influence of drugs (data not shown). Table 3 further explores pre-incarceration risk behaviours among those who were aware and unaware of their status during incarceration (not as a result of this study). Pre-incarceration use of all substances, except sedatives, was significantly more prevalent among those who were aware of their HIV-positive status upon re-entry into the community. Among a number of sexual risk behaviours, having any unprotected sex (p=0.031), with an HIV-positive person (p=0.048) and under the influence of drugs (p=0.016) were each significantly more common before imprisonment among those who were aware of their HIV-seropositive status than among those who were informed only upon prison release. Getting a tattoo from a non-professional did not, however, emerge as significantly different between the groups.
Table 3.
Pre-incarceration substance use and HIV risk-taking behaviours among HIV-positive prisoners in Ukraine who were aware and unaware of their HIV status upon prison entry and prison release (N=78)
| Drug-related behaviours | Comparison groups | ||
|---|---|---|---|
| Aware N=9 (%) | Unaware N=69 (%) | p | |
| Prison entry | |||
| Amphetamine use | 5 (55.6) | 18 (26.1) | 0.114 |
| Opioid use | 9 (100.0) | 38 (55.1) | 0.010 |
| Sedatives use | 5 (55.6) | 19 (27.5) | 0.124 |
| Substance use | 9 (100.0) | 43 (62.3) | 0.025 |
| Multiple-substance use | 8 (88.9) | 32 (46.4) | 0.029 |
| Injected drugs | 9 (100.0) | 46 (66.9) | 0.052 |
| Re-used syringe, container or needle | 8 (88.9) | 30 (43.5) | 0.662 |
| Sex-related risks | |||
| Sex without condom with at least one person | 9 (100.0) | 38 (55.1) | 0.010 |
| Sex without condom with HIV+ person | 4 (44.4) | 7 (10.1) | 0.020 |
| Sex without condom under influence of drugs | 8 (88.9) | 18 (26.1) | 0.030 |
| Other risks | |||
| Tattoo from non-professional | 0 | 12 (17.4) | 0.340 |
| Prison release Aware N=40 (%) | Unaware N=38 (%) | p | |
| Amphetamine use | 18 (45.0) | 5 (13.2) | 0.003 |
| Opioid use | 29 (72.5) | 18 (47.4) | 0.037 |
| Sedatives use | 15 (37.5) | 9 (23.7) | 0.225 |
| Substance use | 33 (82.5) | 19 (50.0) | 0.004 |
| Multiple-substance use | 26 (65.0) | 14 (36.8) | 0.023 |
| Injected drugs | 33 (82.5) | 22 (57.9) | 0.009 |
| Re-used syringe, container or needle | 26 (65.0) | 12 (31.6) | 0.001 |
| Sex-related risks | |||
| Sex without condom with at least one person | 28 (70.0) | 19 (50.0) | 0.031 |
| Sex without condom with HIV+ person | 9 (22.5) | 2 (5.3) | 0.048 |
| Sex without condom under influence of drugs | 20 (50.0) | 6 (15.9) | 0.016 |
| Other risks | |||
| Tattoo from non-professional | 6 (15.0) | 6 (15.8) | 1.000 |
Bold text signifies significant (p<0.05) differences.
Figure 3 depicts the proportion of pre-incarceration HIV risk-taking behaviours in all the HIV-seropositive respondents, stratified by who was aware and who was unaware of their status just prior to prison release. Those who were aware reported consistently more risky behaviours.
Figure 3.
Comparison of pre-incarceration risk behaviours by those who were aware and unaware of their HIV status just prior to prison release (N=78).
