Abstract
Background. Group A streptococcus (GAS) commonly colonizes the oropharynx and nonintact skin. However, colonization has been little studied and the role of biofilm formation is unclear, as biofilm experiments to date have not been conducted under conditions that mimic the host environment.
Methods. In this study we grew GAS biofilms on human keratinocytes under various environmental conditions and used this model to evaluate colonization, invasive disease and natural transformation.
Results. GAS grown on epithelial cells, but not biofilms grown on abiotic surfaces, produced biofilms with characteristics similar to in vivo colonization. These biofilm bacteria showed a 100-fold higher bacterial burden of nasal-associated lymphoid tissue in mice than broth-grown bacteria, and were not virulent during septic infection, which was attributed in part to down-regulation of genes typically involved in localized and invasive disease. We also showed for the first time that GAS were naturally transformable when grown in biofilms and during colonization of NALT in vivo.
Conclusions. These findings provide novel model systems to study biofilm formation of GAS in vitro and in vivo, suggest an important role for biofilm formation during GAS colonization, and provide an explanation for the known genome diversity within the GAS population.
Keywords: carriage, host-pathogen interaction, quorum sensing, Rgg regulator, comptetence, transformation
Group A streptococcus (GAS) is commonly associated with acute pharyngitis and impetigo, as well as a wide variety of severe invasive diseases [1]. Infection begins with colonization of the upper respiratory tract or injured skin surfaces, a critical step in pathogenesis [2]. Although not considered normal flora, asymptomatic GAS colonization varies between 5% and 20% in healthy individuals [3–6] and plays an important role in the spread of this bacterial species. The epidemiology of GAS infections has greatly evolved in the past decade, with the emergence of new emm types [7] and increasing prevalence of antibiotic resistance [8, 9]. Understanding the mechanisms governing long-term asymptomatic carriage is critical as asymptomatic oropharyngeal carriage of GAS continues to confound the results of clinical testing for patients with pharyngitis [10].
GAS exist in large microcolony aggregates in the oropharynx that are closely attached to the epithelial surface [6, 11–14] and have been suggested to organize as biofilms and hide inside nonphagocytic cells as they remain viable even after the administration of appropriate antimicrobial therapy [15–17]. Modeling asymptomatic mucosal carriage of GAS in vitro has been challenging. In vitro biofilm models on abiotic surfaces [18–22] have allowed for important investigations into biofilm formation and composition by GAS, but the overall virulence gene expression profile of these biofilms differs markedly from the virulence gene expression of in vivo tissue communities [21], probably owing to the absence of host factors. Unfortunately, because of the inherent toxicity of broth-grown GAS strains toward epithelial cells [23], no models for long-term epithelial-pathogen coculture have thus far been possible.
We have recently shown that Streptococcus pneumoniae (pneumococcus) colonize the nasopharynx as biofilms, and we have recapitulated this biofilm phenotype in vitro using conditions that mimic the nasopharyngeal environment [24]. Furthermore, we have shown that pneumococcal biofilms, both in vitro and in vivo, provide a preeminent environment for genetic exchange with an observed frequency of natural transformation several orders of magnitude higher than under broth-grown conditions [25]. Recent studies with GAS have identified a quorum-sensing pathway using a peptide pheromone to induce the Com system, leading to activation of competence genes [26, 27]. Although the Com system could be induced, DNA uptake could not be substantiated and transformation was not detected [27].
In the current study, we demonstrated that GAS biofilms formed on human keratinocytes in vitro colonize the oropharynx in vivo significantly better than broth-grown GAS, yet are less likely to cause invasive disease because of their altered expression of virulence determinants. Importantly, we show for the first time that GAS can be naturally transformed in the presence of exogenous DNA when grown as biofilms both in vitro and in vivo.
MATERIALS AND METHODS
Bacterial Strains and Epithelial Cells
Bacterial strains and bacterial and epithelial cells growth conditions are described in detail in the Supplementary Information. As described elsewhere, squamous cell carcinoma (SCC) 13 keratinocytes were grown in keratinocyte-serum free medium [23], and cell viability was determined by trypan blue exclusion [28].
Static Biofilm Assays
Detailed procedures can be found in the Supplementary Information. GAS grown in various media were washed, resuspended in medium, seeded onto confluent paraformaldehyde prefixed SCC13 cells [24], and cultured at 34°C for the indicated times. For maintenance of biofilms on live epithelia, mature biofilms gently pipetted off prefixed epithelial cells were inoculated onto live SCC13 cells and maintained at 34°C. Biofilm biomass and antibiotic resistance were determined as described elsewhere [24]. Intracellular biofilm bacteria were determined from culturing lysates of infected cells after gentamicin or penicillin treatment.
Transformation Assays
Streptococcus pyogenes was transformed either with or without exogenous addition of 10 µmol/L XIP pheromone peptide (EFDWWNLG) [27]. For biofilm transformation, 10 µg/mL of chromosomal DNA from strains MGAS315 ropB::erm, 188 (kan), or MGAS315 murM2::spc was added to together with GAS during the initial seeding of the biofilm. Medium was replaced every 12 hours. After 48 hours, biofilms were mechanically dispersed and plated onto blood agar plates containing the appropriate antibiotic. In parallel, the starting culture with added DNA was grown to mid–log phase and plated on antibiotic selection media to control for potential transformation during planktonic growth. Antibiotic-resistant transformants were isolated and verified by polymerase chain reaction (PCR) for insertion of the donor antibiotic cassette in the correct locus (see primers in Supplementary Figure 1). Transformation efficiency was calculated as the fraction of transformants obtained relative to the total number of cells recovered.
Mouse Colonization Model
Detailed procedures can be found in the Supplementary Information. In short, BALB/cByJ mice were either injected intraperitoneally with 1 × 105 CFU or colonized intranasally with 5 × 106 CFU of either broth-grown or biofilm GAS. For sepsis studies, mice were killed at 24 hours after inoculation or when moribund, as described elsewhere [29]. For colonization studies, nasopharyngeal lavage and nasopharyngeal-associated lymphoid tissue (NALT) was collected from euthanized animals, as described elsewhere [30] and the bacterial burden was determined from homogenized tissue by plate counts.
