Abstract
Pseudouridines are the most abundant and highly conserved modified nucleotides identified in spliceosomal small nuclear RNAs (snRNAs). Most pseudouridines are also clustered in functionally important regions of spliceosomal snRNAs. Experiments carried out in several independent experimental systems show that the pseudouridines in spliceosomal snRNAs are functionally important for pre-messenger RNA (mRNA) splicing. Experimental data also indicate that spliceosomal snRNA pseudouridylation can be catalyzed by both RNA-dependent (box •H/ACA RNPs) and RNA-independent (protein-only enzymes) mechanisms.
INTRODUCTION
When it was first identified in RNA, pseudouridine (Ψ) was considered a fifth nucleoside in addition to uridine, cytidine, guanosine, and adenosine.1–3 Ψ is the 5′-ribosyluracil isomer of uridine (Figure 1), and differs from uridine in two ways. First, Ψ has a C–C linkage between the base and the sugar, rather than the N–C linkage present in virtually all other nucleotides. Second (and perhaps more importantly), Ψ has an extra hydrogen bond donor. In an RNA chain, the presence of Ψ residues results in an increase in base stacking and extra hydrogen bonds between the base and its own phosphate backbone (mediated by a water molecule), thus bringing rigidity to the RNA structure.4–9 Furthermore, it is reported that the Ψ-A pair is more stable than the U-A pair.10,11 Indeed, Ψ contributes to stable intermolecular base-pairing interactions.10–12 Thus, Ψ has unique chemical properties.
FIGURE 1.

Isomerization of uridine into pseudouridine. The reaction begins with the breakage of the glycosidic bond (N1–C1′) between the base and the sugar ring. The base is then rotated 180° along the C6–N3 axis, leading to the formation of a new bond (C5–C1′) linking the base to the sugar. a, hydrogen bond acceptor; d, hydrogen bond donor.
Ψ is one of the most frequently occurring post-transcriptional modifications found in stable RNAs, including ribosomal RNAs (rRNAs),13–16 transfer RNAs (tRNAs),17–20 and spliceosomal small nuclear RNAs (snRNAs).21–25 Because these stable RNAs all play important roles in the pathway of gene expression, pseudouridines and their potential functions in this pathway have drawn a great deal of attention. However, due at least in part to a lack of effective assays, research on RNA pseudouridylation, and RNA modification in general, had not generated fruitful results until relatively recently, when several new assays and experimental systems were developed.26–31 Indeed, the intensive research on RNA modifications carried out in recent years has generated remarkable progress. In this review, we will discuss recent advances in the study of spliceosomal snRNA pseudouridylation, focusing primarily on its functions and mechanisms.
PSEUDOURIDINES ARE IDENTIFIED IN SPLICEOSOMAL snRNAs
Pseudouridines Are Abundant and Conserved in Spliceosomal snRNAs
Since it was first discovered in spliceosomal snRNAs many decades ago,21 pseudouridylation has been known to represent a major type of posttranscriptional modification. In fact, pseudouridines are the most abundant of all modifications in spliceosomal snRNAs.21 For instance, in vertebrate U2 snRNA, about 12% of its ~189 nucleotides are modified, including ~13 pseudouridines, which account for ~60% of the total modifications21,22,32 (Figure 2).
FIGURE 2.
Primary sequences and secondary structures of spliceosomal small nuclear RNAs (snRNAs). Shown are five complete vertebrate spliceosomal snRNA sequences and structures (U1, U2, U4, U5, and U6). The 5′ cap and the internal pseudouridines (boxed) are also shown. Some regions in U1, U2, U5 (loop), and U6 (ΨACAGAG), which are important for splicing, are indicated by the thick lines immediately above or below the sequences. U1 5′ end, 5′ end region of U1; U2 BSRR, branch site recognition region of U2; U5 loop, loop region of U5; U6 (Ψ)ACAGAGA, a U6 region believed to be part of the catalytic center of the spliceosome. The sequences of the 5′ end region of yeast U1, the BSRR of yeast U2, and the loop region of yeast U5 are also shown.
Importantly, some of the pseudouridines in spliceosomal snRNAs are also highly conserved across species.22 For instance, three of the six pseudouridines (Ψ34, Ψ41, and Ψ43) in the vertebrate U2 branch site recognition region have counterparts in Saccharomyces cerevisiae U2 snRNA (Ψ35, Ψ42, and Ψ44) (Figure 2). One of the three pseudouridines in the conserved loop of vertebrate U5 (Ψ43) is also present in yeast U5 at the equivalent site (Ψ99) (Figure 2). Likewise, both vertebrate and S. cerevisiae U1 RNAs contain two pseudouridines at the same positions (Ψ5 and Ψ6) (Figure 2). Remarkably, several of these pseudouridines are even present in plant spliceosomal snRNAs.33 Furthermore, detailed pseudouridine mapping indicates that several pseudouridines that are identified in the major spliceosomal snRNAs (U1, U2, U4, U5, and U6) are also present in the minor spliceosomal snRNAs (U11, U12, U4atac, and U6atac) at the equivalent positions.34 Such phylogenetic conservation of pseudouridines strongly suggests that the modified nucleotides are functionally important.
Pseudouridines Are Clustered in Regions of Functional Importance
Over the years, spliceosomal snRNAs have been extensively studied, resulting in the identification of a number of functionally important sequences.32,35 Inspection of the known modifications in spliceosomal snRNA has led to the observation that modified nucleotides, including pseudouridines, are almost always clustered in these functionally important regions (Figure 2). For instance, the U2 branch site recognition region, which recognizes the branch site of pre-messenger RNA (mRNA) during splicing, is heavily pseudouridylated. The only two pseudouridines (Ψ5 and Ψ6) that have been identified in U1 are located in its 5′ end region, which recognizes the 5′ splice site during the early stages of splicing. The pseudouridines of U5 almost all fall into its conserved loop, which contacts the exon sequences at the 5′ and 3′ splice sites during splicing. Even in U4 and U6, modified nucleotides are found in important regions, including those involved in U4/U6 base-pairing interactions and in a region of U6 that is believed to be part of the catalytic center of the spliceosome (Figure 2).
