Abstract
Sirtuin 6 (SIRT6) is associated with longevity and is also a tumor suppressor. Identification of molecular regulators of SIRT6 might enable its activation therapeutically in cancer patients. In various breast cancer cell lines, we found that SIRT6 was phosphorylated at Ser338 by the kinase AKT1, which induced the interaction and ubiquitination of SIRT6 by MDM2, targeting SIRT6 for protease-dependent degradation. The survival of breast cancer patients positively correlated with the abundance of SIRT6 and inversely correlated with the phosphorylation of SIRT6 at Ser338. In a panel of breast tumor biopsies, SIRT6 abundance inversely correlated with the abundance of phosphorylated AKT. Inhibiting AKT or preventing SIRT6 phosphorylation by mutating Ser338 prevented the degradation of SIRT6 mediated by MDM2, suppressed the proliferation of breast cancer cells in culture, and inhibited the growth of breast tumor xenografts in mice. Overexpressing MDM2 decreased the abundance of SIRT6 in cells, whereas overexpressing an E3 ligase–deficient MDM2 or knocking down endogenous MDM2 increased SIRT6 abundance. Trastuzumab (known as Herceptin) is a drug that targets a specific receptor common in some breast cancers, and knocking down SIRT6 increased the survival of a breast cancer cell exposed to trastuzumab. Overexpression of a nonphosphorylatable SIRT6 mutant increased trastuzumab sensitivity in a resistant breast cancer cell line. Thus, stabilizing SIRT6 may be a clinical strategy for overcoming trastuzumab resistance in breast cancer patients.
INTRODUCTION
Sirtuins are a family of nicotinamide adenine dinucleotide–dependent de-acetylases that are involved in regulating stress resistance, metabolism, and organismal life span (1). They are mammalian homologs of the yeast silent information regulator 2 (Sir2), and there are seven sirtuins in mammals (SIRT1 to 7). SIRT6 was first identified as a chromatin-associated factor that suppresses genomic instability (2). SIRT6 controls cellular senescence by localizing to the telomeres and maintaining the telomere structure by deacetylating histone H3 at Lys9 (3). SIRT6-deficient mice exhibit severe metabolic abnormalities resulting in a degenerative phenotype that includes lymphopenia, osteopenia, loss of subcutaneous fat, and hypoglycemia that is fatal within 10 days (4–6). SIRT6 has both deacetylase and mono–ADP (adenosine 5′-diphosphate)–ribosyltransferase activities that contribute to its role in aging, inflammation, DNA repair, and metabolism (2, 3, 7–12). Loss of SIRT6 expression promotes tumorigenesis of the colon and liver (13, 14). Human breast cancers frequently exhibit loss of heterozygosity (LOH) at chromosome loci 19p13.3, where SIRT6 is located (15–17), suggesting that SIRT6 may function as a tumor suppressor in breast tissue.
There are five phosphorylation sites on SIRT6; the Ser338 residue is critical for the interaction of SIRT6 with a subset of proteins (18), but no biological consequences of this phosphorylation have yet been identified. Also, the kinase (or kinases) that might be responsible for phosphorylating SIRT6 is unknown. Lin et al. identified ubiquitin-specific peptidase 10 (USP10) as a deubiquitinase for SIRT6 and found that USP10 antagonizes c-Myc–dependent transcription through SIRT6 stabilization (19). These studies have begun to shed light on the possible regulation of SIRT6. Here, we investigated the molecular mechanisms that lead to loss of SIRT6 activity or protein abundance in breast cancer and the implications for therapeutic strategies involving trastuzumab (commonly known as Herceptin) in breast cancers.
RESULTS
Activation of AKT1 promotes the degradation of SIRT6
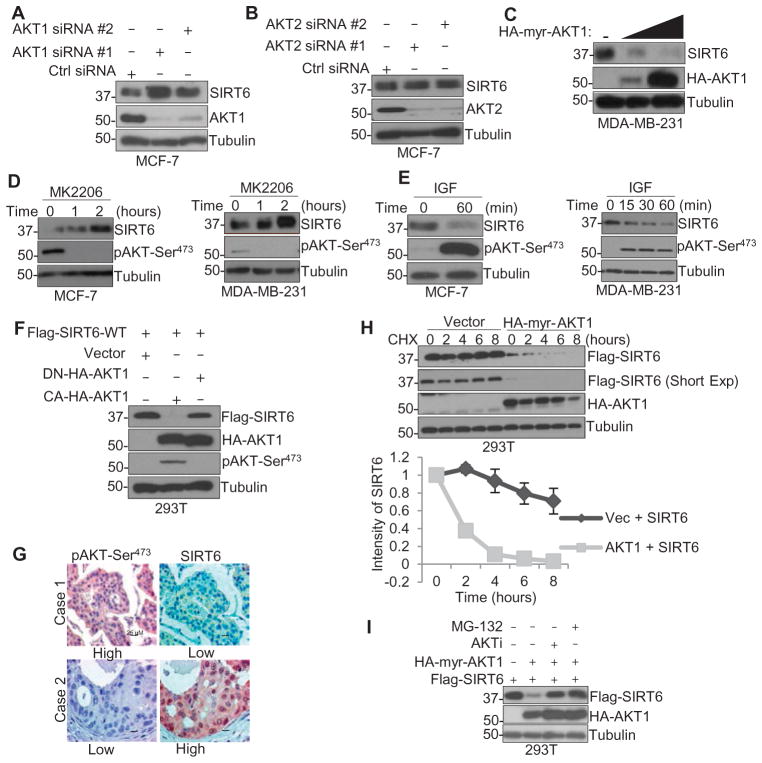
The phenotypes of SIRT6−/− mice, including accelerated aging, cardiac hypertrophy, and reduced life span, are similar to those associated with increased activation of the insulin-like growth factor (IGF)–AKT pathway (20, 21). SIRT6 inhibits IGF-AKT signaling by inhibiting gene transcription and phosphorylation of AKT (22, 23). Because the phosphoinositide 3-kinase (PI3K)–AKT signaling pathway is one of the major oncogenic signaling cascades that result in tumor growth and development (24–26), we speculated that IGF-AKT signaling might also regulate SIRT6. To determine whether AKT signaling regulates SIRT6 expression, AKT1 and AKT2 were knocked down by silencing RNA [small interfering RNA (siRNA)] in MCF-7 (Fig. 1, A and B) and MDA-MB-231 (fig. S1A) human breast cancer cells. Only knockdown of AKT1, but not AKT2, resulted in significant increase in SIRT6 protein abundance. We also observed increased reduction in the endogenous SIRT6 protein abundance with overexpression of constitutively active AKT1 in MDA-MB-231 cells (Fig. 1C) and exogenous SIRT6 abundance in human embryonic kidney (HEK) 293T cells (fig. S1B). Overexpression of constitutively active AKT3 did not decrease SIRT6 protein abundance (fig. S1B), indicating that AKT1 may be the dominant kinase that regulates SIRT6 abundance. Thus, we focused on AKT1 for further experiments. Adding MK2206, an AKT inhibitor, to cultures increased the abundance of SIRT6 in MCF-7, MDA-MB-231, and two additional breast cancer cell lines, HBL-100 and Hs578T (Fig. 1D and fig. S1C). Treatment with growth factors, such as epidermal growth factor (EGF) and IGF, activated AKT1 and decreased SIRT6 abundance in a time-dependent manner (Fig. 1E and fig. S1D). Furthermore, only the expression of constitutively active, but not the dominant-negative, kinase-deficient AKT1 decreased the abundance of Flag-tagged SIRT6 in HEK293T cells (Fig. 1F), suggesting an inverse correlation between AKT activation and SIRT6 abundance. In a panel of breast cancer cell lines (fig. S1E) and 312 patient breast tumor tissue specimens (126 paraffin-embedded samples and 186 samples from tissue microarray) (Fig. 1G and Table 1), we observed a negative correlation between the abundance of SIRT6 and that of AKT phosphorylated at Ser473.