For in vivo transformation, mice colonized with MGAS315 biofilm bacteria were immediately given 20 µL of 10 µg/mL of chromosomal DNA from strain MGAS315::murM2, containing a spectinomycin resistance cassette, intranasally, with or without 10 µmol/L XIP. The DNA treatment was repeated 24 hours after inoculation. At 48 hours, mice were killed and the number of spectinomycin-resistant colonies was determined from homogenized NALT and lavage. Transformants were confirmed by PCR analysis.
Confocal Microscopy
GAS biofilms grown on live SCC13 cells for 48 hours were fixed in 4% paraformaldehyde. To identify internalized bacteria, cells were treated with 3% bovine serum albumin–0.1% Triton X-100 in phosphate-buffered saline, and bacteria were labeled with rabbit anti-GAS antibody (1:200 dilution), and cells were labeled with mouse anti–cytokeratin 14 antibody (1:200 dilution). DAPI (5 µg/mL) staining was also added to visualize cell nuclei. Extracellular GAS biofilms were unable to withstand the washes necessary for successful antibody staining and so were visualized with BacLight Red bacterial stain, (Invitrogen), and epithelial cells were stained with 488-conjugated concanavalin A (Invitrogen) and 4′,6-diamidino-2-phenylindole (DAPI).
RNA Isolation and Quantitative Reverse-Transcription PCR
RNA was isolated from broth-grown and biofilm bacteria and used to determine the relative gene expression by using the 2–ΔΔCT method [31]. See Supplementary Information for details.
Statistical Analysis
The data were analyzed for statistical significance with a 2-tailed Student t test, using Prism 5 software (GraphPad).
RESULTS
Preferential GAS Biofilm Formation on Undifferentiated Keratinocytes
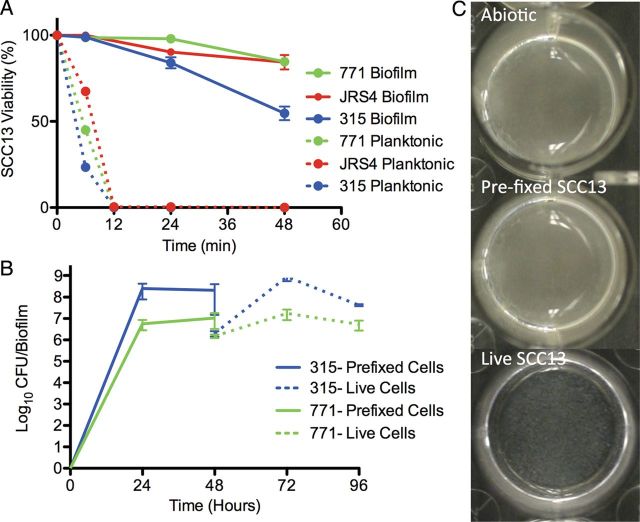
GAS biofilm formation has been exclusively tested using abiotic surfaces in vitro, which they do not naturally colonize. To better understand the characteristics of biofilms formed under more physiological conditions, GAS were first seeded on prefixed epithelial cells under various environmental conditions (Figure 1), because broth-grown GAS are highly toxic to epithelial cells (Figure 2A). Visually, biofilms formed on prefixed epithelial cells appeared similar to those grown on abiotic surfaces (Figure 2C). However, biofilms cultured under conditions that more closely mimicked the in vivo environment (on prefixed epithelial cells at 34°C) were more tolerant to antimicrobial therapy than those grown on abiotic surfaces and/or at higher temperature (37°C; Figure 1A and 1B). This was true even though biofilms grown in the presence of prefixed epithelial cells showed an approximately 10-fold lower biomass (Figure 1C), and it was observed for all GAS strains tested (Figure 1D).
Figure 1.
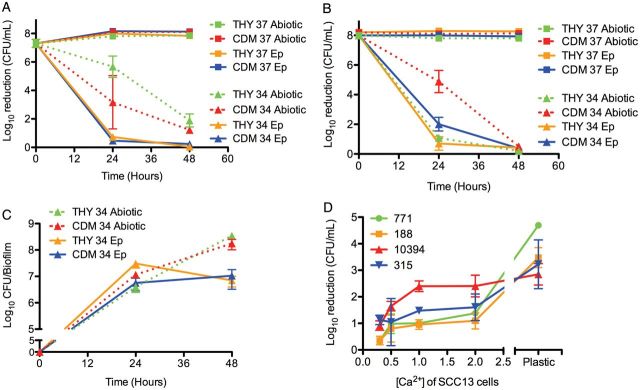
Characteristics of group A streptococcus (GAS) biofilms grown on epithelial cells. A, B, Biofilm-associated antimicrobial death of encapsulated GAS 771 (A) and its unencapsulated derivative 188 (B). Bacterial sensitivity to antibiotics was recorded as bacterial death (in log10 colony-forming units [CFU] per milliliter) after exposure to 500 µg/mL of gentamicin for 3 hours for broth-grown bacteria (time, 0 hours) or after biofilms had been developed for 24 hours or 48 hours on abiotic surfaces (dotted lines), undifferentiated squamous cell carcinoma (SCC) 13 cells (Ep; solid lines) at 34°C (34; circles) or 37°C (37; squares) grown in Todd-Hewitt broth with Yeast extract (THY) (blue) or chemically defined medium (CDM) (red). Both 771 and 188 showed higher antibiotic resistance at 34°C vs 37°C on epithelial cells for either medium (P < .001), and 771 showed higher antibiotic-resistance when grown at 34°C on epithelial cells versus abiotic surface (P < .05). The concentration of broth-grown bacteria exposed at 0 hours was approximately 108 CFU/mL to enable comparison with the bacterial biomass of the biofilms at 24 and 48 hours. C, Biofilm biomass of 771 grown under the same conditions as in A over time. For time, 0 hours represents the start of the experiment when no biofilm formation is present. D, Biofilm-associated antimicrobial death of GAS strains 771, 188, MGAS315, and MGAS10394 grown for 48 hours on SCC13 cells at various stages of differentiation induced by calcium (Ca2+). Strains 771, 188, and MGAS10394 showed significantly increased gentamicin resistance at low calcium concentration (P < .05), and all strains were more sensitive to gentamicin when grown on abiotic surfaces (P < .001 for 771, 188, and MGAS315; P < .05 for MGAS10394).
