Abstract
Paraneoplastic cerebellar degeneration (PCD) is a rare neurological disorder characterized by a widespread loss of Purkinje cells associated with a progressive pancerebellar dysfunction. PCD often precedes the cancer diagnosis by months to years. Here, we report a case of 44-year old postmenopausal woman who presented with PCD symptoms and high levels of anti-Yo antibodies titer since 8 months. We failed to conclude any neoplastic focus after thorough laboratory and imaging study. She minimally responded to methylprednisolone and immunoglobulin therapies. Despite therapy she was severely disabled. Planned abdominal hysterectomy with bilateral salpingo-oophorectomy (AHBSO) was done, histology revealed grade IIA borderline serous papillary carcinoma of ovary. Her neurological deficit responded dramatically to AHBSO. It is first case report who emphasize the response of AHBSO with presentation of anti-Yo antibody-mediated PCD and hidden nidus in post menopausal women.
Keywords: Anti-Yo, abdominal hysterectomy with bilateral salpingo-oophorectomy, paraneoplastic cerebellar degeneration, postmenopausal
Introduction
Paraneoplastic neurological disorders may affect any part of the nervous system.[1] Paraneoplastic cerebellar syndrome is a rare neurological disorder that primarily emerges before the detection of malignancy.[2] Paraneoplastic cerebellar degeneration (PCD) is characterized by cerebellar atrophy with a diffuse loss of Purkinje cells, mediated by a cross-reaction of antibodies with tumor antigens and cerebellar tissue.[3] PCD can present with acute or sub-acute onset. The onset of symptoms and detection of antibodies precede diagnosis of the tumor more than 60% of the time, and in approximately 40% patients, antibodies were not identified. However, this does not exclude the likelihood of occult malignancy.[4] We are reporting a case that showed dramatically response to PCD symptoms after abdominal hysterectomy with bilateral salpingo-oophorectomy (AHBSO).
Case Report
A 44-year-old female presented with sub-acute onset progressive vertigo, nausea, and daily vomiting since 8 months. Since 7 months, she also developed dysarthria and gait and limb (right followed by left side over 2 weeks) ataxia. Her ataxia progressed over a period of 2 months, followed by a more gradual progression making her dependent for activity of daily living (ADL) within 6 months.
During the 8th month of her illness, she presented to us with Modified Rankin Scale (mRS) four. On examination, she was conscious, oriented with normal memory and intelligence. She had staccato speech, gaze evoked nystagmus in all directions, oculomotor dysmetria, slow saccades, broken pursuit, intention tremor, appendicular, and gait ataxia. Rest of the neurological examination was normal.
She was investigated to consider differentials of vasculitis, inflammatory, demyelinating, infective, and immune-mediated cerebellar dysfunction. Investigations revealed bilateral cerebellar atrophy, positive anti-Yo antibody (titer - 1/603 by immunofluorescence; normal <1/500) and high normal CA-125 (29 U/ml; normal range: 0-35 U/ml). Other investigations including complete blood count, blood sugar, electrolytes, urea, creatinine, liver functions test, electrocardiography, chest X-ray, positron emission tomography (PET) scan [Figure 1] cerebral spinal fluid examination, and other specific investigations were normal [Table 1].
Figure 1.
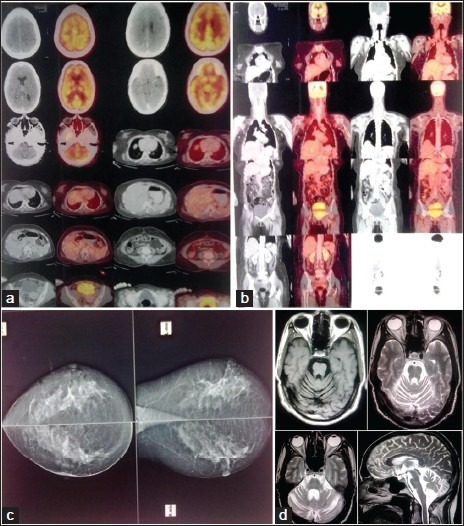
(a and b) PET-CT Brain and whole body showing absence of obvious FDG –avid lesion, (c) Mammography – Normal, D; T1W axial, T2W axial and sagittal section brain showing cerebellar atrophy
Table 1.
