Abstract
Background:
Fatigue contributes significantly to the morbidity and affects the quality of life adversely in Guillain-Barre Syndrome (GBS).
Objective:
To determine the prevalence of fatigue in GBS in neurological rehabilitation setting and to study its clinical correlates.
Materials and Methods:
We performed secondary analysis of data of patients with GBS admitted in neurological rehabilitation ward of a tertiary care centre, recorded at both admission and discharge. Assessment of fatigue was done by Fatigue Severity Scale (FSS), disability-status by Hughe's Disability Scale (HDS), functional-status by Barthel Index, anxiety/depression by Hospital Anxiety Depression Scale, sleep disturbances by Pittsburgh Sleep Quality Index and muscle weakness by Medical Research Council sum scores.
Results:
A total of 90 patients (62 men) with mean age 34 years (95% CI 32.2, 37.7) were included. Median duration of, stay at neurological rehabilitation ward was 30 days, while that of symptoms was 18.5 days. Presence of fatigue at admission (FSS ≥ 4 in 39% patients) was associated with ventilator requirement (P = 0.021) and neuropathic pain (P = 0.03). Presence of fatigue at discharge (FSS ≥ 4 in 12% patients) was associated with disability- HDS (≥3) (P = 0.008), presence of anxiety (P = 0.042) and duration of stay at rehabilitation ward (P = 0.02). Fatigue did not correlate with age, gender, antecedent illness, muscle weakness, depression and sleep disturbances.
Conclusion:
Fatigue is prevalent in GBS during early recovery phase of illness. Despite motor recovery fatigue may persist. Knowledge about fatigue as burden of disease in these patients will improve patient care.
Keywords: Fatigue, Guillain Barre syndrome, rehabilitation
Introduction
The outcome is good in majority of the patients with Guillain-Barre syndrome (GBS) in terms of motor recovery and functional independence.[1] However, about 20% are left with severe motor disability at 1 year.[2,3] Studies have shown that despite relatively good neurological recovery, the majority of patients with GBS remain severely fatigued, independent of severity of residual neurological deficits.[4,5,6] Fatigue contributes significantly to the morbidity and affects the quality of life adversely. Thus, fatigue is an important part of the burden of disease in GBS. Attention in these illnesses is primarily directed towards weakness and sensory disturbances, and it is suggested that fatigue may have been underrecognized.[7]
It is often difficult to assess the severity of fatigue among individuals. The broad range of mechanisms underlying fatigue, its multidimensional character, confounding factors, and different manifestations of fatigue make it difficult to measure. The other factors that influence fatigue includes anemia, hypothyroidism, diabetes mellitus, depression, presence of any infection, inflammation or malignancy, sleep disturbances, and certain drugs like sedatives, antidepressants, etc.[8]
Most of the studies[6,9,10,11] have focused on the prevalence of fatigue, following maximal recovery. After extensive literature search, we could not find any study that has seen prevalence of fatigue in GBS in subacute setting. The aim of the current study was to determine the prevalence of fatigue in patients with GBS in rehabilitation setting and to find clinical correlates of fatigue.
Materials and Methods
The study involved secondary analysis of data that was collected as part of the standard care for the patients with GBS, who were admitted for inpatient rehabilitation. Data of patients diagnosed with GBS (satisfying National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) criteria),[12] who had received treatment based on current practice guidelines with significant disability; Hughes’Disability Scale (HDS) between F4 and F2 with upper limb weakness, in the age range of 16-65 years, admitted in Department of Neurological Rehabilitation for at least 2 weeks, between January 2010 and December 2012 was collected. The records of patients with incomplete data were excluded. The study was approved by institutional ethics committee.
As per the standard guidelines, these patients were evaluated for their clinical status. Demographic data with regards to age and gender was recorded. Enquiry about GBS included duration of illness, weakness, and history of antecedent factors if any, history of respiratory distress or requirement for ventilator assistance, presence of pain and paresthesias, and presence of comorbid conditions. Medical Research Council (MRC) sum-scores scale, HDS, Barthel Index (BI) scale, Fatigue Severity Scale (FSS), Hospital Anxiety and Depression Scale (HADS), and Pittsburgh Sleep Quality Index (PSQI) were administered within 24-48 h of admission. Each patient received individualized inpatient rehabilitation program. The patients were discharged once the set goals of rehabilitation were achieved. The patients were reassessed with above mentioned scales, 24-48 h before discharge.