Figure 2.
Biofilm-growth of group A streptococcus (GAS) on live epithelial cells. A, squamous cell carcinoma (SCC) 13 cell viability after exposure to broth-grown (planktonic) and biofilm-derived GAS 771, JRS4, or MGAS315. B, Biomass during biofilm formation over prefixed undifferentiated SCC13 cells (solid lines) and after transplantation to live SCC13 cells (dotted lines) for MGAS315 and 771. C, Macroscopic view of biofilms of 771 GAS formed over abiotic polystyrene surfaces, prefixed undifferentiated SCC13 cells, or biofilms transplanted to live undifferentiated SCC13 cells. Abbreviation: CFU, colony-forming units.
To investigate the role of epithelial cell differentiation for biofilm formation, biofilms were formed on epithelial cells grown in the presence of different concentrations of calcium, an essential factor in keratinocyte differentiation, before fixation [32]. Although the calcium-induced differentiation did not significantly affect the biofilm biomass, biofilms grown on relatively undifferentiated keratinocytes (in 0.3 mmol/L calcium [Ca2+]) were significantly more resistant to gentamicin than biofilms grown on a more differentiated epithelium (in 2 mmol/L Ca2+; Figure 1D). This may partly explain the observation that GAS is less associated with colonization of intact, differentiated skin surfaces [33, 34] and may suggest a role for epithelial cell differentiation in biofilm organization and functionality.
Biofilm Formation on Live Keratinocytes
As shown in Figure 1, we used prefixed epithelia because broth-grown GAS are highly toxic to epithelial cells ([23] and Figure 2A) and caused detachment of epithelial cells after 4 hours of incubation (Figure 3A). To circumvent this problem, we developed a model system wherein bacterial biofilms formed on a prefixed epithelium for 48 hours were transferred (transplanted) over to live epithelial cells (Figure 2B), similar to the methodology we have recently developed for S. pneumoniae [35]. Transfer of biofilm cultures to live epithelia resulted in recovery of the biomass and antibiotic resistance after 24 hours, with the best effects seen when using epithelial cells grown with 0.3 mmol/L Ca2+ at 34°C, and biofilm bacteria significantly reduced host-cell toxicity over 72 hours, as shown by increased viability (Figure 2A, solid lines) and retained morphology (Figure 3B).
Figure 3.
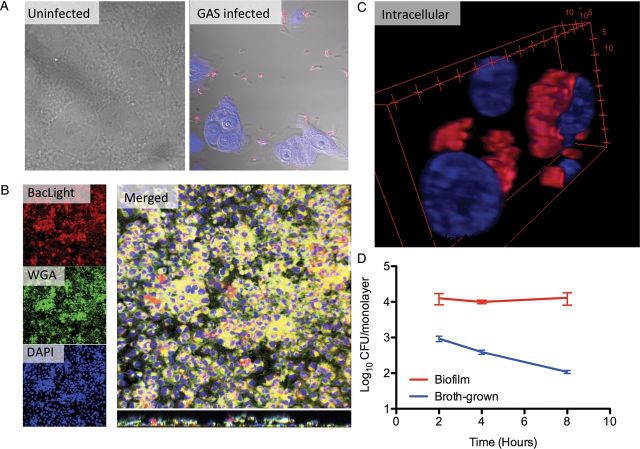
Visualization of group A streptococcus (GAS) biofilms on live epithelial cells. A, Planktonic MGAS315 bacteria (red in the GAS-infected sample) were rapidly toxic to live squamous cell carcinoma (SCC) 13 cells after 4 hours, shown by detachment of epithelial cells compared with the uninfected control. B, MGAS315 (red) were seeded on paraformaldehyde-fixed SCC13 cells. At 48 hours, biofilms were transplanted to live cells and left for another 48 hours. Bacteria are stained red using BacLight Red bacterial stain, epithelia were stained with 488-conjugated wheat germ agglutinin (WGA; green), and bacteria and cell nuclei with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Larger image shows a representative merged 3-dimensional reconstruction of confocal images of this MGAS315 biofilm, with the bottom panel representing a z-section though the biofilm. Individual channels are presented in smaller images on left. C, Three-dimensional reconstruction of nontoxic perinuclear intracellular MGAS315 biofilm communities stained using GAS antibody (red) and DAPI (blue). Numbers represent distance in micrometers. D, Intracellular biofilm and broth-grown GAS recovered from cell lysates at 2, 4, and 8 hours after antibiotic treatment that killed all extracellular bacteria. Data are from triplicate samples and represents as means with standard deviations.
During coculture with live keratinocytes (Figure 2C, bottom), the GAS biofilm morphology changed and appeared less macroscopically dense than that of the biofilms formed over prefixed epithelial cells or abiotic surfaces (Figure 2C). Microscopically, the biofilm layer after 48 hours extended approximately 20–30 µm above the keratinocyte cell layer in large aggregates that could be directly visualized using Baclight Red bacterial stain (Figure 3B). Interestingly, confocal imaging demonstrated that GAS formed both extracellular biofilms and intracellular aggregates (Figure 3C), and viability testing of intracellular bacteria showed increased survival compared with broth-grown bacteria over time (Figure 3D). Intracellular aggregates did not seem to induce apoptosis or necrosis of associated epithelial cells, because the epithelial layer remained intact throughout the experiment (Figure 2A). This pattern of epithelial cell interaction more closely mimics what has been observed during colonization in vivo [6, 14].
Altered Production of Virulence Determinants With GAS Biofilms Formed on Live Undifferentiated Epithelial Cells
Consistent with our observations that biofilm bacteria were less toxic to keratinocytes, we showed that biofilms formed in association with live keratinocytes for 48 hours exhibited significant down-regulation of genes involved in virulence, represented by the genes sagA, speB, slo, hasA, and emm3 (Figure 4) compared with broth-grown bacteria in contact with epithelial cells for 4 hours. In contrast, genes involved in the recently characterized com system [27], here represented by SPY0104 (comGD), was significantly up-regulated in biofilm cultures (2.2-fold; P < .01) compared with mid–log phase planktonic broth-grown bacteria, similar to our observations in pneumococci [25].