Fig. 1. Activation of AKT1 results in SIRT6 degradation.
(A and B) Western blotting for SIRT6 and AKT1 against tubulin (loading control) in lysates from MCF-7 cells transfected with one of two siRNAs against AKT1 (A) or AKT2 (B) or a control (Ctrl) siRNA for 72 hours. (C) Western blotting for SIRT6 or HA in lysates from MDA-MB-231 cells transfected with 2 or 6 μg of HA-tagged, constitutively active AKT1 (HA-myr-AKT1) or a control vector (−). (D and E) Western blotting for SIRT6 or phosphorylated AKT (pAKT-Ser473) in lysates from MCF-7 and MDA-MB-231 cells treated with either 2 μM MK2206 (D) or IGF (50 ng/ml) (E) for the indicated time. (F) Western blotting in lysates from HEK293T cells transfected with HA-tagged dominant-negative AKT1 (DN-HA-AKT1) or constitutively active AKT1 (HA-myr-AKT1; “CA”) and Flag-tagged, wild-type (WT) SIRT6. (G) Immunohistochemical analysis of the abundance of phosphorylated AKT (Ser473) and SIRT6 in tumor sections from two different breast cancer patients (cases 1 and 2). Scale bars, 25 μm. (H) Western blotting in lysates from HEK293T cells transfected with Flag-tagged SIRT6 and either vector or HA-AKT1 in the presence of cycloheximide (CHX) for up to 8 hours. Short Exp, shorter exposure time. (I) Western blotting in lysates from HEK293T cells transfected with constitutively active AKT1 or Flag-tagged SIRT6 and treated with either MG-132 or MK2206 (AKTi). Blots are representative of three experiments.
Table 1. Correlation between phospho-AKT Ser473 and SIRT6 in breast cancer.
The correlation between the abundance of phosphorylated AKT (Ser473) and that of SIRT6 in an array of 186 human breast cancers and 126 formalin-fixed and paraffin-embedded breast carcinoma patient samples, analyzed using χ2 test (P = 0.024).
| SIRT6
|
Total | |||
|---|---|---|---|---|
| Low | High | |||
| Phospho-AKT Ser473 | Low | 51 | 164 | 215 |
| High | 35 | 62 | 97 | |
| Total | 86 | 226 | 312 | |
To determine whether AKT1-mediated SIRT6 suppression was because of changes in protein stability, we measured the half-life of a Flag-tagged SIRT6 in HEK293T cells that overexpressed hemagglutinin (HA)–tagged, constitutively active AKT1. The half-life of SIRT6 was shorter in the presence of active AKT1 than it was in the presence of the vector (Fig. 1H), prompting us to examine whether this decrease was the result of 26S proteasome–mediated degradation. Pretreating HEK293T cells with the proteasome inhibitor MG-132 or the AKT inhibitor MK2206 rescued AKT1-induced suppression of SIRT6 abundance (Fig. 1I). Additionally, overexpression of AKT1 enhanced the ubiquitination of SIRT6 in the presence of MG-132, which was inhibited by either MK2206 or wortmannin, a PI3K inhibitor (fig. S1F). Together, these results suggest that SIRT6 protein abundance is suppressed in a proteasome-dependent manner, and this is dependent on the kinase activity of AKT1.
AKT1 interacts with and phosphorylates SIRT6 on Ser338
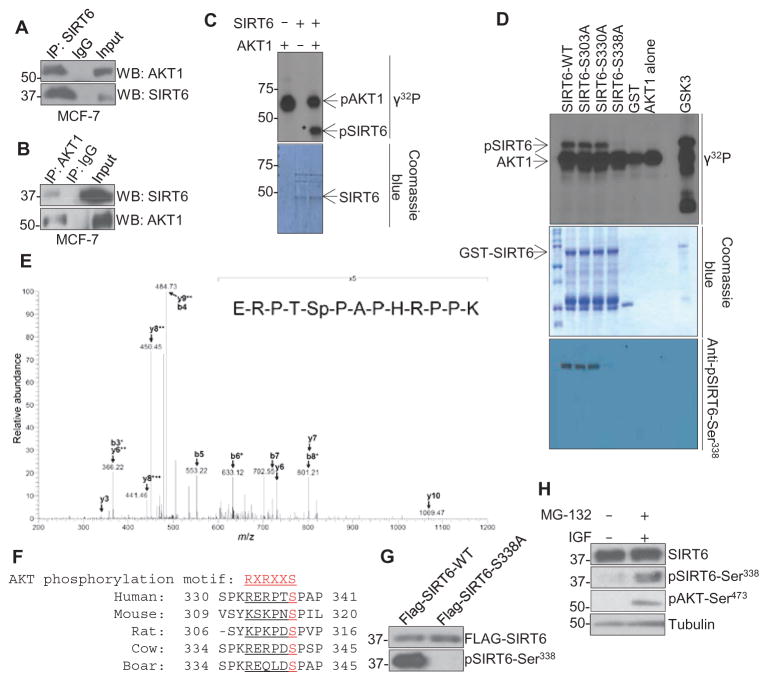
To explore the mechanism of how AKT1 mediates the suppression of SIRT6, we first characterized the interaction between the two proteins. Both endogenous SIRT6 (Fig. 2A) and exogenous Flag-tagged SIRT6 (fig. S2A) physically associated with AKT1 in an immunoprecipitation assay. In addition, endogenous AKT1 interacted with endogenous SIRT6, as shown by reciprocal immunoprecipitation (Fig. 2B), and an in vitro kinase assay showed that full-length recombinant SIRT6 could be directly phosphorylated by recombinant, functionally active AKT1 (Fig. 2C). To further identify the AKT1-mediated phosphorylation sites on SIRT6, we isolated SIRT6 from cells treated with EGF or IGF in the presence of MG-132 and analyzed it by mass spectrometry. Three phosphorylation sites were identified on SIRT6: Ser303, Ser330, and Ser338 (fig. S2B and Fig. 2E). To determine which site (or sites) is phosphorylated by AKT1, we mutated each one to an alanine residue and subjected all three mutants to in vitro kinase assays. Of these three mutants, phosphorylation was abolished in S338A (Fig. 2D), suggesting that AKT1 specifically phosphorylates SIRT6 at this position. A search of the National Center for Biotechnology Information database using the Basic Local Alignment Search Tool (BLAST) revealed that Ser338 of SIRT6, the mass spectrometry profile for which is shown in Fig. 2E, is highly conserved among mammals (Fig. 2F). Ser338 was also identified recently by another independent group (18). To validate whether this site is phosphorylated in cells, we used a commercially available antibody that recognizes SIRT6 phosphorylated at Ser338 and, thus, detected Flag-tagged wild-type but not the nonphosphorylatable S338A mutant SIRT6 in MDA-MB-231 cells (Fig. 2G). In serum-starved MDA-MB-231 cells treated with IGF-1 for 1 hour in the presence of the protease inhibitor MG-132 to stabilize protein abundance, we observed an increase in SIRT6 phosphorylation at Ser338 (Fig. 2H). Together, these results support that Ser338 of SIRT6 is an AKT1 phosphorylation site.
Fig. 2. AKT1 interacts with and phosphorylates SIRT6 in vitro and in vivo.