Study tools
Fatigue was assessed using FSS in our study. It is a self-report questionnaire and measures fatigue by assessing the consequences of fatigue on daily functioning. A mean score on these statements ranges from 1 (no signs of fatigue) to 7 (most disabling fatigue). An average score of 4 and higher is indicative for fatigue and a score of 5 and higher for severe fatigue. This questionnaire has been applied to patients with GBS and shown to be most appropriate for evaluating fatigue. The FSS demonstrated good internal consistency (Cronbach's alpha coefficient = 0.88), test-retest reliability (Cohen's kappa value = 0.84), and discriminative validity in studies among patients with immune mediated polyneuropathies, multiple sclerosis, and Pompe disease.[13,14,15]
HDS is used to assess outcome in GBS patients and focuses mainly on walking. It ranges from grade 0 to grade 6 (grade 0 = healthy; grade 1 = minor signs or symptoms of neuropathy, but capable of manual work; grade 2 = able to walk without support of a stick, but incapable of manual work; grade 3 = able to walk with a stick, appliance, or support; grade 4 = confined to bed or chair bound; grade 5 = requiring assisted ventilation; grade 6 = dead).[16]
MRC sum-score ranges between 0 (paralysis) and 60 (normal strength). It is the sum of MRC grading of three muscles of upper limb (shoulder abductor, elbow flexor, and wrist extensors) and three muscles of lower limb (hip flexor, knee extensor, and ankle dorsiflexor). Score of <48 is considered as significant weakness and score of <36 as severe weakness.[17]
HADS is used to identify caseness (possible and probable) of anxiety disorders and depression among patients in non-psychiatric hospital clinics. It is divided into an anxiety subscale (HADS-anxiety subscale (HADS-A)) and a depression subscale (HADS-depression subscale (HADS-D)), both containing seven intermingled items. It is a self-assessment scale, which is also a valid measure of severity of the emotional disorder.[18]
BI consists of 10 items (bladder and bowel in the preceding week, grooming in preceding 24-48 h, toilet use, feeding, transfers, mobility, dressing, stairs, and bathing) with score ranging from 0 to 100. Scores from 0 to 20 indicate total dependence; 21-60, severe dependence; 61-90, moderate dependence; 91-99, slight dependence; and 100, independence. This has been used earlier in studies on GBS.[19]
PSQI is a self-administered questionnaire which assesses sleep quality and disturbances. Nineteen individual items generate seven “component” scores: Subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. Each component score ranges from 0 (no difficulty) to 3 (severe difficulty). The component scores are summed to produce a global score (range of 0-21). A PSQI global score ≥5 is considered to be suggestive of significant sleep disturbance. The scale has satisfactory internal consistency and adequate test-retest reliability.[20]
Data analysis
Statistical analysis of data was performed by Stata 11 (Stata Corp, 2009. College Station, TX). Cross tables were analyzed using Pearson's chi-squared test or Fisher's exact probability test. Continuous variables were compared (across two categories) using independent samples t-test. The significance of P-value was adjudged against an alpha of 0.05.
In this study, we did comparison between two groups only, that is, those with no fatigue and those with fatigue. This comparison was made at two points of time—at admission and at discharge. At each time point, for example, at admission, we compared for outcomes between those with fatigue and those without fatigue. For continuous variables, we applied independent samples t-test. For categorical variables, we applied chi-squared test of independence.
Results
A total 97 patients satisfied inclusion criteria. Out of these, data for five patients could not be retrieved from medical records, while the records were incomplete for another two patients. Thus, study included 90 patients. The mean age was 34.9 years (95% CI. 32.2, 37.7) and there were 62 (68.8%) men. The median duration of symptoms at the time of admission in neurological rehabilitation was 18.5 days (range 9-103 days) and the median duration of stay was 30 days (range 14-75 days). Electrophysiologically, patients were categorized according to Hadden's criteria[21] (primary demyelinating- 59 (67%), axonal-five (5.6%), inexcitable-nine (10.2%), equivocal-12 (13.6%), normal types-three (3.4%), and refusal for study- 2).
Historically, antecedent events were observed in 35 (39.9%) patients, respiratory distress was present in 14 (15.5%) and six (6.6%) required ventilator assistance. Facial palsy was seen in 43 (47.8%) and bulbar symptoms in 19 (21.1%) patients. Sensory symptoms were prevalent in the form of neuropathic pain in 64 (71.1%) and paresthesias in 54 (60%) patients. Bladder disturbances were present in three (3.3%) and dysautonomia in one (1.1%) patient. Among comorbidities, five (5.56%) patients had diabetes mellitus, 21 (23.3%) hypertension, seven (7.8%) dyslipidemia, seven (7.8%) anemia, and one (1.1%) hypothyroidism.