Laboratory test and imaging data
We kept diagnosis as anti-Yo antibody-mediated PCD. Intravenous methylprednisolone 1 g/d for 5 days was given but due to lack of response, one month later intravenous immunoglobulin (IVIG) 2 g/kg (over 5 days) was administered. After 2 weeks of immunotherapy, her vomiting and tremors subsided but vertigo and ataxia persisted. After 1 month follow up, her general condition improved but she remained almost bed bound.
We advised a repeat IVIG infusion and other second line chemotherapy for further expected improvement, but due to financial constraint and adverse effects, the patient refused. She opted for the alternative offer of AHBSO as a treatment of PCD by anecdotal reports in post-menopausal women. One and a half month after IVIG therapy, she underwent an uneventful AHBSO under general anesthesia. Intraoperatively, no evidence of malignancy was found. Histology revealed grade II-A borderline serous papillary carcinoma of the ovary [Figure 2]. Post surgery, by 2nd week, her dysarthria and vertigo improved and by 1 month, she was ambulatory with support. By 2 months, she had minimal residual appendicular ataxia and ocular findings, and was independent for ADL with mRS grade 2. Post surgery her anti-Yo titer was 1/214.
Figure 2.
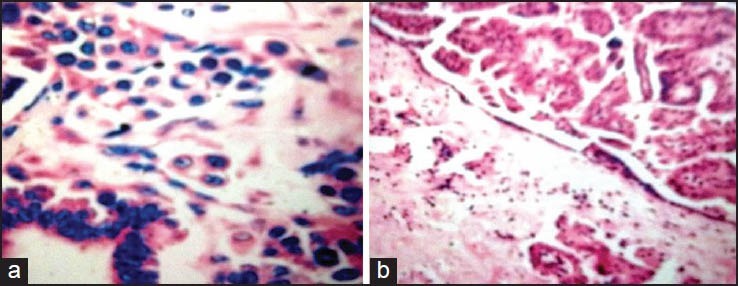
Serous borderline tumor with microinvasion: Eosinophilic cell pattern of microinvasion, characterized by individual cells or small, irregular clusters of cells with abundant eosinophilic cytoplasm within the stromal stalks of serous papillae. The surrounding stroma exhibits no reaction, and the tumor cells are surrounded by clefts (A, H&E, ×200; B, H&E, ×100)
Discussion
PCD is a rare non-metastatic complication of malignancies, and it is believed to be immune mediated. The autoantibodies are considered the result of an immunologic response to tumor and may cross-react with cells of the nervous system, causing neuronal damage. Anti-Yo-related PCD is most commonly found in women with gynecological and breast cancers, but it is also reported in other malignancies. Other PCD-related autoantibodies are anti-Hu and anti-Ri with small cell lung and breast cancer, respectively.[4] The anti-Yo antibodies that specifically damage the Purkinje cells of the cerebellum, also called Purkinje cell cytoplasmic antibody type 1 (PCA-1). The Yo antigen is a cytoplasmic protein (CDR2) that interacts with c-Myc. CDR2 is expressed mostly on the Purkinje cells of the cerebellum and may also be present in neurons of the brain stem. Studies suggest that CDR2 sequesters c-Myc in the neuronal cytoplasm and down regulates its activity. Disruption of this interaction by anti-Yo antibodies may increase c-Myc activity, leading to apoptosis of the Purkinje cells. Antibodies could therefore play an initial pathogenic role in PCD, although the T-cell immune response is believed to be the major effect of neuronal degeneration.[5]
There are no established protocols for the treatment of most paraneoplastic syndromes (PNS).[6] Most investigators have initiated treatment with corticosteroids, plasma exchange, or IVIG.[7] Cyclophosphamide, tacrolimus, rituximab, or possibly mycophenolate mofetil may warrant consideration in patients who fail to stabilize or improve on less aggressive therapies.[8] Radiation therapy has been used as an adjuvant to chemotherapy for Hodgkin's lymphoma with PCD. Although few reports showed improvement with these therapies, generally, there is a minimal effect since the antibodies are intrathecal and unaffected by plasmapheresis or IVIG.