(A and B) Immunoprecipitation (IP) for SIRT6 (A) or AKT1 (B) followed by immunoblotting in lysates from MCF-7 cells against an IgG (immunoglobulin G) control. (C and D) In vitro kinase assay with recombinant, active AKT1 and either (C) recombinant GST-tagged, full-length WT SIRT6 or (D) WT or mutant SIRT6. pSIRT6, phosphorylated SIRT6; GSK3, control AKT substrate. (E) Mass spectrometry analysis of lysates from HeLa cells that had been serum-starved overnight, stimulated with EGF (50 ng/ml) for 30 min, and subjected to immunoprecipitation with a SIRT6 antibody. Spectrum is representative of two experiments. (F) Sequence alignment of the AKT1 phosphorylation motif of SIRT6 from various species. (G) Immunoblot for phosphorylated SIRT6-Ser338 (pSIRT6-Ser338) in MDA-MB-231 cells that stably express Flag-tagged WT or mutant (S338A) SIRT6, demonstrating specificity of the antibody. (H) Western blots for phosphorylated SIRT6 or AKT in lysates from MDA-MB-231 cells serum-starved overnight and then treated with IGF-1 in the presence of MG-132. Blots are representative of three experiments.
MDM2 is required for AKT1-mediated SIRT6 degradation
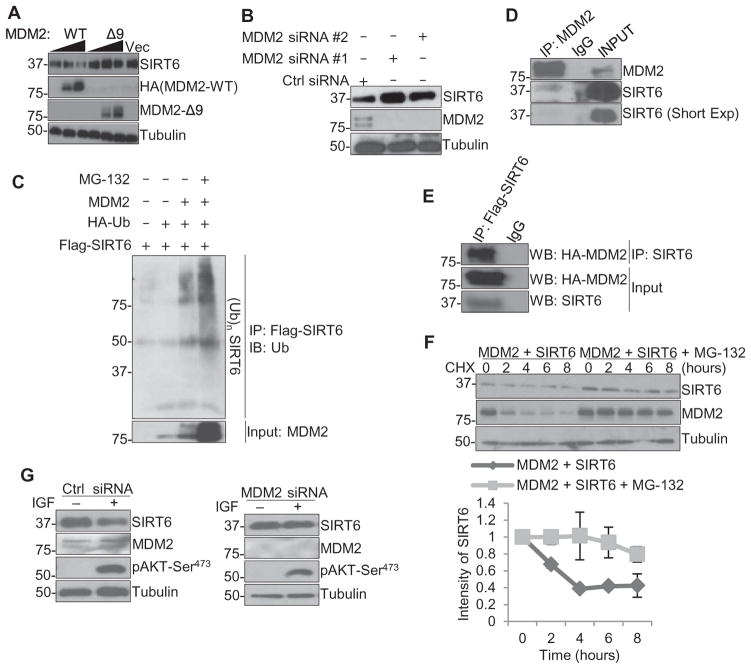
MDM2 is the most well-characterized oncogenic E3 ligase in the PI3K-AKT pathway and is phosphorylated and activated by AKT (27, 28). Because AKT1 suppresses SIRT6 protein abundance by decreasing its stability, we investigated whether MDM2 is involved in this process. First, we found that overexpression of wild-type MDM2 but not the MDM2-Δ9 mutant, which lacks its E3 ligase domain (29), reduced endogenous SIRT6 abundance in HEK293T cells (Fig. 3A). In MCF-7 cells, the abundance of SIRT6 increased when MDM2 was knocked down by siRNA (Fig. 3B). In addition, when ubiquitin was overexpressed concomitantly with MDM2 in HEK293T cells in the presence of MG-132, we observed a polyubiquitination pattern of SIRT6 (Fig. 3C), suggesting that SIRT6 may be polyubiquitinated for subsequent proteasome degradation. Immunoprecipitation showed that MDM2 interacted with endogenous SIRT6 in MCF-7 cells (Fig. 3D) and with exogenous Flag-SIRT6 in HEK293T cells (Fig. 3E). We then analyzed the half-life of SIRT6 by using the protein synthesis inhibitor cycloheximide. Similar to the observations of SIRT6 abundance in HEK293T cells overexpressing a constitutively active AKT1 in the presence of MG-132 (Fig. 1I), exogenous SIRT6 abundance decreased by 50% in the presence of MDM2 after 4 hours in cycloheximide, whereas MG-132 prevented the degradation of SIRT6 even after 8 hours (Fig. 3F). Furthermore, SIRT6 could no longer be suppressed by IGF stimulation when MDM2 is knocked down by siRNA in MCF-7 cells (Fig. 3G). These results suggest that MDM2 degrades SIRT6 in a proteasome-dependent manner.
Fig. 3. MDM2 is required for AKT1-mediated SIRT6 degradation.
(A) Western blotting in lysates from HEK293T cells transfected with 5 or 10 μg of HA-tagged MDM2-WT and MDM2-Δ9 or a control vector. (B) Western blotting in lysates from MCF-7 cells transfected with one of two siRNAs against MDM2 or a control siRNA for 72 hours. (C) Immunoprecipitation (IP) for SIRT6 followed by immunoblotting for ubiquitin (Ub) in lysates from HEK293T cells transfected with the indicated plasmids in the presence of MG-132 (10 μM for 7 hours). (D) Immunoprecipitation for MDM2 followed by immunoblotting for SIRT6 and MDM2 in lysates from MCF-7 cells. Short Exp, shorter exposure time. (E) Immunoprecipitation for Flag followed by immunoblotting (WB) in lysates from HEK293T cells transfected with HA-tagged WT MDM2 (MDM2-WT) and Flag-tagged WT SIRT6 (Flag-SIRT6). (F) Western blotting in lysates from HEK293T cells transfected with WT MDM2 (MDM2-WT) and Flag-tagged SIRT6 with or without MG-132, in the presence of cycloheximide (CHX) for up to 8 hours. (G) Western blotting in lysates from MCF-7 cells transfected with an siRNA against MDM2 or a control siRNA for 48 hours, serum-starved for 16 hours, and then cultured with or without IGF (50 ng/ml) for 1 hour. Blots are representative of three independent experiments.
Phosphorylation of SIRT6 by AKT1 facilitates MDM2-mediated degradation
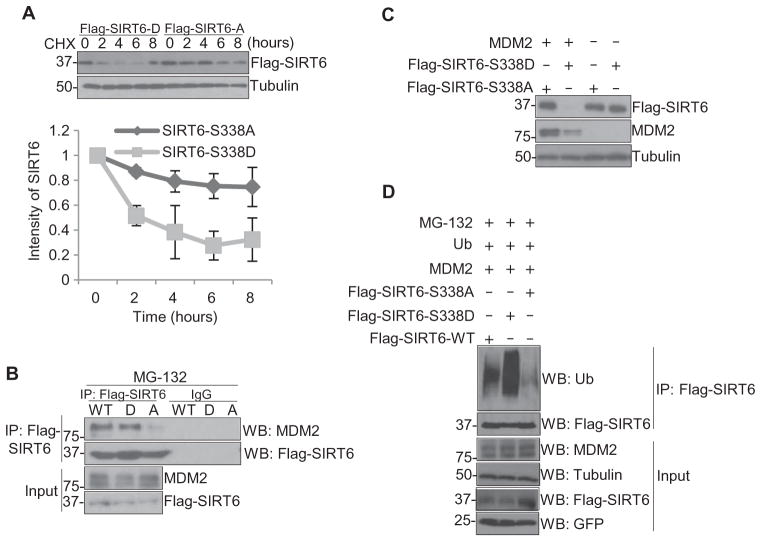
To further show that the phosphorylation of SIRT6 by AKT1 alters its stability, we compared the stability of two SIRT6 mutant proteins: SIRT6-S338A, a nonphosphorylatable mutant, and SIRT6-S338D, a phosphorylation-mimic mutant. Under cycloheximide treatment in MCF-7 cells, the abundance of SIRT6-S338D decreased after 2 hours, whereas SIRT6-S338A abundance remained unsubstantially changed for at least up to 8 hours (Fig. 4A). Consistently, the SIRT6-S338D mutant interacted more strongly with MDM2 in MCF-7 cells than did SIRT6-S338A (Fig. 4B). These results suggest that AKT1-induced phosphorylation of SIRT6 may recruit MDM2 and ubiquitinate SIRT6 to promote its subsequent degradation. To determine whether this interaction indeed promoted SIRT6 degradation, the SIRT6-S338A or SIRT6-S338D mutant was co-transfected with MDM2 into HEK293T cells. As expected, the abundance of SIRT6-S338D, but not SIRT6-S338A, was decreased in the presence of MDM2 (Fig. 4C). Compared with wild-type SIRT6, the SIRT6-S338D mutant was heavily ubiquitinated and the SIRT6-S338A mutant was the least ubiquitinated in the presence of MDM2 and MG-132 in MCF-7 cells (Fig. 4D). Together, these data indicate that MDM2 is the E3 ligase that mediates SIRT6 degradation and that the interaction between MDM2 and SIRT6 is dependent on AKT1-mediated SIRT6 phosphorylation on Ser338.
