Abstract
The ketogenic diet is a high-fat, low-carbohydrate, and restricted protein diet that is useful in patients with refractory epilepsy. The efficacy of the ketogenic diet is better than most of the new antiepileptic drugs. Other modifications of the diet are also beneficial, such as the modified Atkins diet and the low glycemic index treatment. There is a lack of awareness of the ketogenic diet as a treatment modality for epilepsy amongst pediatricians and neurologists. In this review, the use of the ketogenic diet and other dietary treatments in refractory epilepsy is discussed. The Indian experience with the use of these dietary treatments is also briefly reviewed.
Keywords: Infantile spasms, intractable epilepsy, ketogenic diet, the modified Atkins diet
Introduction
The ketogenic diet (KD) is a high fat, low carbohydrate, and restricted protein diet that was initially devised in the 1920's as a treatment for refractory epilepsy. With the discovery of phenytoin and sodium valproate, the use of KD markedly declined as it was considered difficult and unpalatable. Over the last 15 years, however, there has been resurgence in the use of KD in refractory childhood epilepsy, with over 700 peer reviewed publications in the last 15 years. Other modifications of the diet are also being used, such as the modified Atkins diet and the low glycemic index treatment. These dietary treatments are now also being considered for neurological disorders other than epilepsy, such as brain tumors, parkinsonism, Alzheimer's disease, and amyotrophic lateral sclerosis.[1]
There is a lack of awareness and acceptability of KD as a treatment modality for epilepsy amongst pediatricians and neurologists. Perceived difficulties include doubtful acceptability in a predominantly vegetarian population, unavailability of labeled foods, and unfamiliarity of dieticians with KD.[2] In this review, the use of KD and other dietary treatments in refractory epilepsy is discussed. The Indian experience with the use of these dietary treatments is also briefly reviewed.
Composition of KD
The classic KD consists of a 4:1 ratio of grams of fat to grams of protein plus carbohydrate combined; 90% of the calories in the diet come from fat. Lower ratios such as 3:1 may be used in younger children. Calories may be restricted initially to 80%-90% of the daily recommendations for age but are adjusted over time to provide for growth.[3] Fluid restriction is no longer considered necessary, and adequate fluid intake is important for prevention of dehydration (especially in hot weather), constipation, and kidney stones, the last two may occur with KD.
KD has to be calculated individually for each patient and requires strict weighing of foods. Parental counseling which can take many hours is also very important for the success of the diet. Hence, a trained dietician who has the time and interest for this treatment is an essential prerequisite to start KD. The classic KD is quite restrictive, especially for Indian patients, as there is no scope for cereal staples such as chapattis and rice.
Efficacy of KD
The efficacy of KD is better than most of the new antiepileptic drugs. In general, half of all patients treated with KD will have at least a 50% reduction in seizure frequency. In a recent large study in 317 Chinese children,[4] 35.0%, 26.2%, and 18.6% children showed >50% seizure reduction at three, six, and 12 months, respectively. One systematic review showed complete cessation of seizures in 16% of children, greater than 90% reduction in 32%, and greater than 50% reduction in 56% children.[5] Keene et al. reported a systematic review of studies of KD published, with a total collective population of 972.[6] At six months, an average of 15.6% of the patients had become seizure-free, while 33.0% were reported to have more than 50% reduction in seizure frequency after commencing the diet. There have been concerns that all these studies were uncontrolled and hence not good evidence. However, there has been a recent randomized controlled trial of KD in refractory epilepsy. Neal et al. randomized children to receive KD, either after a one-month (treatment group) or four-month delay (control group) with no changes in the anti-epileptic drugs.[7] The seizure frequency after four months was significantly lower in the 54 children in the diet group (38% decrease in seizures) compared to the 49 controls (37% increase in seizures; P < 0.0001). A recent Cochrane review concluded that the KD results in short- to medium-term benefits in seizure control, the effects of which are comparable to modern anti-epileptic drugs.[8]
KD has been found to be feasible and efficacious in the Indian scenario as well. In a prospective study of the use of KD in 27 children with refractory epilepsy, 55% remained on KD at six months, and 37% remained on it at one year;[2] 48% had >50% reduction in seizures and four children (15%) were seizure-free at 6 months. At one year, 37% had >50% reduction in seizures, and five children (18.5%) were seizure-free.