The strategic location of pseudouridines in functional regions, coupled with the frequency at which they appear and the degree to which they are conserved across species, strongly suggests that pseudouridines of spliceosomal snRNAs are functionally important.
PSEUDOURIDINES IN SPLICEOSOMAL snRNAs ARE FUNCTIONALLY IMPORTANT
Although they were discovered more than four decades ago, and have always been suspected to be functionally relevant, spliceosomal snRNA modifications have largely been ignored, due primarily to a lack of experimental systems. In the past decade, however, remarkable progress has been made in this research area. In fact, several labs have contributed to the development of new experimental systems with which to address various questions related to the functions of spliceosomal snRNA modifications, particularly pseudouridylation.30,31,36–43 Important results derived from several different experimental systems are discussed below.
The Mammalian In Vitro System
In the early 1990s, Jeffery Patton pioneered functional analysis of spliceosomal snRNA pseudouridylation using nuclear extracts prepared from mammalian cells.42,43 He found that an in vitro synthesized spliceosomal snRNA, when incubated with Hela cell nuclear extracts under appropriate conditions, could be efficiently pseudouridylated. Interestingly, pseudouridylation of a spliceosomal snRNA could also be site/region specifically blocked upon the addition of a 5-fluorouridine-containing oligoribonucleotide (uridines substituted with 5-fluorouridines) corresponding to the sequence of the respective spliceosomal snRNA.42 Taking advantage of this site/region-specific inhibition scheme, Patton carried out a series of in vitro experiments directed toward addressing the effects of pseudouridylation on snRNP assembly. His results showed that the inhibition of pseudouridylation of a spliceosomal snRNA resulted in disadvantages in snRNP assembly, suggesting that pseudouridines play at least some functional role in spliceosomal snRNP biogenesis.
Direct experimental evidence linking mammalian spliceosomal snRNA (U2) pseudouridylation to pre-mRNA splicing came from functional reconstitution experiments carried out in the Luhrmann lab in 2004.36 In this study, they depleted, using oligonucleotide affinity selection, endogenous U2 snRNP from splicing extracts prepared from Hela cells. They then assembled the U2 snRNP in vitro using in vitro synthesized U2 and snRNP proteins. The resulting U2 snRNP was then added to the U2-depleted splicing extracts for pre-mRNA splicing assay. Given that the U2 RNAs were synthesized in such a way that only one of the natural modification sites in the 5′ end region was left unmodified, and that the splicing extracts were incapable of catalyzing U2 pseudouridylation, any failures of the in vitro synthesized U2 to reconstitute splicing pinpointed specific sites in U2 where pseudouridylation is important for splicing. Using this assay, they identified Ψ6, Ψ7, and Ψ15 as functionally important pseudouridines (Figure 2).
The Xenopus Oocyte Microinjection System
Functional analysis of spliceosomal snRNA modifications was also carried out in Xenopus oocytes.30,37,38 The Xenopus oocyte system relies on the fact that an endogenous spliceosomal snRNA/RNP can be specifically depleted through antisense oligodeoxynucleotide-directed RNase digestion, and that functional spliceosomal snRNP and splicing can be subsequently restored upon addition of the respective snRNA isolated from Xenopus oocytes.
To study U2 pseudouridylation, an antisense U2 DNA oligonucleotide is injected into Xenopus oocytes to hybridize with endogenous U2 snRNA, thereby triggering endogenous RNase H activity, which degrades the RNA strand (U2) of the RNA–DNA hybrid.30 Importantly, by the time the endogenous U2 snRNA is depleted (~4 h), the antisense U2 DNA oligonucleotide itself is degraded by endogenous DNase activity.30 In vitro transcribed U2 (unmodified), cellular U2 (completely modified), or a hybrid of the two (partially modified) is then injected into the U2-depleted oocytes. After a short reconstitution period (3.5 h), snRNP biogenesis and pre-mRNA splicing are analyzed, allowing a detailed assessment of the function of the injected U2 snRNA in these processes.30 Because U2 modifications occur slowly in some regions (no modification or very light modification is observed within 3.5 h),37 the failure of injected U2 to rescue snRNP assembly and pre-mRNA splicing would reflect the importance of modifications in these particular regions.
Using this reconstitution method, Yu et al. identified functionally important modified nucleotides within the first 27 nucleotides of U2 snRNA, including Ψ6, Ψ7, and Ψ15.30 U2 snRNAs lacking these modifications failed to assemble into functional snRNPs; consequently, no splicing complexes were observed.30
While this assay is very good at analyzing the influence of slow modifications, such as the ones that occur in the 5′ end region of U2, it is not as effective for those modifications that occur swiftly.37 For instance, Zhao and Yu discovered that pseudouridine formation in the U2 branch site recognition region, including Ψ34, Ψ37, Ψ39, Ψ41, Ψ43, and Ψ44, occurred rather rapidly.37 In fact, they found that pseudouridylation in the U2 branch site recognition region had already been completed before U2 snRNP biogenesis and pre-mRNA splicing could be assayed (after 3.5 h of reconstitution). Thus, under these conditions (reconstitution for 3.5 h), a functional analysis of rapidly occurring pseudouridylation is impossible.
