Abstract
OBJECTIVE
Folic acid fortification has reduced neural tube defect prevalence by 50% to 70%. It is unlikely that fortification levels will be increased to reduce neural tube defect prevalence further. Therefore, it is important to identify other modifiable risk factors. Vitamin B12 is metabolically related to folate; moreover, previous studies have found low B12 status in mothers of children affected by neural tube defect. Our objective was to quantify the effect of low B12 status on neural tube defect risk in a high-prevalence, unfortified population.
METHODS
We assessed pregnancy vitamin B12 status concentrations in blood samples taken at an average of 15 weeks’ gestation from 3 independent nested case-control groups of Irish women within population-based cohorts, at a time when vitamin supplementation or food fortification was rare. Group 1 blood samples were from 95 women during a neural tube defect–affected pregnancy and 265 control subjects. Group 2 included blood samples from 107 women who had a previous neural tube defect birth but whose current pregnancy was not affected and 414 control subjects. Group 3 samples were from 76 women during an affected pregnancy and 222 control subjects.
RESULTS
Mothers of children affected by neural tube defect had significantly lower B12 status. In all 3 groups those in the lowest B12 quartiles, compared with the highest, had between two and threefold higher adjusted odds ratios for being the mother of a child affected by neural tube defect. Pregnancy blood B12 concentrations of <250 ng/L were associated with the highest risks.
CONCLUSIONS
Deficient or inadequate maternal vitamin B12 status is associated with a significantly increased risk for neural tube defects. We suggest that women have vitamin B12 levels of >300 ng/L (221 pmol/L) before becoming pregnant. Improving B12 status beyond this level may afford a further reduction in risk, but this is uncertain.
Keywords: vitamin B12, cobalamin, neural tube defects, folic acid fortification, folate
Folic acid can prevent up to three fourths of neural tube defects (NTDs).1–3 Folic acid fortification of grain products in the United States was initially reported to reduce the incidence of NTDs by 19%,4 but this was probably an underestimate.5 Recent studies have shown reductions between 35% and 78% since mandatory fortification programs were introduced.6–10 There is debate on whether all folic acid-preventable NTDs are being prevented11–13 and whether the observed range of effectiveness can be explained by underlying ethnic differences in susceptibility10 or differences in completeness of case ascertainment.5 One argument is that insufficient folic acid has been added, and there have been calls to increase the level of fortification in the United States.11–13 Nevertheless, it is generally agreed that not all NTDs are preventable by folic acid. Therefore, to further reduce NTDs, other modifiable risk factors must be found.
Maternal obesity has been identified as 1 modifiable risk factor.14–16 Vitamin B12 (B12) status might be another, given the close metabolic association between B12 and folate and the importance of B12 status as a determinant of plasma homocysteine.17,18 A link between low maternal serum B12 level and anencephaly was suggested as far back as 1980.19 Several studies found differences in maternal B12 status (measured by serum total B12 or by holotranscobalamin) both during20–23 and after21,23–27 an NTD-affected pregnancy (AP). Lower amniotic fluid B12 or lower B12 binding capacity was also reported in NTD-APs.28–32 The 2 largest positive studies, conducted during the introduction of folic acid fortification in the United States26 and postfortification in Canada,22 found a tripling of risk between the lowest and highest quintiles of serum B1226 or quartile of holotranscobalamin.22
In a previous study, undertaken primarily to examine the association of folate status with risk of NTD, we found that low maternal B12 status during an AP was associated with risk, independent of folate status.20 To gain further insight into the role of B12 in the prevention of NTDs, we present results on 2 additional groups, using pregnancy blood samples from over the same years, which predate the era of widespread food fortification and when medical advice was to avoid unnecessary prophylactic supplements during early pregnancy, including vitamin supplements.33 For comparison, we also included hitherto unreported risk analysis from our previous study,20 using B12 data only from women in the study where we had definite information that they did not take vitamin supplements.
MATERIALS AND METHODS
Sample Selection
All 3 of the studies involved a nested case-control design where samples were selected from within 2 large population-based cohorts. Groups 1 and 3 included case blood samples taken from mothers during an NTD-AP. Group 2 case blood samples were taken during pregnancy from mothers who previously delivered an NTD-affected infant but whose current pregnancy was not affected (NAP). All of the samples were collected with institutional ethical approval and in compliance with applicable national ethical standards.
Group 1
Between July 1983 and February 1986, serum samples were made available from first antenatal clinic blood samples collected from all pregnant women as part of the Irish National Rubella Screening Program. Details of the collection source were presented in an earlier study.34 Samples were identified from 129 women who were currently undergoing an AP. This represented 92% of all of the ascertained NTD affected births in the major Dublin maternity hospitals between 1984 and 1986. Sufficient serum was available for analysis of B12 in 95 of these case subjects. Control samples (n = 265) were randomly selected from the same source over the same sampling period, using the sequential sample numbering system of the screening laboratory. All of the control women had normal pregnancies, based on hospital charts. Case and control blood samples were processed in a similar manner and were stored at − 20°C before analysis.
Groups 2 and 3
Between 1986 and 1990, research blood samples were collected from 56 049 women at their first antenatal visit in the 3 major Dublin maternity hospitals. This represents ~70% of women who delivered in these hospitals during the period. Additional details have been published elsewhere.17,20,35–37 An aliquot in 1% ascorbic acid for red cell folate (RCF) and a plasma sample were stored at − 20°C for each participant.
