Abstract
Guillain-Barre syndrome (GBS) rarely complicates pregnancy, but can be associated with high maternal and perinatal morbidity if not properly identified and treated. A high index of suspicion, supportive measures, access to intensive care unit and intravenous immunoglobulin (IVIG) therapy are cornerstones of management in GBS complicating pregnancy. Neurologists and Obstetricians should be aware of the risks of relapsing GBS in the immediate postpartum period. Surgery and anesthesia may be triggers for relapse in association with an overall increase in pro-inflammatory cytokines in the postpartum period. We report a unique case of GBS complicating pregnancy in the third trimester followed by a relapse in the postpartum period. She made a good recovery with supportive measures and a repeat course of IVIG during the relapse.
Keywords: Guillain-Barre syndrome, pregnancy, relapse
Introduction
Guillain-Barre syndrome (GBS) complicating pregnancy is a rare event. Even more unusual is a relapse in the immediate postpartum. We report such a case of GBS complicating the third trimester of pregnancy followed by a relapse in the immediate postpartum after a surgical delivery under spinal anesthesia.
Case Report
This is a case report of a 30-year-old female patient who presented to us at 34 weeks gestation in her second pregnancy with 3 days duration of tingling sensations over her fingers and toes bilaterally. There was an associated weakness of the left side of the face. There was no history suggestive of bulbar, respiratory or autonomic disturbances. At 10 days prior to the onset of neurological symptoms she had an episode of gastro-enteritis that had lasted 2 days. She had received immunization for tetanus in view of pregnancy but otherwise her progress during this pregnancy has been unremarkable.
Neurological examination revealed normal eye movements and optic fundi. She had left lower motor neuron type of facial palsy, but no bulbar weakness. Tone was reduced over all four limbs. Symmetric mild proximal weakness was seen over the lower limbs and she required mild support to get up from the supine position. Sensory system was normal. Stretch reflexes were bilaterally absent and plantars were flexors on both sides. Respiratory examination was within the normal limits.
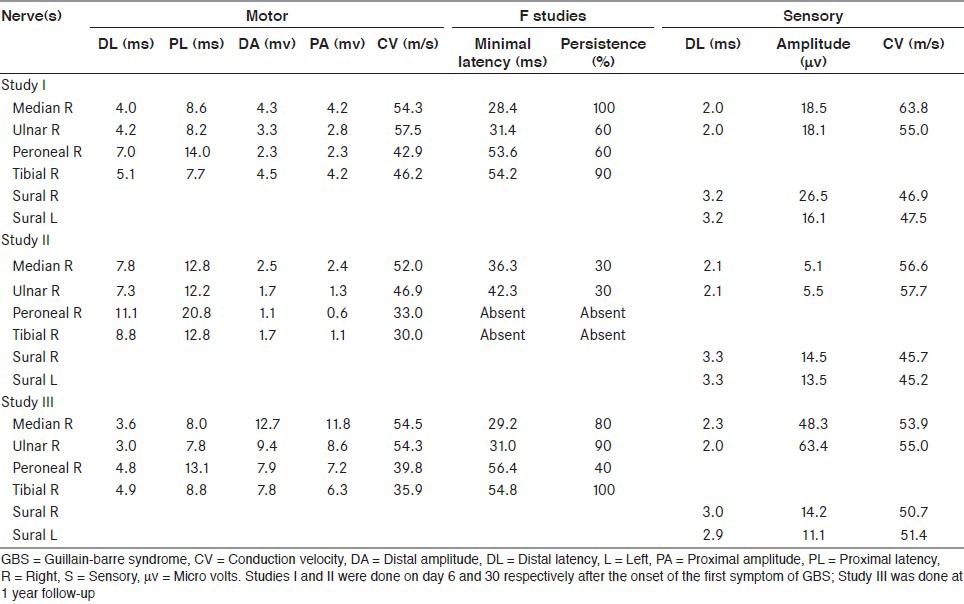
Serum chemistry, hemogram and urinalysis were normal. No atypical cells were seen in the peripheral smear. Erythrocyte sedimentation rate was 65 mm/h. Antinuclear antibodies, rheumatoid factor, C-reactive protein, venereal disease research laboratory, hepatitis B surface antigen, hepatitis C virus, human immunodeficiency virus, anti-neutrophil cytoplasmic antibodies and antiphospholipid antibodies (IgG and IgM) and lupus anticoagulant test were negative. Toxoplasma, rubella, cytomegalovirus and herpes simplex virus antibodies were negative. Thyroid function tests were within normal limits. Cerebral spinal fluid examination could not be done due to lack of consent. Her electrophysiological findings are presented in the Table 1, Study 1.
