Abstract
Objectives:
Changes in lifestyle habits such as diet modification or supplementation have been indicated as probable protective factors for a number of chronic conditions including Alzheimer's disease (AD). With this background, we aim to hypothesize that whether C677T polymorphism of methylenetetrahydrofolate reductase (MTHFR) gene contributes towards the risk of developing AD and its association with vitamin B12 and folate levels.
Materials and Methods:
A case-control study comprising of total 200 subjects, within the age group of 50-85 years. Their blood samples were analyzed for serum folate, vitamin B12 levels, and MTHFR C677T polymorphism by restriction fragment length polymorphism (RFLP).
Results:
The mean plasma levels of vitamin B12 and folate were significantly lower in study group when compared to the control group (P < 0.001). Genotypic and allelic frequency of MTHFR gene in both groups was found to be significant (P < 0.05). The intergenotypic variations of vitamin B12 and folate were found to be significant (P < 0.001).
Conclusion:
We concluded that the subjects with homozygous mutated alleles are more prone to AD and also pointed out the influence of presence/absence of MTHFR T allelic variants on serum folate and vitamin B12 levels.
Keywords: Alzheimer's disease, folate, methylenetetrahydrofolate reductase, vitamin B12
Introduction
Alzheimer's disease (AD) is one of the most common forms of dementia affecting mostly the elderly population. It is a progressive and fatal neurodegenerative disorder with characteristic neuropathology and clinical symptomology. AD is characterized by the presence of two aberrant structures in the brain of the patients, senile plaques and neurofibrillary tangles, together with marked neuronal death.[1] Senile plaques are filamentous aggregates of amyloid-beta peptide[2] and microtubule-associated protein tau is the main component of neurofibrillary tangles in AD.[3] AD is an age-related disease with a strong genetic component, but risk is also likely to be influenced by modifiable factors, including diet.[4,5] B-vitamins including folate, vitamin B6, and vitamin B12 may be related to cognitive health in several ways. Low levels of B-vitamins have been associated with increased homocysteine levels and have been observed in the cognitively impaired in several large population-based studies.[6] In addition, B-vitamins may affect levels of S-adenosyl-methionine (SAM), an important intermediate for key methylation reactions in the brain.[6] Finally, folate is essential for nucleic acid formation and deficiency is associated with the chromosomal breakage.[7]
Vitamin B12 is a cofactor for methionine synthase, the enzyme that transfers a methyl group from 5-methyltetrahydrofolate (5-methyl-THF) to homocysteine to form methionine. The conversion of 5, 10 methylene-THF to 5-methyl-THF requires the enzyme, methyltetrahydrofolate reductase. This reaction is irreversible, therefore deficiency of B12, prevents 5-methyl-THF from being converted to 5, 10 methylene-THF, essential for the synthesis of nucleotides. Low levels of nucleotides may result in misincorporation of nucleotides in dexoyribonucleic acid (DNA) replication and chromosomal breaks, which may facilitate neuronal damage common in patient's brains.[7]
The prevalence of B-vitamin deficiencies, especially B12, is highest among elderly persons. Gastritis and other conditions that inhibit vitamin B12 absorption have been estimated to affect 20-50% of the elderly in the US. Depending on the diagnosis criteria, 24% of elders age 60-69 and 37% of those older than 80 were found to have gastritis in the Framingham Study's cohort.[8] Deficiency of folate is also supposed to be higher among the elderly and may increase with age due to decreased absorption caused by changes in the gastrointestinal tract. Folate metabolism, also known as one-carbon metabolism, plays a fundamental role in DNA synthesis and integrity, in chromosome stability, in DNA and protein methylation, as well as in antioxidant defense mechanisms, and impairments of this pathway have been often linked to AD risk.[9,10,11,12]
The human methylenetetrahydrofolate reductase (MTHFR) gene is 20 kb long (20, 336 bp) and located on the short (p) arm of chromosome 1 at position 36.3, having 11 exons. More than 40 polymorphisms have been described in MTHFR, but the most common and clinically important variants are C677T in exon 4 and A1298C in exon 7. Several studies have investigated the 677C → T polymorphism of the MTHFR gene as a risk factor for these conditions, as the C-to-T transition causes reduced enzyme activity, and alters the folate and vitamins B12 levels.[13]
In the coming years, the number of individuals with AD will augment as the elderly population worldwide is anticipated to grow significantly, thus, putting an added strain on healthcare systems. Thus it has been suggested that early intervention strategies, which delay or halt the disease progression will have a strong impact on clinical outcomes. Changes in lifestyle habits such as diet modification or supplementation have been indicated as probable protective factors for a number of chronic conditions including AD. With this background, we aim to hypothesize that whether C677T polymorphism of MTHFR gene contributes towards the risk of developing AD and its association with vitamin B12 and folate levels.
