Abstract
Background
The extent to which relative contributions of traditional cardiovascular factors risk to incident cardiovascular disease (CVD) may have changed over time remains unclear.
Methods and Results
We studied 13,541 participants (56% women, 26% black) in the Atherosclerosis Risk in Communities Study, aged 52-66 years and free of CVD at exams in 1987-89, 1990-92, 1993-95, or 1996-98. At each exam, we estimated the population attributable risks (PAR) of traditional risk factors (hypertension, diabetes, obesity, hypercholesterolemia, and smoking) for the 10-year incidence of CVD. Overall, the PAR of all risk factors combined appeared to decrease from 1987-89 to 1996-98 (0.58 to 0.53). The combined PAR was higher in women than men in 1987-89 (0.68 vs. 0.51, P<0.001) but not by 1996-98 (0.58 vs. 0.48, P=0.08). The combined PAR was higher in blacks than whites in 1987-89 (0.67 vs. 0.57, P=0.049), and this difference was more pronounced by 1996-98 (0.67 vs. 0.48, P=0.002). By 1996-98, the PAR of hypertension had become higher in women than men (P=0.02) and also appeared higher in blacks than whites (P=0.08). By 1996-98, the PAR of diabetes remained higher in women than men (P<0.0001) and in blacks than whites (P<0.0001).
Conclusions
The contribution to CVD of all traditional risk factors combined is greater in blacks than whites, and this difference may be increasing. The contributions of hypertension and diabetes remain especially high, in women as well as blacks. These findings underscore the continued need for individual as well as population approaches to CVD risk factor modification.
Keywords: cardiovascular disease risk factors, cardiovascular disease prevention, gender differences, race and ethnicity
INTRODUCTION
Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality in the United States, with an overall economic burden in excess of $440 billion annually.1,2 Data from prior studies suggest that the incidence of CVD has been decreasing over time,3-7 in association with increasing public health emphasis on the prevention and treatment of modifiable risk factors such as hypertension and hypercholesterolemia.8-13 However, several studies indicate that improvements in CVD incidence are lagging in certain subgroups, including women and blacks.3-6 The degree to which these trends may be due to differences between subgroups in the relative contribution of traditional risk factors to incident CVD is not known. To better understand changes over time in the contribution of risk factors to incident CVD in the population at large, as well as in important subgroups, we examined the proportion of CVD risk attributable to traditional risk factors in a large bi-racial sample of men and women living in the community with longitudinal data available and outcomes surveillance spanning over 2 decades.
METHODS
Study Sample
The study design and sampling of the Atherosclerosis Risk in Communities (ARIC) Study has been described (Supplement).14 Of all 15,792 participants who were enrolled in ARIC at the first exam, a total of 13,541 participants (56% women, 26% black) contributed any person observations for the current analyses. Specifically, a total of 8,240 participants had no known CVD (coronary heart disease, heart failure, or prior cerebrovascular event), had no missing data on traditional cardiovascular risk factors (i.e. obesity, hypertension, diabetes, hypercholesterolemia, and smoking status), and were aged 52 to 66 years at exam 1 (1987-1989). The number of participants from the study sample who, due to aging, were similarly 52 to 66 years and free of CVD at the subsequent exams was 8915 (exam 2, 1990-1992), 8467 (exam 3, 1993-1995), and 6884 (exam 4, 1996-1998). Institutional review boards approved the original study protocol at each ARIC site. Participants all provided written informed consent and study procedures were in accordance with institutional guidelines regarding the protection of human subjects.
Clinical Assessment
We used data collected from each exam to define presence of the following traditional risk factors: obesity (body mass index ≥30 kg/m2), hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or taking anti-hypertensive medication), hypercholesterolemia (total cholesterol ≥200 mg/dL or taking cholesterol lowering medication), diabetes (fasting glucose ≥126 mg/dL, non-fasting glucose ≥200 mg/dL, or report of clinical diagnosis or treatment of diabetes), and current smoking status (self-reported active smoking within 1 year prior to the exam). Individuals identified as having hypertension or diabetes at a given exam were considered as having hypertension or diabetes, respectively, at all subsequent exams. Otherwise, individuals with missing covariate data at a subsequent exam were coded as missing that variable for that particular exam. All participants were followed through December 31, 2008 for the incidence of cardiovascular events (coronary heart disease, heart failure, stroke) or CVD death, ascertained according to previously-defined criteria (Supplement).
Statistical Analyses
At each of the exams 1 through 4 (i.e. the ‘baseline’ exams), we estimated the population attributable risk (PAR) % of each primary risk exposure (obesity, hypertension, diabetes, hypercholesterolemia, and smoking status) for the 10-year incidence of CVD using a method that is considered internally valid when adjusted relative risks must be used to account for possible confounding (Supplement).15 We performed all PAR calculations using the risk factor prevalence at each exam and the multivariable-adjusted hazards ratio estimate for 10 years after each exam, derived from Cox proportional hazards models adjusting for all primary risk factors. All Cox models were also stratified by sex, race, study center, and age (i.e. controlled for these 4 variables without requiring the standard proportional hazards assumption). We performed all analyses using STATA version 12.1 (College Station, TX), with a 2-tailed P value <0.05 considered statistically significant.
RESULTS
The sample characteristics are shown in Table 1 and Supplemental Table 1. Of the participants who contributed observations to this analysis, 56% were women and 26% were black. Mean body mass index slightly but significantly increased from 1987-1989 through 1996-1998 (P<0.001), and the proportion of individuals with obesity increased from 26% to 33% over this same time period (P<0.001). The frequencies of hypertension (39% to 45%) and diabetes (12% to 14%) also increased from 1987-1989 to 1996-1998, whereas the frequencies of smoking (24% to 16%) and hypercholesterolemia (68% to 55%) decreased (P<0.001 for all).
Table 1.