Group 2 includes blood samples within the above biobank that were collected from women with a history of NTD-APs but who had an NAP between 1986 and 1990. From the EUROCAT birth defects registry38 and hospital records we ascertained that there were 303 such women during the time period in the 3 hospitals. Of these, 187 women had given research blood samples. We excluded 65 women because they were taking vitamins, mainly as participants in the Irish Trial for the prevention of NTD.39 A further 9 women had insufficient sample for analysis. We had blood samples for 1 woman during both an AP and an NAP pregnancy. We included her AP blood in group 3 below and excluded her NAP sample. Control subjects were obtained for each case by selecting 4 to 5 women who attended the antenatal clinic in the same hospital on the same day. Hospital charts for these women were scrutinized for vitamin supplementation and other demographic details. From this, samples from 439 nonsupplemented women were eligible for analysis. Laboratory B12 results were missing for 5 case subjects and 25 control subjects, leaving a final data set of 107 case subjects and 414 control subjects.
Group 3 represents a previously unpublished analysis from our earlier study on maternal folate and B12 in NTD-APs.20 These blood samples were also obtained from the 1986–1990 biobank described above. For the current analysis, all of the known vitamin supplement users were removed, leaving data from 76 case mothers and 222 control mothers.
Laboratory Methods
B12, serum folate, and RCF were measured by microbiologic methods, as described previously.20 All of the vitamin analyses were completed between 3 and 9 years from the sample collection, with each group analyzed as a batch in a continuous run of assays. Case and control samples were randomly mixed in every assay, and operators were not aware of the sample status. Interassay and intra-assay coefficients of variation were within 10.4% and 12.0% for folate and B12, respectively. An ongoing laboratory quality-control system ensured long-term performance of the assays within established limits over the time scale of analysis.
Statistical Methods
Data were not normally distributed and are presented as medians and interquartile ranges. Case-control comparisons were conducted using Wilcoxon 2-sample tests. Logistic regression models were used to test whether decreasing levels of B12 were a significant risk for NTD in each of the groups. B12 was entered as a continuous variable or as quartiles of the control population. Adjustments were made for year of sampling and for folate status. Analysis of risk by maternal B12 status was conducted using cutoffs for B12 that represented deficient (0–149 ng/L), borderline-deficient (150–199 ng/L), low-adequate (200–299 ng/L), adequate-good (300–399 ng/L) and good (≥400 ng/L) status. Based on NTD prevalence data in the Dublin maternity hospitals, an average NTD rate of 2.9 per 1000 births was used for group 1, collected between 1984 and 1986, and 1.9 per 1000 births for groups 2 and 3, collected between 1986 and 1990. Confidence limits for risk estimates were calculated by assigning each control a representative weight (based on assumed overall risk) and assuming no sampling variability in the population and a Poisson distribution for the number of cases. Comparison of risk for the B12 status categories within each study group was based on an analysis of maximum likelihood odds ratio (OR) estimates, using the highest B12 category as the reference. All of the analyses were done using SAS 9 (SAS Institute, Cary, NC). Significant effects were those with 2-tailed P values <.05.
RESULTS
Table 1 gives available characteristics of the 3 groups. Median gestation was 15 weeks. Group 2 case mothers tended to be older than control subjects and had more pregnancies (P < .001), which is consistent with these case subjects having a previous pregnancy history. In all of the groups, the median B12 concentration was between 13% and 19% lower in case subjects (P < .002; Table 2). RCF was significantly lower in group 3 case subjects (238 vs 315 ng/mL; P < .0001) and marginally lower in group 2 case subjects (median: 255 vs 291 ng/mL; P = .079). RCF concentrations were not available for group 1, but the serum folate was not different between case and control subjects (2.85 vs 3.3 µg/L; P = .42). There was a weak correlation between B12 and RCF in group 3 samples (r = 0.16; P = .006; N = 298) but little correlation between the B12 and RCF in group 2 (r = 0.07; P = .11; N = 514). There was little correlation between serum/plasma folate and B12 in any of the 3 groups (r = 0.09, 0.07, and 0.10 for groups 1, 2, and 3, respectively). The median B12 of control subjects in group 1, sampled between 1984 and 1986, was some 20% lower than groups 2 and 3, sampled between 1986 and 1990 (P < .0001). When the median B12 was plotted by year, there was weak general trend toward increased status over the period of the study (data not shown). We noted a 4% difference (P = .023) in median B12 among control subjects in groups 2 and 3, both of which were sampled from the same population of 56 049 blood samples. This was probably because of differences in the year of analysis and minor fluctuations in assay performance but was well within the interassay coefficients of variation of our laboratory.
TABLE 1.