Discussion
Though reviews of publicly available data suggest that HIV, drug use and incarceration are inextricably intertwined in this region [26, 27], to our knowledge, this is the first study to directly collect data that examines correlates of HIV infection among prisoners in any country of the FSU, a region with a volatile HIV epidemic concentrated among high-risk groups [29, 30]. This sentinel biobehavioural survey of prisoners confirms this epidemiological impression and provides new insights that inform HIV prevention and treatment strategies. First, HIV prevalence in this representative sample of soon-to-be-released Ukrainian prisoners is substantially higher than recorded in official statistics [28] and several-fold higher (19.4% vs. 1.1%) than in the community [3, 29]. Second, this survey includes only soon-to-be-released prisoners, which provides a window into how effective current voluntary testing strategies are performing. In this case, nearly half of the prisoners would have transitioned to the community remaining unaware of their HIV status. Third, the comparison of those who were aware and unaware of being HIV seropositive near release identified marked differences in risks, pointing to the need to move away from risk-based testing in favour of routine testing strategies.
Meta-analyses and systematic reviews suggest that identification of HIV-seropositive status markedly reduces HIV risk behaviours [30, 31]. Identifying HIV alone, along with self-imposed risk reduction, could markedly contribute to reducing onward HIV transmission after release. Recent data from Ukraine of recently released HIV-positive prisoners suggest that their HIV injection behaviours were lower post-release compared to pre-incarceration behaviours [15]. Though there was evidence that VCT efforts correctly identified 39.7% of HIV-seropositives, a 2009 assessment showed that VCT tested only 1.08% of Ukrainian prisoners [4]. Of the 40 prisoners aware of their status, most (77.5%) were identified during this incarceration. More disturbing, however, is that nearly all (88.5%) participants had been missed by community-based HIV testing strategies. This finding conflicts with national estimates where only 50% of cases, much like we found by the end of imprisonment, were unaware of being HIV seropositive [32]. The increased identification of HIV during imprisonment suggests that in the absence of alternatives to incarceration or improved community-based treatment, prisons can serve as important sites for HIV diagnosis and treatment [33], especially when linked to effective transitional care services [34]. In the absence of diagnosis, people living with HIV/AIDS (PLWHA) often present to care with advanced HIV resulting in increased morbidity and mortality. Early HIV diagnosis improves ART access, which not only benefits individual health but also facilitates secondary prevention [35, 36].
Particularly troubling is the high prevalence of pre-incarceration HIV risk behaviours reported, especially among the HIV-seropositives. Drug use immediately preceding incarceration was independently correlated with being aware of HIV infection. Therefore, using injection risk alone to test patients would have missed a third of all HIV infections. Of the HIV-positive prisoners, those less likely to report risk behavior were significantly less likely to be tested for HIV, indicating that risk-based testing had failed to identify a substantial portion of PLWHA. Not only is HIV testing a hallmark of combined HIV prevention strategies [37, 38], it is also the first essential principle of the Seek, Test and Treat strategy that requires identifying most PLWHA (>90%) to reduce HIV transmission [39]. In many settings, routine HIV testing has been recommended as a cost-effective public health strategy to reduce barriers to testing, including empiric evidence from testing in jails and prisons [23, 24].
Examining drug use, a central driver of HIV transmission among prisoners, provided important insights for future prevention. While any substance use was significantly associated with HIV seropositivity, even when controlling for sexual risks, drug injection independently portended more than a four-fold increased association with HIV and nearly all (87%) of HIV-positive PWIDs injected primarily opioids. For opioid injectors, opioid substitution therapy (OST) with either methadone or buprenorphine remains the single most effective therapy to reduce HIV transmission. Recent data from Ukraine suggest that in the absence of prison-based OST, the prevalence of within-prison drug injection among PLWHA is extraordinarily high and often involves multiple sharing partners [15, 16]. Our data support within-prison HIV transmission since we minimally identify three instances of seroconversion during the current incarceration. Although the actual number of within-prison transmission is potentially much higher since, for 66 of the HIV-positive respondents, we cannot definitively conclude whether they were infected during incarceration or in the community. Even after nearly a decade since OST was introduced in Ukraine [40], it remains absent from the CJS despite plans for pilot study introduction into pre-trial detention settings which have been aborted since political unrest in Ukraine began. Lack of OST within the CJS persists despite the breadth of evidence showing the effectiveness of prison-initiated methadone [41], its benefits for prisoners transitioning to the community [42, 43] and, specifically, evidence that OST reduces HIV transmission risk among PWIDs in Ukraine [44].