In addition to seizure control, cognition and alertness have been seen to improve because of KD. Whether this is due improvement in seizure control, decreased anti-epileptic medication, or a nonspecific effect of the diet (or a combination of all) is uncertain. Developmental quotients, attention, and social function were also noted to improve in a prospective study.[9]
Mechanisms of Action
The actual mechanism by which the KD helps suppress epilepsy remains unclear despite decades of research. A consideration of the spectrum of activity of the diet in acute animal seizure models suggests that the diet acts in a mechanically distinct way from clinically used anti-epileptic drugs.[10,11] Acetone and acetoacetate both have anticonvulsant properties in animal models. Epileptic patients who responded well to KD have been shown to have elevations in brain acetone levels by magnetic resonance spectroscopy.[12]
Recent research has focused on the role of polyunsaturated fatty acids (PUFAs). After KD treatment, specific PUFAs, including arachidonic acid and docosahexenoic acid, have been found to be elevated in both serum and brain.[13] PUFAs induce the expression of mitochondrial uncoupling proteins.[11] The activation of mitochondrial uncoupling proteins results in reduction of the proton gradient across the inner mitochondrial membrane, which reduces reactive oxygen species production. There are emerging data that seizures may be precipitated by oxidative stress, and that a reduction of free radical formation may prevent seizure activity.[14]
Improved cerebral energetic has also been postulated as a mechanism. Devivo et al. proposed that KD results in an enhanced energy reserve in the brain, which provides an increased resistance to seizures.[15] This hypothesis of an increase in energy change has been supported by recent experiments in patients with Lennox Gastaut syndrome. Using 31P spectroscopic imaging, it was shown that the ketogenic diet was associated with an improvement in energy metabolism.[16] Various proposed mechanisms have been summarized in Table 1.
Table 1.
Postulated mechanisms of action of ketogenic diet

Patient Selection for the Diet
KD should be considered in patients with refractory epilepsy, after the failure of two or three appropriate anti-epileptic drugs. Some epilepsy syndromes, such as infantile spasms, myoclonic astatic epilepsy, and Dravet syndrome, respond especially well to KD [Table 2].[17] In some centers, KD has been used even as a first-line treatment for children with infantile spasms.[18] KD is a good option for Lennox Gastatut syndrome as well.[19]
Table 2.
Patient selection for ketogenic diet
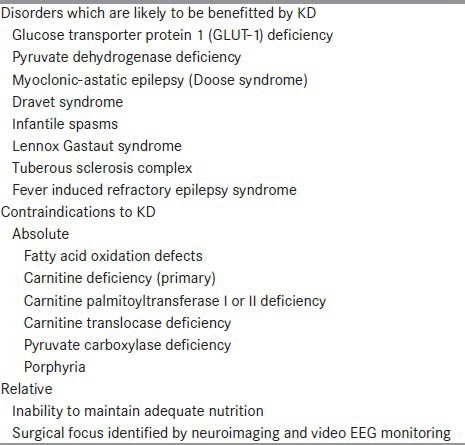
KD is the treatment of choice in glucose transporter-1 defect and pyruvate dehydrogenase deficiency. Other conditions which may potentially benefit with KD include Rett syndrome, tuberous sclerosis complex, and Lafora body disease.[17] KD has also been found to be of use in the ICU setting for refractory status epilepticus[20] and in the recently described febrile infection related epilepsy syndrome (FIRES).[21]
KD is contraindicated in fatty acid oxidation defects, pyruvate carboxylase deficiency, primary carnitine deficiency, porphyria, and several mitochondrial disorders.[17] Children with partial seizures are less likely to respond to the KD. This is especially true if the child is a candidate for epilepsy surgery.[22] However, while the patient is awaiting the epilepsy surgery work-up (which may take many months in our scenario), KD may be tried, especially if there is an increase in the seizure frequency.[23]
Pre-Diet Preparation
After the candidacy of the patient for dietary treatment has been established, the family needs counseling and pre-diet preparation. Inborn errors of metabolism should be screened for, especially in patients with unknown etiology of epilepsy. Family history of renal stones must be enquired for, as this is an important complication of KD. The parents must be counseled that the diet requires a significant commitment, and all members of the family, especially siblings and grandparents, must understand and cooperate with the patient.