Fig. 4. Phosphorylation of SIRT6 by AKT1 facilitates MDM2-mediated degradation.
(A) Western blotting in lysates from MCF-7 cells that stably express Flag-tagged SIRT6-S338A (Flag-SIRT6-A) or SIRT6-S338D (Flag-SIRT6-D) in the presence of cycloheximide (CHX) for up to 8 hours. (B) Immunoprecipitation (IP) with a Flag antibody followed by immunoblotting (WB) in lysates from MCF-7 cells that stably express Flag-tagged, WT SIRT6 (WT), SIRT6-S338A (A), or SIRT6-S338D (D) treated with MG-132 for 7 hours. (C) Western blotting in lysates from HEK293T cells transfected with Flag-tagged SIRT6-S338A (Flag-SIRT6-S338A) or Flag-tagged SIRT6-S338D (Flag-SIRT6-S338D) and WT MDM2, harvested 72 hours after transfection. (D) Immunoprecipitation with a Flag antibody followed by immunoblotting for ubiquitin in lysates from HEK293T cells transfected with either Flag-tagged WT SIRT6 (Flag-SIRT6-WT) or mutant SIRT6 (S338A or S338D) and WT MDM2 and ubiquitin (Ub) and treated with MG-132 for 7 hours. Blots are representative of three experiments.
Nonphosphorylatable SIRT6 inhibits breast cancer tumorigenesis
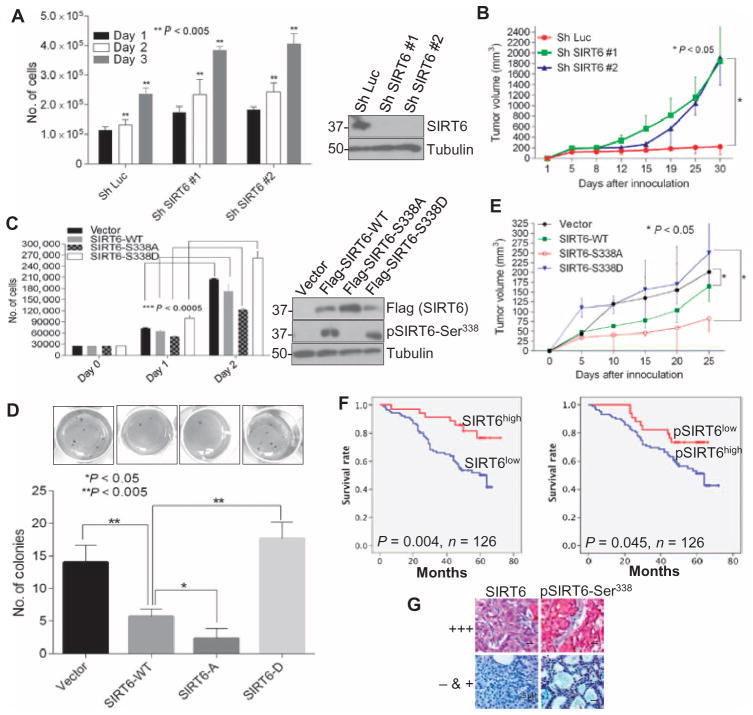
Because the nonphosphorylatable SIRT6 mutant had enhanced stability and the phosphorylation-mimic mutant had less stability compared to the wild-type SIRT6, we examined the function of SIRT6-WT, SIRT6-S338A, and SIRT6-S338D in cellular proliferation and breast cancer tumorigenesis. Knockdown of endogenous SIRT6 by short hairpin RNA (shRNA) increased the proliferation of MDA-MB-231 cells in culture, as determined by a cell counting assay (Fig. 5A), and enhanced the growth of MDA-MB-231 xenografts in the mammary fat pads of nude mice (Fig. 5B). We further examined the function of the phosphorylation of SIRT6 at Ser338 in cell proliferation and tumori-genesis by expressing wild-type or either mutant SIRT6 in MDA-MB-231 cells. Expression of the nonphosphorylatable SIRT6-S338A mutant suppressed cell proliferation (Fig. 5C) and colony formation on soft agar (Fig. 5D) more than did wild-type SIRT6 or the phosphorylation-mimic SIRT6-S338D mutant compared to the vector control. To further test the tumor-suppressive activity of SIRT6 mutants in vivo, we injected MDA-MB-231 cells stably expressing the control vector, wild-type SIRT6, or either mutant SIRT6 into the mammary fat pads of nude mice and monitored tumor development. We found that tumor volume in mice injected with MDA-MB-231 cells stably expressing wild-type SIRT6 was smaller than those injected with cells expressing the control vector. The growth of tumors expressing the SIRT6-S338A mutant was significantly decreased compared with those expressing the control vector or the phosphorylation-mimic SIRT6-S338D mutant (Fig. 5E).
Fig. 5. SIRT6-S338A, but not SIRT6-S338D, inhibits tumorigenesis in breast cancer.
(A) Proliferation and immunoblot of MDA-MB-231 cells transfected with either shRNA against luciferase or one of two shRNAs against SIRT6. Data are means ± SE from three experiments. (B) Growth of mammary fat pad xenografts derived from MDA-MB-231 cells transfected with either luciferase shRNA or one of two SIRT6 shRNAs. Data are means ± SE from five mice per group. (C) Proliferation and immunoblot of MDA-MB-231 cells infected with lentiviral vector, WT SIRT6 (SIRT6-WT), SIRT6-S338A, or SIRT6-S338D. Data are means ± SE from three experiments. (D) Soft agar colony formation by MDA-MB-231 cells infected with lentiviral vector, SIRT6-WT, SIRT6-S338A (SIRT6-A), or SIRT6-S338D (SIRT6-D). (E) Tumor growth of orthotopically transplanted MDA-MB-231 cells infected with lentiviral vector, SIRT6-WT, SIRT6-S338A, or SIRT6-S338D. Data are means ± SE from five mice per group. (F) Survival curves of patients with breast tumors that have high or low abundance of total or phosphorylated SIRT6. (G) Immunohistochemistry for SIRT6 and phosphorylated SIRT6 in representative tumor tissues from patients in (F). +++, high expression; − & +, low or no expression. Scale bars, 25 μm. P values for (A) to (E) were calculated by ANOVA test and for (F) by log-rank test.