Characteristics of Pregnant Case and Control Women in the 3 Study Groups
| Characteristic | Group 1 |
Group 2 |
Group 3 |
|||
|---|---|---|---|---|---|---|
| Case Subjects |
Control Subjects |
Case Subjects |
Control Subjects |
Case Subjects |
Control Subjects |
|
| Median age (IQR), y | 27.0 (22–32) | 28.0 (24–32) | 32.1 (29–36)a | 27.9 (24–32)a | 26.5 (24–33) | 28.0 (24–33) |
| Median weeks’ gestation at sampling (IQR) | 16.0 (12–21) | 15.0 (11–20) | 15.3 (11–21) | 14.5 (12–19) | 14.4 (12–20) | 15.1 (12–20) |
| Median No. of pregnancies (range) | 1 (0–9) | 1 (0–8) | 4 (1–11)a | 1 (0–10)a | 0 (0–14) | 1 (0–13) |
| NTD type, n (%) | ||||||
| Spina bifida only | 47 (49.5) | — | 47 (43.9) | — | 36 (47.4) | — |
| Anencephaly only | 27 (28.4) | — | 35 (32.7) | — | 24 (31.6) | — |
| Spina bifida + anencephaly | 6 (6.3) | — | 16 (15.0) | — | 9 (11.8) | — |
| Encephalocele only | 9 (9.5) | — | 7 (6.5) | — | 3 (3.9) | — |
| Other | 6 (6.3) | — | 2 (1.8) | — | 4 (5.3) | — |
| Sample year, n (%) | ||||||
| 1983 | 16 (16.8) | 37 (14.0) | — | — | — | — |
| 1984 | 49 (51.6) | 122 (46.0) | — | — | — | — |
| 1985 | 28 (29.5) | 103 (38.9) | — | — | — | — |
| 1986 | 2 (2.1) | 3 (1.1) | 36 (33.6) | 151 (36.5) | 22 (28.9) | 50 (22.5) |
| 1987 | — | — | 28 (26.2) | 114 (27.5) | 20 (26.3) | 66 (29.7) |
| 1988 | — | — | 31 (29.0) | 108 (26.1) | 16 (21.1) | 44 (19.8) |
| 1989 and 1990 | — | — | 12 (11.2) | 41 (9.9) | 18 (23.7) | 62 (27.9) |
IQR indicates interquartile range, denoting the 25th to 75th percentile values; —, no data.
P < .001.
TABLE 2.
Serum B12 Concentrations During Pregnancy in Mothers With a History of Pregnancies Affected by NTD (Cases) and Nonaffected Mothers (Controls) Matched for Group
| Variable | Group 1 (AP) | Group 2 (NAP) | Group 3 (AP) |
|---|---|---|---|
| Case B12, median (IQR), ng/L | 210 (162–252) | 270 (208–360) | 244 (208–330) |
| No. | 95 | 107 | 76 |
| Control B12, median (IQR), ng/L | 242 (190–297) | 314 (253–404) | 300 (237–366) |
| No. | 265 | 414 | 222 |
| Wilcoxon test, P | .0003 | .0004 | .0018 |
IQR indicates interquartile range, denoting the 25th to 75th percentile values; AP, case samples were taken during an AP; NAP, case samples were taken during an NAP from women who previously had an NTD-AP.
To determine the independent contributions of B12 and RCF to risk of NTD, we used logistic regression analysis with B12 and RCF (groups 2 and 3) as continuous variables. There were highly significant associations (Table 3), such that each unit increase in B12 concentration provided an ~0.3% reduction in risk, independent of RCF. RCF was a significant factor only in group 3.
TABLE 3.
ORs for B12 and RCF as Independent Risk Factors for Being a Mother of an NTD-Affected Child Using a Continuous Logistic Regression Model
| Group | Effect | ORa | 95% CI | P |
|---|---|---|---|---|
| 1 (AP) | B12, ng/L | 0.995 | 0.992–0.998 | .0005 |
| 2 (NAP) | B12, ng/L | 0.997 | 0.995–0.999 | .0030 |
| RCF, µg/L | 1.000 | 0.999–1.001 | .71 | |
| 3 (AP) | B12, ng/L | 0.997 | 0.994–1.000 | .0320 |
| RCF, µg/L | 0.995 | 0.993–0.998 | .0002 |
OR indicates odds ratio; CI, confidence interval.
Odds ratios are calculated per unit increase in concentration of B12 or RCF.
To explore whether the associations with risk were confined to particular sectors of the B12 distribution, we categorized the data by quartile of B12 concentration among control subjects. Logistic regression analysis, using the highest B12 quartile as the reference group and adjusting for RCF and year of sampling (Table 4), showed that risk of NTD was significantly increased only in the lowest quartile for groups 2 and 3 but extended to the second quartile for group 1 (adjusted for serum folate). The upper cutoffs for the second quartile in group 1 (242 ng/L) and the lowest quartile in group 2 (252 ng/L) and group 3 (237 ng/L) were remarkably similar. In all 3 of the groups, those with B12 concentrations of <250 ng/L had a 2.5- to 3-fold higher risk of being the mother of an NTD-affected child, after adjusting for folate. Adjusting for gestation did not substantially change the magnitude of the effects (data not shown).
TABLE 4.