Characteristics of the Baseline Sample (Aged 52-66 years) at Each Exam
| Characteristic | Exam 1 1987-1989 | Exam 2 1990-1992 | Exam 3 1993-1995 | Exam 4 1996-1998 |
|---|---|---|---|---|
| No. participants | 8214 | 8956 | 8514 | 6872 |
| Age, years | 58±4 | 59±4 | 59±4 | 60±4 |
| Women, % | 54 | 56 | 58 | 59 |
| Black, % | 23 | 23 | 23 | 23 |
| Body mass index, kg/m2 | 27.4±5.1 | 27.8±5.2 | 28.4±5.4 | 28.9±5.6 |
| Systolic blood pressure, mm Hg | 124±19 | 122±19 | 123±18 | 125±18 |
| Diastolic blood pressure, mmHg | 73±11 | 72±10 | 72±10 | 72±10 |
| Hypertension medication use, % | 29 | 29 | 31 | 35 |
| Cholesterol-lowering medication use, % | 3 | 6 | 8 | 10 |
| Obesity, % | 26 | 27 | 33 | 36 |
| Current smoker, % | 24 | 21 | 18 | 16 |
| Hypertension, % | 39 | 38 | 40 | 45 |
| Hypercholesterolemia, % | 68 | 61 | 59 | 55 |
| Diabetes, % | 12 | 14 | 13 | 14 |
Values are displayed as means±standard deviation or percent frequencies.
The 10-year crude CVD event rate was 1.51 (95% CI 1.43-1.60) per 100 person-years for middle-aged participants (52-66 years old) who attended the first exam in 1987-1989, with similar rates observed for those attending exams in 1990-1992 (1.59 [1.51-1.68]), 1993-1995 (1.52 [1.44-1.61], and 1996-1998 (1.45 [1.36-1.55]). For participants attending the initial 1987-1989 exam, crude 10-year event rates were higher with increasing number of CVD risk factors present: 0.8, 0.9, 1.6, 2.5, 4.4, and 7.5 per 100 person-years for individuals with 0 to 5 risk factors, respectively; this trend was similar across time (Supplemental Table 2).
Temporal Trends in the Total Sample
Overall, the single largest and most consistent contributor to population CVD risk over time was hypertension (Table 2); the population risk attributable to hypertension was not significantly different in 1987-1989 compared with 1996-1998 (PAR 0.25 vs. 0.25, P=0.82). Hypercholesterolemia was a major contributor to CVD risk in 1987-1989, but this contribution appeared to have decreased by 1996-1998 (PAR 0.18 vs. 0.09, P=0.08) in the setting of both lower prevalence and lower associated hazard by exam 4 (Table 2). Although the prevalence of smoking decreased over time, this was accompanied by an associated hazard that remained high; thus, the overall population attributable risk for smoking did not significantly decrease from 1987-1989 to 1996-1998 (PAR 0.15 vs. 0.13, P=0.16). The CVD risks attributable to diabetes (PAR 0.15 vs 0.17, P=0.17) and obesity (PAR 0.06 vs. 0.06, P=0.83) also remained similar from 1987-1989 through 1996-1998. In the total study sample, well over 50% of the CVD events in the population was explained by all the risk factors combined. The PAR for all 5 major risk factors appeared higher in 1987-1989 (PAR 0.58 [95% CI 0.53, 0.62]) than in 1996-1998 (PAR 0.53 [95% CI 0.47, 0.58]) although this difference in PARs was not statistically significant (P=0.13).
Table 2.
Temporal Trends in Population Attributable Risk in the Total Sample*
| Risk Factor | Prevalence, % (95% CI) | Adjusted HR (95% CI) | Adjusted PAR (95% CI) |
|---|---|---|---|
| Hypertension | |||
| Exam 1 | 39 (38, 41) | 1.82 (1.61, 2.07) | 0.25 (0.21, 0.29) |
| Exam 2 | 38 (37, 39) | 1.89 (1.68, 2.12) | 0.26 (0.23, 0.29) |
| Exam 3 | 40 (39, 41) | 1.64 (1.45, 1.86) | 0.22 (0.17, 0.26) |
| Exam 4 | 45 (43, 46) | 1.68 (1.46, 1.94) | 0.25 (0.19, 0.29) |
| P value | – | – | 0.82 |
| Diabetes | |||
| Exam 1 | 12 (11, 12) | 2.45 (2.12, 2.83) | 0.15 (0.13, 0.16) |
| Exam 2 | 14 (13, 15) | 2.15 (1.89, 2.45) | 0.15 (0.13, 0.17) |
| Exam 3 | 13 (13, 14) | 2.52 (2.20, 2.89) | 0.17 (0.16, 0.19) |
| Exam 4 | 14 (13, 15) | 2.44 (2.09, 2.85) | 0.17 (0.15, 0.19) |
| P value | – | – | 0.17 |
| Obesity | |||
| Exam 1 | 26 (25, 27) | 1.24 (1.08, 1.41) | 0.06 (0.03, 0.10) |
| Exam 2 | 27 (27, 28) | 1.17 (1.04, 1.33) | 0.05 (0.01, 0.09) |
| Exam 3 | 33 (32, 34) | 1.20 (1.06, 1.37) | 0.06 (0.02, 0.11) |
| Exam 4 | 36 (35, 37) | 1.15 (1.00, 1.33) | 0.06 (0.00, 0.11) |
| P value | – | – | 0.83 |
| Hypercholesterolemia | |||
| Exam 1 | 68 (67, 69) | 1.34 (1.18, 1.53) | 0.18 (0.11, 0.25) |
| Exam 2 | 61 (60, 62) | 1.26 (1.12, 1.42) | 0.13 (0.07, 0.19) |
| Exam 3 | 59 (58, 60) | 1.34 (1.18, 1.51) | 0.16 (0.10, 0.22) |
| Exam 4 | 55 (54, 57) | 1.20 (1.04, 1.37) | 0.09 (0.03, 0.16) |
| P value | – | – | 0.08 |
| Smoking | |||
| Exam 1 | 24 (23, 25) | 1.84 (1.62, 2.10) | 0.15 (0.12, 0.17) |
| Exam 2 | 21 (21, 22) | 1.83 (1.61, 2.07) | 0.13 (0.11, 0.15) |
| Exam 3 | 18 (17, 19) | 2.23 (1.95, 2.54) | 0.16 (0.14, 0.17) |
| Exam 4 | 16 (15, 17) | 2.07 (1.77, 2.43) | 0.13 (0.11, 0.14) |
| P value | – | – | 0.16 |
All results shown are from stratified Cox models, with age, sex, race, and study center as stratification factors. All analyses are adjusted for age, hypertension, diabetes, obesity, hypercholesterolemia, and smoking.