Figure 4.
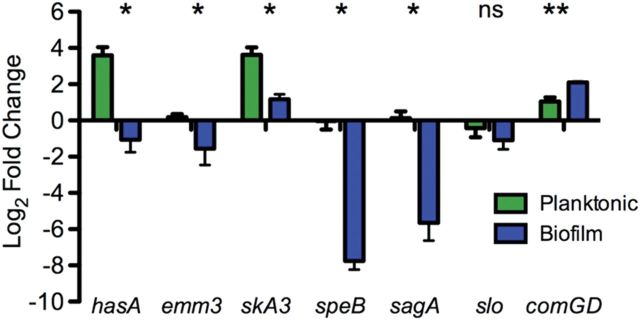
Changed gene expression of virulent determinants in biofilm bacteria. The gene expression of key virulence genes in broth-grown bacteria, broth-grown bacteria in contact with squamous cell carcinoma (SCC) 13 cells for 4 hours, and biofilm bacteria grown on epithelial cells for 48 hours were determined with quantitative reverse-transcription polymerase chain reaction and normalized to gyrA expression. Results are expressed as ratios of broth-grown bacteria grown in contact with epithelial cells and broth-grown cells grown in broth (planktonic) or ratios of biofilm bacteria and broth-grown group A streptococcus (biofilm). *P < .05 for all comparisons (Student t test). Abbreviation: NS, not significant.
Priming of GAS Biofilms for Murine Colonization and Toxicity During Septic Infection
Based on their differential host cell toxicity and gene expression of virulence determinants, we tested the virulence of broth-grown and biofilm bacteria in murine colonization and infection models. After intranasal challenge of mice with equivalent CFUs of biofilm and broth-grown GAS strains, biofilm bacteria were found to have a significantly enhanced ability to colonize the NALT (bacterial burden approximately 2 log10 higher) with a slightly decreased dissemination to the lungs compared with broth-grown organisms (Figure 5A and 5B). Confocal imaging of NALT removed from biofilm-colonized mice demonstrated large microcolony formation overlying the epithelial surface (Figure 6; red). In NALT tissue inoculated with broth-grown bacteria we were unable to detect any microcolonies, which may reflect the 100-fold lower bacterial burden and the sensitivity of the microscopy. Furthermore, biofilm bacteria were able to persist at the same bacterial burden for up to 9 days after colonization (Figure 5C), when broth-grown bacteria had cleared (not shown).
Figure 5.
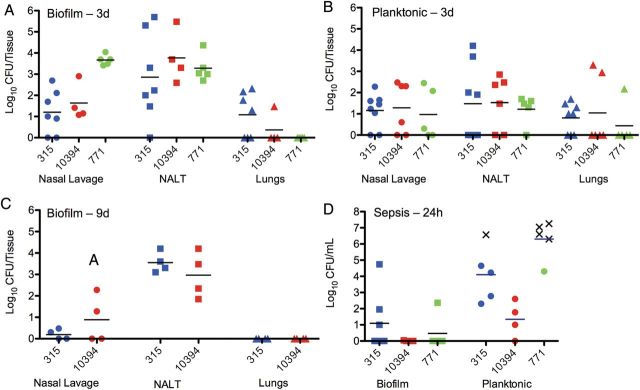
Colonization and dissemination of streptococcal populations after intranasal inoculation or intraperitoneal inoculation. A, B, Individual 8-week old female BALB/cByJ mice were inoculated with either biofilm (A) or broth-grown, planktonic populations (B) of MGAS315, MGAS10394, or 771 intranasally without anesthesia; 72 hours after inoculation, oropharyngeal colonization was measured by the bacterial burden in oropharyngeal or nasal lavage samples and nasal-associated lymphoid tissue (NALT). Dissemination to the lungs was determined by the bacterial burden in lung tissue homogenates. Biofilm bacteria showed significantly higher colonization burden than broth-grown bacteria (P < .01 for 771, P < .05 for MGAS10394, and P = .07 for MGAS315). C, Mice were also inoculated with biofilm populations of MGAS315 and MGAS10394 for 9 days to evaluate long-term colonization. D, Mice were injected intraperitoneally with equal inocula of biofilm and planktonic (broth-grown) MGAS315, MGAS10394, or 771 group A streptococcus (GAS) to determine their respective virulence in the vascular compartment. For intraperitoneal challenge, blood was collected 24 hours after infection. Each dot in the graphs represents an individual mouse. X's represent mice that became moribund and required euthanasia before the end of the experiment. Each experiment included ≥4 mice. Bacteremia developed in all mice receiving broth-grown bacteria intraperitoneally, with significantly higher bacterial burden in the bloodstream (P < .05 for MGAS315, P < .01 for MGAS10394, and P < .001 for 771).
Figure 6.
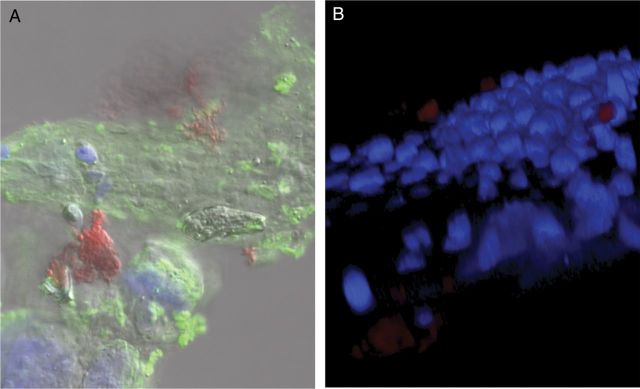
Confocal analysis of MGAS315 biofilm colonization of nasal-associated lymphoid tissue (NALT). Eight-week old female BALB/cByJ mice were infected intranasally with biofilm MGAS315 that was formed for 48 hours on paraformaldehyde-fixed squamous cell carcinoma (SCC) 13 cells. At 72 hours after inoculation, mice were euthanized, and NALT was removed and fixed in 4% neutral buffered formalin. A, NALT was stained with 488-conjugated wheat germ agglutinin (green), nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI), and group A streptococcus (GAS) stained with anti-GAS antibody (red). B, Representative 3-dimensional reconstruction of the specimen, using confocal images stained with DAPI (blue) and anti-GAS antibody (red).