To examine whether pseudouridines in the U2 branch site recognition region contribute to pre-mRNA splicing, Zhao and Yu then utilized 5-fluorouridine-containing U2 to selectively block rapidly occurring U2 pseudouridylation in this region.37 They injected into U2-depleted Xenopus oocytes an artificial U2 RNA, in which the uridines in the branch site recognition region were substituted with 5-fluorouridines. The presence of this RNA had no effect on the ability of cellularly derived U2 (containing all modifications), injected at a later time, to restore splicing. However, the 5-fluorouridine-containing U2 site specifically inhibited the pseudouridylation of in vitro transcribed U2 RNA. After a prolonged incubation (~16 h), the rescuing U2 RNA (in vitro transcribed) was modified at all natural sites except for the sites within the branch site recognition region. Importantly, the resultant U2 RNA, lacking pseudouridines in the branch site recognition region, failed to restore pre-mRNA splicing, indicating that the six pseudouridines in the U2 branch site recognition region are also important for pre-mRNA splicing.37
To further study the functional importance of the pseudouridines of U2 snRNA in pre-mRNA splicing, Zhao and Yu treated Hela cells with 5-fluorouracil (5-FU, a widely used anticancer drug and a suspected pseudouridylation inhibitor) and subsequently analyzed cellular U2 RNA.38 Remarkably, U2 RNA isolated from 5-FU-treated cells lost pseudouridines at natural pseudouridylation sites, including those in the branch site recognition region. In contrast, U2 RNA isolated from uracil-treated control Hela cells retained pseudouridines at these sites. U2 RNAs from both groups were then separately injected into U2-depleted Xenopus oocytes, and reconstitution of splicing was assayed. While U2 RNA isolated from uracil-treated cells fully rescued pre-mRNA splicing, U2 RNA isolated from 5-FU-treated cells failed to reconstitute pre-mRNA splicing in Xenopus oocytes.38 Thus, these results reinforced the notion that at least some pseudouridines within U2 snRNA play an important role in pre-mRNA splicing.
The Yeast System
The yeast system is also used to study the function of spliceosomal snRNA modifications. By 2005, pseudouridylases responsible for the formation of all three pseudouridines in the branch site recognition region of yeast U2 snRNA were identified,12,44,45 making it possible to study the functional importance of U2 pseudouridines in yeast cells. Although no obvious phenotype was observed when any one of the three pseudouridylases was deleted, combined deletions of these pseudouridylases resulted in growth defect phenotype (Wu and Yu, unpublished data), suggesting once again that pseudouridines in U2 are functionally important.
To carry out a detailed analysis, Yang et al. used a genetic synthetic lethal screen to test the functional role of Ψ35, the pseudouridine that pairs with the nucleotide next to the pre-mRNA branch point adenosine during splicing.31 As expected, when PUS7 (the pseudouridylase responsible for position 35) was deleted, no Ψ35 formation was detected; however, the deletion resulted only in an extremely mild growth disadvantage phenotype. Similarly, no growth defect phenotype was detected when a number of mutant strains, each containing a point mutation in the U2 gene, were tested. However, a combination of the pus7 deletion and a U2 point mutation at position 40 (either a deletion or substitution) resulted in a temperature-sensitive phenotype. While quite healthy at 30°C, the mutant strain could not grow at all at 37°C. Further analysis indicated that the mutant strain was deficient in pre-mRNA splicing at 37°C, indicating that under certain conditions, Ψ35 in the U2 branch site recognition region contributes to pre-mRNA splicing in S. cerevisiae.31 These results are also consistent with the results of similar analyses conducted in the Xenopus oocyte microinjection system (see above).
Other Systems
Functional analyses of spliceosomal snRNA modifications have also been carried out in other systems. For instance, using a biophysical system, •NMR, Newby and Greenbaum determined solution structures of the duplex of the branch site sequence–U2 branch site recognition sequence either in the presence or absence of Ψ35.39 They showed that, when paired with the pre-mRNA branch site, Ψ35 is favored over uridine for maintaining the bulge of the branch point nucleotide adenosine, which is used in a nucleophilic attack in the first step of splicing.39 More recent work by Lin and Kielkopf solved the crystal structure of the duplex of the branch site sequence–U2 branch site recognition sequence in the presence of Ψ35.41 They observed that either the branch point adenosine or a preceding purine residue assumed a bulged conformation in which the 2′-OH group of either nucleotide was available to initiate the first step of splicing. Thus, it appears that Ψ35 plays a role in promoting the bulged conformation, which is functionally relevant for catalysis.41
The importance of Ψ35 in splicing is further supported by the work of Valadkhan and Manley,40,46 who developed a cell- and protein-free system in which a splicing-related reaction, involving the U2 branch site recognition region, can be assayed. This assay showed that the presence of Ψ35 greatly enhanced the production of the product of the splicing-related reaction.40
Furthermore, experiments carried out in two independent systems suggested that the pseudouridines (Ψ5 and Ψ6) in U1 snRNA might contribute to 5′ splice site recognition. Using an in vitro splicing system, in which two 5′ splice sites were in competition with each other, Roca et al. suggested that the U1 pseudouridines might provide an advantage in 5′ splice site selection.47 Likewise, by transiently transfecting Hela cells with a reporter gene derived from human immunodeficiency virus-1 (HIV-1), Freund et al. suggested that a Ψ-G base pair is important for the interaction between U1 and the 5′ splice site during splicing.48
In summary, results from several experimental systems have established the functional importance of pseudouridines in U2 snRNA (and perhaps U1 and other spliceosomal snRNAs) in pre-mRNA splicing. However, the detailed mechanism by which these pseudouridines contribute to U2 function in splicing remains unclear.
SPLICEOSOMAL snRNA PSEUDOURIDYLATION IS CATALYZED BY TWO INDEPENDENT MECHANISMS
Box H/ACA RNP-Catalyzed (or RNA-Dependent) Mechanism
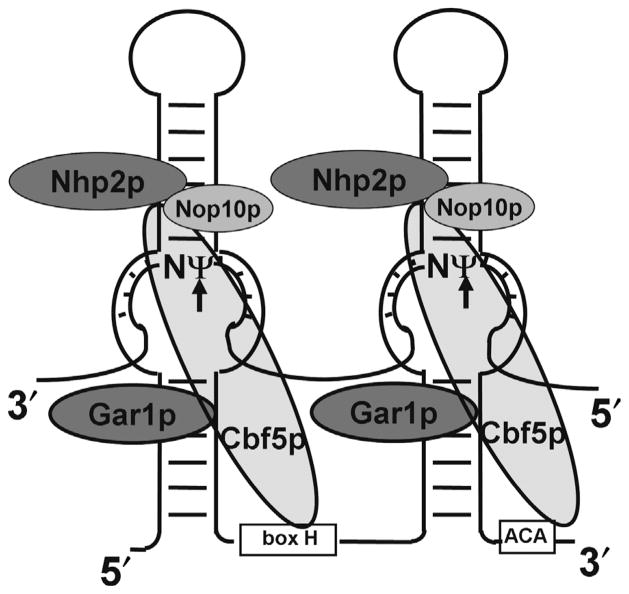
The mechanism of spliceosomal snRNA pseudouridylation eluded researchers for more than three decades, until, in 1997, several groups made a major breakthrough in the study of rRNA pseudouridylation. They reported that eukaryotic rRNA pseudouridylation was catalyzed by box H/ACA snoRNPs, RNA–protein complexes each consisting of one unique small non-coding guide RNA (box H/ACA RNA) and four common core proteins [Cbf5 (NAP57/dyskerin), Nhp2, Gar1, and Nop10].49–53 In a box H/ACA snoRNP complex, the box H/ACA guide RNA forms a unique ‘hairpin-hinge-hairpin-tail’ structure, in which each of the two hairpins contains an internal loop (pseudouridylation pocket) that base pairs with the target RNA, positioning the target uridine at the base of the upper stem (Figure 3). Subsequently, Cbf5 (NAP57/dyskerin), a catalytic component of box H/ACA RNP,52–54 converts the target uridine to pseudouridine in the substrate (Figure 3). This mechanism has been experimentally confirmed in rRNAs.