Numbers of CVD cases per total individuals at risk (i.e. sample sizes) were as follows: 1136 of 8214 at Exam 1, 1293 of 8956 at Exam 2, 1178 of 8514 at Exam3, and 908 of 6872 at Exam 4. P values are for comparisons of estimates between Exam 1 and Exam 4.
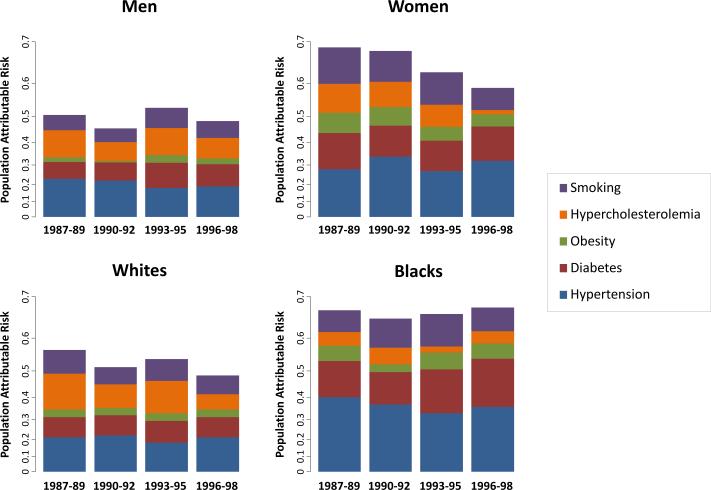
Temporal Trends by Sex
Sex-based variation in estimated attributable risks for CVD existed initially in 1987-1989 as well as subsequent time points (Table 3 and Figure 1). In analyses stratified by sex (Supplemental Figure 1), the attributable contribution of all 5 risk factors combined was significantly higher in women than in men in 1987-1989 (PAR 0.68 [0.62, 0.74] vs. 0.51 [0.44, 0.57], P<0.001) but not by 1996-1998 (PAR 0.58 [0.49, 0.65] vs. 0.48 [0.41, 0.55], P=0.08). The extent to which hypertension was a greater CVD risk contributor in women compared with men was more evident later in 1996-1998 (PAR 0.32 vs 0.19, P=0.02) than earlier in 1987-1989 (PAR 0.28 vs 0.23, P=0.23) (Supplemental Figure 2). Diabetes was a greater contributor to CVD risk in women compared with men, and this trend remained constant over time: PAR 0.22 vs. 0.11 (P<0.001) in 1987-1989, and PAR 0.21 vs. 0.14 (P<0.001) in 1996-1998. Smoking was a greater contributor to CVD risk in women compared to men early on in 1987-1989 (PAR 0.22 vs 0.10, P<0.001) and this gender gap narrowed by 1996-1998 (PAR 0.14 vs. 0.11, P=0.07). Similarly, obesity was a more important contributor to CVD risk in women than men earlier on in 1987-1989 (PAR 0.13 vs. 0.03, P=0.016) but not later on by 1996-1998 (PAR 0.08 vs. 0.04, P=0.54). Although more prevalent in women than men, the contribution of hypercholesterolemia to CVD risk was similar between the women and men at all exams. Except for obesity, the reason women often had higher PARs than men was because the HRs for CVD tended to be greater for risk factors, rather than the prevalence of risk factors (Supplemental Figure 2).
Table 3.
Temporal Trends in Population Attributable Risk by Sex
| Men* | Women† | |||||
|---|---|---|---|---|---|---|
| Risk Factor | Prevalence, % (95% CI) | Adjusted HR (95% CI) | Adjusted PAR (95% CI) | Prevalence, % (95% CI) | Adjusted HR (95% CI) | Adjusted PAR (95% CI) |
| Hypertension | ||||||
| Exam 1 | 37 (35, 38) | 1.81 (1.54, 2.12) | 0.23 (0.18, 0.27) | 42 (40, 43) | 1.82 (1.48, 2.24) | 0.28 (0.21, 0.35) |
| Exam 2 | 37 (35, 38) | 1.75 (1.51, 2.03) | 0.22 (0.17, 0.26) | 40 (38, 41) | 2.15 (1.77, 2.61) | 0.34 (0.28, 0.39) |
| Exam 3 | 38 (36, 39) | 1.56 (1.33, 1.83) | 0.18 (0.13, 0.24) | 41 (40, 43) | 1.79 (1.47, 2.18) | 0.27 (0.20, 0.33) |
| Exam 4 | 42 (40, 44) | 1.54 (1.27, 1.86) | 0.19 (0.12, 0.26) | 46 (45, 48) | 1.91 (1.53, 2.38) | 0.32 (0.23, 0.39) |
| P value | – | – | 0.39 | – | – | 0.56 |
| Diabetes | ||||||
| Exam 1 | 11 (10, 12) | 2.12 (1.75, 2.57) | 0.11 (0.09, 0.13) | 12 (11, 13) | 2.98 (2.40, 3.70) | 0.22 (0.20, 0.25) |
| Exam 2 | 14 (13, 15) | 1.97 (1.66, 2.35) | 0.12 (0.10, 0.14) | 14 (13, 15) | 2.39 (1.95, 2.93) | 0.19 (0.16, 0.22) |
| Exam 3 | 14 (13, 15) | 2.43 (2.03, 2.90) | 0.16 (0.14, 0.18) | 13 (12, 14) | 2.65 (2.14, 3.29) | 0.19 (0.16, 0.21) |
| Exam 4 | 15 (14, 17) | 2.13 (1.72, 2.64) | 0.14 (0.11, 0.16) | 13 (12, 15) | 2.88 (2.30, 3.61) | 0.21 (0.19, 0.24) |
| P value | – | – | 0.14 | – | – | 0.64 |
| Obesity | ||||||
| Exam 1 | 22 (21, 23) | 1.14 (0.95, 1.36) | 0.03 (−0.01, 0.07) | 29 (28, 30) | 1.42 (1.15, 1.74) | 0.13 (0.06, 0.19) |
| Exam 2 | 24 (22, 25) | 1.05 (0.89, 1.24) | 0.01 (−0.03, 0.06) | 30 (29, 32) | 1.39 (1.13, 1.69) | 0.12 (0.06, 0.18) |
| Exam 3 | 29 (28, 31) | 1.17 (0.99, 1.39) | 0.05 (0.00, 0.10) | 35 (34, 36) | 1.25 (1.02, 1.53) | 0.09 (0.01, 0.16) |
| Exam 4 | 33 (31, 35) | 1.12 (0.93, 1.37) | 0.04 (−0.03, 0.10) | 38 (37, 40) | 1.19 (0.96, 1.47) | 0.08 (−0.01, 0.16) |