Materials and Methods
Study population and design
The study was conducted during the period February 2010-August 2013. A total of 200 subjects were enrolled for the study, belonging to the State of Delhi and other surrounding states of North India. All the subjects were between 50 and 85 years of age. A detailed history was taken from each subject and/or his family. The sample size was calculated (α = 0.05, β = 0.20, and power = 0.80) by using PS software, version 3.0.14.
Study Group comprised of 100 patients within the age group of 50-85 years with complaint of memory or other cognitive impairment, who were subjected to define by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA)[14] for AD and a magnetic resonance imaging/computed tomography/positron emission tomography (MRI/CT/PET) brain scan supporting the clinical diagnosis of AD. Additional inclusion criteria were a score of < 23 on the Mini-Mental State Examination (MMSE) and a Clinical Dementia Rating score of ≥ 0.5. Control group comprised of healthy volunteers within the age group of 50-85 years and all of them came to the hospital for routine health checkup.
Subjects were excluded in both case and control groups if there was no consent for participation in the study, history of cerebral stroke, epilepsy, a history of head trauma, and other concomitant disease potentially associated with dementia, chronic intake of drugs affecting cognitive processes, moderate to severe depressive episode, and familial history of any kind of cognitive/behavioral abnormality. The study protocol and informed consent form (ICF) were reviewed and approved by the Institutional Ethics Committee.
Estimation of Vitamin B12
Serum vitamin B12 were estimated in samples collected after overnight fasting by chemiluminescent analyzer (Roch, Elecsys 2010) using commercially available kit purchased from Roche Diagnostics GmbH, USA.
Estimation of folate
Serum folate levels were estimated in samples collected after overnight fasting by chemiluminescent analyzer (Roch, Elecsys 2010) using commercially available kit purchased from Roche Diagnostics GmbH, USA.
Genetic analysis
DNA extraction
DNA extraction was performed using GeneJET genomic DNA purification kit purchased from Fermentas Life Science, EU. The kit utilizes silica-based membrane technology in the form of a convenient spin column; eliminating the need for expensive resins, toxic phenol-chloroform extraction, or time-consuming alcohol precipitation. The standard procedure takes less than 20 min following the cell lysis and yields purified DNA of more than 30 kb in size.
MTHFR genotyping
This polymorphism arises due to the single nucleotide substitution mutation (C → T) at 677th position of the MTHFR gene. The fourth exon which encodes amino acid residues 222 was amplified by using the specific primers 5’-TGAAGGAGAAGGTGTATGAGGGA-3’ and 5’-AG GACGGTGCGGTGAGAGTG-3’[15] (Dorszewska et al., 2007). Polymerase chain reaction (PCR) reaction mixture containing 1΄ high-fidelity master mix (0.04 U/μl DNA polymerase, 1.5 mM MgCl2, and 200 μM dNTPs), 10 ng of genomic DNA, and 0.25 μM of each primer in 20.0 μl volume was used. PCR profile consisted of a 1 min hold at 98°C followed by 35 cycles of 98°C (30 s), 61°C (30 s), and 72°C (30 s) and final extension 72°C for 3 min. PCR product (198 bp) was digested with 10 units of Hinf1 and run on 12% polyacrylamide gel followed by the ethidium bromide staining. Allelic sizes were compared with known molecular weight marker. The CC (Ala/Ala) genotype contained 198 bp fragment; the CT (Ala/Val) genotype contained 198, 175, and 23 bp fragment; and the TT (Val/Val) genotype contained 175 and 23 bp fragment [Figure 1].