FIGURE 3.
Schematic representation of eukaryotic box H/ACA RNP. The core components of a box H/ACA RNP, including a box H/ACA RNA, and four proteins (Nhp2p, Cbf5p, Gar1p, and Nop10p), are shown. Also shown is an RNA substrate paired with the two internal loops of the box H/ACA RNA. The arrows indicate the target nucleotides for pseudouridylation. The H box and ACA box of the box H/ACA RNA are indicated.
Because spliceosomal snRNAs also contain a number of pseudouridines, the same type of modification seen in rRNA, it was proposed that spliceosomal snRNA pseudouridylation might be catalyzed by box H/ACA snoRNPs in a manner identical to rRNA pseudouridylation. Indeed, after an intense search for snoRNA guides, including experimental (RNomics) and computational methods, a number of potential box H/ACA guide RNAs containing spliceosomal snRNA-specific guide sequences were identified in mammalian cells.55–57 Several guide activities have been experimentally confirmed.57
Meanwhile, an effort was also made to search for U2 pseudouridylation guides in Xenopus oocytes using biotinylated 5-fluorouridine-containing U2 snRNA. It is well known that 5-fluorouridine-containing U2 inhibits U2 pseudouridylation by binding tightly to pseudouridylases, thereby sequestering these enzymes.30,42 Thus, it seemed possible to use affinity selection to purify pseudouridylases associated with the inhibitor, biotinylated 5-fluorouridine-containing U2 snRNA. Based on this hypothesis, Zhao et al. carried out the affinity-selection experiment. RNAs copurifying with the inhibitor were eluted and separated on a denaturing gel. One of the RNAs was directly sequenced, yielding a perfect box H/ACA RNA potentially capable of directing U2 snRNA pseudouridylation at positions 34 and 44.58
It is relatively easy to deplete an RNA in oocytes (by DNA oligonucleotide-directed RNase H targeting) and to subsequently reconstitute function (by injecting a synthetic version of this RNA into depleted oocytes). Using this approach, the ability of this guide RNA to direct U2 Ψ34 and Ψ44 formation was tested and verified in vivo.58 Given that not just one, but a number of RNAs copurified with the biotinylated pseudouridylation inhibitor (as expected), the RNA selected by 5-fluorouridine-containing U2 appears to be a rich source from which to identify U2 snRNA-specific guide RNAs in Xenopus oocytes.
These results, coupled with the fact that a nearly complete set of box H/ACA RNAs has been identified for known pseudouridylation sites within mammalian rRNAs and snRNAs, strongly suggest that RNA-guided (or RNA-dependent) pseudouridylation is a major (if not the only) mechanism for snRNA and rRNA pseudouridylation in mammals (and perhaps all vertebrates).
It is worth noting that the human disease dyskeratosis congenita (DC), a bone marrow failure syndrome, is found to predominantly affect box H/ACA RNPs. Indeed, a number of mutations have been identified within core components of the box H/ACA RNPs from patients suffering from X-linked and autosomal recessive DC.59,60 Currently, it is believed that a defect in human telomerase (a special type of box H/ACA RNP) and/or ribosome biogenesis and function may be the cause of DC.59,60 However, given that box H/ACA RNPs also play an important role in spliceosomal snRNA modification and pre-mRNA splicing, it is not impossible that defective spliceosomal snRNA pseudouridylation and splicing also contribute to this disease.
RNA-Independent Mechanism
Great efforts have also been made to identify spliceosomal snRNA-specific modifying activities in S. cerevisiae. Surprisingly, however, early work indicated that pseudouridylation of spliceosomal snRNAs in S. cerevisiae was catalyzed by protein-only enzymes (an RNA-independent mechanism).12,44
Using known yeast pseudouridylases, Branlant and colleagues found that one pseudouridylase, Pus1p, previously identified as a tRNA pseudouridylase, also catalyzed U44-to-Ψ44 conversion in yeast U2.12 Specifically, when incubated with Pus1p, in vitro synthesized U2 became pseudouridylated specifically at position 44. Deletion of PUS1 from yeast cells resulted in the loss of Ψ44 in U2 snRNA. These results showed, both in vitro and in vivo, that Pus1p has a Ψ44-specific pseudouridylase activity.
Rapid progress was also made with the help of the •GST-ORF fusion protein library.61 To screen the library for pseudouridylase activity specific for Ψ35 formation, Ma et al. constructed a synthetic U2 RNA in which the phosphorus atom (P) between position 34 and position 35 was substituted with 32P.44 Upon incubation with the GST-ORF fusion library, the singly radiolabeled U2 was recovered, digested with nuclease P1, and subjected to TLC analysis, thus allowing an accurate assessment of [32P]U35-to-[32P] Ψ35 conversion. The screen identified a Y35-specific pseudouridylase activity associated with YOR243c ORF, which was later named PUS7. Deletion of PUS7 from yeast cells led to a complete loss of Y35 in U2. Expression of PUS7 restored Y35 formation. Furthermore, Escherichia coli-expressed Pus7p was also fully functional in catalyzing U2 pseudouridylation at position 35 in vitro, further showing that Pus7p alone is the Y35-specific pseudouridylase.44 Interestingly, Pus7p, like Pus1p, is capable of pseudouridylating both U2 and tRNA.62
Taken together, these results suggested that the pseudouridylation process in yeast spliceosomal snRNA (or at least that of Y35 and Y44 in U2), which relies on an RNA-independent mechanism, might be different from that of higher eukaryotes (which calls on box H/ACA RNPs).