| P value | – | – | 0.79 | – | – | 0.35 |
| Hypercholesterolemia | ||||||
| Exam 1 | 61 (60, 63) | 1.35 (1.15, 1.58) | 0.17 (0.09, 0.25) | 73 (72, 74) | 1.29 (1.03, 1.63) | 0.18 (0.02, 0.31) |
| Exam 2 | 55 (53, 56) | 1.25 (1.08, 1.45) | 0.12 (0.05, 0.19) | 66 (65, 68) | 1.27 (1.05, 1.55) | 0.16 (0.04, 0.26) |
| Exam 3 | 52 (51, 54) | 1.39 (1.19, 1.62) | 0.17 (0.10, 0.23) | 64 (63, 65) | 1.24 (1.02, 1.51) | 0.14 (0.02, 0.24) |
| Exam 4 | 47 (45, 49) | 1.31 (1.09, 1.57) | 0.13 (0.05, 0.19) | 61 (59, 63) | 1.04 (0.85, 1.28) | 0.03 (−0.11, 0.14) |
| P value | – | – | 0.39 | – | – | 0.12 |
| Smoking | ||||||
| Exam 1 | 25 (24, 27) | 1.51 (1.28, 1.80) | 0.10 (0.07, 0.13) | 23 (22, 24) | 2.50 (2.04, 3.06) | 0.22 (0.19, 0.24) |
| Exam 2 | 23 (22, 25) | 1.50 (1.27, 1.77) | 0.09 (0.06, 0.12) | 20 (19, 21) | 2.48 (2.04, 3.02) | 0.19 (0.16, 0.21) |
| Exam 3 | 20 (18, 21) | 1.85 (1.56, 2.21) | 0.13 (0.10, 0.15) | 17 (16, 18) | 2.94 (2.40, 3.59) | 0.20 (0.18, 0.22) |
| Exam 4 | 18 (17, 19) | 1.82 (1.47, 2.26) | 0.11 (0.08, 0.14) | 15 (14, 16) | 2.45 (1.94, 3.11) | 0.14 (0.12, 0.17) |
| P value | – | – | 0.69 | – | – | <0.001 |
All results shown are from stratified Cox models, with age, race, and study center as stratification factors. All analyses are adjusted for age, hypertension, diabetes, obesity, hypercholesterolemia, and smoking.
Numbers of CVD cases per total men at risk (i.e. sample sizes) were as follows: 693 of 3742 at Exam 1, 785 of 3955 at Exam 2, 687 of 3615 at Exam3, and 501 of 2800 at Exam 4.
Numbers of CVD cases per total women at risk (i.e. sample sizes) were as follows: 443 of 4472 at Exam 1, 508 of 5001 at Exam 2, 491 of 4899 at Exam3, and 407 of 4072 at Exam 4.
P values are for comparisons of estimates between Exam 1 and Exam 4.
Figure 1.
Population attributable risks by sex and race. The population attributable risk (PAR) of major risk factors for the 10-year incidence of cardiovascular disease is shown by sex and race for each of the 4 baseline exams. Units of PAR are shown on the logarithmic scale to allow for comparisons within and across exams.
Temporal Trends by Race
There were marked differences in the attributable risks for CVD between blacks and whites (Table 4 and Figure 1). As show in Supplemental Figure 1, the contribution of all risk factors combined was higher in blacks than whites in 1987-1989 (PAR 0.67 [0.57, 0.74] vs. 0.56 [0.50, 0.61], P=0.049) and this difference was more pronounced by 1996-1998 (PAR 0.67 [0.57, 0.75] vs. 0.48 [0.41, 0.54], P=0.002). For the most part, blacks compared with whites had higher prevalences of risk factors but not greatly different associated hazards. The extent to which hypertension was a greater CVD risk contributor in blacks compared with whites was more apparent in 1987-1989 (PAR 0.40 vs. 0.21, P=0.002) than in 1996-1998 (PAR 0.36 vs. 0.21, P=0.08) (Supplemental Figure 3). However, diabetes remained a significantly greater contributor to CVD risk in blacks than whites over time, with PARs of 0.22 vs. 0.13 (P<0.001) in 1987-1989 and PARs of 0.28 vs. 0.13 (P<0.001) in 1996-1998. Even further, the contribution of diabetes to CVD risk increased in blacks (PAR 0.22 vs. 0.28, P=0.03) but stayed constant in whites (PAR 0.13 vs. 0.13, P=0.80) over the time period from 1987-1989 to 1996-1998; this increase in diabetes-related CVD risk was driven by both increasing prevalence as well as increasing associated hazards (Table 4 and Supplemental Figure 3). Hypercholesterolemia conferred similar risks in whites and blacks, although this contribution decreased over time in whites (PAR 0.22 vs. 0.10, P=0.04). Although more prevalent in blacks than whites, contributions of obesity and smoking to CVD risk were not significantly different between races at all time points.