ORs for B12 as a Risk Factor for Being a Mother of an NTD-Affected Child According to Quartile of Control Mother B12 Concentrations
| Effect | Group 1: AP |
Group2: NAP |
Group 3: AP |
|||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| B12 quartile unadjusted | ||||||
| 1 | 3.19 | 1.49–6.82a | 2.89 | 1.51–5.53b | 2.99 | 1.41–6.35c |
| 2 | 2.77 | 1.28–5.98d | 1.40 | 0.68–2.87 | 1.49 | 0.65–3.41 |
| 3 | 1.82 | 0.81–4.09 | 1.89 | 0.95–3.73 | 1.16 | 0.49–2.77 |
| 4 | Ref | — | Ref | — | Ref | — |
| Adjustede | ||||||
| 1 | 3.14 | 1.46–6.72f | 2.75 | 1.43–5.28g | 2.45 | 1.12–5.32h |
| 2 | 2.63 | 1.21–5.72i | 1.34 | 0.65–2.77 | 1.55 | 0.66–3.61 |
| 3 | 1.79 | 0.80–4.03 | 1.80 | 0.91–3.57 | 1.09 | 0.45–2.65 |
| 4 | Ref | — | Ref | — | Ref | — |
Ref indicates reference; —, no data.
P = .003.
P= .001.
P = .005.
P = .010.
Data are adjusted for year of assay and serum folate (group 1) or RCF (groups 2 and 3).
P = .003.
P = .003.
P = .024.
P= .015.
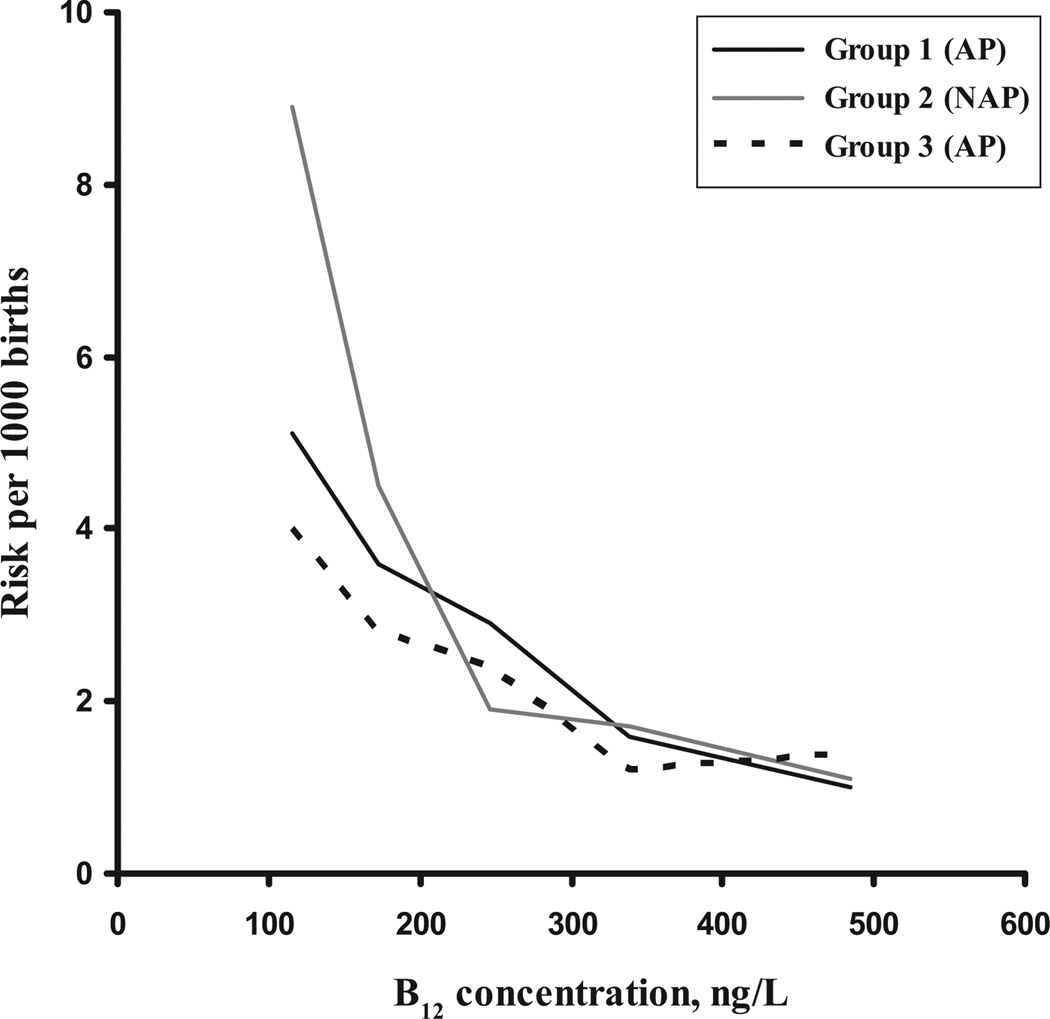
We then divided the B12 levels into 5 nutritionally relevant groups (deficient, borderline deficient, etc) and calculated relative risks for each group, based on the known NTD prevalence rates at the time of sampling. Point estimates with SEs are shown on Table 5. These data show clear trends of risk reduction across the 5 categories in all of the groups, with those in the lowest category (pregnancy B12 concentrations of <150 ng/L) having ~ 5-times higher risk compared with those with pregnancy B12 levels of >400 ng/L. The effects are significant below a concentration of 200 ng/L in groups 1 and 2. Effects were not significant for group 3, but smaller numbers are likely to have been a factor. Figure 1 shows trends across the B12 distributions. These suggest that some further reduction in risk may be afforded by having a B12 status >320 to 350 ng/L, but there is no statistically significant effect.