The patient's growth and nutritional parameters should be reviewed. The dietician should calculate the caloric requirements and the daily intake of the patient. Children with severe neurological impairments should be assessed for ability to chew and swallow and any evidence of gastroesophageal reflux.[3] The family's food preferences and cultural taboos, such as vegetarianism, should be studied, and the diet recipes should be prepared to be as similar to the family's usual diet to maximize the compliance. For vegetarians, soya products are useful, as they contain high-quality protein with minimal carbohydrates. The carbohydrate content of the child's medications should be reviewed and changed to carbohydrate-free preparations. Syrups and dispersible tablets should not be used. Corticosteroids and ACTH should be stopped at least one week prior to diet initiation.
Baseline investigations should include hemogram, serum electrolytes, calcium, phosphate, liver and kidney function tests, fasting lipid profile, and spot urinary calcium to creatinine ratio [Table 3].[17] Parents must be taught how to measure urine ketones, and document and also to maintain seizure diaries.
Table 3.
Laboratory monitoring of KD at baseline and follow up

Diet Initiation
Fasting initiation
In the original protocol, which is still being followed at many centers, the diet is initiated using fasting.[24] Patients are admitted for KD initiation and fasted (water is allowed) with blood glucose checks every six hours. Glucose levels as low as 25-40 mg/dl do not need treatment unless the patient becomes symptomatic (e.g., extreme lethargy, severe emesis). Patients are fasted for 36 hours or till urine ketones become positive. On the first day of feeding, the patient is given 1/3rd of the planned caloric intake; on the second day, 2/3rd of the total calories are administered, and on third day, the full caloric intake is administered. The patient is discharged by the fifth day.
Advantages of fasting include a shorter time to onset of ketosis, improved screening for underlying metabolic disorders, and the need for hospital admission period, which allows teaching the family about preparation of meals and the monitoring of ketosis.[11] Some patients also experience a rapid reduction in seizures after fasting, akin to a “loading dose” of intravenous anti-convulsant medication.[25] Disadvantages of fasting include psychological stress, the risk of hypoglycemia and dehydration, the cost and inconvenience of hospitalization, and repeated blood tests. Recently, “intermittent fasting” regimen in combination with KD was used in a small pediatric population, which had failed to achieve the goal for seizure control on KD.[26]
Non-fasting initiation
Non-fasting initiation has been found to be equally effective. Bergqvist et al. conducted a prospective randomized clinical trial comparing fasting versus gradual initiation of KD.[27] The gradual initiation protocol began with a 1:1 ratio (fat: Carbohydrate + protein) by weight, full calorie meals, and then daily advanced to a 2:1, 3:1, and finally to a 4:1 ratio. There was no difference in efficacy at 3 months between the fasting and non-fasting groups; children in the non-fasting group were less likely to lose weight, become hypoglycemic, require treatment for acidosis, or become dehydrated. This protocol was found to be safe and well-tolerated in Indian patients as well.[2]
Nathan et al. have described another approach for non-fasting initiation; the carbohydrate washout diet.[28] In this, the patients receive a minimal (almost zero) carbohydrate diet for a few days till they develop ketosis, and then they are started on KD.
Advantages of the non-fasting initiation are that the diet can be initiated even in the outpatient setting. However, hospitalization has the advantages that the patient may be observed for any deterioration (because of unmasking of an undetected inborn error of metabolism), and it provides ample time and opportunities for parent counseling.
