Abstract
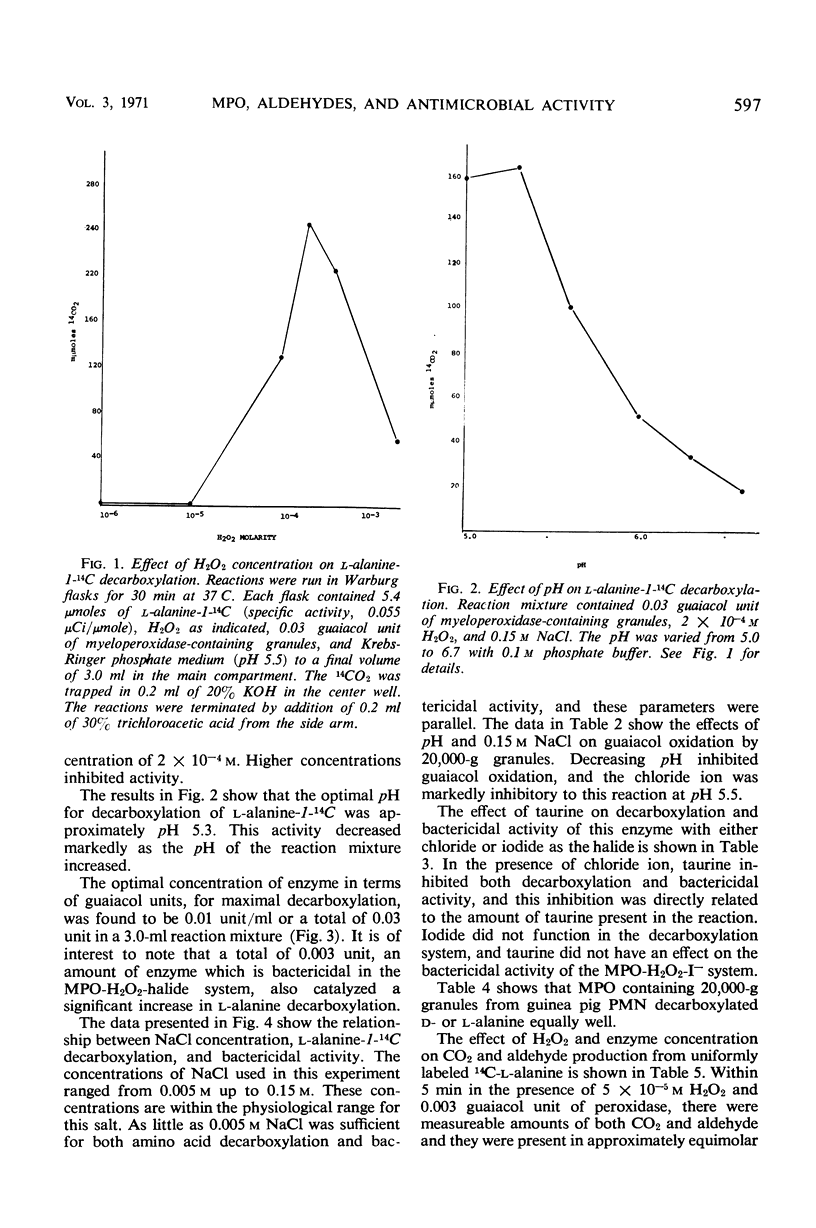
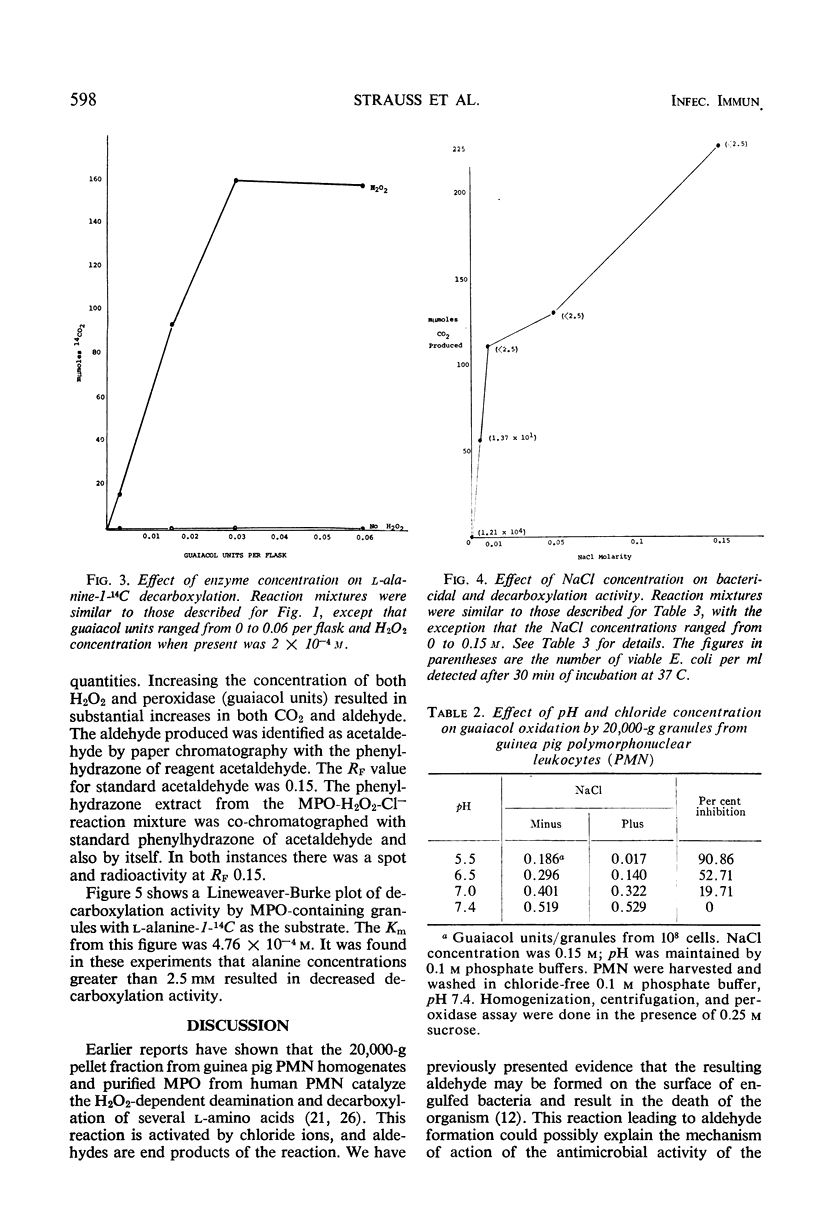
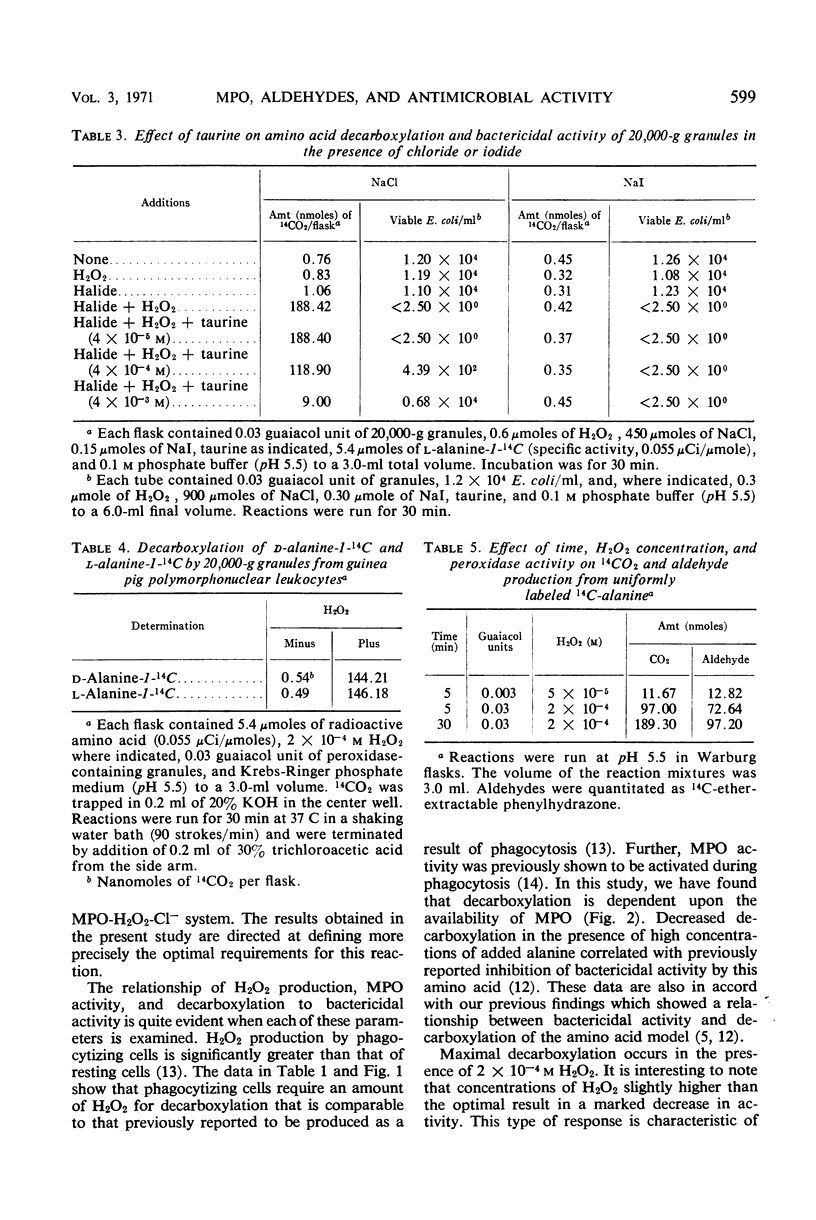
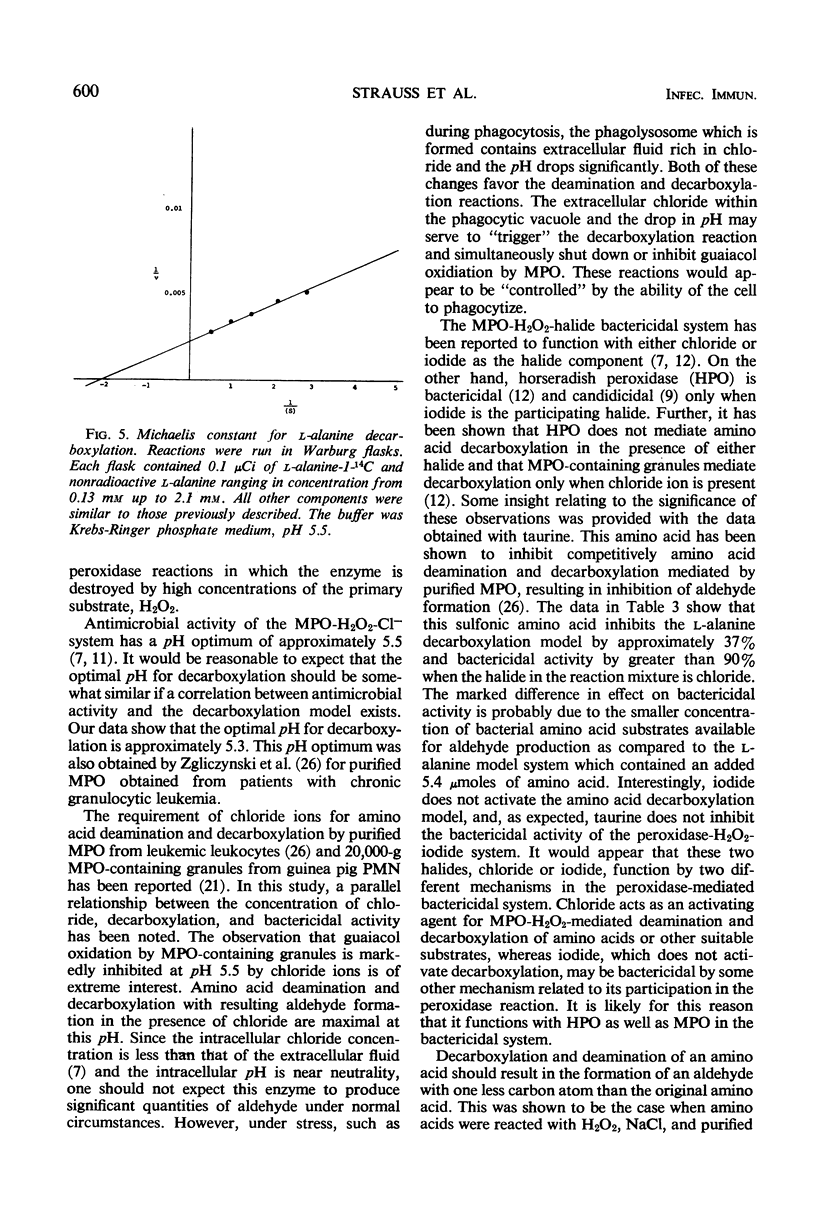
Evidence is presented which suggests that the mechanism of action of the myeloperoxidase-H2O2-Cl− antimicrobial system in the phagocyte is by the formation of aldehydes. Aldehyde production resulting from myeloperoxidase-mediated decarboxylation and deamination of alanine was quantitated with 20,000-g granules from guinea pig polymorphonuclear leukocytes serving as the enzyme. Equimolar quantities of acetaldehyde and CO2 were obtained. There was an absolute requirement for both H2O2 and Cl− for decarboxylation by the myeloperoxidase-containing granules. The myeloperoxidase-H2O2-Cl− system decarboxylated both d- or l-alanine equally and had a pH optimum of 5.3. Decarboxylation of l-alanine by intact guinea pig polymorphonuclear leukocytes was increased 2.5-fold by phagocytosis. Guaiacol peroxidation by the granules was inhibited 90% in the presence of Cl− at acid pH. Under these conditions, decarboxylation and deamination of amino acids by myeloperoxidase were significantly stimulated, resulting in