To further investigate whether the expression of SIRT6 phosphomutants affects the endogenous expression of known SIRT6 target genes that are involved in promoting tumorigenesis, we performed a quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis of MDA-MB-231 cells expressing vector control, SIRT6-WT, SIRT6-S338A, or SIRT6-S338D. We found that the SIRT6-S338A mutant suppressed the mRNA abundance of a panel of target genes more significantly (AKT1, AKT3, IGF-1R, PDK1, MTOR, and LDHA) than others (GSK3B and PFKM), whereas the SIRT6-S338D mutant had no inhibitory effect on the target genes compared to SIRT6-WT (fig. S3).
SIRT6-deficient mice exhibit increased phosphorylation of AKT compared with controls and subsequently have severe hypoglycemia because of enhanced basal and insulin-stimulated glucose uptake (5). On the other hand, SIRT6-deficient mouse embryonic fibroblasts (MEFs) showed similar amounts of phosphorylated AKT to wild-type MEFs (14). Thus, we investigated the phosphorylation of AKT in MDA-MB-231 breast cancer cell line that expressed vector, SIRT6-WT, A-SIRT6, or D-SIRT6. Clones were chosen in such a way that the expression of wild-type and mutant SIRT6 were similar, which would make the phosphorylation of AKT comparable. In our system, although there was a slight decrease in the abundance of phosphorylated AKT in the presence of wild-type SIRT6 as previously reported (5), there was no significant difference between the mutants and the wild-type SIRT6 (fig. S4), suggesting that the Ser338 mutation on SIRT6 might not contribute to SIRT6-mediated suppression of AKT activation.
To determine the correlation between SIRT6 phosphorylation and breast cancer patient survival or disease progression, immunohistochemical staining was performed for total and phosphorylated SIRT6 in biopsy tissues from 126 breast cancer patients. Patients whose tumors had high SIRT6 abundance had better overall survival than those whose tumors had low SIRT6 abundance. However, patients whose tumors had high abundance of phosphorylated SIRT6 had poorer overall survival than those whose tumors had low abundance of phosphorylated SIRT6 (Fig. 5, F and G). These results suggest that SIRT6 and its phosphorylation status may have the potential to be predictive of breast cancer patient survival.
Loss of SIRT6 results in trastuzumab resistance in breast cancer cells overexpressing HER2
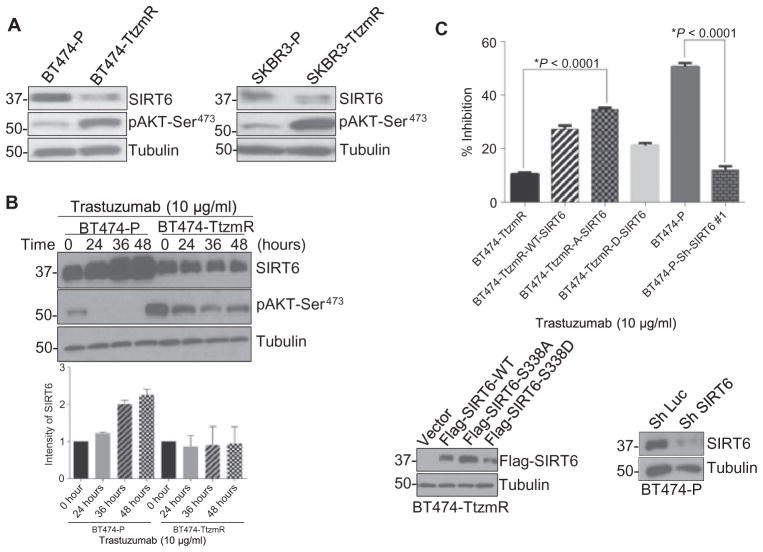
Trastuzumab is a standard treatment for patients with HER2-positive breast cancer. However, intrinsic or acquired resistance to this treatment is observed (30, 31). Increased or constitutive activation of AKT appears to be a key factor in trastuzumab resistance (32–35). Because we found that AKT1 decreased SIRT6 stability through phosphorylation and subsequent proteasome-dependent degradation, we speculated that SIRT6 might also play a role in trastuzumab resistance. Indeed, SIRT6 abundance was lower in two trastuzumab-resistant, HER2-positive breast cancer cell lines (BT474-TtzmR and SKBR3-TtzmR) compared with trastuzumab-sensitive parental lines (BT474-P and SKBR3-P) (Fig. 6A). The abundance of SIRT6 increased after the addition of trastuzumab in BT474-P, but not in BT474-TtzmR, cells (Fig. 6B). In the BT474-P cells, 24-hour treatment with trastuzumab inhibited the phosphorylation of AKT at Ser473, whereas in the BT474-TtzmR cells, there was residual phosphorylation of AKTeven after 48 hours of trastuzumab treatment. This persistent activation of AKT appeared to attenuate the increase in SIRT6 protein abundance seen in the parental cells, suggesting that the induction of SIRT6 contributes to the therapeutic effect of trastuzumab.
Fig. 6. Loss of SIRT6 results in trastuzumab resistance in breast cancer cells overexpressing HER2.
(A) Western blotting in cell lysates from BT474 parental (BT474-P), BT474 trastuzumab-resistant (BT474-TtzmR), SKBR3 parental (SKBR3-P), and SKBR3-trastuzumab–resistant (SKBR3-TtzmR) cells. Blots are representative of three experiments. (B) Western blots for SIRT6 and phosphorylated AKT at Ser473 (pAKT-Ser473) in BT474-P and BT474-TtzmR cells treated with trastuzumab for up to 48 hours. Blot is representative, and data in graph are means ± SE abundance of SIRT6 normalized to tu-bulin from three independent experiments. (C) Cell viability, assessed by relative proliferation by an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay, in BT474-TtzmR cells that stably expressed SIRT6-WT, SIRT6-S338A, or SIRT6-S338D, and in BT474-P cells transfected with luciferase or SIRT6 shRNA, each treated with trastuzumab (10 μg/ml) for 4 days. Blots show representative transfection or knockdown, respectively. Data are means ± SE from three independent experiments. *P < 0.0001, Student’s t test.
To further validate the above findings, we knocked down SIRT6 in BT474-P cells and cultured them in trastuzumab for 4 days. Loss of SIRT6 in trastuzumab-sensitive BT474-P cells decreased cell sensitivity to trastuzumab to a similar sensitivity seen in BT474-TtzmR cells as measured by relative metabolic activity in an MTT assay (Fig. 6C). Expression of the nonphosphorylatable SIRT6-S338A mutant considerably resensitized BT474-TtzmR cells to trastuzumab compared with expression of either wild-type SIRT6 or the phosphorylation-mimic SIRT6-S338D mutant (Fig. 6C). These data suggest a mechanism by which trastuzumab inhibits breast cancer cell proliferation through the induction of SIRT6 and that loss of SIRT6 mediated by AKT1 and MDM2 contributes to trastuzumab resistance.