TABLE 5.
Risk of NTD per 1000 Births According to Maternal B12 Status
| B12 Category, ng/L | Group 1 (AP)a |
Group 2 (NAP)b |
Group 3 (AP)c |
|||
|---|---|---|---|---|---|---|
| Risk (CI) | P | Risk (CI) | P | Risk (CI) | P | |
| 0–149 | 5.1 (3.1–8.0) | .014 | 8.9 (4.5–15.9) | <.0001 | 4.0 (1.3–9.2) | .14 |
| 150–199 | 3.6 (2.2–5.5) | .050 | 4.5 (2.4–7.5) | .0008 | 2.8 (1.2–5.5) | .25 |
| 200–299 | 2.9 (2.1–3.9) | .082 | 1.9 (1.3–2.6) | .082 | 2.4 (1.7–3.3) | .21 |
| 300–399 | 1.6 (0.7–3.1) | .502 | 1.7 (1.2–2.5) | .134 | 1.2 (0.7–1.9) | .65 |
| ≥400 | 1.0 (0.2–2.8) | Ref | 1.1 (0.6–1.7) | Ref | 1.4 (0.7–2.7) | Ref |
Data are the relative risk (with confidence limits)of being a case mother compared with being a control mother, given a B12 concentration With in the designated category. CI indicates confidence limit; Ref, reference.
Case samples were taken during an AP at a time when the underlying prevalence of NTD was 2.9 per 1000 births.
Case samples were taken during an NAP at a time when the underlying prevalence of NTD was 1.9 per 1000 births.
Case samples were taken during an AP at a time when the underlying prevalence of NTD was 1.9 per 1000 births.
FIGURE 1.
Risk of being the mother of an NTD-affected child according to pregnancy vitamin B12 status during an AP or NAP. Risks were calculated from the proportion of case and control subjects in defined B12 categories, multiplied by the prevalence of NTD at the time of sampling. x-axis values are the median B12 values within each category.
DISCUSSION
We have shown, in 3 separate groups, that low B12 status is an independent maternal risk factor for having an NTD-AP. Moreover, our study is the first to examine the risk by concentration of B12. Our data indicate that women with pregnancy B12 concentrations of <200 ng/L are at 3 times greater risk than those with levels of >400 ng/L. These results agree remarkably with effects observed by others.22,26 Our analysis indicates that the majority of risk is confined to B12 levels below ~250 ng/L (Tables 4 and 5), although the trend line in Fig 1 suggests that further risk reduction might be achieved by having a B12 status >320 to 350 ng/L. Considering that these women were sampled at an average of 15 weeks’ gestation and, by that time, there is a natural physiologic drop of ~20% to 25% in serum B12 from the prepregnancy level,40,41 our data indicate that women should aim to enter pregnancy with serum B12 concentrations of >300 ng/L (221 pmol/L) and that levels above 400 ng/L (295 pmol/L) might be desirable, although we found no statistically significant benefit.
It is uncertain whether further reduction in NTDs can be achieved in the United States by increasing the level of grain fortification with folic acid. Moreover, recent reports on possible adverse effects of high folic acid consumption make such a strategy unlikely.42–44 The addition of B12 in conjunction with folic acid has been proposed but mainly to protect individuals with low B12 status.12,13,45,46 There is little information on the proportion of women who enter pregnancy with B12 levels of <300 ng/L, although a recent National Health and Nutrition Examination Survey report found a mean serum B12 of just over 400 ng/L (300 pmol/L) in women between 20 and 39 years old.47 Our study suggests that the addition of B12 to fortified grains may be a useful and acceptable way to further reduce the prevalence of NTD, but more studies are needed to establish the safety of fortifying with B12 and the dose of B12 that might be required to reach an effective level of protection.
Previous case-control reports revealed lower B12 status in women with a history of NTD-APs who were not pregnant at the time of study.21,23–27 Our study is unusual in that we observed lower B12 concentrations during an NAP in such mothers (our group 2 case subjects). There are 2 possible explanations for this finding. One is that B12 is merely marking low folate status. However, we found little interaction between B12 and folate in any of our groups. This is not surprising, because in a nonfortified, nonsupplemented population, vitamin status is determined by dietary sources of folate and B12, and the 2 are quite different. The second, more likely explanation, is that long-term low B12 status may act in synergy with low folate status to precipitate an NTD-AP. B12 status, along with genetic differences, may help to explain the low NTD rates seen in some ethnic groups and may also help in understanding why low maternal folate status alone usually does not result in NTD-APs. For example, blacks, who have both lower NTD rates and lower folate levels than other ethnic groups in the United States, have significantly higher B12 status,48,49 and this high status is also seen during pregnancy.50 Our case mothers were all of Irish descent (ie, white) and lived in a region of traditionally high NTD prevalence, suggesting a moderately high genetic predisposition. The importance of B12 as a synergistic factor is also supported by our previous observation20 that women in the bottom quartile of both plasma folate and B12 had >5 times higher ORs of a birth affected by NTD than those in the highest quartile of both vitamins. Women in the bottom quartile of plasma folate but the highest B12 quartile had less than half that risk.