In a septicemia model, bacteremia developed in all mice receiving broth-grown bacteria intraperitoneally, and 80% of 771 and 20% of MGAS315 inoculated mice had to be euthanized before the end of the 24-hour study period based on the rapid progression of the disease (Figure 5D). In contrast, mice infected with equal inocula of biofilm bacteria showed partial or complete clearance of the infection at 24 hours (Figure 5D), with no deaths, and they appeared visibly healthier (ie, more active with less ruffled fur), and the bacterial burden further declined during the next 24 hours, indicating that the biofilm phenotype was attenuated for invasive disease.
ComR as Requirement for GAS Biofilm Formation and Natural Transformation In Vitro
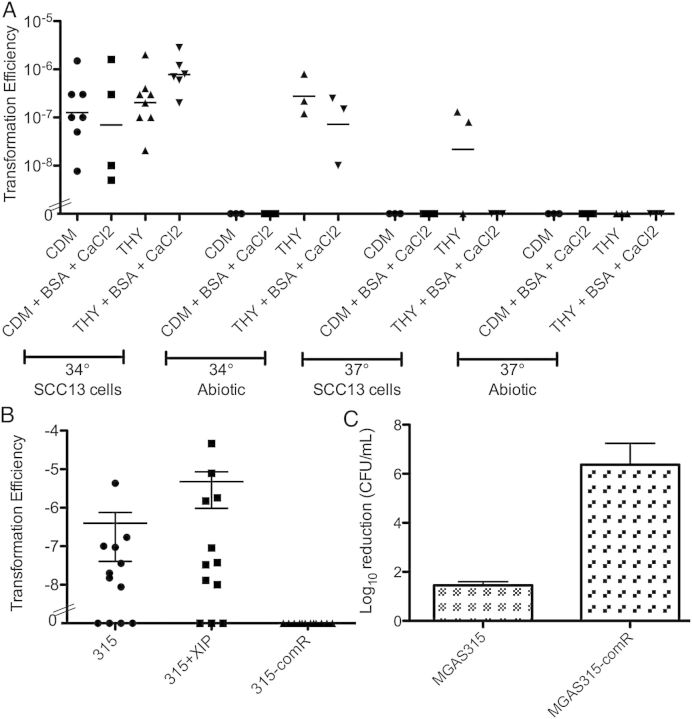
The Com system has recently been identified and described to induce competence-related gene expression in GAS [26, 27], but natural transformation has not been observed. Based on our experiences with enhanced transformation of pneumococci grown in biofilms [25], we investigated the role of biofilm formation on transformation efficiency. GAS 771 was grown on epithelial cells in the presence of 10 µg/mL of chromosomal DNA from strain 188, harboring a kanamycin cassette in the capsule locus. After 48 hours, the biofilms were dispersed and plated on selective agar to determine transformation efficiency. The biofilm biomass averaged 2.4 × 108 CFU per biofilm, somewhat higher than in our initial experiments in Figure 1C. Using this model system, natural transformation was indeed observed (Figure 7A), although not surprisingly, the efficiencies observed were lower than obtained in similar experiments with S. pneumoniae [25]. Transformants were validated for insertion of the kanamycin cassette by PCR of genomic DNA, and they also had an acapsular appearance on the plates. Natural transformation was found to be variable and dependent on media, temperature, calcium chloride concentration, and the presence of bovine serum albumin and was highest when biofilms were grown at 34°C on epithelial cells in THY media (Figure 7A). The number of transformants varied between 2 and 100 in separate biofilm cultures.
Figure 7.
A, Transformation efficiency of group A streptococcus (GAS) 771 with 188 chromosomal DNA carrying a kanamycin cassette in the capsule locus under different environmental conditions. B, Transformation efficiency of MGAS315 biofilms alone or with the addition of exogenous XIP, or MGAS315-comR biofilms transformed with MGAS315-murm2 DNA carrying a spectinomycin cassette in the murM locus. C, Biofilm-specific antimicrobial sensitivity of MGAS315 and MGAS315-comR biofilms formed for 48 hours in CDM at 34°C over undifferentiated squamous cell carcinoma (SCC) 13 cells after exposure to 500 µg/mL gentamicin for 3 hours. Abbreviations: BSA, bovine serum albumin; CaCl2, calcium chloride; CDM, chemically defined medium; THY, Todd-Hewitt medium with Yeast extract.
To further analyze the role of the competence activation pathway in GAS, MGAS315 and its ComR-deficient mutant was studied. MGAS315 biofilms, produced under optimal conditions as described above, integrated chromosomal DNA from MGAS315-murM2::spc carrying a spectinomycin cassette in the murM locus (Figure 7B) and DNA from MGAS315-ropB::erm carrying an erythromycin cassette (not shown) with an efficiency similar to 771 in Figure 7A. Addition of the competence-activating peptide pheromone, XIP, increased the transformation efficiency approximately 10-fold (Figure 7B), and between 7 and 273 transformants were detected per biofilm. As expected, the MGAS315 ComR-deficient strain was unable to integrate exogenous DNA during biofilm formation (Figure 7B). Interestingly, this strain was also impaired for biofilm formation, showing reduced biomass (not shown) and significantly lower antimicrobial resistance than the wild-type strain (Figure 7C), suggesting a partial overlap in biofilm and competence signaling. These results suggest that S. pyogenes strains are naturally transformable during biofilm growth and that activation of ComR contributes to the regulation of this process.