Whereas PWID comprise 69% of all PLWHA in Ukraine, they have limited ART access [45]. Central to increasing PWIDs’ access to ART and achieving maximal viral suppression is OST expansion [40, 46, 47], which is recommended by international guidelines [48]. Overcoming stigma faced by PWIDs and reducing structural ART access obstacles is a priority for curbing the epidemic in this high-risk group [49]. Particularly, it is essential to establish effective transitional programmes to community settings to maintain ART benefits [50] which is an evidence-based HIV prevention tool [51]. Of concern here is the fact that half of the HIV-seropositive respondents were unaware of their status, highlighting a critical gap in HIV testing which could be scaled up through routine testing. Our findings suggest high acceptability of routine HIV testing given that very few approached for this study actually refused. Identifying HIV, however, should be linked to care, including ART. Only five patients here received ART; this represents 6.4% of HIV-seropositives, 12.5% of those aware of being HIV seropositive and 22.4% of those meeting ART treatment guidelines (CD4<350).
This study attests to the disproportionate impact HIV has on incarcerated women, portending a two-fold elevated risk. Across Ukraine, evidence suggests that the generalizing HIV epidemic is disproportionately impacting women [2]. Moreover, emerging data from Ukraine suggest that female PWIDs experience increased violence, which restricts access to services, safer sex and injection practices, and, consequently, increases HIV risk [52]. In this study, however, transactional sex was not significantly associated with HIV seropositivity. Even so, women's unique needs often remain unmet by prison services [53], necessitating gender-tailored interventions to address inequalities in care, prevention and rehabilitation services [54, 55].
Breaking the cycle of incarceration in Ukraine is central to HIV prevention efforts [56]. In our sample, having been previously imprisoned increased HIV risk more than two-fold. This underscores the risk posed to the general population, especially considering that an estimated 592 HIV-positive inmates are released to the community each month [29], with about half not knowing their HIV status, suggesting that ongoing sexual risk is contributing to an increasing proportion of new reported HIV cases in Ukraine [4]. Though recent reports document heterosexual transmission's increased contribution in Ukraine's expanding HIV epidemic, data point primarily to transmission to sexual partners of PWIDs [57], causing concern for a generalized epidemic [58].
Nearly all participants became aware of being HIV-positive only after incarceration, thereby limiting exploration of risk behaviours to the pre-incarceration period to ensure inmate safety and confidentiality. Six participants, however, knew their HIV-positive status before incarceration. All of them reported sex without a condom and five had re-used injection equipment and had unprotected sex under the influence of drugs. These data suggest that serosorting may have occurred among sexual partners since knowing one's status markedly reduces risk to others, but further data are needed. Irrespective of whether serosorting occurred or not, the magnitude of risky behaviours, especially under the influence of drugs by those aware of their HIV status remains a concern, and may point to inadequate access to evidence-based HIV risk reduction programmes that effectively curb secondary transmission [59]. At a minimum, recent data point to low coverage of harm reduction programmes such as needle/syringe exchange (NSP) and OST programmes as reasons for increased drug-related risk behaviours [8, 60, 61]. Most studies among HIV-positive PWIDs show reduced sexual risk after learning of one's HIV-seropositive status [62, 63], but this requires further exploration in the current context.
Though these findings provide insights into HIV-related risk and HIV testing strategies for Ukrainian prisoners, this study has limitations. First, the cross-sectional assessment does not determine causality. Second, to protect inmate safety, our study could not directly confirm within-prison HIV risk-taking. Third, recall bias and under-reporting could have occurred, but the use of CASI reduces, although it does not eliminate, reporting bias. Despite these limitations, the findings are a matter of concern and provide insights into future HIV prevention and treatment interventions.