It is not known how folate and B12 might interact to affect neural tube formation, but several mechanisms are possible. As cofactor to the enzyme methionine synthase, B12 influences both the incorporation of folates into the cellular pool and the flux of folate derived 1-carbon units destined for DNA synthesis or for methylation reactions. DNA synthesis is an essential feature of embryonic development, but other factors that trigger developmental changes, such as cell-signaling events that lead to differential gene expression, are partially controlled by methylation reactions. Impairment of either of these systems could be involved in folate or B12-responsive NTDs.
Our study has several strengths. First, our groups were large enough to detect an average B12 difference of 15%, enabling us to get a good estimate of the critical level of B12 required to prevent NTDs. Several other studies that found no difference were limited to ≤60 case subjects51 or were conducted in an area of very low NTD prevalence with high reported B12 levels.52 Our study samples were taken from a population of high NTD risk, at a time when women were not exposed to prenatal vitamins before the blood draw. Such populations are difficult to find nowadays, but they have the advantage that the observed concentrations are more likely to reflect the blood vitamin level at the time of neural tube closure, because they have not been confounded by early pregnancy intake of vitamin supplements. Samples from all of our groups were assayed using the same methodology, and our collection strategy ensured that case and control subjects were matched for gestational, temporal, and storage variables likely to affect the B12 content. One limitation is the fact that we could not also control for maternal age and number of pregnancies. However, we had no evidence that these variables would affect maternal B12 status. The study is also limited by sparse demographic data on participants and by the lack of RCF data for group 1, which, because of its greater stability, may have been more informative than serum folate.
CONCLUSIONS
We have confirmed that low B12 status is an important maternal risk factor for having an NTD-AP. More importantly, our study is first to address the public health question of what B12 concentrations might be protective, although our calculations are limited by extrapolation from pregnancy values to a nonpregnant condition. Our logistic regression analysis suggests that women who start pregnancy with serum B12 concentrations of <300 ng/L (221 pmol/L) are at significantly higher risk for NTDs. Improving B12 status beyond 300 ng/L might offer further risk reduction, but this is unclear.
What’s Known on This Subject
Folic acid can prevent many, but not all, NTDs. Vitamin B12 interacts closely with folate metabolism and may play a role in NTD prevention. Some studies have found low vitamin B12 status in mothers of NTD-affected children.
What This Study Adds
This study confirms that low maternal vitamin B12 status is an independent risk factor for having an NTD-affected pregnancy and is the first to address the public health question of what B12 level might be protective for women entering pregnancy.
ACKNOWLEDGMENTS
This work was supported by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, and the Health Research Board (Ireland).
We gratefully acknowledge the cooperation of the masters and nursing staff of the Dublin Maternity hospitals who enabled the 1986–1990 blood collections. We also thank the director and staff of the Virus Reference Laboratory, Department of Medical Microbiology (University College Dublin), who made the Irish National Rubella Screening Program samples available during 1983–1986.
Abbreviations
- NTD
neural tube defect
- B12
vitamin B12
- AP
affected pregnancy
- NAP
nonaffected pregnancy
- RCF
red cell folate
- OR
odds ratio
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338(8760):131–137. [PubMed] [Google Scholar]
- 2.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327(26):1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 3.Berry RJ, Li Z, Erickson JD, et al. Prevention of neural-tube defects with folic acid in China: China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341(20):1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- 4.Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285(23):2981–2986. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- 5.Mills JL, Signore C. Neural tube defect rates before and after food fortification with folic acid. Birth Defects Res A Clin Mol Teratol. 2004;70(11):844–845. doi: 10.1002/bdra.20075. [DOI] [PubMed] [Google Scholar]
- 6.Ray JG, Meier C, Vermeulen MJ, Boss S, Wyatt PR, Cole DE. Association of neural tube defects and folic acid food fortification in Canada. Lancet. 2002;360(9350):2047–2048. doi: 10.1016/S0140-6736(02)11994-5. [DOI] [PubMed] [Google Scholar]