Figure 1.
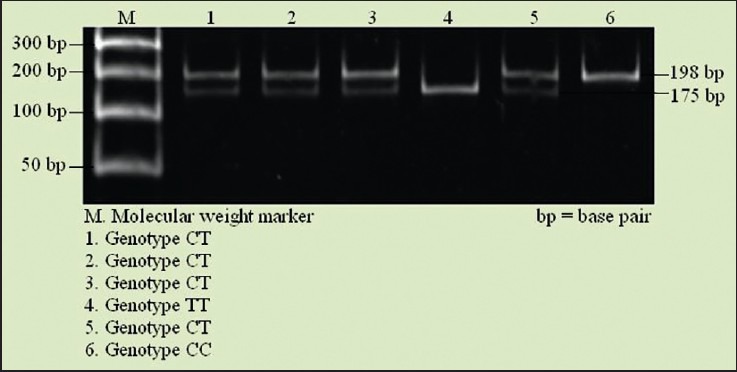
Polyacrylamide gel picture show the methylenetetrahydrofolate reductase (MTHFR) gene polymorphism
Statistical analysis
All statistical analyses were performed by using Statistical Package for Social Sciences (SPSS, version 17.0). Mean±standard errors were calculated to describe the quantitative data, whereas, percentages were calculated to describe qualitative data. The sociodemographic categorical variables were compared by the chi-square tests (χ2) tests. The Student's t-test was used for normally distributed continuous variables. Kruskal-Wallis test was used to assess the intergenotypic levels of variables on prediction of risk for AD. Genotype distributions between AD and controls were compared by χ2 tests. Odds ratio (OR) and their 95% confidential intervals (CIs) were calculated by 2 × 2 contingency tables. All tests were two-tailed and P < 0.05 was considered as significant for result interpretation.
Results
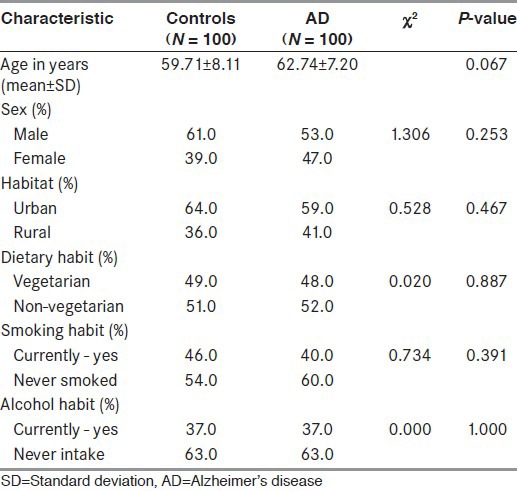
No significant difference was found between cases and controls in terms of demographic characteristics. Cases and controls were homogenous in terms of sex, habitat, dietary habit, smoking habit, and alcoholic intake [Table 1]
Table 1.
Demographic characteristics of the study population
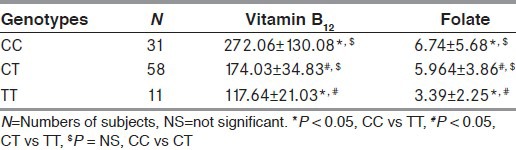
The comparative levels of serum vitamin B12 and folate in healthy subjects and AD are presented in Table 2. The observed values of vitamin B12 were found to be significantly lower in AD as compared to healthy subjects. Similarly, significant lower levels of folate were found in AD as compared to healthy subjects (P < 0.001).
Table 2.