Natural Transformation of GAS Biofilms In Vivo
As in vitro biofilm bacteria were naturally competent, and biofilm formation was associated with colonization, we tested whether natural transformation occurred during in vivo colonization. Mice were colonized intranasally with MGAS315 biofilm bacteria and given 20 µL of 10 µg/mL chromosomal DNA from strain MGAS315-murM2::spc, containing a streptomycin resistance cassette at the time of colonization and again 24 hours after inoculation. At 48 hours, mice were killed and nasopharyngeal lavage samples and NALT tissue was collected. Of the 5 mice given chromosomal DNA, 2 had spectinomycin-resistant β-hemolytic transformants (2 and 3 colonies, validated by PCR). These results were encouraging, considering that the total biomass in the NALT tissue was much lower than in the in vitro biofilm cultures (approximately 1 × 104 CFU per tissue compared with approximately 1 × 108 CFU per in vitro biofilm). Addition of the competence pheromone XIP together with the chromosomal DNA did not significantly increase the proportion of mice (3 of 8) with recoverable transformants, but it did increase the number of transformed colonies that were recovered from each mouse (1, 23, and 38 colonies, validated by PCR). All transformants were recovered exclusively from the NALT. These results validate that horizontal gene transfer can occur at low frequencies under in vivo conditions associated with GAS biofilm colonization.
DISCUSSION
Bacterial growth conditions in vitro are strikingly different from those encountered during in vivo colonization and infection. In vivo, streptococcal colonization is the manifestation of a dynamic series of interactions between the host and pathogen that involves changes in both bacterial and host gene expression [36–38]. Therefore, the most informative in vitro model systems of colonization and disease are likely to be those that most closely mimic the physiological environment and in which both the pathogen and host can be manipulated.
This study describes a biofilm model system that recapitulates the in vivo colonization phenotype to a greater degree than earlier in vitro biofilm models by integrating aspects of the colonizing environment. We demonstrated that GAS can form nontoxic biofilms in association with live human keratinocytes that organized as smaller microcolonies rather than the dense mucoid sheet formed over abiotic surfaces [20] and were more resistant to antibiotic exposure, a typical trait of functional biofilms. These observations corroborate histological analyses of colonization of GAS on the tonsils of children [6] or on skin [14]. Our results also indicated that GAS, generally considered an extracellular pathogen, can invade and persist in epithelial cells without host cell damage, owing to their biofilm lifestyle. This corroborates and extends findings from the last 2 decades that have provided increasing evidence that GAS can invade and persist in nonphagocytic cells [39, 40] and suggested that internalization of GAS by host cells may be an important aspect of the carrier state [41].
The role of biofilm bacteria during colonization was further supported in murine colonization and infection studies where in vitro produced biofilm bacteria were shown to cause significantly higher bacterial persistence in the NALT than broth-grown organisms; conversely, they showed a lower ability to persist during systemic infection of mice after intraperitoneal infection, which better mimics the dissemination process to the bloodstream than direct intravenous injection. This may well be explained by the differences in virulence gene expression observed, wherein biofilm bacteria down-regulate genes associated with dissemination and invasive disease (eg, capsule, M protein, and toxins) to adapt to asymptomatic oropharyngeal colonization of the NALT in mice and the tonsil and adenoid tissue in humans by being less toxic to epithelial cells and inducing less inflammation. Infection may therefore be a consequence of bacteria recognizing changes in the colonizing environment that results in up-regulation of virulence factors and release from biofilm structures. It is possible, even likely, that GAS reacting to a changing and potentially hostile environment result in more virulent bacteria than those grown in broth, similar to our results with pneumococci [39]. This is an exciting avenue for further study.
Another major finding in this study was the observation that GAS are naturally competent when grown in organized biofilms on epithelial cells in vitro as well as during biofilm colonization in vivo. This is the first description of natural transformation of a bacterial species in the pyogenic streptococcal group. Earlier studies have identified the ComR quorum-sensing system in GAS and the peptide pheromone involved in activation of the competence system [27]. Here we showed that ComR was required both for growth of organized biofilms and for successful natural transformation, which suggests that the abilities to form biofilms and to exchange genetic material are closely related. The level of transformation obtained in the biofilms was approximately 100 000 fold lower than those observed in biofilm cultures of the highly competent species S. pneumoniae [25] and was mirrored by lower competence induction in the biofilm cultures. This may well be the reason that standard competence assays in liquid medium have not been successful in inducing genetic exchange in GAS. It may also apply to many other streptococcal species that are considered nontransformable. The genes required for transformation, including the pheromone peptides, their receptors and the alternative sigma factors are conserved in essentially all streptococcal species [26]. It may therefore be a matter of using an appropriate, more physiological model system, such as a biofilm system, to evaluate competence induction in these species. It is likely that the biofilm microenvironment is more conducive to horizontal gene transfer based on the proximity of the cells within the community, the access to DNA, and other aspects of the biofilm microenvironment that result in induction of competence genes.
Our results also support the current genomic information that indicates strong evidence for horizontal gene transfer in GAS. Although streptococcal populations circulating in school-age children tend to be highly related [3], comparative genomics approaches undertaken have suggested that as much as 35% of the GAS genome, including many housekeeping genes, vary owing to recombination events [42–44]. Further reports suggest that virulence determinants have disseminated by lateral gene transfer to genetically diverse strains of GAS [45, 46], and niche separation seems to have had minimal effects on neutral gene divergence, with clear evidence of genetic recombination between throat and skin strains [44].
In aggregate, results of prior studies of biofilm formation and genetic plasticity of GAS combined with our current results of this study suggest that oropharyngeal carriage of GAS in biofilm communities may play a role in asymptomatic colonization and be critical for the transmission of important virulence genes and antibiotic resistance elements between colonizing bacteria. This underscores the importance of better understanding GAS colonization and continuing surveillance efforts to track the changing epidemiology of GAS.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are grateful to Donald Morrison, PhD, University of Illinois, Chicago, for critical reading of the manuscript and insightful suggestions, and Michael Wessels, MD. Children's Hospital Boston, Boston, Massachusetts, for providing strains 771 and 188 for our studies.
Financial support. This work was supported in part by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant AI091779 to M. J. F.), and the Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Disease for M.J.F.