Yeast U2 snRNA Pseudouridylation Is Catalyzed by Both RNA-Dependent and RNA-Independent Mechanisms
Yeast U2 snRNA contains three pseudouridines (Ψ35, Ψ42, and Ψ44) in the branch site recognition region (Figure 2). The identification of Pus1p and Pus7p as pseudouridylases responsible for the formation of Ψ44 and Ψ35, respectively, left only one pseudouridine (Ψ42) uncharacterized. By analogy, it was initially widely believed that Ψ42 formation was also catalyzed by a protein enzyme alone. Using the powerful yeast GST-ORF fusion protein library, Ma et al. constructed a yeast U2 RNA containing a single [32P] at position 42, and screened the GST-ORF library for the enzyme responsible for this remaining pseudouridine.45
Surprisingly, the screen identified a Ψ42-specific pseudouridylase activity that was associated with Nhp2p, one of the four core protein components of box H/ACA RNPs (Figure 2). However, E. coli-expressed recombinant Nhp2p alone had no activity, suggesting that a box H/ACA RNP, including several proteins and a guide RNA, might be involved in Ψ42 formation. Indeed, the other box H/ACA RNP proteins, when isolated by tandem affinity purification (TAP), copurified with the pseudouridylase activity. To directly test whether box H/ACA RNA was required for activity, Ma et al. treated the TAP preparations (e.g., Gar1-TAP-tagging preparation) with micrococcal nuclease, yielding preparations devoid of pseudouridylase activity. The activity was restored, however, upon subsequent addition of RNAs extracted from TAP preparations, showing a requirement for box H/ACA RNA.45
To identify the specific box H/ACA RNA, Ma et al. first gel fractionated total box H/ACA RNA isolated from the Gar1p-TAP-tagging preparation.45 They then added individual fractions to the micrococcal nuclease-treated Gar1p-TAP-tagging preparation to test their ability to reconstitute pseudouridylation in vitro. One RNA fraction (~180–200 nucleotides) was successful in this regard, suggesting that the Ψ42-specific guide RNA was present in this fraction. However, it appeared as though there were many different box H/ACA RNAs in this fraction, as a smeared polyacrylamide gel signal ranging from ~180–200 nucleotides was observed when the RNA fraction was 3′-labeled with [32P]pCp. To isolate the Ψ42-specific RNA, they constructed an RNA library using this particular RNA fraction and the ‘tailing-RT-PCR-cloning’ method.63,64 Using the reconstitution system, they then screened individual RNAs derived from the RNA library for guide activity.45 Somewhat surprisingly, the Ψ42-specific guide RNA was identified as snR81, a genuine box H/ACA snoRNA whose 3′ pseudouridylation pocket had already been assigned guide activity for 25S rRNA at position 1501.65 Apparently, the 5′ pseudouridylation pocket is specific for Ψ42 of U2, and the 3′ pocket is specific for Ψ1501 of 25S rRNA (Figure 4).
FIGURE 4.
Pseudouridylation of U2 small nuclear RNA (snRNA) and 25S ribosomal rRNA (rRNA) by snR81 box H/ACA RNP. The sequence and structure of snR81 box H/ACA RNA are shown. The H box and ACA box are indicated (shaded boxes). As depicted by arrows, the internal loop (pseudouridylation pocket) within the 5′ stem of snR81 RNA is specific for Ψ42 of U2, and the internal loop within the 3′ stem is specific for Ψ1051 of 25S rRNA. The base-pairing interactions between the snR81 internal loops and the substrate sequences (U2 and 25S rRNA) are also shown.
To further confirm the results of the screening, Ma et al. also constructed an snr81Δ strain and nhp2 or cbf5 conditional depletion strains (all core box H/ACA RNP proteins are essential for growth) or used a slow-growth cbf5 point mutation strain54 to show that the pseudouridylase activity is in fact dependent on snR81 snoRNA and the box H/ACA RNP proteins in vivo.45 The results indicated that the conversion of U42 to Ψ42 in yeast U2 is indeed catalyzed by snR81 snoRNP via an RNA-guided mechanism, which differs from the previously identified protein-only mechanism catalyzing yeast U2 pseudouridylation at the other two positions, Ψ3544 and Ψ44.12 These data thus indicated for the first time that spliceosomal snRNA pseudouridylation can be catalyzed by an RNA-dependent mechanism as well as an RNA-independent mechanism, and that a box H/ACA snoRNA can guide both spliceosomal snRNA (nucleoplasmic) and rRNA (nucleolar) for pseudouridylation (Figure 4).
CONCLUDING REMARKS
There are a number of pseudouridines in spliceosomal snRNAs. Many of them are conserved across species and are clustered in functionally important regions, suggesting that these pseudouridines are functionally important.22,66,67 Indeed, functional analyses in a number of experimental systems show that the pseudouridines in spliceosomal snRNAs contribute to pre-mRNA splicing.30,31,36–43,47,48 However, the details surrounding the mechanisms by which these pseudouridines affect splicing remain unclear. Understanding the mechanisms of action of pseudouridines during splicing is therefore an important aspect of ongoing research concerning spliceosomal snRNA pseudouridylation. It appears that, in order to achieve this goal, a combination of genetic, biochemical, and biophysical approaches is necessary. In addition, besides the currently available experimental systems, it is desirable to develop an in vitro system in which the effect of pseudouridines on spliceosome assembly and structures can be assessed.