Intergenotypic variations of vitamin B12 and folate in AD patients
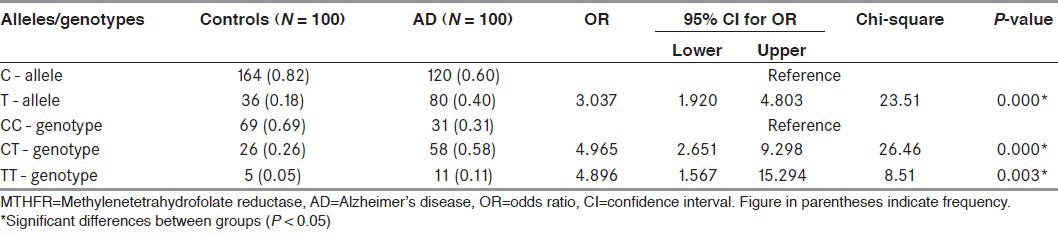
Significant differences were found among genotype and allele frequencies of the MTHFR C677T gene in the study and the control groups and are shown in Table 3. The frequency of C allele was 60% in study group and 82% in control group, while the frequency of T allele was 40 and 18% in study and control group, respectively. In our study we found that, subjects homozygous for the T677 allele were 3.0 times (95% CI, 1.9-4.8; P < 0.001) more likely to develop AD compared with carriers for the C677 allele.
Table 3.
Frequencies and genotype distribution of MTHFR gene in control and AD patients
When the study groups were further subdivided on the basis of the presence of CC, CT, and TT genotypes, we have seen significant intergenotypic variations of vitamin B12 and folate between both study as well as control group (P < 0.001), while we have not found any significant difference of folate levels among study group [Table 2].
Discussion
This was an in-depth study in which we aimed to demonstrate that AD is associated with MTHFR gene polymorphism and significantly decreasing folate and vitamin B12 levels, causing neurodegeneration. In the present study, we found significant association between 677C > T polymorphism and AD, which is supported by recent meta-analysis, which showed that the MTHFR 677C > T polymorphism can cause AD susceptibility in east Asians.[16] In one of the study, MTHFR gene mutations were related to schizophrenia, depression, and bipolar disorder.[17] Even Roffman et al., showed that MTHFR C677T mutation was associated with functional deficiency in operational memory and schizophrenia.[18]
The result of our study showed that 69.0% of AD patients carried at least one copy of MTHFR T allele, which is significant when comparing with control (31.0 %). The patients suffering from AD are 4.95 times more susceptible when having MTHFR T allele. Among the genotypes, CT and TT show a strong significant association with AD recording an odd ratio 4.96 and 4.89 respectively. Interestingly, a study showed that no significant associations of MTHFR C677T allele and genotype with AD were observed, but significant associations of T allele and TT genotype with AD were identified in APOE ε4 carrier subgroup, suggesting that MTHFR 677 T allele and APOE ε4 allele may synergistically act to increase AD risk.[16]
AD and other neurodegenerative disorder are all associated with chronic neuroinflammation and oxidative stress. It is possible that these clinical associations reflect compromised vitamin B12 metabolism due to such stress.[19] Folate deficiency is a risk factor for neural tube defects and late in life for cognitive decline and AD, during embryogenesis. It induces several Alzheimer pathomechanisms like oxidative stress, Ca (++) influx, and accumulation of hyperphosphorylated tau and β-amyloid.[20]
In the present study, we found significant lower levels of folate and vitamin B12 in AD as compared to healthy controls. Coppede et al.,[21] also observed significantly decreased serum folate levels in AD subjects with respect to controls, which is in accordance to our study. Folates appear to be of elementary importance in brain growth, differentiation, development, repair, mood, cognition, and ageing.[22,23,24] These functions and their breakdown in folate and vitamin B12 deficiency are perhaps primarily mediated through nucleotide synthesis, DNA integrity, and transcription.[24] Neuropsychological studies have found broad and precise impairments of intellectual function including concentration and episodic memory and present a theoretical reasoning that credited to folate deficiency.[22,25] Studies in mice has established that slighter degrees of gestational folate deficiency resulted in a slaughter of progenitor cells, a total decline of cells in the fetal brain, reduced brain weight, and anxiety-related behavior in the offspring; suggesting an elementary outcome of prenatal folate status on neurodevelopment and behavioral recital later in life.[26,27] Hinterberger and Fischer[20] have recently reported that low folate and high cortisol are the only predictors of AD after the age of 75 years old. Moore et al.,[28] showed that low serum levels of vitamin B12 are allied with neurodegenerative disease and cognitive impairment and that vitamin B12 therapy does not improve cognition in patients without preexisting deficiency. Folate and vitamin B12 are involved in processes important for central nervous system function and have been associated with a diversity of diseases (Burdge et al., 2012).[13,29] The vitamin B12 and folate, both are of diagnostic importance for the related causes.