Supplements to be given with KD
All children on KD must receive supplements with multivitamin with minerals (including trace minerals) and preparations containing calcium with vitamin D. Optional supplements include oral citrates (to prevent kidney stones, see below), and carnitine.[17]
Adverse effects
As with all other treatments for epilepsy, KD has a defined adverse event profile, consisting of possible complications seen during initiation or maintenance. Dehydration may occur during the initiation phase. Fluids should not be restricted. Blood glucose levels may fall during fasting but mostly the children are asymptomatic. If the child is symptomatic, 30 ml of orange juice should be given and the blood glucose rechecked. Vomiting is also a common side effect during the initiation phase.
Constipation is the one of the most common side effects in the maintenance phase.[2] This can be managed by increasing the dietary fiber intake and by use of lactulose. The diet is deficient in vitamins and minerals. Hence, supplementation with magnesium, zinc, selenium, and vitamin D and B complex vitamins is recommended to avoid deficiency-related disease states. The high fat and low roughage may contribute to exacerbation of pre-existing gastroesophageal reflux disease.
Growth problems have been reported with KD. Significant reduction in both height and weight gain among children with epilepsy after prolonged KD has been reported. After diet discontinuation, significant catch-up growth was observed.[29] A recent study advocated a protein-to-energy ratio of 1.5 g protein/100 kcal for children on KD to prevent growth retardation.[30]
Kidney stones occur in 3%-10% and can be minimized by allowing adequate hydration and avoidance of medicines (i.e., topiramate, zonisamide, and acetazolamide) that increase their risk.[31] Renal sonography should be performed if there is hematuria, pain, or crystalluria. Periodic spot urine calcium to creatinine ratios can also help screen for this condition. Recently, a study evaluated the preventive use of potassium citrate in children on KD.[32] The results demonstrated that the use of oral potassium citrate significantly decreased the prevalence of stones (3.2% vs. 10%, P = 0.049).
Serum cholesterol and triglyceride levels may increase, especially during the first six months, and tend to plateau after six months; they need to be monitored.[33] Kwiterivoch et al. performed a study to determine the effect of KD on lipid profile.[34] At six months, KD had significantly increased the mean plasma levels of total cholesterol (58 mg/dl), LDL (50 mg/dl), VLDL (8 mg/dl), and triglycerides (58 mg/dl) [P < 0.001]; and significantly decreased HDL levels (P < 0.001). However, in a recent small study, the diet was successfully used in children with pre-existing hyperlipidemia with diet modifications.[35]
Prolonged QT interval and cardiac arrhythmias have been rarely reported with the diet.[36] However, a prospective study of QT intervals of 27 children started on KD did not reveal any QT prolongation or rhythm disturbances.[37]
Monitoring and follow up
Children started on KD should be followed up monthly for the first three months, and every 3-6 months thereafter. The anti-epileptic drugs are initially continued along with KD. If the patient becomes seizure-free, or there is a significant improvement in the seizure frequency, the anti-epileptic drugs can gradually be tapered. Parents should be asked to maintain a daily seizure and urine ketone chart. During each visit, the compliance should be checked, and growth parameters reviewed. The diet may need to be fine-tuned to maximize the urinary ketones and provide adequate calories for growth. The blood tests (hematological and biochemical tests, and fasting lipid profile) and urinary spot calcium to creatinine ratio should be measured every six months.
KD Discontinuation
KD should be tried for at least three months to check for efficacy. For children who remain on the diet and achieve >50% seizure response, KD should be continued for at least two years. KD can be weaned immediately in cases of emergency or more slowly over weeks/months by reducing the ratio gradually in those who have been treated for years.[3] Seizure recurrence may occur following stopping of the diet and may be managed by re-initiation of diet or anti-convulsant drugs.[38] Some children may require long-term dietary management. Transition plans to adult neurology care should be in place when managing adolescents with epilepsy on dietary therapies.[39]
Modifications of the KDs
The medium-chain triglyceride (MCT) diet
The MCT diet has been shown to have similar efficacy and tolerability to the classic KDs.[40] A greater allowance of carbohydrate and protein, and 10%-20% lesser calories from fat is provided because medium-chain triglycerides are more ketogenic compared to long-chain triglycerides.[3] Adverse effects like renal stones, acidosis, hypoglycemia, constipation, and growth retardation are less common. The MCT oil, however, is expensive.