Table 1.
Electrophysiological observations in our patient with relapsing GB syndrome
Weakness evolved and by day 2 of admission (day 5 of illness). She had worsening of proximal weakness requiring significant support for walking and worsening of difficulty in getting up. Intravenous immunoglobulin (IVIG) was instituted at a dose of 0.4 g kg/day over next 5 days. Weakness further evolved and by day 3 of admission (day 6 of illness) right facial and neck flexor weakness also developed. By day 4 of admission she also had requirement of assistive device (walker) for walking. By day 5 of admission (day 8 of illness) she however started improving with no further progression and mild improvement in neck flexion. No drop in single breath count was ever noticed during her hospital stay. Blood pressure (BP) during hospital stay was detected to be high (systolic 130-150 mm Hg, diastolic 90-100 mm Hg). Alpha-methyldopa was started and carefully titrated to avoid precipitous fall of BP considering the dysautonomia of GBS. Limb power gradually improved. By day 9 of admission (day 12 of illness) neck flexor weakness had improved significantly to normal power. She could walk unsupported though with mild unsteadiness, when she was discharged.
She remained neurologically stable subsequently and was admitted in her 36th week of gestation with labor pain. She underwent cesarean section under spinal anesthesia. Indication for cesarean section was floating head despite labor pain in the 36th week of gestation. Neurological examination a day prior to delivery (day 17 after the onset of the first symptom of GBS) did not reveal any limb weakness. There was only residual left facial weakness. No significant limb weakness was observed during her stay at the hospital following delivery and she was discharged on the seventh postpartum day (25th day after the onset of the first symptom of GBS).
She developed recurrence of weakness of limbs postpartum, noticed since the 29th day of onset of the first symptom of GBS. She was readmitted a day later with rapidly worsening weakness and requirement of support for walking. Neurological examination revealed bilateral symmetrical weakness over all 4 limbs. She had diffuse areflexia and normal sensory examination. Results of repeat electrophysiological studies are presented in the Table 1, Study II.
A repeat course of IVIG (0.4 Kg/day for 5 days) was administered. She gradually improved in her motor power and could walk unsupported after 7 days when she was discharged. She remained neurologically asymptomatic subsequently. Repeat electrophysiological studies done at 1 year follow-up showed significant improvement [Table 1, Study III].
Discussion
The incidence of GBS has been reported to be very low during pregnancy.[1,2] The risk of GBS increases after delivery, particularly during the first 2 weeks postpartum.[3,4] We report a patient who manifested with clinical and electrophysiological features of GBS during the last trimester of pregnancy, who had recurrence of weakness in her postpartum period, requiring re-administration of immunomodulatory therapy. To the best of our knowledge, this is the first report of a patient with GBS in pregnancy, who significantly improved and had a relapse during the postpartum period. Such a presentation is unique and might be due to underlying immunological alterations.
Relapse in GBS has been reported to occur in 5.5-6.8% of patients.[5,6] Such a relapse could be a true relapse when there is an actual recurrence of the disease or an apparent progression related to the natural history of the disease or a pharmacologic relapse due to wearing off effect of immunomodulatory therapy. Treatment-related clinical fluctuation is well-known in GBS where patients may deteriorate after initial stabilization, a phenomenon seen in 5-10% of patients, which may have accounted for this relapse. In our patient, the recurrence of weakness was noted after 29 days and therefore it was not possible for us to say whether this was a progression of a single episode of GBS (monophasic course) or there were two separate episodes as these are often indistinguishable clinically and electro physiologically.