Looking to the future for soon-to-be-released prisoners, the time immediately after release from prison is associated with numerous adverse health consequences [34], including overdose and death [64, 65]. Effective transitional care during this period is crucial since former prisoners return to unstable environments that trigger substance use relapse and increase drug-related death [66]. Optimal transitional care should include linkage to integrated care sites to address medical, psychiatric and addiction needs. Such sites already exist in Ukraine, which document improved health outcomes for HIV-positive PWIDs [67]. Risk reduction programmes are also essential during transition to reduce HIV-risk-taking behaviours [34] and curb HIV transmission to partners of PWID [68] and ultimately to the community [69]. This is especially crucial since low ART coverage among HIV-positive prisoners leaves many without virologic suppression.
Conclusions
This study is the first to determine the correlates of HIV infection among prisoners in Ukraine, where HIV is concentrated within the epicentre of Europe's worst HIV epidemic. Drug injection, primarily of opioids, is most highly correlated with HIV infection in a representative sample of soon-to-be-released prisoners. Being female and having been previously incarcerated were also associated with HIV infection. Of concern is the fact that half of the HIV-positive prisoners were unaware of their status and this group did not report HIV-related risk behaviours to the same extent as those who were aware. Our findings strongly reinforce the need for five evidence-based practices for prisoners: 1) routine rather than risk-based HIV testing in an expanding and generalized HIV epidemic; 2) OST; 3) expanded prescription of ART to all eligible prisoners; 4) NSPs; and 5) effective transitional care from the prison to the community setting. Though diseases that contribute to health disparities are concentrated within prisons, such as HIV and addiction, good prisoner health is good public health since most prisoners transition to the community and bring with them their medical and social co-morbidity. Coordinated efforts that align health and safety are urgently needed in Ukraine.
Acknowledgements
The authors are grateful to the staff at the State Penitentiary Service of Ukraine for their support. We also thank Olena Chernova for her help in facilitating this study. We are grateful to the inmates who participated in this research and the Ukrainian NGOs who took part in its implementation.
Competing interests
This research was supported by grants from the National Institute on Drug Abuse for research (R01 DA029910, Altice, PI) and career development (K24 DA017072, Altice, PI). The authors have declared that no competing interests exist.
Authors' contributions
Conceived and designed the study: FLA, SD, JAW, YG, LA; performed the experiments: LA, YG; analyzed the data: FLA, JAW, LA; wrote the manuscript: LA; revised the manuscript: FLA, SD, JAW. All authors have read and approved the final version.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS report on the global AIDS epidemic 2010; Geneva, Switzerland: UNAIDS; 2010. [Google Scholar]
- 2.Burruano L, Kruglov Y. HIV/AIDS epidemic in Eastern Europe: recent developments in the Russian Federation and Ukraine among women. Gend Med. 2009;6(1):277–89. doi: 10.1016/j.genm.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Kruglov YV, Kobyshcha YV, Salyuk T, Varetska O, Shakarishvili A, Saldanha VP. The most severe HIV epidemic in Europe: Ukraine's national HIV prevalence estimates for 2007. Sex Transm Infect. 2008;84:I37–41. doi: 10.1136/sti.2008.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ministry of Health of Ukraine. Ukraine: National report on monitoring progress towards the UNGASS declaration of commitment on HIV/AIDS; Kyiv: UNAIDS; 2010. Reporting Period: January 2008–December 2009. [Google Scholar]
- 5.Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1564–74. doi: 10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- 6.Amirkhanian YA. Review of HIV vulnerability and condom use in central and eastern Europe. Sex Health. 2012;9(1):34–43. doi: 10.1071/SH11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–45. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376(9738):355–66. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe D. Paradoxes in antiretroviral treatment for injecting drug users: access, adherence and structural barriers in Asia and the former Soviet Union. Int J Drug Policy. 2007;18(4):246–54. doi: 10.1016/j.drugpo.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 10.International Harm Reduction Program. Harm reduction developments 2005: countries with injection driven epidemics; New York:: Open Society Institute; 2006. [Google Scholar]
- 11.International Centre for Prison Studies King's College London. World prison brief 2012 [Internet] [cited 2012 Mar 2]. Available from: http://www.prisonstudies.org/info/worldbrief/wpb_country.php?country=168.