Thirty-five patients (38.9%) reported fatigue (FSS ≥4) at the time of admission, out of which 15 patients had severe fatigue (FSS ≥5). Eleven patients (12.2%) had fatigue at the time of discharge, out of which four (4.4%) had severe fatigue. On comparing the presence of fatigue at time of discharge with fatigue at admission, a significant correlation (P = 0.002) was found.
Comparison of various parameters among patients with GBS with and without fatigue, at time of admission and discharge are shown in Tables 1 and 2. It was seen that requirement for ventilator and presence of neuropathic pain were significantly associated with the presence of fatigue at the time of admission. None of the other factors were significantly associated. At the time of discharge, HDS score (≥ 3) and presence of anxiety was significantly correlated.
Table 1.
Clinical correlates of fatigue at admission in neurological rehabilitation
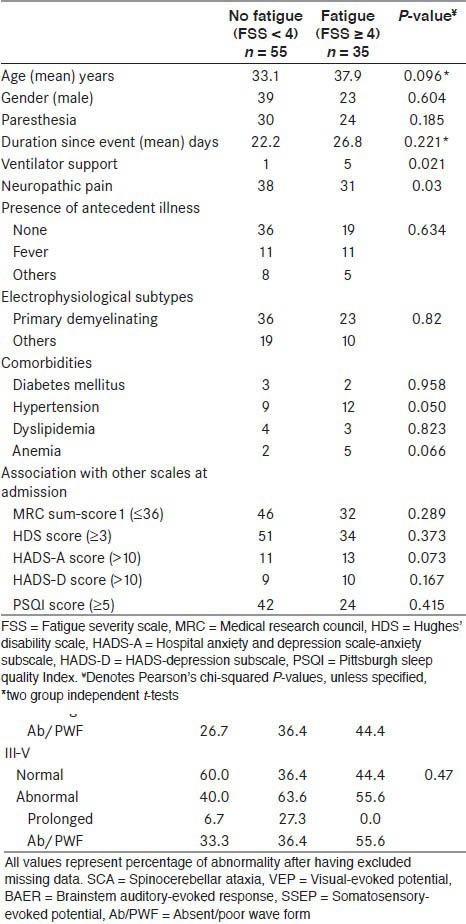
Table 2.
Clinical correlates of fatigue at discharge from neurological rehabilitation
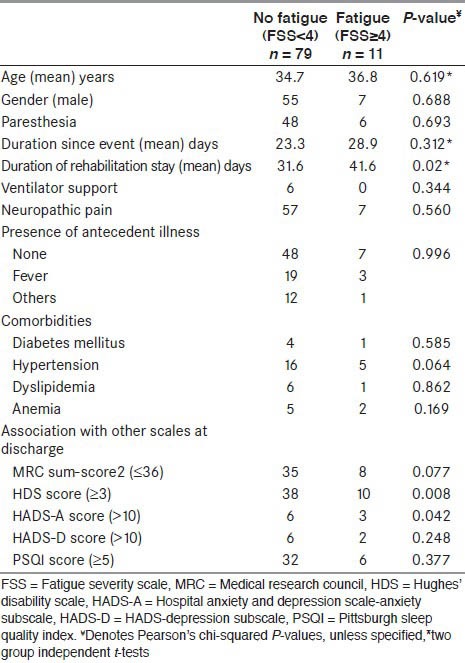
The mean duration of stay at neurological rehabilitation ward was 32.8 days. The mean duration of stay for those without fatigue was approximately lesser by 10 days compared to those with fatigue. At 5% level of significance, this was significant at P = 0.02.
Discussion
Out of the 90 patients, prevalence of fatigue (FSS ≥ 4) decreased from 38.9% at admission to 12.2% at the time of discharge from neurological rehabilitation. The prevalence of fatigue in patients with neuromuscular disorders ranges from 38 to 86%.[10] However, one recent study shows prevalence of severe fatigue using FSS in 30% of the patients of GBS, after more than 1 year of onset of disease.[22]
The lower prevalence of severe fatigue in our study could be due to the fact that the patients were assessed early in the recovery phase, when patients had significant disability. During this period, fatigue might not be the primary concern as compared to functional disability due to motor weakness. Other studies have been done in patients following motor recovery where patients were independent in ambulation. It may be assumed that with motor recovery burden of fatigue might be more forthcoming.