Lower ketogenic ratio diets
Lower ratios such as 2:1 and 2.5:1 allow more carbohydrate intake, and approximation with the traditional Indian diets. Two studies from India have shown the efficacy of lower ratio diets in children with refractory epilepsy. Nathan et al. reported on the use of ketogenic ratios ranging from 2:1 to 4:1 in 105 children with refractory epilepsy. Though they did not compare the ratios, they reported an overall efficacy of seizure freedom in 37% and a more than 50% reduction in 81% of the patients.[28] Raju et al. conducted a randomized open-labeled study comparing the use of 2.5:1 versus the classic 4:1 ketogenic ratio diets in young children with refractory epilepsy.[41] They found comparable efficacy and tolerability of the two diets.
The modified Atkins diet
The modified Atkins diet has been advocated as a less restrictive alternative to KD.[42] In this diet, carbohydrates are restricted to 10-20 g/day. Fats are actively encouraged, and proteins can be given unlimited. A number of uncontrolled studies in the last five years have shown a similar efficacy to the ketogenic diet.[43,44,45] Approximately, half of the patients show more than 50% improvement in seizures with this diet.
This diet has the advantages of non-fasting initiation. Also, it can be used in resource constraint settings with limited dietician support as it does not require tedious calculations.[46] The counseling time is reduced to 30-60 minutes. In the West, this diet has predominantly been advocated for adolescents and adults. However, in India, this diet has been found useful in young children as well. In a study of 15 children, aged 6 months-3 years, with infantile spasms refractory to hormonal therapy and/or vigabatrin, the modified Atkins diet was found to render six children (40%) spasm-free with EEG resolution of hypsarrhythmia at 3 months.[47] The diet was well tolerated in these young children.
In a recent randomized controlled trial,[48] 102 children aged 2-14 years who had daily seizures despite the appropriate use of at least three anti-epileptic drugs were randomized to either receive the modified Atkins diet (n = 50), or no dietary intervention (n = 52) for a period of three months. The ongoing anti-epileptic medications were continued unchanged in both the groups. Four patients discontinued the diet before the study endpoint, and three patients in the control group were lost to follow up. The median seizure frequency at three months, expressed as a percentage of the baseline was significantly less in the diet group (37.3% vs. 100%, P = 0.003). The proportion of children with >90% seizure reduction (30% vs. 7.7%, P = 0.005) and >50% seizure reduction was significantly higher in the diet group (52% vs. 11.5%, P < 0.001). Constipation was the commonest adverse effect (46%) among children on the diet.
Despite its advantages, the modified Atkins diet becomes very restrictive for Indians, all the more so for vegetarians. This is usually not a problem in young children or cognitively impaired children. Older children who are able to express their preferences complain about the diet's restrictiveness and absence of cereal staples of Indian food such as rice and chapattis.
The low glycemic index treatment
The low glycemic index treatment (LGIT) is another less restrictive alternative to KD. LGIT allows liberalization of total daily carbohydrate intake to approximately 40-60 g/day, but regulates the type of carbohydrate, favoring those with glycemic indices less than 50.[17] A few uncontrolled studies have shown good tolerability with reasonable efficacy.[49,50] A recently concluded randomized controlled trial (NCT01645072), Kannan et al. (manuscript in preparation; personal communication) found significantly higher proportion of children with refractory epilepsy with >50% seizure reduction compared to baseline seizure frequency at three months on LGIT compared to children on standard anti-convulsant therapy.