- 12.Azbel L, Wickersham JA, Grishaev Y, Dvoryak S, Altice FL. Burden of infectious diseases, substance use disorders, and mental illness among Ukrainian prisoners transitioning to the community. PLoS One. 2013;8(3):e59643. doi: 10.1371/journal.pone.0059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montaner JS. Treatment as prevention – a double hat-trick. Lancet. 2011;378(9787):208–9. doi: 10.1016/S0140-6736(11)60821-0. [DOI] [PubMed] [Google Scholar]
- 14.Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376(9740):532–9. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izenberg J, Bachireddy C, Wickersham JA, Soule M, Kiriazova T, Dvoriak S, et al. Within-prison drug injection among HIV-infected Ukrainian prisoners: prevalence and correlates of an extremely high-risk behaviour. Int J Drug Policy. doi: 10.1016/j.drugpo.2014.02.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donestk Memorial. Donetsk: Donestkii Memorial; 2012. Sobliudenie prav zakliuchennykh v Ukraine. [Google Scholar]
- 17.Hunt N, Tyrell S. Stratified sampling 2001 [Internet] [cited 2012 Oct 4]. Available from: http://www.coventry.ac.uk/ec/~unt/meths/strati.html.
- 18.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203–14. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 19.Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res. 2007;31(2):185–99. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 20.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption – II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 21.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–14. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 23.Kavasery R, Maru DS, Sylla LN, Smith D, Altice FL. A prospective controlled trial of routine opt-out HIV testing in a men's jail. PLoS One. 2009;4(11):e8056. doi: 10.1371/journal.pone.0008056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kavasery R, Maru DS, Cornman-Homonoff J, Sylla LN, Smith D, Altice FL. Routine opt-out HIV testing strategies in a female jail setting: a prospective controlled trial. PLoS One. 2009;4(11):e7648. doi: 10.1371/journal.pone.0007648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kavasery R, Altice FL. Observations on implementing routine HIV testing in jails. AIDS Patient Care STDS. 2007;21(10):715–16. doi: 10.1089/apc.2007.0039. author reply 7. [DOI] [PubMed] [Google Scholar]
- 26.Bridge J, Lazarus JV, Atun R. HIV epidemics and prevention responses in Asia and Eastern Europe: lessons to be learned? AIDS. 2010;24(Suppl 3):S86–94. doi: 10.1097/01.aids.0000390094.91176.d8. [DOI] [PubMed] [Google Scholar]
- 27.Vagenas P, Azbel L, Polonsky M, Kerimi N, Mamyrov M, Dvoryak S, et al. A review of medical and substance use co-morbidities in Central Asian prisons: implications for HIV prevention and treatment. Drug Alcohol Depend. 2013;32(Suppl 1):S25–31. doi: 10.1016/j.drugalcdep.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.United Nations Development Programme (UNDP) VIL/SNID ta prava liudyny u penitentsiarnii systemi; Kyiv: State Penitentiary Service of Ukraine; 2011. [Google Scholar]
- 29.Azbel L, Wickersham JA, Grishaev Y, Dvoryak S, Altice FL. Burden of infectious diseases, substance use disorders, and mental illness among Ukrainian prisoners: implications for transition to the community. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinhardt LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a meta-analytic review of published research, 1985–1997. Am J Public Health. 1999;89(9):1397–405. doi: 10.2105/ajph.89.9.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fonner VA, Denison J, Kennedy CE, O'Reilly K, Sweat M. Voluntary counselling and testing (VCT) for changing HIV-related risk behavior in developing countries (Review) Cochrane Database Syst Rev. 2012;9:CD001224. doi: 10.1002/14651858.CD001224.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.State Service of Ukraine on HIV/AIDS. National HIV/AIDS estimates in Ukraine as of beginning of 2012; Kyiv, Ukraine: Ministry of Health of Ukraine; 2012. [Google Scholar]