DISCUSSION
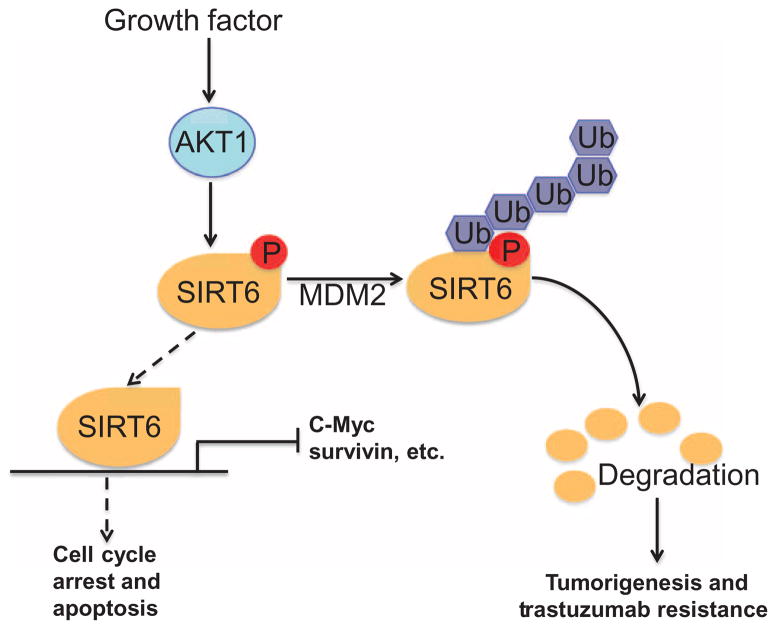
Our study suggests a model in which the tumor suppressor SIRT6 is inhibited by oncoprotein AKT1 through phosphorylation and subsequent degradation by the MDM2-dependent ubiquitin proteasome pathway (Fig. 7). We identified an AKT1 phosphorylation site on SIRT6 (Ser338), the phosphorylation of which increased susceptibility to interaction with and degradation by MDM2, a bona fide E3 ligase mediating SIRT6 degradation. Ronnebaum et al. identified C terminus of Hsc70-interacting protein (CHIP) as an E3 ligase that stabilizes SIRT6 through noncanonical ubiquitination (36). They observed canonical ubiquitination of SIRT6 even in the absence of CHIP, indicating that other E3 ligases like MDM2 might also ubiquitinate and degrade SIRT6. Also, recently, USP10 was shown to interact with, deubiquitinate, and stabilize SIRT6 in colon cancer cells (19), indicating that SIRT6 could be destabilized through E3 ligase–mediated ubiquitination. USP10 has been shown to deubiquitinate and stabilize p53, a well-known substrate of MDM2 (37), suggesting a mechanism whereby SIRT6 is ubiquitinated and destabilized by MDM2, which could be reversed by USP10-mediated deubiquitination.
Fig. 7. AKT1 promotes tumorigenesis by inhibiting SIRT6 through MDM2-mediated degradation.
Schematic representation showing that AKT1 interacts with and phosphorylates SIRT6 at Ser338. Phosphorylation at this residue increases the interaction between SIRT6 and MDM2 and enhances the degradation of SIRT6 through an MDM2-dependent ubiquitin proteasome pathway, contributing to tumorigenesis and trastuzumab resistance in breast cancer.
We showed that the nonphosphorylatable SIRT6-S338A mutant is resistant to MDM2-mediated degradation, is more stable than wild-type SIRT6, and exhibits enhanced suppression of cell proliferation and tumor growth. Together with previously reported studies, our results further strengthen the role of SIRT6 as a tumor suppressor in numerous cancers, and loss of SIRT6 is a critical promoter of cancer cell survival.
In addition to promoting tumorigenesis, increased AKT activation is associated with the development of trastuzumab resistance in breast tumors with increased HER2 expression (30, 31, 38). Consequently, several alternate treatment modifications and combination therapies have been designed to address this issue, including combining trastuzumab with the SRC inhibitor saracatinib (39) or an AKT inhibitor (40) and, most recently, trastuzumab-DM1, in which a cytotoxic agent mertansine is linked to the monoclonal antibody against HER2 (41). Because SIRT6 abundance was lower in trastuzumab-resistant cells than in trastuzumab-sensitive cells, and manipulating SIRT6 abundance modulates sensitivity, the loss of SIRT6 might be one of the mechanisms that enable acquired resistance to trastuzumab, suggesting that histochemical analysis of SIRT6 might be used as a biomarker to determine drug sensitivity in breast cancer patients undergoing trastuzumab treatment.
MATERIALS AND METHODS
Cell lines
All cell lines used were purchased from the American Type Culture Collection. They included HEK293T, a HEK cell line; MCF-7, a human mammary adenocarcinoma cell line from pleural effusion; MDA-MB-231, a human mammary adenocarcinoma cell line from pleural effusion; Hs578T, a human mammary carcinoma cell line; and HBL-100, a human mammary epithelial carcinoma. The BT474 cell line and its trastuzumab-resistant counterpart (BT474-TtzmR) were gifts from D. Yu at The University of Texas MD Anderson Cancer Center (Houston, TX). All cells were grown on tissue culture dishes in Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and penicillin and streptomycin (100 U, 100 ng/ml) at 37°C in a humidified atmosphere with 5% CO2, unless specified otherwise. Before IGF (50 ng/ml) or EGF (50 ng/ml) treatment, the cells were serum-starved overnight. The concentrations and treatment durations of each chemical were as follows: MK2206 (2 μM, 1 hour), MG-132 (10 μM, 7 to 10 hours), and cycloheximide (1 μg/ml). Stable puromycin-resistant cell lines were maintained in DMEM/F12 medium that contained puromycin (1 μg/ml).
Antibodies
Commercial antibodies against SIRT6 (1:1000), AKT1 (1:1000), AKT2 (1:1000), AKT3 (1:1000), and phospho-AKT Ser473 (1:1000) were purchased from Cell Signaling Technology; antibody against phosphorylated SIRT6 Ser338 (1:500) was from Abnova; and antibodies against HA (1:2000) and Flag (1:2000) were from Sigma.
Reagents
The AKT inhibitor MK2206 was purchased from Selleck Chemicals. MG-132, cycloheximide, EGF, and IGF were purchased from Sigma.
Plasmids
DNA plasmids encoding Flag-SIRT6 (plasmid 13817), HA-myr-AKT1 (plasmid 9008), and HA-myr-AKT3 (plasmid 9017) were from Addgene. Wild-type MDM2 and the MDM2 deletion mutant (MDM2-Δ9) were gifts from J. Chen (H. Lee Moffitt Cancer Center, Tampa, FL). SIRT6-S338A and SIRT6-S338D point mutants were generated using the QuickChange Site-Directed Mutagenesis Kit from Stratagene using the following primers: SIRT6-S338D, 5′-GCGGCCCACCGACCCTGCCCCCCACAG-3′ (forward) and 5′-GTGGGGGGCAGGGTCGGTGGGCCGCTC-3′ (reverse). All lentiviral pLKO.1 expression and shRNA-encoding plasmids were purchased from Sigma. SIRT6 shRNA 1 clone ID: TRCN0000232532: CCGGCTCCCTGGT-CTCCAGCTTAAACTCGAGTTTAAGCTGGAGACCAGGGAGTTTTTG; SIRT6 shRNA 2 clone ID: TRCN0000050473:CCGGTGGAAGAATGTGC-CAAGTGTACTCGAGTACACTTGGCACATTCTTCCATTTTTG. Wild-type MDM2 and MDM2 deletion mutants were gifts from J. Chen (H. Lee Moffitt Cancer Center, Tampa, FL). AKT1, AKT2, and MDM2 siRNAs were purchased from Sigma.
Immunoblotting, immunoprecipitation, and ubiquitination assays
Immunoblotting, immunoprecipitation, and ubiquitination assays were performed as previously described (25), using antibodies against SIRT6, AKT1, AKT2, phosphorylated AKT at Ser473 (Cell Signaling Technology), tubulin, and actin (Sigma). For glutathione S-transferase (GST) pull-down assays, GST-SIRT6 protein (10 μg) was incubated with 2 mg of MCF-7 cell extract overnight at 4°C. GST-tagged proteins were recovered by incubating the reaction mixture with 20 μl of glutathione Sepharose beads at 4°C overnight. The bead pellet was washed three times in 1× phosphate-buffered saline. The boiled samples were then subjected to 10% SDS–polyacrylamide gel electrophoresis (SDS-PAGE).