Conclusion
KD is an effective and well-tolerated treatment for refractory childhood epilepsy, especially in children who are not candidates for epilepsy surgery. It is effective in various seizure types and many epilepsy syndromes. There is a need for increased awareness of KD amongst physicians and dieticians. The modified Atkins diet is a good option in resource constraint settings with limited dietician support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59. doi: 10.3389/fphar.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma S, Gulati S, Kalra V, Agarwala A, Kabra M. Seizure control and biochemical profile on the ketogenic diet in young children with refractory epilepsy--Indian experience. Seizure. 2009;18:446–9. doi: 10.1016/j.seizure.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Kossoff EH, Zupec-Kania BA, Rho JM. Ketogenic diets: An update for child neurologists. J Child Neurol. 2009;24:979–88. doi: 10.1177/0883073809337162. [DOI] [PubMed] [Google Scholar]
- 4.Suo C, Liao J, Lu X, Fang K, Hu Y, Chen L, et al. Efficacy and safety of the ketogenic diet in Chinese children. Seizure. 2013;22:174–8. doi: 10.1016/j.seizure.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Lefevre F, Aronson N. Ketogenic diet for the treatment of refractory epilepsy in children: A systematic review of efficacy. Pediatrics. 2000;105:E46. doi: 10.1542/peds.105.4.e46. [DOI] [PubMed] [Google Scholar]
- 6.Keene DL. A systematic review of the use of the ketogenic diet in childhood epilepsy. Pediatr Neurol. 2006;35:1–5. doi: 10.1016/j.pediatrneurol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. The ketogenic diet for the treatment of childhood epilepsy: A randomised controlled trial. Lancet Neurol. 2008;7:500–6. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 8.Levy RG, Cooper PN, Giri P. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. 2012;3:CD001903. doi: 10.1002/14651858.CD001903.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Pulsifer MB, Gordon JM, Brandt J, Vining EP, Freeman JM. Effects of ketogenic diet on development and behavior: Preliminary report of a prospective study. Dev Med Child Neurol. 2001;43:301–6. doi: 10.1017/s0012162201000573. [DOI] [PubMed] [Google Scholar]
- 10.Hartman AL, Gasior M, Vining EP, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatr Neurol. 2007;36:281–92. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman J, Veggiotti P, Lanzi G, Tagliabue A, Perucca E Institute of Neurology IRCCS C. Mondino Foundation. The ketogenic diet: From molecular mechanisms to clinical effects. Epilepsy Res. 2006;68:145–80. doi: 10.1016/j.eplepsyres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Seymour KJ, Bluml S, Sutherling J, Sutherling W, Ross BD. Identification of cerebral acetone by 1H-MRS in patients with epilepsy controlled by ketogenic diet. MAGMA. 1999;8:33–42. doi: 10.1007/BF02590633. [DOI] [PubMed] [Google Scholar]
- 13.Taha AY, Ryan MA, Cunnane SC. Despite transient ketosis, the classic high-fat ketogenic diet induces marked changes in fatty acid metabolism in rats. Metabolism. 2005;54:1127–32. doi: 10.1016/j.metabol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55:576–80. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- 15.DeVivo DC, Leckie MP, Ferrendelli JS, McDougal DB., Jr Chronic ketosis and cerebral metabolism. Ann Neurol. 1978;3:331–7. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- 16.Pan JW, Bebin EM, Chu WJ, Hetherington HP. Ketosis and epilepsy: 31P spectroscopic imaging at 4.1 T. Epilepsia. 1999;40:703–7. doi: 10.1111/j.1528-1157.1999.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 17.Kossoff EH, Zupec-Kania BA, Amark PE, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Charlie Foundation, Practice Committee of the Child Neurology Society, Practice Committee of the Child Neurology Society, International Ketogenic Diet Study Group. Optimal clinical management of children receiving the ketogenic diet: Recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009;50:304–17. doi: 10.1111/j.1528-1167.