- 33.Flanigan T, Zaller N, Beckwith CG, Bazerman LB, Rana A, Gardner A, et al. Testing for HIV, sexually transmitted infections, and viral hepatitis in jails: still a missed opportunity for public health and HIV prevention. J Acquir Immune Defic Syndr. 2010;55(Suppl 2):S78–83. doi: 10.1097/QAI.0b013e3181fbc94f. [DOI] [PubMed] [Google Scholar]
- 34.Springer SA, Spaulding A, Meyer JP, Altice FL. Public health implications for adequate transitional care for HIV-infected prisoners: five essential components. Clin Infect Dis. 2011;53(5):469–79. doi: 10.1093/cid/cir446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gustafson R, Montaner J, Sibbald B. Seek and treat to optimize HIV and AIDS prevention. Can Med Assoc J. 2012;184(18):1971. doi: 10.1503/cmaj.121810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–64. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 37.Dutta A, Wirtz A, Stanciole A, Olerichs R, Semini I, Baral S, et al. Washington, DC: The World Bank; 2013. The global HIV epidemics among people who inject drugs. [Google Scholar]
- 38.Centers for Disease Control and Prevention. Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: summary guidance from CDC and the U.S. Department of Health and Human Services; Atlanta, GA: Centers for Disease Control and Prevention; 2012. [PubMed] [Google Scholar]
- 39.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 40.Bruce RD, Dvoryak S, Sylla L, Altice FL. HIV treatment access and scale-up for delivery of opiate substitution therapy with buprenorphine for IDUs in Ukraine – programme description and policy implications. Int J Drug Policy. 2007;18(4):326–8. doi: 10.1016/j.drugpo.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O'Grady KE. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months postrelease. J Subst Abuse Treat. 2009;37(3):277–85. doi: 10.1016/j.jsat.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wickersham JA, Marcus R, Kamarulzaman A, Zahari MM, Altice FL. Implementing methadone maintenance treatment in prisons in Malaysia. Bull World Health Organ. 2013;91:124–9. doi: 10.2471/BLT.12.109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wickersham JA, Zahari MM, Azar MM, Kamarulzaman A, Altice FL. Methadone dose at the time of release from prison significantly influences retention in treatment: implications from a pilot study of HIV-infected prisoners transitioning to the community in Malaysia. Drug Alcohol Depend. 2013;132(1–2):378–82. doi: 10.1016/j.drugalcdep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawrinson P, Ali R, Buavirat A, Chiamwongpaet S, Dvoryak S, Habrat B, et al. Key findings from the WHO collaborative study on substitution therapy for opioid dependence and HIV/AIDS. Addiction. 2008;103(9):1484–92. doi: 10.1111/j.1360-0443.2008.02249.x. [DOI] [PubMed] [Google Scholar]
- 45.Joint United Nations Programme on HIV/AIDS (UNAIDS) Ukraine – National report on monitoring progress towards the UNGASS declaration of commitment on HIV/AIDS; Geneva: WHO; 2012. [Google Scholar]
- 46.Springer SA, Chen S, Altice FL. Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: the impact of buprenorphine treatment. J Urban Health. 2010;87(4):592–602. doi: 10.1007/s11524-010-9438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Springer SA, Qiu J, Saber-Tehrani AS, Altice FL. Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners. PLoS One. 2012;7(5):e38335. doi: 10.1371/journal.pone.0038335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156(11):817–33. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Izenberg JM, Altice FL. Next steps for Ukraine abolition of HIV registries, implementation of routine Human Immunodeficiency Virus testing and expansion of services. Addiction. 2010;105(3):569–70. doi: 10.1111/j.1360-0443.2009.02881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Springer SA, Pesanti E, Hodges J, Macura T, Doros G, Altice FL. Effectiveness of antiretroviral therapy among HIV-infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clin Infect Dis. 2004;38(12):1754–60. doi: 10.1086/421392. [DOI] [PubMed] [Google Scholar]