Table 4.
Temporal Trends in Population Attributable Risk by Race
| Blacks* | Whites† | |||||
|---|---|---|---|---|---|---|
| Risk Factor | Prevalence, % (95% CI) | Adjusted HR (95% CI) | Adjusted PAR (95% CI) | Prevalence, % (95% CI) | Adjusted HR (95% CI) | Adjusted PAR (95% CI) |
| Hypertension | ||||||
| Exam 1 | 61 (59, 63) | 2.06 (1.57, 2.70) | 0.40 (0.29, 0.49) | 33 (32, 34) | 1.77 (1.53, 2.04) | 0.21 (0.17, 0.25) |
| Exam 2 | 58 (55, 60) | 2.01 (1.57, 2.58) | 0.37 (0.27, 0.45) | 33 (32, 34) | 1.84 (1.61, 2.10) | 0.22 (0.19, 0.26) |
| Exam 3 | 59 (57, 61) | 1.79 (1.36, 2.37) | 0.33 (0.20, 0.43) | 34 (33, 35) | 1.61 (1.40, 1.84) | 0.18 (0.14, 0.22) |
| Exam 4 | 65 (63, 67) | 1.82 (1.31, 2.51) | 0.36 (0.20, 0.48) | 38 (37, 40) | 1.66 (1.41, 1.95) | 0.21 (0.16, 0.26) |
| P value | – | – | 0.65 | – | – | 0.90 |
| Diabetes | ||||||
| Exam 1 | 21 (19, 23) | 2.39 (1.87, 3.04) | 0.22 (0.18, 0.26) | 9 (8, 10) | 2.48 (2.07, 2.96) | 0.13 (0.11, 0.14) |
| Exam 2 | 25 (23, 27) | 2.09 (1.66, 2.63) | 0.20 (0.16, 0.25) | 11 (10, 12) | 2.18 (1.86, 2.56) | 0.13 (0.11, 0.14) |
| Exam 3 | 22 (20, 24) | 2.69 (2.09, 3.45) | 0.26 (0.22, 0.29) | 11 (10, 12) | 2.47 (2.09, 2.91) | 0.14 (0.13, 0.16) |
| Exam 4 | 24 (22, 26) | 2.74 (2.09, 3.59) | 0.28 (0.24, 0.33) | 11 (10, 12) | 2.30 (1.90, 2.79) | 0.13 (0.11, 0.15) |
| P value | – | – | 0.03 | – | – | 0.80 |
| Obesity | ||||||
| Exam 1 | 39 (37, 41) | 1.28 (1.01, 1.64) | 0.10 (0.01, 0.19) | 22 (21, 23) | 1.21 (1.03, 1.43) | 0.05 (0.01, 0.08) |
| Exam 2 | 43 (41, 45) | 1.12 (0.88, 1.42) | 0.05 (−0.05, 0.14) | 23 (22, 24) | 1.20 (1.04, 1.40) | 0.05 (0.01, 0.09) |
| Exam 3 | 46 (44, 48) | 1.28 (0.99, 1.65) | 0.11 (0.01, 0.21) | 29 (28, 30) | 1.18 (1.01, 1.37) | 0.05 (0.01, 0.09) |
| Exam 4 | 49 (47, 52) | 1.22 (0.93, 1.61) | 0.10 (−0.03, 0.21) | 32 (31, 33) | 1.13 (0.96, 1.34) | 0.05 (−0.01, 0.10) |
| P value | – | – | 0.95 | – | – | 0.94 |
| Hypercholesterolemia | ||||||
| Exam 1 | 65 (63, 67) | 1.15 (0.90, 1.47) | 0.09 (−0.06, 0.22) | 69 (68, 70) | 1.42 (1.22, 1.67) | 0.22 (0.13, 0.30) |
| Exam 2 | 60 (57, 62) | 1.20 (0.96, 1.51) | 0.11 (−0.02, 0.21) | 61 (60, 63) | 1.29 (1.12, 1.48) | 0.15 (0.07, 0.21) |
| Exam 3 | 55 (53, 58) | 1.08 (0.85, 1.37) | 0.04 (−0.10, 0.16) | 60 (59, 61) | 1.44 (1.25, 1.67) | 0.20 (0.13, 0.27) |
| Exam 4 | 50 (47, 52) | 1.18 (0.91, 1.54) | 0.08 (−0.04, 0.20) | 57 (56, 58) | 1.20 (1.02, 1.41) | 0.10 (0.02, 0.17) |
| P value | – | – | 0.95 | – | – | 0.04 |
| Smoking | ||||||
| Exam 1 | 28 (26, 30) | 1.63 (1.28, 2.09) | 0.14 (0.08, 0.19) | 23 (22, 24) | 1.92 (1.65, 2.24) | 0.15 (0.12, 0.17) |
| Exam 2 | 25 (23, 27) | 2.03 (1.59, 2.58) | 0.18 (0.14, 0.23) | 20 (19, 21) | 1.75 (1.51, 2.03) | 0.11 (0.09, 0.14) |
| Exam 3 | 22 (20, 24) | 2.45 (1.89, 3.16) | 0.20 (0.17, 0.24) | 17 (16, 18) | 2.16 (1.85, 2.52) | 0.14 (0.12, 0.16) |
| Exam 4 | 19 (17, 21) | 2.24 (1.65, 3.04) | 0.15 (0.11, 0.19) | 15 (14, 16) | 2.02 (1.68, 2.43) | 0.12 (0.10, 0.14) |
| P value | – | – | 0.62 | – | – | 0.06 |
All results shown are from stratified Cox models, with age, sex, and study center as stratification factors. All analyses are adjusted for age, hypertension, diabetes, obesity, hypercholesterolemia, and smoking.