Potential Conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ralph AP, Carapetis JR. Group a streptococcal diseases and their global burden. Curr Top Microbiol Immunol. 2013;368:1–27. doi: 10.1007/82_2012_280. [DOI] [PubMed] [Google Scholar]
- 2.Muotiala A, Seppala H, Huovinen P, Vuopio-Varkila J. Molecular comparison of group A streptococci of T1M1 serotype from invasive and noninvasive infections in Finland. J Infect Dis. 1997;175:392–9. doi: 10.1093/infdis/175.2.392. [DOI] [PubMed] [Google Scholar]
- 3.Durmaz R, Durmaz B, Bayraktar M, et al. Prevalence of group A streptococcal carriers in asymptomatic children and clonal relatedness among isolates in Malatya, Turkey. J Clin Microbiol. 2003;41:5285–7. doi: 10.1128/JCM.41.11.5285-5287.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Lama Z, Gonzalez JJ, Lupiola P, Tejedor MT. Carriers of beta hemolytic streptococci from groups A, B, and C among schoolchildren in Las Palmas. Enferm Infecc Microbiol Clin. 2000;18:271–3. [PubMed] [Google Scholar]
- 5.de Melo MC, Sa Figueiredo AM, Ferreira-Carvalho BT. Antimicrobial susceptibility patterns and genomic diversity in strains of Streptococcus pyogenes isolated in 1978–1997 in different Brazilian cities. J Med Microbiol. 2003;52:251–8. doi: 10.1099/jmm.0.04938-0. [DOI] [PubMed] [Google Scholar]
- 6.Roberts AL, Connolly KL, Kirse DJ, et al. Detection of group A Streptococcus in tonsils from pediatric patients reveals high rate of asymptomatic streptococcal carriage. BMC Pediatr. 2012;12:3. doi: 10.1186/1471-2431-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikebe T, Murai N, Endo M, et al. Changing prevalent T serotypes and emm genotypes of Streptococcus pyogenes isolates from streptococcal toxic shock-like syndrome (TSLS) patients in Japan. Epidemiol Infect. 2003;130:569–72. [PMC free article] [PubMed] [Google Scholar]
- 8.Halpern MT, Schmier JK, Snyder LM, et al. Meta-analysis of bacterial resistance to macrolides. J Antimicrob Chemother. 2005;55:748–57. doi: 10.1093/jac/dki060. [DOI] [PubMed] [Google Scholar]
- 9.Jones RN, Wilson ML, Weinstein MP, Stilwell MG, Mendes RE. Contemporary potencies of minocycline and tetracycline HCL tested against Gram-positive pathogens: SENTRY Program results using CLSI and EUCAST breakpoint criteria. Diagn Microbiol Infect Dis. 2013;75:402–5. doi: 10.1016/j.diagmicrobio.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Kurien M, Stanis A, Job A, Brahmadathan, Thomas K. Throat swab in the chronic tonsillitis: how reliable and valid is it? Singapore Med J. 2000;41:324–6. [PubMed] [Google Scholar]
- 11.Woo JH, Kim ST, Kang IG, Lee JH, Cha HE, Kim DY. Comparison of tonsillar biofilms between patients with recurrent tonsillitis and a control group. Acta Otolaryngol. 2012;132:1115–20. doi: 10.3109/00016489.2012.689859. [DOI] [PubMed] [Google Scholar]
- 12.Diaz RR, Picciafuoco S, Paraje MG, et al. Relevance of biofilms in pediatric tonsillar disease. Eur J Clin Microbiol Infect Dis. 2011;30:1503–9. doi: 10.1007/s10096-011-1249-3. [DOI] [PubMed] [Google Scholar]
- 13.Torretta S, Marchisio P, Drago L, et al. Nasopharyngeal biofilm-producing otopathogens in children with nonsevere recurrent acute otitis media. Otolaryngol Head Neck Surg. 2012;146:991–6. doi: 10.1177/0194599812438169. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama H, Morizane S, Yamasaki O, Oono T, Iwatsuki K. Assessment of Streptococcus pyogenes microcolony formation in infected skin by confocal laser scanning microscopy. J Dermatol Sci. 2003;32:193–9. doi: 10.1016/s0923-1811(03)00096-3. [DOI] [PubMed] [Google Scholar]
- 15.Gillespie SH. Failure of penicillin in Streptococcus pyogenes pharyngeal infection. Lancet. 1998;352:1954–6. doi: 10.1016/s0140-6736(05)61327-x. [DOI] [PubMed] [Google Scholar]
- 16.Thulin P, Johansson L, Low DE, et al. Viable group A streptococci in macrophages during acute soft tissue infection. PLoS Med. 2006;3:e53. doi: 10.1371/journal.pmed.0030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conley J, Olson ME, Cook LS, Ceri H, Phan V, Davies HD. Biofilm formation by group a streptococci: is there a relationship with treatment failure? J Clin Microbiol. 2003;41:4043–8. doi: 10.1128/JCM.41.9.4043-4048.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang JC, LaSarre B, Jimenez JC, Aggarwal C, Federle MJ. Two group A streptococcal peptide pheromones act through opposing Rgg regulators to control biofilm development. PLoS Pathog. 2011;7:e1002190. doi: 10.1371/journal.ppat.1002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doern CD, Roberts AL, Hong W, et al. Biofilm formation by group A Streptococcus: a role for the streptococcal regulator of virulence (Srv) and streptococcal cysteine protease (SpeB) Microbiology. 2009;155:46–52. doi: 10.1099/mic.0.021048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lembke C, Podbielski A, Hidalgo-Grass C, Jonas L, Hanski E, Kreikemeyer B. Characterization of biofilm formation by clinically relevant serotypes of group A streptococci. Appl Environ Microbiol. 2006;72:2864–75. doi: 10.1128/AEM.72.4.2864-2875.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho KH, Caparon MG. Patterns of virulence gene expression differ between biofilm and tissue communities of Streptococcus pyogenes. Mol Microbiol. 2005;57:1545–56. doi: 10.1111/j.1365-2958.2005.04786.x. [DOI] [PubMed] [Google Scholar]