We did not find any association between fatigue and age, as in study by Drory et al.,[10] although one study[11] reported that fatigue was more common in patients with age more than 50 years. While the studies by Garssen et al.,[11] and Merkies et al.,[4] showed higher prevalence of fatigue in females, we found no association with gender, similar to study by Drory et al.[10] In accordance with study by Garssen et al.,[11] there was no association of fatigue with presence of antecedent events or any electrophysiological subtype. Six patients required ventilator assistance in acute phase and it was significantly related to presence of fatigue at time of admission in neurological rehabilitation. This could imply that fatigue was more prevalent in severe form of disease.
In our study, among 27 patients who had one or more comorbidities (such as, diabetes mellitus, hypertension, dyslipidemia, and anemia), only the presence of hypertension showed an association at a P-value of 0.05; though with this P-value, we could not establish any significant association for presence of hypertension with fatigue in GBS patients; however, we believe that it provides an indication to consider hypertension as an important variable for future studies. Thus, comorbidities could be contributing to fatigue in these patients.
Anxiety and fear may accompany depression in patients with GBS, due to sudden onset of symptoms in patients who are in relatively good health.[23] Majority of our patients did not have symptoms of anxiety or depression, since they might have passed through mood disturbances during acute phase of illness. The study found association of fatigue at time of discharge and presence of anxiety.
There are conflicting reports regarding the effect of motor weakness on the occurrence of fatigue. Rekand et al.,[6] had observed higher prevalence of fatigue in patients with increased muscle weakness. We used MRC sum-score to assess severity of weakness in our study and found no association between fatigue and weakness as in study by Garssen et al.[11] We found a positive correlation between fatigue at the time of discharge and HDS scores ≥3. Other studies[6,9,10] have also found correlation between fatigue and disability.
Majority (73%) of our patients had sleep disturbance at the time of admission that could be due to high prevalence of neuropathic pain (77%) and paresthesias (60%). Significant association between fatigue and presence of neuropathic pain was found — like in study by Rekand et al.[6] Though sleep disturbances can attribute to fatigue, we did not find any association of fatigue with sleep disturbance in our study. Hagemans et al.,[24] also observed that fatigue was highly prevalent among adult patients with Pompe disease, in both with or without sleep disturbances.
Study by Garssen et al.,[25] showed that that physical training improves fatigue, fitness, and quality of life in patients with GBS. We also observed that with physical training and improvement in motor scores, fatigue scores decreased at the time of discharge.
Limitations of the study
It was a retrospective, single center, hospital-based study, conducted in rehabilitation setting, and thus may not represent the actual population of GBS. There was heterogeneity in the day of illness when the patients were assessed for fatigue, as admission was dependent on severity of GBS and when patient was medically stable. The cohort of patients was highly selective and there was no follow-up.
Conclusions
The study showed that fatigue was prevalent in patients with GBS, during recovery from the acute illness and was independent of severity of motor weakness, depression, and sleep disturbance. Presence of fatigue was associated with requirement for ventilator during acute phase, presence of neuropathic pain, and presence of anxiety and higher disability scores. Among patients with GBS in our study, the rehabilitation program improved functional outcome as well as symptoms of fatigue, anxiety-depression, and sleep disturbances. However, despite motor recovery, fatigue persisted among the study participants. More attention in this direction would improve the rehabilitation outcome and quality of life of these patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Dornonville de la Cour C, Jakobsen J. Residual neuropathy in long-term population-based follow-up of Guillain-Barré syndrome. Neurology. 2005;64:246–53. doi: 10.1212/01.WNL.0000149521.65474.83. [DOI] [PubMed] [Google Scholar]