With regard to the mechanisms of spliceosomal snRNA pseudouridylation, although two types of modifying enzymes (box H/ACA RNP and protein-only pseudouridylases) have been identified (both act on a number of sites within spliceosomal snRNAs),12,44,45,49,50 there are still some natural pseudouridylation sites to which no enzymes have yet been assigned. Because pseudouridylation of yeast U2 is catalyzed by both RNA-dependent (box H/ACA RNP) and RNA-independent (protein-only) mechanisms,44,45 it is of particular interest to focus on the remaining three natural pseudouridylation sites in yeast spliceosomal snRNAs, including Ψ5 and Ψ6 in U1 and Ψ99 in U5 (Figure 2). Characterizing the mechanisms (RNA-dependent and/or RNA-independent) by which pseudouridines are introduced into these sites will not only help to understand, from an evolutionary perspective, the relationship between the two mechanisms but will also greatly facilitate the functional analysis of these pseudouridines. Given that a number of techniques are currently available, it is expected that a clear and complete picture, with all pseudouridylases assigned to their target sites, will soon emerge.
Acknowledgments
We thank the members of the Yu laboratory for valuable discussions.
References
- 1.Cohn WE, Volkin W. Nucleoside-5′-phosphates from ribonucleic acid. Nature (London) 1951;167:483–484. [Google Scholar]
- 2.Davis FF, Allen FW. Ribonucleic acids from yeast which contain a fifth nucleotide. J Biol Chem. 1957;227:907–915. [PubMed] [Google Scholar]
- 3.Lane BG. Historical perspectives on RNA nucleoside modifications. In: Grosjean H, Benne R, editors. Modification and Editing of RNA. Washington, DC: ASM Press; 1998. pp. 1–20. [Google Scholar]
- 4.Arnez JG, Steitz TA. Crystal structure of unmodified tRNA(Gln) complexed with glutaminyl-tRNA synthetase and ATP suggests a possible role for pseudouridines in stabilization of RNA structure. Biochemistry. 1994;33:7560–7567. doi: 10.1021/bi00190a008. [DOI] [PubMed] [Google Scholar]
- 5.Davis DR. Stabilization of RNA stacking by pseudouridine. Nucleic Acids Res. 1995;23:5020–5026. doi: 10.1093/nar/23.24.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolev NG, Steitz JA. In vivo assembly of functional U7 snRNP requires RNA backbone flexibility within the Sm-binding site. Nat Struct Mol Biol. 2006;13:347–353. doi: 10.1038/nsmb1075. [DOI] [PubMed] [Google Scholar]
- 7.Charette M, Gray MW. Pseudouridine in RNA: what, where, how, and why. IUBMB Life. 2000;49:341–351. doi: 10.1080/152165400410182. [DOI] [PubMed] [Google Scholar]
- 8.Auffinger P, Westhof E. RNA hydration: three nanoseconds of multiple molecular dynamics simulations of the solvated tRNA(Asp) anticodon hairpin. J Mol Biol. 1997;269:326–341. doi: 10.1006/jmbi.1997.1022. [DOI] [PubMed] [Google Scholar]
- 9.Westhof E, Dumas P, Moras D. Hydration of transfer RNA molecules: a crystallographic study. Biochimie. 1988;70:145–165. doi: 10.1016/0300-9084(88)90056-9. [DOI] [PubMed] [Google Scholar]
- 10.Newby MI, Greenbaum NL. Investigation of Overhauser effects between pseudouridine and water protons in RNA helices. Proc Natl Acad Sci U S A. 2002;99:12697–12702. doi: 10.1073/pnas.202477199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall KB, McLaughlin LW. Properties of pseudouridine N1 imino protons located in the major groove of an A-form RNA duplex. Nucleic Acids Res. 1992;20:1883–1889. doi: 10.1093/nar/20.8.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massenet S, Motorin Y, Lafontaine DL, Hurt EC, Grosjean H, Branlant C. Pseudouridine mapping in the Saccharomyces cerevisiae spliceosomal U small nuclear RNAs (snRNAs) reveals that pseudouridine synthase pus1p exhibits a dual substrate specificity for U2 snRNA and tRNA. Mol Cell Biol. 1999;19:2142–2154. doi: 10.1128/mcb.19.3.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branlant C, Krol A, Machatt MA, Pouyet J, Ebel JP, Edwards K, Kossel H. Primary and secondary structures of Escherichia coli MRE 600 23S ribosomal RNA. Comparison with models of secondary structure for maize chloroplast 23S rRNA and for large portions of mouse and human 16S mitochondrial rRNAs. Nucleic Acids Res. 1981;9:4303–4324. doi: 10.1093/nar/9.17.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ofengand J, Fournier MJ. The pseudouridine residues of rRNA: number, location, biosynthesis, and function. In: Grosjean H, editor. Modification and Editing of RNA. Washington, DC: ASM Press; 1998. pp. 229–253. [Google Scholar]
- 15.Maden BE. The numerous modified nucleotides in eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1990;39:241–303. doi: 10.1016/s0079-6603(08)60629-7. [DOI] [PubMed] [Google Scholar]
- 16.Bachellerie J-P, Cavaille J. Small nucleolar RNAs guide the ribose methylations of eukaryotic rRNAs. In: Grosjean H, Benne R, editors. Modification and Editing of RNA. Washington, DC: ASM Press; 1998. pp. 255–272. [Google Scholar]
- 17.Bjork GR. Biosynthesis and function of modified nucleotides. In: Soll D, RajBhandary U, editors. tRNA: Structure, Biosynthesis, and Function. Washington, DC: ASM Press; 1995. pp. 165–205. [Google Scholar]
- 18.Grosjean H, Sprinzl M, Steinberg S. Posttranscriptionally modified nucleosides in transfer RNA: their locations and frequencies. Biochimie. 1995;77:139–141. doi: 10.1016/0300-9084(96)88117-x. [DOI] [PubMed] [Google Scholar]
- 19.Sprinzl M, Horn C, Brown M, Ioudovitch A, Steinberg S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1998;26:148–153. doi: 10.1093/nar/26.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopper AK, Phizicky EM. tRNA transfers to the limelight. Genes Dev. 2003;17:162–180. doi: 10.1101/gad.1049103. [DOI] [PubMed] [Google Scholar]