Numbers of CVD cases per total blacks at risk (i.e. sample sizes) were as follows: 320 of 1916 at Exam 1, 346 of 2016 at Exam 2, 298 of 1939 at Exam3, and 244 of 1601 at Exam 4.
Numbers of CVD cases per total whites at risk (i.e. sample sizes) were as follows: 816 of 6298 at Exam 1, 947 of 6940 at Exam 2, 880 of 6575 at Exam3, and 664 of 5271 at Exam 4.
P values are for comparisons of estimates between Exam 1 and Exam 4.
In secondary analyses conducted using a hard CHD endpoint (defined as including only hospitalized myocardial infarction or CHD death) as part of the composite CVD outcome, we observed results that were similar to those of the main analyses. These results were similar for the total sample as well as by sex and race (data not shown). In analyses repeated separately for each study center, we observed geographic variation that was not completely reflected by between-center differences in racial distributions (Supplemental Table 3).
DISCUSSION
We investigated temporal trends in contributions of risk factors to CVD in a large multi-center and bi-racial community-based cohort, with clinical assessments conducted over 12 years and outcomes surveillance spanning over 2 decades. We observed a trend of decreasing overall population risk attributable to traditional risk factors over time, largely due to the decreasing prevalence of hypercholesterolemia and smoking. However, the combined contribution of all traditional risk factors has remained substantially higher in women compared with men, and in blacks compared with whites. These sex- and race-based differences remain especially pronounced for hypertension and diabetes. Together, these data highlight the continued need for targeted risk factor modification despite recent improvements in the public health efforts to reduce the overall burden of CVD risk.
In our total study sample, the CVD risk attributable to all the 5 major traditional risk factors that we analyzed steadily decreased over the time period extending from the late 1980s through the last decade. These overall trends may be related to concomitant changes in the definitions of select CVD risk factors, and their effects on practice in the community,16 along with increasing rates of treatment and control for select risk factors.17 Specifically, we observed marked declines in the prevalence of hypercholesterolemia and particularly smoking, which have been reported previously.17 Interestingly, the hazard associated with hypercholesterolemia also decreased, whereas the hazard associated with smoking actually increased over time. The trends observed for hypercholesterolemia may well have been related to substantially increased use of cholesterol-lowering medication over time in the population at large; further research is warranted to better understand time trends of the population risk attributable to statin use as well as other medications used to modify cardiovascular risk. These observations may also be related to birth cohort effects or differing dose-response relationships (not captured by the binary variables we studied) in the magnitude of CVD risk associated with having persistently high cholesterol or being an active smoker. Accordingly, with respect to hypertension, secular trends and improvements in blood pressure control are a likely explanation for the finding that the hazard associated with hypertension decreased even though hypertension prevalence increased over time.8,18 Although it is well known that the prevalence of obesity is increasing nationwide,19,20 we observed that the CVD risk attributable to obesity has remained relatively low as well as constant over time. It is possible that obesity may have longer term effects than could be seen within 10 years of follow up,21 and that such longer term effects may well manifest as risk attributable to diabetes. Indeed, consistent with national and worldwide trends,22 the overall prevalence of diabetes increased over the study period; this increase was associated with hazards for CVD that remained high overall, with some notable variations by subgroup.
We observed substantial differences between women and men in population attributable risks for CVD over time. At exam 1, the proportion of CVD risk attributable to all risk factors was significantly higher in women than men. Prior studies that have noted similar sexual dimorphism in attributable CVD risks associated with major risk factors such as hypertension and diabetes.23-25 These sex-based differences may be related to the possibility that nontraditional risk factors contribute more to CVD in risk in men than women, or that women were historically less likely to have prevalent risk factors controlled.26,27 We observed that the overall sex difference in PARs had substantially narrowed by 1996-1998, potentially related to the success of public health effects aimed at increasing the awareness of CVD risk in women.28 Indeed, prevalence of hypertension was higher in women than men in 1987-1989, and this difference decreased over time. However, women still tended to have more prevalent hypertension as well as a higher associated CVD risk, even by 1996-1998; this finding could be related to the greater proportion of isolated systolic hypertension and arterial stiffness in women than men.29-31 Despite similar trends to men in the decreasing prevalence of smoking and increasing prevalence of obesity, women tended to display consistently higher hazards of CVD risk associated with both of these risk factors over time. Most importantly, the CVD risk attributable to diabetes remained significantly higher in women than men over time, driven by higher associated hazards despite lower prevalence rates. Prior studies have reported similar sex-based differences in the risks associated with smoking,32 obesity,33 and diabetes34-36 although without specifically temporal trends in both prevalence and associated hazards in the context of other risk factors. The reasons for persistent sex differences in attributable CVD risks over time are not yet clear,37 and could be related to greater clustering of risk factors in women than men,38,39 continued under-recognition or under-treatment of prevalent risk factors in women,28 or hormonal or non-hormonal biological variation that has yet to identified.40 Additionally, men could have more inherent unexplained risk, which could be accounted for by non-traditional risk factors.