aldehyde production. Taurine, a competitive inhibitor of amino acid decarboxylation, inhibited bactericidal activity of the myeloperoxidase-H2O2-Cl− system but had no effect on the myeloperoxidase-H2O2-I− bactericidal system. Since the myeloperoxidase-H2O2-I− system does not participate in amino acid decarboxylation, its mechanism of antimicrobial action appears to be different from that found with Cl−.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAGAN R. H., KARNOVSKY M. L. ENZYMATIC BASIS OF THE RESPIRATORY STIMULATION DURING PHAGOCYTOSIS. Nature. 1964 Oct 17;204:255–257. doi: 10.1038/204255a0. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. D-amino acid oxidase in leukocytes: a possible D-amino-acid-linked antimicrobial system. Proc Natl Acad Sci U S A. 1969 Mar;62(3):756–763. doi: 10.1073/pnas.62.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med. 1956 May 1;103(5):589–611. doi: 10.1084/jem.103.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER G. Y., QUESTEL J. H. NADPH and NADH oxidation by guinea pig polymorphonuclear leucocytes. Can J Biochem Physiol. 1963 Feb;41:427–434. [PubMed] [Google Scholar]
- Jacobs A. A., Paul B. B., Strauss R. R., Sbarra A. J. The role of the phagocyte in host-parasite interactions. 23. Relation of bactericidal activity to peroxidase-associated decarboxylation and deamination. Biochem Biophys Res Commun. 1970 Apr 24;39(2):284–289. doi: 10.1016/0006-291x(70)90791-6. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Antifungal effects of peroxidase systems. J Bacteriol. 1969 Aug;99(2):361–365. doi: 10.1128/jb.99.2.361-365.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XI. Relationship between stimulated oxidative metabolism and hydrogen peroxide formation, and intracellular killing. J Bacteriol. 1967 Nov;94(5):1417–1424. doi: 10.1128/jb.94.5.1417-1424.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B. B., Jacobs A. A., Strauss R. R., Sbarra A. J. Role of the Phagocyte in Host-Parasite Interactions XXIV. Aldehyde Generation by the Myeloperoxidase-H(2)O(2)-Chloride Antimicrobial System: a Possible In Vivo Mechanism of Action. Infect Immun. 1970 Oct;2(4):414–418. doi: 10.1128/iai.2.4.414-418.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B. B., Strauss R. R., Jacobs A. A., Sbarra A. J. Function of h(2)o(2), myeloperoxidase, and hexose monophosphate shunt enzymes in phagocytizing cells from different species. Infect Immun. 