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 18.Kossoff EH, Hedderick EF, Turner Z, Freeman JM. A case-control evaluation of the ketogenic diet versus ACTH for new-onset infantile spasms. Epilepsia. 2008;49:1504–9. doi: 10.1111/j.1528-1167.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 19.Lemmon ME, Terao NN, Ng YT, Reisig W, Rubenstein JE, Kossoff EH. Efficacy of the ketogenic diet in Lennox-Gastaut syndrome: A retrospective review of one institution's experience and summary of the literature. Dev Med Child Neurol. 2012;54:464–8. doi: 10.1111/j.1469-8749.2012.04233.x. [DOI] [PubMed] [Google Scholar]
- 20.Kossoff EH, Nabbout R. Use of dietary therapy for status epilepticus. J Child Neurol. 2013;28:1049–51. doi: 10.1177/0883073813487601. [DOI] [PubMed] [Google Scholar]
- 21.Nabbout R, Mazzuca M, Hubert P, Peudennier S, Allaire C, Flurin V, et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES) Epilepsia. 2010;51:2033–7. doi: 10.1111/j.1528-1167.2010.02703.x. [DOI] [PubMed] [Google Scholar]
- 22.Stainman RS, Turner Z, Rubenstein JE, Kossoff EH. Decreased relative efficacy of the ketogenic diet for children with surgically approachable epilepsy. Seizure. 2007;16:615–9. doi: 10.1016/j.seizure.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Villeneuve N, Pinton F, Bahi-Buisson N, Dulac O, Chiron C, Nabbout R. The ketogenic diet improves recently worsened focal epilepsy. Dev Med Child Neurol. 2009;51:276–81. doi: 10.1111/j.1469-8749.2008.03216.x. [DOI] [PubMed] [Google Scholar]
- 24.Freeman JM, Vining EP, Pillas DJ, Pyzik PL, Casey JC, Kelly LM. The efficacy of the ketogenic diet-1998: A prospective evaluation of intervention in 150 children. Pediatrics. 1998;102:1358–63. doi: 10.1542/peds.102.6.1358. [DOI] [PubMed] [Google Scholar]
- 25.Hartman AL, Vining EP. Clinical aspects of the ketogenic diet. Epilepsia. 2007;48:31–42. doi: 10.1111/j.1528-1167.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- 26.Hartman AL, Rubenstein JE, Kossoff EH. Intermittent fasting: A “new” historical strategy for controlling seizures? Epilepsy Res. 2013;104:275–9. doi: 10.1016/j.eplepsyres.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergqvist AG, Schall JI, Gallagher PR, Cnaan A, Stallings VA. Fasting versus gradual initiation of the ketogenic diet: A prospective, randomized clinical trial of efficacy. Epilepsia. 2005;46:1810–9. doi: 10.1111/j.1528-1167.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 28.Nathan JK, Purandare AS, Parekh ZB, Manohar HV. Ketogenic diet in Indian children with uncontrolled epilepsy. Indian Pediatr. 2009;46:669–73. [PubMed] [Google Scholar]
- 29.Kim JT, Kang HC, Song JE, Lee MJ, Lee YJ, Lee EJ, et al. Catch-up growth after long-term implementation and weaning from ketogenic diet in pediatric epileptic patients. Clin Nutr. 2013;32:98–103. doi: 10.1016/j.clnu.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Nation J, Humphrey M, Mackay M, Boneh A. Linear growth of children on a ketogenic diet: Does the protein-to-energy ratio matter? J Child Neurol. 2013 doi: 10.1177/0883073813508222. [DOI] [PubMed] [Google Scholar]
- 31.Kossoff EH, Pyzik PL, Furth SL, Hladky HD, Freeman JM, Vining EP. Kidney stones, carbonic anhydrase inhibitors, and the ketogenic diet. Epilepsia. 2002;43:1168–71. doi: 10.1046/j.1528-1157.2002.11302.x. [DOI] [PubMed] [Google Scholar]
- 32.Sampath A, Kossoff EH, Furth SL, Pyzik PL, Vining EP. Kidney stones and the ketogenic diet: Risk factors and prevention. J Child Neurol. 2007;22:375–8. doi: 10.1177/0883073807301926. [DOI] [PubMed] [Google Scholar]