- 7.Persad VL, Van den Hof MC, Dube JM, Zimmer P. Incidence of open neural tube defects in Nova Scotia after folic acid fortification. CMAJ. 2002;167(3):241–245. [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, West R, Randell E, et al. A comprehensive evaluation of food fortification with folic acid for the primary prevention of neural tube defects. BMC Pregnancy Childbirth. 2004;4(1):20. doi: 10.1186/1471-2393-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Camelo JS, Orioli IM, da Graca Dutra M, et al. Reduction of birth prevalence rates of neural tube defects after folic acid fortification in Chile. Am J Med Genet A. 2005;135(2):120–125. doi: 10.1002/ajmg.a.30651. [DOI] [PubMed] [Google Scholar]
- 10.Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995–2002. Pediatrics. 2005;116(3):580–586. doi: 10.1542/peds.2005-0592. [DOI] [PubMed] [Google Scholar]
- 11.Brent RL, Oakley GP, Jr, Mattison DR. The unnecessary epidemic of folic acid-preventable spina bifida and anencephaly. Pediatrics. 2000;106(4):825–827. doi: 10.1542/peds.106.4.825. [DOI] [PubMed] [Google Scholar]
- 12.Brent RL, Oakley GP., Jr Triumph and/or tragedy: the present Food and Drug Administration program of enriching grains with folic acid. Pediatrics. 2006;117(3):930–932. doi: 10.1542/peds.2005-2557. [DOI] [PubMed] [Google Scholar]
- 13.Brent RL, Oakley GP., Jr The Food and Drug Administration must require the addition of more folic acid in “enriched” flour and other grains. Pediatrics. 2005;116(3):753–755. doi: 10.1542/peds.2005-1536. [DOI] [PubMed] [Google Scholar]
- 14.Werler MM, Louik C, Shapiro S, Mitchell AA. Prepregnant weight in relation to risk of neural tube defects. JAMA. 1996;275(14):1089–1092. doi: 10.1001/jama.1996.03530380031027. [DOI] [PubMed] [Google Scholar]
- 15.Watkins ML, Rasmussen SA, Honein MA, Botto LD, Moore CA. Maternal obesity and risk for birth defects. Pediatrics. 2003;111(5 pt 2):1152–1158. [PubMed] [Google Scholar]
- 16.Waller DK, Mills JL, Simpson JL, et al. Are obese women at higher risk for producing malformed offspring? Am J Obstet Gynecol. 1994;170(2):541–548. doi: 10.1016/s0002-9378(94)70224-1. [DOI] [PubMed] [Google Scholar]
- 17.Mills JL, McPartlin JM, Kirke PN, et al. Homocysteine metabolism in pregnancies complicated by neural-tube defects. Lancet. 1995;345(8943):149–151. doi: 10.1016/s0140-6736(95)90165-5. [DOI] [PubMed] [Google Scholar]
- 18.Steegers-Theunissen RP, Boers GH, Trijbels FJ, Eskes TK. Neural-tube defects and derangement of homocysteine metabolism. N Engl J Med. 1991;324(3):199–200. doi: 10.1056/NEJM199101173240315. [DOI] [PubMed] [Google Scholar]
- 19.Schorah CJ, Smithells RW, Scott J. Vitamin B12 and anencephaly. Lancet. 1980;1(8173):880. doi: 10.1016/s0140-6736(80)91381-1. [DOI] [PubMed] [Google Scholar]
- 20.Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med. 1993;86(11):703–708. [PubMed] [Google Scholar]
- 21.Hao L, Liu M, Liu X, Chen X, Tang Y, Li Z. Relationship between folate, vitamin B12, total plasma homocysteine and mutation of reductase [in Chinese] Zhonghua Yu Fang Yi Xue Za Zhi. 2000;34(1):22–24. [PubMed] [Google Scholar]
- 22.Ray JG, Wyatt PR, Thompson MD, et al. Vitamin B12 and the risk of neural tube defects in a folic-acid-fortified population. Epidemiology. 2007;18(3):362–366. doi: 10.1097/01.ede.0000257063.77411.e9. [DOI] [PubMed] [Google Scholar]
- 23.Gaber KR, Farag MK, Soliman SE, El-Bassyouni HT, El-Kamah G. Maternal vitamin B12 and the risk of fetal neural tube defects in Egyptian patients. Clin Lab. 2007;53(1–2):69–75. [PubMed] [Google Scholar]
- 24.Zhang T, Xin R, Gu X, et al. Maternal serum vitamin B12, folate and homocysteine and the risk of neural tube defects in the offspring in a high-risk area of China. Public Health Nutr. 2008 doi: 10.1017/S1368980008002735. In press. [DOI] [PubMed] [Google Scholar]
- 25.Wright ME. A case-control study of maternal nutrition and neural tube defects in Northern Ireland. Midwifery. 1995;11(3):146–152. doi: 10.1016/0266-6138(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 26.Suarez L, Hendricks K, Felkner M, Gunter E. Maternal serum B12 levels and risk for neural tube defects in a Texas-Mexico border population. Ann Epidemiol. 2003;13(2):81–88. doi: 10.1016/s1047-2797(02)00267-3. [DOI] [PubMed] [Google Scholar]
- 27.Groenen PM, van Rooij IA, Peer PG, Gooskens RH, Zielhuis GA, Steegers-Theunissen RP. Marginal maternal vitamin B12 status increases the risk of offspring with spina bifida. Am J Obstet Gynecol. 2004;191(1):11–17. doi: 10.1016/j.ajog.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 28.Gardiki-Kouidou P, Seller MJ. Amniotic fluid folate, vitamin B12 and transcobalamins in neural tube defects. Clin Genet. 1988;33(6):441–448. doi: 10.1111/j.1399-0004.1988.tb03478.x. [DOI] [PubMed] [Google Scholar]
- 29.Dawson EB, Evans DR, Van Hook JW. Amniotic fluid B12 and folate levels associated with neural tube defects. Am J Perinatol. 1998;15(9):511–514. doi: 10.1055/s-2007-993975. [DOI] [PubMed] [Google Scholar]