- 2.Rees JH, Thompson RD, Smeeton NC, Hughes RA. Epidemiological study of Guillain-Barré syndrome in south east England. J Neurol Neurosurg Psychiatry. 1998;64:74–7. doi: 10.1136/jnnp.64.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visser LH, Schmitz PI, Meulstee J, van Doorn PA, van der Meché FG. Prognostic factors of Guillain-Barré syndrome after intravenous immunoglobulin or plasma exchange. Dutch Guillain-Barré Study Group. Neurology. 1999;53:598–604. doi: 10.1212/wnl.53.3.598. [DOI] [PubMed] [Google Scholar]
- 4.Merkies IS, Schmitz PI, Samijn JP, van der Meché FG, van Doorn PA. Fatigue in immune-mediated polyneuropathies. European Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Neurology. 1999;53:1648–54. doi: 10.1212/wnl.53.8.1648. [DOI] [PubMed] [Google Scholar]
- 5.Garssen MP, van Doorn PA, Visser GH. Nerve conduction studies in relation to residual fatigue in Guillain-Barré syndrome. J Neurol. 2006;253:851–6. doi: 10.1007/s00415-006-0962-9. [DOI] [PubMed] [Google Scholar]
- 6.Rekand T, Gramstad A, Vedeler CA. Fatigue, pain and muscle weakness are frequent after Guillain-Barré syndrome and poliomyelitis. J Neurol. 2009;256:349–54. doi: 10.1007/s00415-009-0018-z. [DOI] [PubMed] [Google Scholar]
- 7.Wokke JH. Fatigue is part of the burden of neuromuscular diseases. J Neurol. 2007;254:948–9. doi: 10.1007/s00415-006-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 9.Forsberg A, Press R, Holmqvist LW. Residual disability 10 years after falling ill in Guillain-Barré syndrome: A prospective follow-up study. J Neurol Sci. 2012;317:74–9. doi: 10.1016/j.jns.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Drory VE, Bronipolsky T, Bluvshtein V, Catz A, Korczyn AD. Occurrence of fatigue over 20 years after recovery from Guillain-Barré syndrome. J Neurol Sci. 2012;316:72–5. doi: 10.1016/j.jns.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Garssen MP, Van Koningsveld R, Van Doorn PA. Residual fatigue is independent of antecedent events and disease severity in Guillain-Barré syndrome. J Neurol. 2006;253:1143–6. doi: 10.1007/s00415-006-0163-6. [DOI] [PubMed] [Google Scholar]
- 12.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27(Suppl):S21–4. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 13.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 14.Merkies IS, Schmitz PI, van der Meché FG, Samijn JP, van Doorn PA Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Connecting impairment, disability, and handicap in immune mediated polyneuropathies. J Neurol Neurosurg Psychiatry. 2003;74:99–104. doi: 10.1136/jnnp.74.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: A practical guide for clinicians and researchers. J Psychosom Res. 2004;56:157–70. doi: 10.1016/S0022-3999(03)00371-4. [DOI] [PubMed] [Google Scholar]
- 16.Hughes RA, Newsom-Davis JM, Perkin GD, Pierce JM. Steroids in acute polyneuropathy. Lancet. 1978;2:1383. doi: 10.1016/s0140-6736(78)92022-6. [DOI] [PubMed] [Google Scholar]
- 17.Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in theassessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. 1991;14:1103–9. doi: 10.1002/mus.880141111. [DOI] [PubMed] [Google Scholar]
- 18.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 19.Ng YS, Lo YL, Lim PA. Characteristics and acute rehabilitation in Guillain-Barre syndrome in Singapore. Ann Acad Med Singapore. 2004;33:314–9. [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Hadden RD, Cornblath DR, Hughes RA, Zielasek J, Hartung HP, Toyka KV, et al. Electrophysiological classification of Guillain-Barré syndrome: Clinical associations and outcome. Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Ann Neurol. 1998;44:780–8. doi: 10.1002/ana.410440512. [DOI] [PubMed] [Google Scholar]
- 22.Witsch J, Galldiks N, Bender A, Kollmar R, Bösel J, Hobohm C, et al. Long-term outcome in patients with Guillain-Barré syndrome requiring mechanical ventilation. J Neurol. 2013;260:1367–74. doi: 10.1007/s00415-012-6806-x. [DOI] [PubMed] [Google Scholar]
- 23.Khan F, Amatya B. Rehabilitation interventions in patients with acute demyelinating inflammatory polyneuropathy: A systematic review. Eur J Phys Rehabil Med. 2012;48:507–22. [PubMed] [Google Scholar]
- 24.Hagemans ML, van Schie SP, Janssens AC, van Doorn PA, Reuser AJ, van der Ploeg AT. Fatigue: An important feature of late-onset Pompe disease. J Neurol. 2007;254:941–5. doi: 10.1007/s00415-006-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garssen MP, Bussmann JB, Schmitz PI, Zandbergen A, Welter TG, Merkies IS, et al. Physical training and fatigue, fitness, and quality of life in Guillain-Barré syndrome and CIDP. Neurology. 2004;63:2393–5. doi: 10.1212/01.wnl.0000148589.87107.9c. [DOI] [PubMed] [Google Scholar]