In vitro kinase assay
Purified GST-SIRT6 (wild-type or mutant) fragments were incubated with active AKT1 (Millipore) and 50 mM ATP (adenosine 5′-triphosphate) in a kinase buffer containing [32P]ATP (5 μCi) for 30 min at 30°C. The reaction products were resolved via SDS-PAGE, and 32P-labeled products were detected using autoradiography.
Identification of phosphorylation sites by mass spectrometry analysis
HeLa cell lysates were immunoprecipitated with an antibody against SIRT6 to identify the phosphorylation sites of SIRT6 in cells. In vitro, the phosphorylation site of SIRT6 was identified using an in vitro kinase assay with recombinant, active AKT1 kinase and full-length GST-SIRT6. After protein gel electrophoresis, the bands were excised and subjected to digestion with trypsin. The enriched phosphopeptides were isolated using immobilized metal affinity chromatography and analyzed by micro–liquid chromatography–tandem mass spectrometry using an UltiMate Capillary LC system (LC Packings) coupled to a QSTAR XL quadruple time-of-flight mass spectrometer (Applied Biosystems). The product ion spectra, generated by nanoscale capillary spectrometry, were searched against National Center for Biotechnology Information databases for exact matches using the ProID (Applied Biosystems) and MASCOT search programs. Carbamidomethyl cysteine was set as a fixed modification, and serine, threonine, and tyrosine phosphorylation were set as variable modifications. All phosphopeptides identified were confirmed by manual interpretation of the spectra.
Cell growth, soft agar, and cell viability assays
Cell growth was determined by cell counting. Cells (1 × 105) were plated in triplicate in 12-well plates. They were then trypsinized at the indicated time points and counted. For the soft agar transformation assay, 2.5 × 104 cells were seeded in 1 ml of DMEM with 10% FBS and 0.4% agarose and overlaid on 1 ml of DMEM with 10% FBS and 0.8% agarose in each well of a six-well plate. After 2 to 3 weeks, colonies larger than 2 mm in diameter were counted.
Animal studies
MDA-MB-231 cells (2 × 106) with lentiviral-stable expression of SIRT6-WT, SIRT6-S338A, or SIRT6-S338D and Sh SIRT6 or Sh Luc control cells were injected into the mammary fat pads of nude mice (five per group). Tumor size was measured every 3 days with a caliper, and tumor volume was determined using the formula L × W2 × 0.52, where L is the longest diameter and W is the shortest diameter. All animal procedures were conducted under regulations of Division of Laboratory Animal Medicine at The University of Texas MD Anderson Cancer Center. Animal protocols (protocol number 06-87-06139) were reviewed and approved by the Institutional Animal Care and Use Committee at The University of Texas MD Anderson Cancer Center.
Breast tumor tissue specimens
One hundred twenty-six formalin-fixed and paraffin-embedded infiltrating breast carcinoma patient samples were obtained from the Department of Pathology, Shanghai East Breast Disease Hospital, People’s Republic of China. Breast cancer tissue microarray containing 186 cases was purchased from Pantomics (BRC2281).
Immunohistochemical staining
A modified immunoperoxidase staining was used as described previously (42) for staining with SIRT6 (Novus, NB100-2522), phospho-AKT Ser473 (Cell Signaling Technology, 3787S), and phospho-SIRT6 (Bioss, bs-5634R-bio).
Statistical analysis
SAS software (version 8.1) was used for the statistical analysis (SAS Institute). A univariate analysis was used to determine the variable distributions. Categorical variables among the groups were compared using the χ2 test or Fisher’s exact test if 20% of the expected values were less than 5. Continuous variables were analyzed using Student’s t test. A P value <0.05 was considered statistically significant.
Supplementary Material
Fig. S1. Activation of AKT results in SIRT6 degradation.
Fig. S2. AKT1 interacts with and phosphorylates SIRT6 in vitro and in vivo.
Fig. S3. SIRT6-S338A, but not SIRT6-S338D, inhibits tumorigenesis in breast cancer.
Fig. S4. SIRT6 mutants affect the phosphorylation of AKT similar to wild-type SIRT6.
Acknowledgments
Funding: This study was funded in part by the following grants: NIH (CA109311, CA099031, and CCSG CA16672); National Breast Cancer Foundation Inc.; the Breast Cancer Research Foundation; The University of Texas MD Anderson–China Medical University and Hospital Sister Institution Fund (to M.-C.H.); Ministry of Health and Welfare, China Medical University Hospital Cancer Research Center of Excellence (MOHW103-TD-B-111-03; Taiwan), the Program for Stem Cell and Regenerative Medicine Frontier Research (NSC102-2321-B-039-001; Taiwan), International Research-Intensive Centers of Excellence in Taiwan (NSC103-2911-I-002-303), Center for Biological Pathways, and Genomics Research Center, Academia Sinica (to C.-H.C.).
Footnotes
Author contributions: U.T. and M.-C.H. designed and conceived the study; U.T. and M.-C.H. wrote the manuscript; and A.M.L. showed the initial inverse relationship between AKT activation and SIRT6 protein level. U.T., J.S., W.X., Y.W., A.M.L., and C.-W.L. performed experiments. W.-C.C. and C.-H.C. performed the mass spectrometric analysis. D.Y. provided the trastuzumab-sensitive and trastuzumab-resistant cell lines. Both D.Y. and H.-K.L. provided inputs toward trastuzumab resistance experiments.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: There are material transfer agreements or patents restricting use of plasmids encoding AKT1, 2, and 3, obtained from Addgene. The mass spectrometry proteomics data are deposited to the ProteomeXchange Consortium database PRIDE, identifier PXD001154.