We also observed marked differences between blacks and whites in the temporal trends of population attributable risks for CVD. The risk attributable to all 5 traditional risk factors combined was higher in blacks than whites in 1987-1989 and this difference was even larger by 1996-1998, indicating that efforts to improve CVD risk factor modification are not benefitting blacks as much as whites. The two primary contributors to race-based differences in CVD risk over time were hypertension and diabetes. The prevalence of hypertension increased in both blacks and whites, but both prevalence rates and associated hazards were consistently higher in blacks than whites at all exams. Race differences for diabetes were even more profound. In whites, the prevalence of diabetes remained constant while the associated hazard tended to decrease over time; in blacks, the prevalence of diabetes was consistently over double that in whites and the diabetes-associated hazards actually increased over time in blacks. Consequently, the attributable CVD risk of diabetes was significantly higher in 1996-1998 than 1987-1989 in blacks. Although numerous prior studies have reported differences by race in the prevalence of CVD risk factors including hypertension and diabetes,41 fewer have investigated race differences in associated hazards,42-44 and none have previously examined race variation in both prevalence and hazards over time. Prior work in the ARIC cohort has noted that the proportion of CVD risk explained by traditional risk factors is much larger in blacks compared to whites, particularly when considering borderline risk factors;45 therefore, race-based differences over time may be even more pronounced when accounting for borderline clinical risk factors. Because the vast majority of blacks in this study were enrolled at the Jackson study center, it is important to note that our race-based analyses may have been particularly influenced by geography even in analyses stratified by study center. Notwithstanding the multiple additional socio-economic, clinical, and biological factors that may be implicated,44 our results highlight the magnitude and trajectory of differences between blacks and whites in the contribution of risk factors to CVD.
Several limitations of the study merit consideration. Our composite CVD outcome included coronary revascularization events; although these procedures may have been performed less frequently in certain subgroups, the main study findings were similar for subgroups when analyses were repeated using the CVD outcome that did not include coronary revascularization. It is known that precision in the estimate of the PAR is suboptimal for highly prevalent exposures;15 thus, PAR estimates for very prevalent exposures within a given sample or subgroup should be interpreted with caution. Because PAR estimates can be calculated only for binary variables, we were not able to analyze continuous variables that may contribute residual CVD risk but are without widely established definitions of normal versus abnormal; thus, we focused on presence versus absence of traditional risk factors that are easily assessed and are also generally considered to be modifiable. Because we used conventional definitions for binary risk factor variables, PARs reported herein are lower than estimates that could be calculated using risk factor definitions based on “ideal” thresholds.12 It is important to note that the PAR estimate provides a comparison between the observed data and a hypothetical scenario where a given risk factor has been eliminated; despite the impracticality of such an assumption, PAR estimates can provide a common scale for comparing the potential population-level impact of targeting interventions for preventing or treating a given risk factor. Although the time period studied was relatively short, it included an interval that captured major changes in risk factors. Indeed, the observed time trends are likely related to a combination of effects from aging, birth cohort differences, and secular changes that cannot be completely measured and accounted for in our models. In addition, sampling at the more recent exams may yet have been influenced by survivor bias. To facilitate comparisons across time intervals, we restricted analyses to a sample that was middle-aged as well as predominantly either white or black with respect to racial/ethnic group. Generalizability of our findings to other age and racial/ethnic groups or to populations beyond the 4 ARIC communities is unknown.
We examined the temporal trends in the attributable contributions of traditional risk factors to incident CVD in a large community-based cohort comprising middle-aged men and women, representing both blacks and whites. The overall PAR for all risk factors remains well over 50%, indicating that preventing or removing these risk factors might eliminate a large proportion of CVD in the community. In fact, if the whole population could maintain low risk status through primordial prevention, most CVD events might be prevented.12,20,46,47 Indeed, we observed that the overall 10-year rate of CVD in a middle-aged sample is ~1.5 per 100 person-years and among those with no major risk factors, the CVD rate is only ~0.7. In addition to persistently high PAR for the total sample, we observed that the contribution to incident CVD of all traditional risk factors combined is greater in blacks than whites, and this difference may be increasing with time. The risks attributable to hypertension and diabetes remain especially high, in women as well as blacks. Together, these findings underscore the continued need for individual as well as population approaches to CVD risk factor modification.
Supplementary Material
Acknowledgements
The authors thank the participants and staff of the ARIC Study for their important contributions to this research.
Funding Sources: The ARIC Study is a collaborative study supported by the National Heart, Lung, and Blood Institute contracts: HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. Dr. Cheng is supported in part by R00-HL-107642 and a grant from the Ellison Foundation. Dr. Shah is supported in part by K08-HL-116792.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the united states: A policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 2.The state of us health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom AR. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 us communities, 1987-2008. Circulation. 2012;125:1848–1857. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Normand SL, Wang Y, Drye EE, Schreiner GC, Krumholz HM. Recent declines in hospitalizations for acute myocardial infarction for medicare fee-for-service beneficiaries: Progress and continuing challenges. Circulation. 2010;121:1322–1328. doi: 10.1161/CIRCULATIONAHA.109.862094. [DOI] [PubMed] [Google Scholar]