Four cases of GBS have been reported occurring 1-2 weeks after epidural anesthesia.[7] Though similar reports have not been documented following spinal anesthesia the possibility cannot be entirely excluded. Furthermore surgery per se of any nature has been reported to be a trigger for GBS. While the first episode occurred following a diarrheal illness no similar preceding event was noted prior to the second worsening. Thus, there may be a case to consider the postpartum period per se or surgery (cesarean section) or anesthesia as potential triggers for the relapse of the symptoms.
Clinical remission is known in immune-mediated diseases like multiple sclerosis and experimental autoimmune encephalomyelitis during pregnancy. Of the three sets of helper T cells, Th1 cells secrete pro-inflammatory cytotoxic cytokines, Th2 cells secrete anti-inflammatory non-cytotoxic cytokines and Treg (regulatory T cells) cells play a role in induction of tolerance to fetal alloantigen. During pregnancy Th2 cytokines predominate over Th1 cytokines and Treg activity increases during the first and second trimester. These changes are regulated by hormones. This may explain why GBS is rare in pregnancy and may also account for a relative increase in the incidence of the disease in the third trimester of pregnancy compared with the first and second trimesters. In the postpartum period there is an overall increase in pro-inflammatory cytokines and this could underlie the increased incidence of the disease in postpartum period.[8]
To summarize, GBS is a rare occurrence in pregnancy but can be associated with severe co-morbidities if unrecognized, especially respiratory muscle involvement and dysautonomia. Obstetricians should therefore have a high index of suspicion if a pregnant woman complains of muscle weakness or breathlessness in the context of recent diarrheal illness or a viral infection. Postpartum relapses can occur, as in our case. The efficacy of IVIG is well-established and can be safely given during pregnancy. Early diagnosis, multidisciplinary input and prompt immune-modulatory therapy are the cornerstones in management of GBS during pregnancy and postpartum to improve outcomes for the mother and fetus.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Chan LY, Tsui MH, Leung TN. Guillain-Barré syndrome in pregnancy. Acta Obstet Gynecol Scand. 2004;83:319–25. [PubMed] [Google Scholar]
- 2.Brooks H, Christian AS, May AE. Pregnancy, anaesthesia and Guillain Barré syndrome. Anaesthesia. 2000;55:894–8. doi: 10.1046/j.1365-2044.2000.01367.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheng Q, Jiang GX, Fredrikson S, Link H, de Pedro-Cuesta J. Increased incidence of Guillain-Barré syndrome postpartum. Epidemiology. 1998;9:601–4. [PubMed] [Google Scholar]
- 4.Jiang GX, de Pedro-Cuesta J, Strigård K, Olsson T, Link H. Pregnancy and Guillain-Barré syndrome: A nationwide register cohort study. Neuroepidemiology. 1996;15:192–200. doi: 10.1159/000109907. [DOI] [PubMed] [Google Scholar]
- 5.Taly AB, Gupta SK, Anisya V, Shankar SK, Rao S, Das KB, et al. Recurrent Guillain Barre’ Syndrome: A clinical, electrophysiological and morphological study. J Assoc Physicians India. 1995;43:249–52. [PubMed] [Google Scholar]
- 6.Taly AB, Gupta SK, Anisya V, Shankar SK, Rao S, Das KB, et al. Recurrent Guillain Barre’ Syndrome: A clinical, electrophysiological and morphological study. J Assoc Physicians India. 1995;43:249–52. [PubMed] [Google Scholar]
- 7.Steiner I, Argov Z, Cahan C, Abramsky O. Guillain-Barré syndrome after epidural anesthesia: Direct nerve root damage may trigger disease. Neurology. 1985;35:1473–5. doi: 10.1212/wnl.35.10.1473. [DOI] [PubMed] [Google Scholar]
- 8.Lee LK. Physiological adaptations of pregnancy affecting the nervous system. Semin Neurol. 2007;27:405–10. doi: 10.1055/s-2007-991124. [DOI] [PubMed] [Google Scholar]