- 51.Porco TC, Martin JN, Page-Shafer KA, Cheng A, Charlebois E, Grant RM, et al. Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS. 2004;18(1):81–8. doi: 10.1097/01.aids.0000096872.36052.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Platt L, Jolley E, Hope V, Latypov A, Hickson F, Reynolds L, et al. Washington, DC: World Bank; 2013. HIV in the European Region: using evidence to strengthen policy and programmes – vulnerability and response: synthesis report. [Google Scholar]
- 53.van den Bergh BJ, Gatherer A, Fraser A, Moller L. Imprisonment and women's health: concerns about gender sensitivity, human rights and public health. Bull World Health Organ. 2011;89(9):689–94. doi: 10.2471/BLT.10.082842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yorick R, Skipalska H, Suvorova S, Sukovatova O, Zakharov K, Hodgdon S. HIV Prevention and rehabilitation models for women who inject drugs in Russia and Ukraine. Adv Prev Med. 2012;2012:316871. doi: 10.1155/2012/316871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyer JP, Springer SA, Altice FL. Substance abuse, violence, and HIV in women: a literature review of the syndemic. J Womens Health. 2011;20(7):991–1006. doi: 10.1089/jwh.2010.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maru DS, Basu S, Altice FL. HIV control efforts should directly address incarceration. Lancet Infect Dis. 2007;7(9):568–9. doi: 10.1016/S1473-3099(07)70190-1. [DOI] [PubMed] [Google Scholar]
- 57.Joint United Nations Programme on HIV/AIDS. AIDS epidemic update; Geneva, Switzerland: WHO; 2009. [Google Scholar]
- 58.DeBell D, Carter R. Impact of transition on public health in Ukraine: case study of the HIV/AIDS epidemic. BMJ. 2005;331(7510):216–19. doi: 10.1136/bmj.331.7510.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taran YS, Johnston LG, Pohorila NB, Saliuk TO. Correlates of HIV risk among injecting drug users in sixteen Ukrainian cities. AIDS Behav. 2011;15(1):65–74. doi: 10.1007/s10461-010-9817-6. [DOI] [PubMed] [Google Scholar]
- 60.Degenhardt L, Mathers B, Vickerman P, Rhodes T, Latkin C, Hickman M. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet. 2010;376(9737):285–301. doi: 10.1016/S0140-6736(10)60742-8. [DOI] [PubMed] [Google Scholar]
- 61.Strathdee SA, Hallett TB, Bobrova N, Rhodes T, Booth R, Abdool R, et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376(9737):268–84. doi: 10.1016/S0140-6736(10)60743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crepaz N, Lyles CM, Wolitski RJ, Passin WF, Rama SM, Herbst JH, et al. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta-analytic review of controlled trials. AIDS. 2006;20(2):143–57. doi: 10.1097/01.aids.0000196166.48518.a0. [DOI] [PubMed] [Google Scholar]
- 63.Bunnell R, Opio A, Musinguzi J, Kirungi W, Ekwaru P, Mishra V, et al. HIV transmission risk behavior among HIV-infected adults in Uganda: results of a nationally representative survey. AIDS. 2008;22(5):617–24. doi: 10.1097/QAD.0b013e3282f56b53. [DOI] [PubMed] [Google Scholar]
- 64.Binswanger IA, Blatchford PJ, Mueller SR, Stern MF. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592–600. doi: 10.7326/0003-4819-159-9-201311050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, et al. Release from prison – a high risk of death for former inmates. N Engl J Med. 2007;356(2):157–65. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merrall EL, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction. 2010;105(9):1545–54. doi: 10.1111/j.1360-0443.2010.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bachireddy C, Soule MC, Izenberg JM, Dvoryak S, Dumchev K, Altice FL. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug Alcohol Depend. 2014;134:106–14. doi: 10.1016/j.drugalcdep.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339(6122):966–71. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]