- 21.Reddy R, Busch H. Small nuclear RNAs: RNA sequences, structure, and modifications. In: Birnsteil ML, editor. Structure and Function of Major and Minor Small Nuclear Ribonucleoprotein Particles. Heidelberg: Sringer-Verlag Press; 1988. pp. 1–37. [Google Scholar]
- 22.Massenet S, Mougin A, Branlant C. Posttranscriptional modifications in the U small nuclear RNAs. In: Grosjean H, editor. Modification and Editing of RNA. Washington, DC: ASM Press; 1998. pp. 201–228. [Google Scholar]
- 23.Yu YT, Terns RM, Terns MP. Mechanisms and functions of RNA-guided RNA modification. In: Grosjean H, editor. Fine-Tuning of RNA Functions by Modification and Editing. New York: Springer-Verlag Press; 2005. pp. 223–262. [Google Scholar]
- 24.Stephenson D, Karijolich J, Yu YT. Functional roles of spliceosomal snRNA modifications in pre-mRNA splicing. In: Smith H, editor. RNA and DNA Editing: Molecular Mechanisms and Their Integration into Biological Systems. Hoboken, NJ: Wiley-Interscience; 2008. pp. 175–189. [Google Scholar]
- 25.Karijolich J, Yu YT. Spliceosomal snRNA modifications and their function. •RNA Biol. 7:192–204. doi: 10.4161/rna.7.2.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakin AV, Ofengand J. Mapping of pseudouridine residues in RNA to nucleotide resolution. Methods Mol Biol. 1998;77:297–309. doi: 10.1385/0-89603-397-X:297. [DOI] [PubMed] [Google Scholar]
- 27.Zhao X, Yu YT. Detection and quantitation of RNA base modifications. RNA. 2004;10:996–1002. doi: 10.1261/rna.7110804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai Q, Fong R, Saikia M, Stephenson D, Yu YT, Pan T, Piccirilli JA. Identification of recognition residues for ligation-based detection and quantitation of pseudouridine and N6-methyladenosine. Nucleic Acids Res. 2007;35:6322–6329. doi: 10.1093/nar/gkm657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segault V, Will CL, Sproat BS, Luhrmann R. In vitro reconstitution of mammalian U2 and U5 snRNPs active in splicing: Sm proteins are functionally interchangeable and are essential for the formation of functional U2 and U5 snRNPs. EMBO J. 1995;14:4010–4021. doi: 10.1002/j.1460-2075.1995.tb00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu YT, Shu MD, Steitz JA. Modifications of U2 snRNA are required for snRNP assembly and pre-mRNA splicing. EMBO J. 1998;17:5783–5795. doi: 10.1093/emboj/17.19.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang C, McPheeters DS, Yu YT. Psi35 in the branch site recognition region of U2 small nuclear RNA is important for pre-mRNA splicing in Saccharomyces cerevisiae. J Biol Chem. 2005;280:6655–6662. doi: 10.1074/jbc.M413288200. [DOI] [PubMed] [Google Scholar]
- 32.Yu YT, Scharl EC, Smith CM, Steitz JA. The growing world of small nuclear ribonucleoproteins. In: Gesteland RF, Cech TR, Atkins JF, editors. The RNA World. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999. pp. 487–524. [Google Scholar]
- 33.Solymosy F, Pollak T. Uridylate-rich small nuclear RNAs (usnRNAs), their genes and pseudogenes, and usnRNPs in plants: structure and function. A comparative approach. Crit Rev Plant Sci. 1993;12:275–369. [Google Scholar]
- 34.Massenet S, Branlant C. A limited number of pseudouridine residues in the human atac spliceosomal UsnRNAs as compared to human major spliceosomal UsnRNAs. RNA. 1999;5:1495–1503. doi: 10.1017/s1355838299991537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burge CB, Tuschl T, Sharp PA. Splicing of precursors to mRNAs by the spliceosome. In: Gesteland RF, Cech TR, Atkins JF, editors. The RNA World. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999. pp. 525–560. [Google Scholar]
- 36.Donmez G, Hartmuth K, Luhrmann R. Modified nucleotides in the 5′ end of the human U2 snRNA are required for early spliceosome (E complex) formation in vitro. The 2004 RNA meeting abstract. 2004:92. doi: 10.1261/rna.7186504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao X, Yu YT. Pseudouridines in and near the branch site recognition region of U2 snRNA are required for snRNP biogenesis and pre-mRNA splicing in Xenopus oocytes. RNA. 2004;10:681–690. doi: 10.1261/rna.5159504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao X, Yu YT. Incorporation of 5-fluorouracil into U2 snRNA blocks pseudouridylation and pre-mRNA splicing in vivo. Nucleic Acids Res. 2007;35:550–558. doi: 10.1093/nar/gkl1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newby MI, Greenbaum NL. Sculpting of the spliceosomal branch site recognition motif by a conserved pseudouridine. Nat Struct Biol. 2002;9:958–965. doi: 10.1038/nsb873. [DOI] [PubMed] [Google Scholar]
- 40.Valadkhan S, Manley JL. Characterization of the catalytic activity of U2 and U6 snRNAs. RNA. 2003;9:892–904. doi: 10.1261/rna.5440303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Y, Kielkopf CL. X-ray structures of U2 snRNA-branchpoint duplexes containing conserved pseudouridines. Biochemistry. 2008;47:5503–5514. doi: 10.1021/bi7022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patton JR. Ribonucleoprotein particle assembly and modification of U2 small nuclear RNA containing 5-fluorouridine. Biochemistry. 1993;32:8939–8944. doi: 10.1021/bi00085a027. [DOI] [PubMed] [Google Scholar]