1970 Apr;1(4):338–344. doi: 10.1128/iai.1.4.338-344.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B., Sbarra A. J. The role of the phagocyte in host-parasite interactions. 13. The direct quantitative estimation of H2O2 in phagocytizing cells. Biochim Biophys Acta. 1968 Feb 1;156(1):168–178. doi: 10.1016/0304-4165(68)90116-5. [DOI] [PubMed] [Google Scholar]
- Reed P. W. Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem. 1969 May 10;244(9):2459–2464. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Sbarra A. J., Jacobs A. A., Strauss R. R., Paul B. B., Mitchell G. W., Jr The biochemical and antimicrobial activities of phagocytizing cells. Am J Clin Nutr. 1971 Feb;24(2):272–281. doi: 10.1093/ajcn/24.2.272. [DOI] [PubMed] [Google Scholar]
- Skarnes R. C. Leukin, a bactericidal agent from rabbit polymorphonuclear leucocytes. Nature. 1967 Nov 25;216(5117):806–808. doi: 10.1038/216806a0. [DOI] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Jacobs A. A., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XXII. H2O2-dependent decarbosylation and deamination by myeloperoxidase and its relationship to antimicrobial activity. J Reticuloendothel Soc. 1970 Jun;7(6):754–761. [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Jacobs A. A., Sbarra A. J. The role of the phagocyte in host-parasite interactions. XIX. Leukocytic glutathione reductase and its involvement in phagocytosis. Arch Biochem Biophys. 1969 Dec;135(1):265–271. doi: 10.1016/0003-9861(69)90539-6. [DOI] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Sbarra A. J. Effect of phenylbutazone on phagocytosis and intracellular killing by guinea pig polymorphonuclear leukocytes. J Bacteriol. 1968 Dec;96(6):1982–1990. doi: 10.1128/jb.96.6.1982-1990.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEYA H. I., SPITZNAGEL J. K. ANTIBACTERIAL AND ENZYMIC BASIC PROTEINS FROM LEUKOCYTE LYSOSOMES: SEPARATION AND IDENTIFICATION. Science. 1963 Nov 22;142(3595):1085–1087. doi: 10.1126/science.142.3595.1085. [DOI] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Ostrowiski W., Naskalski J., Sznajd J. Myeloperoxidase of human leukaemic leucocytes. Oxidation of amino acids in the presence of hydrogen peroxide. Eur J Biochem. 1968 May;4(4):540–547. doi: 10.1111/j.1432-1033.1968.tb00246.x. [DOI] [PubMed] [Google Scholar]