- 5.Ovbiagele B. National sex-specific trends in hospital-based stroke rates. J Stroke Cerebrovasc Dis. 2011;20:537–540. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for medicare beneficiaries, 1998-2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 8.Egan BM, Zhao Y, Axon RN. Us trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 9.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in united states adults between 1988-1994 and 1999-2004. Hypertension. 2008;52:818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 10.Mann D, Reynolds K, Smith D, Muntner P. Trends in statin use and low-density lipoprotein cholesterol levels among us adults: Impact of the 2001 national cholesterol education program guidelines. Ann Pharmacother. 2008;42:1208–1215. doi: 10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg BA, Bhatt DL, Mehta S, Poole-Wilson PA, O'Hagan P, Montalescot G, Ballantyne CM, Cannon CP. Nine-year trends in achievement of risk factor goals in the us and european outpatients with cardiovascular disease. Am Heart J. 2008;156:719–727. doi: 10.1016/j.ahj.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: The american heart association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 13.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, Narayan KM, Williamson DF. Secular trends in cardiovascular disease risk factors according to body mass index in us adults. JAMA. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 14.The atherosclerosis risk in communities (ARIC) study: Design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz LM, Woloshin S. Changing disease definitions: Implications for disease prevalence. Analysis of the third national health and nutrition examination survey, 1988-1994. Eff Clin Pract. 1999;2:76–85. [PubMed] [Google Scholar]
- 17.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in u.S. Deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 18.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among united states adults 1999-2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 19.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among us adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all-cause and cvd mortality among us adults. JAMA. 2012;307:1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daviglus ML, Liu K, Yan LL, Pirzada A, Manheim L, Manning W, Garside DB, Wang R, Dyer AR, Greenland P, Stamler J. Relation of body mass index in young adulthood and middle age to medicare expenditures in older age. JAMA. 2004;292:2743–2749. doi: 10.1001/jama.292.22.2743. [DOI] [PubMed] [Google Scholar]
- 22.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 23.Wong ND, Thakral G, Franklin SS, L'Italien GJ, Jacobs MJ, Whyte JL, Lapuerta P. Preventing heart disease by controlling hypertension: Impact of hypertensive subtype, stage, age, and sex. Am Heart J. 2003;145:888–895. doi: 10.1016/S0002-8703(02)94787-3. [DOI] [PubMed] [Google Scholar]
- 24.Stegmayr B, Asplund K. Diabetes as a risk factor for stroke. A population perspective. Diabetologia. 1995;38:1061–1068. doi: 10.1007/BF00402176. [DOI] [PubMed] [Google Scholar]
- 25.Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S. Risk factors for myocardial infarction in women and men: Insights from the interheart study. Eur Heart J. 2008;29:932–940. doi: 10.1093/eurheartj/ehn018. [DOI] [PubMed] [Google Scholar]
- 26.Journath G, Hellenius ML, Petersson U, Theobald H, Nilsson PM. Sex differences in risk factor control of treated hypertensives: A national primary healthcare-based study in sweden. Eur J Cardiovasc Prev Rehabil. 2008;15:258–262. doi: 10.1097/HJR.0b013e3282f37a45. [DOI] [PubMed] [Google Scholar]
- 27.Gouni-Berthold I, Berthold HK, Mantzoros CS, Bohm M, Krone W. Sex disparities in the treatment and control of cardiovascular risk factors in type 2 diabetes. Diabetes Care. 2008;31:1389–1391. doi: 10.2337/dc08-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosca L, Ferris A, Fabunmi R, Robertson RM. Tracking women's awareness of heart disease: An American Heart Association national study. Circulation. 2004;109:573–579. doi: 10.1161/01.CIR.0000115222.69428.C9. [DOI] [PubMed] [Google Scholar]
- 29.Crescioni M, Gorina Y, Bilheimer L, Gillum RF. Trends in health status and health care use among older men. Natl Health Stat Report. 2010:1–18. [PubMed] [Google Scholar]
- 30.Cheng S, Xanthakis V, Sullivan LM, Vasan RS. Blood pressure tracking over the adult life course: Patterns and correlates in the framingham heart study. Hypertension. 2012;60:1393–1399. doi: 10.1161/HYPERTENSIONAHA.112.201780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng S, Gupta DK, Claggett B, Sharrett AR, Shah AM, Skali H, Takeuchi M, Ni H, Solomon SD. Differential influence of distinct components of increased blood pressure on cardiovascular outcomes: From the atherosclerosis risk in communities study. Hypertension. 2013;62:492–498. doi: 10.1161/HYPERTENSIONAHA.113.01561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: A systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378:1297–1305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- 33.Masters RK, Reither EN, Powers DA, Yang YC, Burger AE, Link BG. The impact of obesity on us mortality levels: The importance of age and cohort factors in population estimates. Am J Public Health. 2013;103:1895–1901. doi: 10.2105/AJPH.2013.301379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med. 2007;147:149–155. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 35.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: Meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norhammar A, Schenck-Gustafsson K. Type 2 diabetes and cardiovascular disease in women. Diabetologia. 2013;56:1–9. doi: 10.1007/s00125-012-2694-y. [DOI] [PubMed] [Google Scholar]
- 37.Jonsdottir LS, Sigfusson N, Gudnason V, Sigvaldason H, Thorgeirsson G. Do lipids, blood pressure, diabetes, and smoking confer equal risk of myocardial infarction in women as in men? The reykjavik study. J Cardiovasc Risk. 2002;9:67–76. [PubMed] [Google Scholar]
- 38.Mansur AP, Gomes EP, Avakian SD, Favarato D, Cesar LA, Aldrighi JM, Ramires JA. Clustering of traditional risk factors and precocity of coronary disease in women. Int J Cardiol. 2001;81:205–209. doi: 10.1016/s0167-5273(01)00568-x. [DOI] [PubMed] [Google Scholar]
- 39.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among us adults: Findings from the third national health and nutrition examination survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 40.Stock EO, Redberg R. Cardiovascular disease in women. Curr Probl Cardiol. 2012;37:450–526. doi: 10.1016/j.cpcardiol.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: A report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carnethon MR, Lynch EB, Dyer AR, Lloyd-Jones DM, Wang R, Garside DB, Greenland P. Comparison of risk factors for cardiovascular mortality in black and white adults. Arch Intern Med. 2006;166:1196–1202. doi: 10.1001/archinte.166.11.1196. [DOI] [PubMed] [Google Scholar]
- 43.Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, Safford MM, Cushman M, Glasser SP, Howard VJ. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med. 2013;173:46–51. doi: 10.1001/2013.jamainternmed.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas AJ, Eberly LE, Davey Smith G, Neaton JD, Stamler J. Race/ethnicity, income, major risk factors, and cardiovascular disease mortality. Am J Public Health. 2005;95:1417–1423. doi: 10.2105/AJPH.2004.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: Comparison of african american with white subjects--atherosclerosis risk in communities study. Arch Intern Med. 2007;167:573–579. doi: 10.1001/archinte.167.6.573. [DOI] [PubMed] [Google Scholar]
- 46.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. Us population data. Arch Intern Med. 1993;153:598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.