- 30.Economides DL, Ferguson J, Mackenzie IZ, Darley J, Ware II, Holmes-Siedle M. Folate and vitamin B12 concentrations in maternal and fetal blood, and amniotic fluid in second trimester pregnancies complicated by neural tube defects. Br J Obstet Gynaecol. 1992;99(1):23–25. doi: 10.1111/j.1471-0528.1992.tb14386.x. [DOI] [PubMed] [Google Scholar]
- 31.Steen MT, Boddie AM, Fisher AJ, et al. Neural-tube defects are associated with low concentrations of cobalamin (vitamin B12) in amniotic fluid. Prenat Diagn. 1998;18(6):545–555. [PubMed] [Google Scholar]
- 32.Weekes EW, Tamura T, Davis RO, et al. Nutrient levels in amniotic fluid from women with normal and neural tube defect pregnancies. Biol Neonate. 1992;61(4):226–231. doi: 10.1159/000243747. [DOI] [PubMed] [Google Scholar]
- 33.Murphy JF. Drugs and pregnancy. Ir Med J. 1984;77(2):52–56. [PubMed] [Google Scholar]
- 34.Molloy AM, Kirke P, Hillary I, Weir DG, Scott JM. Maternal serum folate and vitamin B12 concentrations in pregnancies associated with neural tube defects. Arch Dis Child. 1985;60(7):660–665. doi: 10.1136/adc.60.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM. Folate levels neural tube defects. Implications for prevention. JAMA. 1995;274(21):1698–1702. doi: 10.1001/jama.1995.03530210052030. [DOI] [PubMed] [Google Scholar]
- 36.Cotter AM, Molloy AM, Scott JM, Daly SF. Elevated plasma homocysteine in early pregnancy: a risk factor for the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185(4):781–785. doi: 10.1067/mob.2001.117304. [DOI] [PubMed] [Google Scholar]
- 37.Cotter AM, Molloy AM, Scott JM, Daly SF. Elevated plasma homocysteine in early pregnancy: a risk factor for the development of nonsevere preeclampsia. Am J Obstet Gynecol. 2003;189(2):391–394. doi: 10.1067/s0002-9378(03)00669-0. [DOI] [PubMed] [Google Scholar]
- 38.Eastern Regional Health Authority. Congenital Anomalies in the Eastern Region of Ireland 1980–1999. Report of the Dublin EURO-CAT Registry on Twenty Years of Congenital Anomaly Surveillance. Dublin, Ireland: Eastern Regional Health Authority; 2001. [Google Scholar]
- 39.Kirke PN, Daly LE, Elwood JH. A randomised trial of low dose folic acid to prevent neural tube defects. The Irish Vitamin Study Group. Arch Dis Child. 1992;67(12):1442–1446. doi: 10.1136/adc.67.12.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy MM, Molloy AM, Ueland PM, et al. Longitudinal study of the effect of pregnancy on maternal and fetal cobalamin status in healthy women and their offspring. J Nutr. 2007;137(8):1863–1867. doi: 10.1093/jn/137.8.1863. [DOI] [PubMed] [Google Scholar]
- 41.Milman N, Byg KE, Bergholt T, Eriksen L, Hvas AM. Cobalamin status during normal pregnancy and postpartum: a longitudinal study comprising 406 Danish women. Eur J Haematol. 2006;76(6):521–525. doi: 10.1111/j.0902-4441.2006.t01-1-EJH2550.x. [DOI] [PubMed] [Google Scholar]
- 42.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297(21):2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 43.Morris MC, Evans DA, Bienias JL, et al. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol. 2005;62(4):641–645. doi: 10.1001/archneur.62.4.641. [DOI] [PubMed] [Google Scholar]
- 44.Troen AM, Mitchell B, Sorensen B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006;136(1):189–194. doi: 10.1093/jn/136.1.189. [DOI] [PubMed] [Google Scholar]
- 45.Herbert V, Bigaouette J. Call for endorsement of a petition to the Food and Drug Administration to always add vitamin B-12 to any folate fortification or supplement. Am J Clin Nutr. 1997;65(2):572–573. doi: 10.1093/ajcn/65.2.572. [DOI] [PubMed] [Google Scholar]
- 46.Oakley GP., Jr Let’s increase folic acid fortification and include vitamin B-12. Am J Clin Nutr. 1997;65(6):1889–1890. doi: 10.1093/ajcn/65.6.1889. [DOI] [PubMed] [Google Scholar]
- 47.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr. 2005;82(2):442–450. doi: 10.1093/ajcn.82.2.442. [DOI] [PubMed] [Google Scholar]
- 48.Saxena S, Carmel R. Racial differences in vitamin B12 levels in the United States. Am J Clin Pathol. 1987;88(1):95–97. doi: 10.1093/ajcp/88.1.95. [DOI] [PubMed] [Google Scholar]
- 49.Pfeiffer CM, Johnson CL, Jain RB, et al. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988 2004. Am J Clin Nutr. 2007;86(3):718–727. doi: 10.1093/ajcn/86.3.718. [DOI] [PubMed] [Google Scholar]
- 50.Knight EM, Spurlock BG, Edwards CH, et al. Biochemical profile of African American women during three trimesters of pregnancy and at delivery. J Nutr. 1994;124(6 suppl):943S–953S. doi: 10.1093/jn/124.suppl_6.943S. [DOI] [PubMed] [Google Scholar]
- 51.Ray JG, Blom HJ. Vitamin B12 insufficiency and the risk of fetal neural tube defects. QJM. 2003;96(4):289–295. doi: 10.1093/qjmed/hcg043. [DOI] [PubMed] [Google Scholar]
- 52.Mills JL, Tuomilehto J, Yu KF, et al. Maternal vitamin levels during pregnancies producing infants with neural tube defects. J Pediatr. 1992;120(6):863–871. doi: 10.1016/s0022-3476(05)81951-1. [DOI] [PubMed] [Google Scholar]