- 22.Courtney HS, Ofek I, Penfound T, et al. Relationship between expression of the family of M proteins and lipoteichoic acid to hydrophobicity and biofilm formation in Streptococcus pyogenes. PLoS One. 2009;4:e4166. doi: 10.1371/journal.pone.0004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hakansson A, Bentley CC, Shakhnovic EA, Wessels MR. Cytolysin-dependent evasion of lysosomal killing. Proc Natl Acad Sci U S A. 2005;102:5192–7. doi: 10.1073/pnas.0408721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marks LR, Parameswaran GI, Hakansson AP. Pneumococcal interactions with epithelial cells are crucial for optimal biofilm formation and colonization in vitro and in vivo. Infect Immun. 2012;80:2744–60. doi: 10.1128/IAI.00488-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marks LR, Reddinger RM, Hakansson AP. High levels of genetic recombination during nasopharyngeal carriage and biofilm formation in Streptococcus pneumoniae. MBio. 2012;3:e00200–12. doi: 10.1128/mBio.00200-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mashburn-Warren L, Morrison DA, Federle MJ. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol Microbiol. 2010;78:589–606. doi: 10.1111/j.1365-2958.2010.07361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mashburn-Warren L, Morrison DA, Federle MJ. The cryptic competence pathway in Streptococcus pyogenes is controlled by a peptide pheromone. J Bacteriol. 2012;194:4589–600. doi: 10.1128/JB.00830-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schommer NN, Christner M, Hentschke M, Ruckdeschel K, Aepfelbacher M, Rohde H. Staphylococcus epidermidis uses distinct mechanisms of biofilm formation to interfere with phagocytosis and activation of mouse macrophage-like cells 774A.1. Infect Immun. 2011;79:2267–76. doi: 10.1128/IAI.01142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bast DJ, Yue M, Chen X, et al. Novel murine model of pneumococcal pneumonia: use of temperature as a measure of disease severity to compare the efficacies of moxifloxacin and levofloxacin. Antimicrob Agents Chemother. 2004;48:3343–8. doi: 10.1128/AAC.48.9.3343-3348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu HY, Nguyen HH, Russell MW. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand J Immunol. 1997;46:506–13. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]
- 31.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 32.Ponec M, Weerheim A, Kempenaar J, Elias PM, Williams ML. Differentiation of cultured human keratinocytes: effect of culture conditions on lipid composition of normal vs. malignant cells. In Vitro Cell Dev Biol. 1989;25:689–96. doi: 10.1007/BF02623721. [DOI] [PubMed] [Google Scholar]
- 33.Leyden JJ, Stewart R, Kligman AM. Experimental infections with group A streptococci in humans. J Invest Dermatol. 1980;75:196–201. doi: 10.1111/1523-1747.ep12522655. [DOI] [PubMed] [Google Scholar]
- 34.Dajani AS, Wannamaker LW. Experimental infection of the skin in the hamster simulating human impetigo. I. Natural history of the infection. J Infect Dis. 1970;122:196–204. doi: 10.1093/infdis/122.3.196. [DOI] [PubMed] [Google Scholar]
- 35.Marks LR, Davidson BA, Knight PR, Hakansson AP. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. MBio. 2013;4:e00438–13. doi: 10.1128/mBio.00438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreikemeyer B, McIver KS, Podbielski A. Virulence factor regulation and regulatory networks in Streptococcus pyogenes and their impact on pathogen-host interactions. Trends Microbiol. 2003;11:224–32. doi: 10.1016/s0966-842x(03)00098-2. [DOI] [PubMed] [Google Scholar]
- 37.Virtaneva K, Porcella SF, Graham MR, et al. Longitudinal analysis of the group A Streptococcus transcriptome in experimental pharyngitis in cynomolgus macaques. Proc Natl Acad Sci U S A. 2005;102:9014–9. doi: 10.1073/pnas.0503671102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Persson J, Vance RE. Genetics-squared: combining host and pathogen genetics in the analysis of innate immunity and bacterial virulence. Immunogenetics. 2007;59:761–78. doi: 10.1007/s00251-007-0248-0. [DOI] [PubMed] [Google Scholar]
- 39.Kreikemeyer B, Klenk M, Podbielski A. The intracellular status of Streptococcus pyogenes: role of extracellular matrix-binding proteins and their regulation. Int J Med Microbiol. 2004;294:177–88. doi: 10.1016/j.ijmm.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Hertzen E, Johansson L, Kansal R, et al. Intracellular Streptococcus pyogenes in human macrophages display an altered gene expression profile. PLoS One. 2012;7:e35218. doi: 10.1371/journal.pone.0035218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson L, Norrby-Teglund A. Immunopathogenesis of streptococcal deep tissue infections. Curr Top Microbiol Immunol. 2013;368:173–88. doi: 10.1007/82_2012_282. [DOI] [PubMed] [Google Scholar]
- 42.Lefebure T, Stanhope MJ. Evolution of the core and pan-genome of Streptococcus: positive selection, recombination, and genome composition. Genome Biol. 2007;8:R71. doi: 10.1186/gb-2007-8-5-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feil EJ, Holmes EC, Bessen DE, et al. Recombination within natural populations of pathogenic bacteria: short-term empirical estimates and long-term phylogenetic consequences. Proc Natl Acad Sci U S A. 2001;98:182–7. doi: 10.1073/pnas.98.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalia A, Spratt BG, Enright MC, Bessen DE. Influence of recombination and niche separation on the population genetic structure of the pathogen Streptococcus pyogenes. Infect Immun. 2002;70:1971–83. doi: 10.1128/IAI.70.4.1971-1983.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sitkiewicz I, Green NM, Guo N, Mereghetti L, Musser JM. Lateral gene transfer of streptococcal ICE element RD2 (region of difference 2) encoding secreted proteins. BMC Microbiol. 2011;11:65. doi: 10.1186/1471-2180-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers S, Commons R, Danchin MH, et al. Strain prevalence, rather than innate virulence potential, is the major factor responsible for an increase in serious group A streptococcus infections. J Infect Dis. 2007;195:1625–33. doi: 10.1086/513875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.