REFERENCES AND NOTES
- 1.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 3.Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TLA, Barrett JC, Chang HY, Bohr VA, Ried T, Gozani O, Chua KF. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HS, Xiao C, Wang RH, Lahusen T, Xu X, Vassilopoulos A, Vazquez-Ortiz G, Jeong WI, Park O, Ki SH, Gao B, Deng CX. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 2010;12:224–236. doi: 10.1016/j.cmet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao C, Kim HS, Lahusen T, Wang RH, Xu X, Gavrilova O, Jou W, Gius D, Deng CX. SIRT6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice. J Biol Chem. 2010;285:36776–36784. doi: 10.1074/jbc.M110.168039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhong L, D’Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, Mostoslavsky R. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1α. Cell. 2010;140:280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaidi A, Weinert BT, Choudhary C, Jackson SP. Human SIRT6 promotes DNA end resection through CtIP deacetylation. Science. 2010;329:1348–1353. doi: 10.1126/science.1192049. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Mao ZY, Hine C, Tian X, Van Meter M, Au M, Vaidya A, Seluanov A, Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332:1443–1446. doi: 10.1126/science.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCord RA, Michishita E, Hong T, Berber E, Boxer LD, Kusumoto R, Guan SH, Shi XB, Gozani O, Burlingame AL, Bohr VA, Chua KF. SIRT6 stabilizes DNA-dependent protein kinase at chromatin for DNA double-strand break repair. Aging. 2009;1:109–121. doi: 10.18632/aging.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michishita E, McCord RA, Boxer LD, Barber MF, Hong T, Gozani O, Chua KF. Cell cycle-dependent deacetylation of telomeric histone H3 lysine K56 by human SIRT6. Cell Cycle. 2009;8:2664–2666. doi: 10.4161/cc.8.16.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tennen RI, Bua DJ, Wright WE, Chua KF. SIRT6 is required for maintenance of telomere position effect in human cells. Nat Commun. 2011;2:433. doi: 10.1038/ncomms1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang B, Zwaans BMM, Eckersdorff M, Lombard DB. The sirtuin SIRT6 deacetylates H3 K56Ac in vivo to promote genomic stability. Cell Cycle. 2009;8:2662–2663. doi: 10.4161/cc.8.16.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Min LH, Ji Y, Bakiri L, Qiu ZX, Cen J, Chen XT, Chen LL, Scheuch H, Zheng H, Qin LX, Zatloukal K, Hui LJ, Wagner EF. Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nat Cell Biol. 2012;14:1203–1211. doi: 10.1038/ncb2590. [DOI] [PubMed] [Google Scholar]
- 14.Sebastián C, Zwaans BMM, Silberman DM, Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber D, Cosentino C, Greenson JK, MacDonald AI, McGlynn L, Maxwell F, Edwards J, Giacosa S, Guccione E, Weissleder R, Bernstein BE, Regev A, Shiels PG, Lombard DB, Mostoslavsky R. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151:1185–1199. doi: 10.1016/j.cell.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oesterreich S, Allredl DC, Mohsin SK, Zhang Q, Wong H, Lee AV, Osborne CK, O’Connell P. High rates of loss of heterozygosity on chromosome 19p13 in human breast cancer. Br J Cancer. 2001;84:493–498. doi: 10.1054/bjoc.2000.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobottka SB, Haase M, Fitze G, Hahn M, Schackert HK, Schackert G. Frequent loss of heterozygosity at the 19p13.3 locus without LKB1/STK11 mutations in human carcinoma metastases to the brain. J Neurooncol. 2000;49:187–195. doi: 10.1023/a:1006442024874. [DOI] [PubMed] [Google Scholar]
- 17.Yang TL, Su YR, Huang CS, Yu JC, Lo YL, Wu PE, Shen CY. High-resolution 19p13.2–13.3 allelotyping of breast carcinomas demonstrates frequent loss of heterozygosity. Gene Chromosomes Cancer. 2004;41:250–256. doi: 10.1002/gcc.20080. [DOI] [PubMed] [Google Scholar]
- 18.Miteva YV, Cristea IM. A proteomic perspective of Sirtuin 6 (SIRT6) phosphorylation and interactions and their dependence on its catalytic activity. Mol Cell Proteomics. 2014;13:168–183. doi: 10.1074/mcp.M113.032847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Z, Yang H, Tan C, Li J, Liu Z, Quan Q, Kong S, Ye J, Gao B, Fang D. USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. Cell Rep. 2013;5:1639–1649. doi: 10.1016/j.celrep.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 22.Kawahara TLA, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KCL, Boxer LD, Chang HY, Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundaresan NR, Vasudevan P, Zhong L, Kim G, Samant S, Parekh V, Pillai VB, Ravindra PV, Gupta M, Jeevanandam V, Cunningham JM, Deng CX, Lombard DB, Mostoslavsky R, Gupta MP. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med. 2012;18:1643–1650. doi: 10.1038/nm.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 25.Hu CL, Cowan RG, Harman RM, Quirk SM. Cell cycle progression and activation of Akt kinase are required for insulin-like growth factor I-mediated suppression of apoptosis in granulosa cells. Mol Endocrinol. 2004;18:326–338. doi: 10.1210/me.2003-0178. [DOI] [PubMed] [Google Scholar]
- 26.Larsson O, Girnita A, Girnita L. Role of insulin-like growth factor 1 receptor signalling in cancer. Br J Cancer. 2005;92:2097–2101. doi: 10.1038/sj.bjc.6602627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou BP, Liaq Y, Xia WY, Zou YY, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–982. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 28.Ashcroft M, Ludwig RL, Woods DB, Copeland TD, Weber HO, MacRae EJ, Vousden KH. Phosphorylation of HDM2 by Akt. Oncogene. 2002;21:1955–1962. doi: 10.1038/sj.onc.1205276. [DOI] [PubMed] [Google Scholar]
- 29.Honda R, Yasuda H. Activity of MDM2, a ubiquitin ligase, toward p53 or itself is dependent on the RING finger domain of the ligase. Oncogene. 2000;19:1473–1476. doi: 10.1038/sj.onc.1203464. [DOI] [PubMed] [Google Scholar]
- 30.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: Understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 31.She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, DeFeo-Jones D, Huber HE, Rosen N. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLOS One. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandarlapaty S, Sakr RA, Giri D, Patil S, Heguy A, Morrow M, Modi S, Norton L, Rosen N, Hudis C, King TA. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin Cancer Res. 2012;18:6784–6791. doi: 10.1158/1078-0432.CCR-12-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grell P, Fabian P, Khoylou M, Radova L, Slaby O, Hrstka R, Vyzula R, Hajduch M, Svoboda M. Akt expression and compartmentalization in prediction of clinical outcome in HER2-positive metastatic breast cancer patients treated with trastuzumab. Int J Oncol. 2012;41:1204–1212. doi: 10.3892/ijo.2012.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan CH, Li CF, Yang WL, Gao Y, Lee SW, Feng ZZ, Huang HY, Tsai KKC, Flores LG, Shao YP, Hazle JD, Yu DH, Wei WY, Sarbassov D, Hung MC, Nakayama KI, Lin HK. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, Herceptin sensitivity, and tumorigenesis. Cell. 2012;149:1098–1111. doi: 10.1016/j.cell.2012.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallardo A, Lerma E, Escuin D, Tibau A, Muñoz J, Ojeda B, Barnadas A, Adrover E, Sánchez-Tejada L, Giner D, Ortiz-Martínez F, Peiró G. Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br J Cancer. 2012;106:1367–1373. doi: 10.1038/bjc.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronnebaum SM, Wu Y, McDonough H, Patterson C. The ubiquitin ligase CHIP prevents SirT6 degradation through noncanonical ubiquitination. Mol Cell Biol. 2013;33:4461–4472. doi: 10.1128/MCB.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan J, Luo K, Zhang L, Cheville JC, Lou Z. USP10 regulates p53 localization and stability by deubiquitinating p53. Cell. 2010;140:384–396. doi: 10.1016/j.cell.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nahta R, O’Regan RM. Evolving strategies for overcoming resistance to HER2-directed therapy: Targeting the PI3K/Akt/mTOR pathway. Clin Breast Cancer. 2010;10(Suppl 3):S72–S78. doi: 10.3816/CBC.2010.s.015. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW, Xiong Y, Tseng LM, Li SH, Ding Z, Sahin AA, Esteva FJ, Hortobagyi GN, Yu D. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17:461–469. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Junttila TT, Akita RW, Parsons K, Fields C, Phillips GDL, Friedman LS, Sampath D, Sliwkowski MX. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Poon KA, Flagella K, Beyer J, Tibbitts J, Kaur S, Saad O, Yi JH, Girish S, Dybdal N, Reynolds T. Preclinical safety profile of trastuzumab emtansine (T-DM1): Mechanism of action of its cytotoxic component retained with improved tolerability. Toxicol Appl Pharmacol. 2013;273:298–313. doi: 10.1016/j.taap.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Xia W, Wei Y, Du Y, Liu J, Chang B, Yu YL, Huo LF, Miller S, Hung MC. Nuclear expression of epidermal growth factor receptor is a novel prognostic value in patients with ovarian cancer. Mol Carcinog. 2009;48:610–617. doi: 10.1002/mc.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Activation of AKT results in SIRT6 degradation.
Fig. S2. AKT1 interacts with and phosphorylates SIRT6 in vitro and in vivo.
Fig. S3. SIRT6-S338A, but not SIRT6-S338D, inhibits tumorigenesis in breast cancer.
Fig. S4. SIRT6 mutants affect the phosphorylation of AKT similar to wild-type SIRT6.