In this study, we aimed to determine the intergenotypic levels of folate and vitamin B12 in study subjects, as those are our main areas for examining the correlation of this polymorphism with these indicators. Subjects with the TT genotype demonstrated decreased vitamin B12 and folate levels as compared with subjects with the CC genotype, also significant difference was seen in subjects having the CT genotype when compared with subjects with the CC genotype. This points towards the fact that subjects with homozygous mutated alleles are more prone to AD.
Our study has also pointed out the influence of presence of MTHFR T allelic variants on serum folate and vitamin B12 levels.
Acknowledgment
We are grateful to the Indian Council of Medical Research (ICMR), New Delhi, India, for the financial assistance during the study as Senior Research Fellow.
Footnotes
Source of Support: We are grateful to the Indian Council of Medical Research (ICMR), New Delhi, India, for the financial assistance during the study as Senior Research Fellow
Conflict of Interest: None declared
References
- 1.Alzheimer A. Uber eine eigenartige Erkrankung der Hirninde. Zeitschrift fur Psychiatrie und Psychisch-Gerichtliche Medizin. 1907;64:146–8. [Google Scholar]
- 2.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–9. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–9. [PubMed] [Google Scholar]
- 4.Pope SK, Shue VM, Beck C. Will a healthy lifestyle help prevent Alzheimer's disease? Annu Rev Public Health. 2003;24:111–32. doi: 10.1146/annurev.publhealth.24.100901.141015. [DOI] [PubMed] [Google Scholar]
- 5.Munoz DG, Feldman H. Causes of Alzheimer's disease. CMAJ. 2000;162:65–72. [PMC free article] [PubMed] [Google Scholar]
- 6.Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. 2002;6:39–42. [PubMed] [Google Scholar]
- 7.Blount BC, Mack MM, Wehr CM, MacGregor JT, Hiatt RA, Wang G, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94:3290–5. doi: 10.1073/pnas.94.7.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selhub J, Bagley LC, Miller J, Rosenberg IH. B vitamins, homocysteine, and neurocognitive function in the elderly. Am J Clin Nutr. 2000;71:614S–20S. doi: 10.1093/ajcn/71.2.614s. [DOI] [PubMed] [Google Scholar]
- 9.Van Dam F, Van Gool WA. Hyperhomocysteinemia and Alzheimer's disease: A systematic review. Arch Gerontol Geriatr. 2009;48:425–30. doi: 10.1016/j.archger.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Coppedè F. One-carbon metabolism and Alzheimer's disease: Focus on epigenetics. Curr Genomics. 2010;11:246–60. doi: 10.2174/138920210791233090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wald DS, Kasturiratne A, Simmonds M. Serum homocysteine and dementia: Meta-analysis of eight cohort studies including 8669 participants. Alzheimers Dement. 2011;7:412–7. doi: 10.1016/j.jalz.2010.08.234. [DOI] [PubMed] [Google Scholar]
- 12.Ho RC, Cheung MW, Fu E, Win HH, Zaw MH, Ng A, et al. Is high homocysteine level a risk factor for cognitive decline in elderly. A systematic review, meta-analysis, and meta-regression? Am J Geriatr Psychiatry. 2011;19:607–17. doi: 10.1097/JGP.0b013e3181f17eed. [DOI] [PubMed] [Google Scholar]