- 33.Wheless JW. The ketogenic diet: An effective medical therapy with side effects. J Child Neurol. 2001;16:633–5. doi: 10.1177/088307380101600901. [DOI] [PubMed] [Google Scholar]
- 34.Kwiterovich PO, Jr, Vining EP, Pyzik P, Skolasky R, Jr, Freeman JM. Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA. 2003;290:912–20. doi: 10.1001/jama.290.7.912. [DOI] [PubMed] [Google Scholar]
- 35.Liu YM, Lowe H, Zak MM, Kobayashi J, Chan VW, Donner EJ. Can children with hyperlipidemia receive ketogenic diet for medication-resistant epilepsy? J Child Neurol. 2013;28:479–83. doi: 10.1177/0883073813476140. [DOI] [PubMed] [Google Scholar]
- 36.Best TH, Franz DN, Gilbert DL, Nelson DP, Epstein MR. Cardiac complications in pediatric patients on the ketogenic diet. Neurology. 2000;54:2328–30. doi: 10.1212/wnl.54.12.2328. [DOI] [PubMed] [Google Scholar]
- 37.Sharma S, Gulati S. The ketogenic diet and the QT interval. J Clin Neurosci. 2012;19:181–2. doi: 10.1016/j.jocn.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Martinez CC, Pyzik PL, Kossoff EH. Discontinuing the ketogenic diet in seizure-free children: Recurrence and risk factors. Epilepsia. 2007;48:187–90. doi: 10.1111/j.1528-1167.2006.00911.x. [DOI] [PubMed] [Google Scholar]
- 39.Kossoff EH, Henry BJ, Cervenka MC. Transitioning pediatric patients receiving ketogenic diets for epilepsy into adulthood. Seizure. 2013;22:487–9. doi: 10.1016/j.seizure.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia. 2009;50:1109–17. doi: 10.1111/j.1528-1167.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 41.Raju KN, Gulati S, Kabra M, Agarwala A, Sharma S, Pandey RM, et al. Efficacy of 4:1 (classic) versus 2.5: 1 ketogenic ratio diets in refractory epilepsy in young children: A randomized open labeled study. Epilepsy Res. 2011;96:96–100. doi: 10.1016/j.eplepsyres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Sharma S, Jain P. The modified Atkins diet in refractory epilepsy. Epilepsy Res Treat 2014. 2014:404202. doi: 10.1155/2014/404202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kossoff EH, McGrogan JR, Bluml RM, Pillas DJ, Rubenstein JE, Vining EP. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. 2006;47:421–4. doi: 10.1111/j.1528-1167.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 44.Kossoff EH, Rowley H, Sinha SR, Vining EP. A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia. 2008;49:316–9. doi: 10.1111/j.1528-1167.2007.01256.x. [DOI] [PubMed] [Google Scholar]
- 45.Kang HC, Lee HS, You SJ, Kang DC, Ko TS, Kim HD. Use of a modified Atkins diet in intractable childhood epilepsy. Epilepsia. 2007;48:182–6. doi: 10.1111/j.1528-1167.2006.00910.x. [DOI] [PubMed] [Google Scholar]
- 46.Kossoff EH, Dorward JL, Molinero MR, Holden KR. The modified Atkins diet: A potential treatment for developing countries. Epilepsia. 2008;49:1646–7. doi: 10.1111/j.1528-1167.2008.01580_6.x. [DOI] [PubMed] [Google Scholar]
- 47.Sharma S, Sankhyan N, Gulati S, Agarwala A. Use of the modified Atkins diet in infantile spasms refractory to first-line treatment. Seizure. 2012;21:45–8. doi: 10.1016/j.seizure.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Sharma S, Sankhyan N, Gulati S, Agarwala A. Use of the modified Atkins diet for treatment of refractory childhood epilepsy: A randomized controlled trial. Epilepsia. 2013;54:481–6. doi: 10.1111/epi.12069. [DOI] [PubMed] [Google Scholar]
- 49.Muzykewicz DA, Lyczkowski DA, Memon N, Conant KD, Pfeifer HH, Thiele EA. Efficacy, safety, and tolerability of the low glycemic index treatment in pediatric epilepsy. Epilepsia. 2009;50:1118–26. doi: 10.1111/j.1528-1167.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- 50.Coppola G, D’Aniello A, Messana T, Di Pasquale F, della Corte R, Pascotto A, et al. Low glycemic index diet in children and young adults with refractory epilepsy: First Italian experience. Seizure. 2011;20:526–8. doi: 10.1016/j.seizure.2011.03.008. [DOI] [PubMed] [Google Scholar]