- 43.Patton JR. Multiple pseudouridine synthase activities for small nuclear RNAs. Biochem J. 1993;290(Pt 2):595–600. doi: 10.1042/bj2900595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma X, Zhao X, Yu YT. Pseudouridylation (Psi) of U2 snRNA in S. cerevisiae is catalyzed by an RNA-independent mechanism. EMBO J. 2003;22:1889–1897. doi: 10.1093/emboj/cdg191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma X, Yang C, Alexandrov A, Grayhack EJ, Behm-Ansmant I, Yu YT. Pseudouridylation of yeast U2 snRNA is catalyzed by either an RNA-guided or RNA-independent mechanism. EMBO J. 2005;24:2403–2413. doi: 10.1038/sj.emboj.7600718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valadkhan S, Manley JL. Splicing-related catalysis by protein-free snRNAs. Nature. 2001;413:701–707. doi: 10.1038/35099500. [DOI] [PubMed] [Google Scholar]
- 47.Roca X, Sachidanandam R, Krainer AR. Determinants of the inherent strength of human 5′ splice sites. RNA. 2005;11:683–698. doi: 10.1261/rna.2040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freund M, Asang C, Kammler S, Konermann C, Krummheuer J, Hipp M, Meyer I, Gierling W, Theiss S, Preuss T, et al. A novel approach to describe a U1 snRNA binding site. Nucleic Acids Res. 2003;31:6963–6975. doi: 10.1093/nar/gkg901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganot P, Bortolin ML, Kiss T. Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell. 1997;89:799–809. doi: 10.1016/s0092-8674(00)80263-9. [DOI] [PubMed] [Google Scholar]
- 50.Ni J, Tien AL, Fournier MJ. Small nucleolar RNAs direct site-specific synthesis of pseudouridine in ribosomal RNA. Cell. 1997;89:565–573. doi: 10.1016/s0092-8674(00)80238-x. [DOI] [PubMed] [Google Scholar]
- 51.Balakin AG, Smith L, Fournier MJ. The RNA world of the nucleolus: two major families of small RNAs defined by different box elements with related functions. Cell. 1996;86:823–834. doi: 10.1016/s0092-8674(00)80156-7. [DOI] [PubMed] [Google Scholar]
- 52.Grozdanov P, Meier UT. Multicomponent machines in RNA modification: H/ACA ribonucleoproteins. In: Grosjean H, editor. DNA and RNA Modification Enzymes: Structure, Mechanism, Function and Evolution. Austin, TX: Landes Bioscience; 2009. pp. 450–460. [Google Scholar]
- 53.Meier UT, Blobel G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J Cell Biol. 1994;127(6 Pt 1):1505–1514. doi: 10.1083/jcb.127.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zebarjadian Y, King T, Fournier MJ, Clarke L, Carbon J. Point mutations in yeast CBF5 can abolish in vivo pseudouridylation of rRNA. Mol Cell Biol. 1999;19:7461–7472. doi: 10.1128/mcb.19.11.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiss AM, Jady BE, Bertrand E, Kiss T. Human box H/ACA pseudouridylation guide RNA machinery. Mol Cell Biol. 2004;24:5797–5807. doi: 10.1128/MCB.24.13.5797-5807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huttenhofer A, Kiefmann M, Meier-Ewert S, O’Brien J, Lehrach H, Bachellerie JP, Brosius J. RNomics: an experimental approach that identifies 201 candidates for novel, small, non-messenger RNAs in mouse. EMBO J. 2001;20:2943–2953. doi: 10.1093/emboj/20.11.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jady BE, Kiss T. A small nucleolar guide RNA functions both in 2′-O-ribose methylation and pseudouridylation of the U5 spliceosomal RNA. EMBO J. 2001;20:541–551. doi: 10.1093/emboj/20.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao X, Li ZH, Terns RM, Terns MP, Yu YT. An H/ACA guide RNA directs U2 pseudouridylation at two different sites in the branchpoint recognition region in Xenopus oocytes. RNA. 2002;8:1515–1525. [PMC free article] [PubMed] [Google Scholar]
- 59.Meier UT. The many facets of H/ACA ribonucleoproteins. Chromosoma. 2005;114:1–14. doi: 10.1007/s00412-005-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meier UT. Dissecting dyskeratosis. Nat Genet. 2003;33:116–117. doi: 10.1038/ng0203-116. [DOI] [PubMed] [Google Scholar]
- 61.Phizicky EM, Martzen MR, McCraith SM, Spinelli SL, Xing F, Shull NP, Van Slyke C, Montagne RK, Torres FM, Fields S, et al. Biochemical genomics approach to map activities to genes. Methods Enzymol. 2002;350:546–559. doi: 10.1016/s0076-6879(02)50984-8. [DOI] [PubMed] [Google Scholar]
- 62.Behm-Ansmant I, Urban A, Ma X, Yu YT, Motorin Y, Branlant C. The Saccharomyces cerevisiae U2 snRNA:pseudouridine-synthase Pus7p is a novel multisite-multisubstrate RNA:Psi-synthase also acting on tRNAs. RNA. 2003;9:1371–1382. doi: 10.1261/rna.5520403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Brien CA, Wolin SL. A possible role for the 60-kD Ro autoantigen in a discard pathway for defective 5S rRNA precursors. Genes Dev. 1994;8:2891–2903. doi: 10.1101/gad.8.23.2891. [DOI] [PubMed] [Google Scholar]
- 64.Yu YT, Tarn WY, Yario TA, Steitz JA. More Sm snRNAs from vertebrate cells. Exp Cell Res. 1996;229:276–281. doi: 10.1006/excr.1996.0372. [DOI] [PubMed] [Google Scholar]
- 65.Schattner P, Decatur WA, Davis CA, Ares M, Jr, Fournier MJ, Lowe TM. Genome-wide searching for pseudouridylation guide snoRNAs: analysis of the Saccharomyces cerevisiae genome. Nucleic Acids Res. 2004;32:4281–4296. doi: 10.1093/nar/gkh768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reddy R, Busch H. Small nuclear RNAs: RNA sequences, structure, and modifications. In: Birnstiel ML, editor. Structure and Function of Major and Minor Small Nuclear Ribonucleoprotein Particles. Heidelberg: Springer-Verlag Press; 1988. pp. 1–37. [Google Scholar]
- 67.Yu YT, Terns RM, Terns MP. Mechanisms and functions of RNA-guided RNA modification. In: Grosjean H, editor. Topics in Current Genetics. New York: Springer-Verlag; 2005. pp. 223–262. [Google Scholar]