- 13.Stover PJ. Polymorphisms in 1-carbon metabolism, epigenetics and folate-related pathologies. J Nutrigenet Nutrigenomics. 2011;4:293–305. doi: 10.1159/000334586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Dorszewska J, Florczak J, Rozycka A, Kempisty B, Jaroszewska-Kolecka J, Chojnacka K, et al. Oxidative DNA damage and level of thiols as related to polymorphisms of MTHFR, MTR, MTHFD1 in Alzheimer's and Parkinson's diseases. Acta Neurobiol Exp (Wars) 2007;67:113–29. doi: 10.55782/ane-2007-1639. [DOI] [PubMed] [Google Scholar]
- 16.Bi XH, Zhao HL, Zhang ZX, Zhang JW. Association of RFC1 A80G and MTHFR C677T polymorphisms with Alzheimer's disease. Neurobiol Aging. 2009;30:1601–7. doi: 10.1016/j.neurobiolaging.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: A HuGE review. Am J Epidemiol. 2007;165:1–13. doi: 10.1093/aje/kwj347. [DOI] [PubMed] [Google Scholar]
- 18.Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Purcell S, et al. Effects of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism on executive function in schizophrenia. Schizophr Res. 2007;92:181–8. doi: 10.1016/j.schres.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 19.McCaddon A. Vitamin B12 in neurology and ageing; clinical and genetic aspects. Biochimie. 2013;95:1066–76. doi: 10.1016/j.biochi.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Hinterberger M, Fischer P. Folate and Alzheimer: When time matters. J Neural Transm. 2013;120:211–24. doi: 10.1007/s00702-012-0822-y. [DOI] [PubMed] [Google Scholar]
- 21.Coppede F, Tannorella P, Pezzini I, Migheli F, Ricci G, Caldarazzo lenco E, et al. Folate, homocysteine, vitamin B12, and polymorphisms of genes participating in one-carbon metabolism in late-onset Alzheimer's disease patients and healthy controls. Antioxid Redox Signal. 2012;17:195–204. doi: 10.1089/ars.2011.4368. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds EH. Folic acid, ageing, depression, and dementia. BMJ. 2002;324:1512–5. doi: 10.1136/bmj.324.7352.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iskandar BJ, Nelson A, Resnick D, Skene JH, Gao P, Johnson C, et al. Folic acid supplementation enhances repair in the adult central nervous system. Ann Neurol. 2004;56:221–7. doi: 10.1002/ana.20174. [DOI] [PubMed] [Google Scholar]
- 24.Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–46. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 25.Goodwin JS, Goodwin JM, Garry PJ. Association between nutritional status and cognitive functioning in a healthy elderly population. JAMA. 1983;249:2917–21. [PubMed] [Google Scholar]
- 26.Craciunescu CN, Brown EC, Mar MH, Albright CD, Nadeau MR, Zeisel SH. Folic acid deficiency during late gestation decreases progenitor cell proliferation and increases apoptosis in fetal mouse brain. J Nutr. 2004;134:162–6. doi: 10.1093/jn/134.1.162. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson SA, Berry KJ, Hansen DK, Wall KS, White G, Antony AC. Behavioral effects of prenatal folate deficiency in mice. Birth Defects Res A Clin Mol Teratol. 2005;73:249–52. doi: 10.1002/bdra.20111. [DOI] [PubMed] [Google Scholar]
- 28.Moore E, Mander A, Ames D, Carne R, Sanders K, Watters D. Cognitive impairment and vitamin B12: A review. Int Psychogeriatr. 2012;24:541–56. doi: 10.1017/S1041610211002511. [DOI] [PubMed] [Google Scholar]
- 29.Burdge GC, Hoile SP, Lillycrop KA. Epigenetics: Are there implications for personalised nutrition? Curr Opin Clin Nutr Metab Care. 2012;15:442–7. doi: 10.1097/MCO.0b013e3283567dd2. [DOI] [PubMed] [Google Scholar]