[7,8] However, it has been demonstrated that early therapy of PCD may improve neurological status, accordingly we started immunomodulatory treatment regimes with corticosteroids followed by IVIG 4 weeks later. By immunotherapy, she showed improvement in vomiting and tremor but remarkably responded to AHBSO and achieved independence. Due to the borderline nature and early stage of the disease, we could not find tumor on imaging study. The only clue to opt AHBSO was a high normal level of CA 125. Tumor occurrence is preceded months or even years by cerebellar signs,[7,8] likewise we observed that PCD emerged without appearance of a primary tumor till AHBSO and was revealed at histology. Yo antibodies were not correlated with specific histological subgroups of ovarian cancer but are related to the staging of ovarian cancer.[9] Borderline tumors, as with other ovarian tumors, are difficult to detect clinically until they are advanced in size or stage. In patients with PNS, the sensitivity (83.33%) and specificity (25%) of PET scanning to detect tumors has been estimated to be low.[10]
In conclusion, post-menopausal and younger women who have completed their family and are diagnosed with anti-Yo antibody-mediated PCD without any substantiate features of other malignancies on non-invasive testing, AHBSO may be assumed to stabilize symptoms of PCD. Further investigations on the pathogenesis of PCD are required to identify more effective therapies, which can stabilize or reverse the neurological symptoms.
Acknowledgment
Dr. Isha Sood, Senior Resident, Department of Medicine, Dr. Sampurnanand Medical College, Jodhpur, Rajasthan, India
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Bataller L, Dalmau J. Paraneoplastic neurologic syndromes. Neurol Clin. 2003;21:221–47. doi: 10.1016/s0733-8619(02)00037-3. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein BH, Birk CL, Van Houten M, Veve R, Brown JV, Rettenmaier MA, et al. Ovarian cancer and late onset paraneoplastic cerebellar degeneration. Arch Gynecol Obstet. 2009;280:99–101. doi: 10.1007/s00404-008-0822-1. [DOI] [PubMed] [Google Scholar]
- 3.McKeon A, Pittock SJ. Paraneoplastic encephalomyelopathies pathology and mechanisms. Acta Neuropathol. 2011;122:381–400. doi: 10.1007/s00401-011-0876-1. [DOI] [PubMed] [Google Scholar]
- 4.Peterson K, Rosenblum MK, Kotanides H, Posner JB. Paraneoplastic cerebellar degeneration. I. A. clinical analysis of 55 anti-Yo antibody positive patients. Neurology. 1992;42:1931–7. doi: 10.1212/wnl.42.10.1931. [DOI] [PubMed] [Google Scholar]
- 5.Okano HJ, Park WY, Corradi JP, Darnell RB. The cytoplasmic Purkinje onconeural antigen cdr2 down-regulates c-Myc function: Implications for neuronal and tumor cell survival. Genes Dev. 1999;13:2087–97. doi: 10.1101/gad.13.16.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vedeler CA, Antoine JC, Giometto B. Management of paraneoplastic neurological syndromes: Report of an EFNS Task Force. Eur J Neurol. 2006;13:682–90. doi: 10.1111/j.1468-1331.2006.01266.x. [DOI] [PubMed] [Google Scholar]
- 7.Thone J, Hohaus A, Lamprecht S, Bickel A, Erbguth F. Effective immunosuppressant therapy with cyclophosphamide and corticosteroids in paraneoplastic cerebellar degeneration. J Neurol Sci. 2008;272:171–3. doi: 10.1016/j.jns.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Widdess Walsh P, Tavee JO, Schuele S, Stevens GH. Response to intravenous immunoglobulin in anti-Yo associated paraneoplastic cerebellar degeneration: Case report and review of the literature. J Neuro Oncol. 2003;63:187–90. doi: 10.1023/a:1023931501503. [DOI] [PubMed] [Google Scholar]
- 9.Monstad SE, Storstein A, Dorum A, Knudsen A, Lonning PE, Salvesen PE, et al. Yo antibodies in ovarian and breast cancer patients detected by a sensitive immunoprecipitation technique. Clin Exp Immunol. 2006;144:53–8. doi: 10.1111/j.1365-2249.2006.03031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younes-Mhenni S, Janier MF, Cinotti L, Antoine JC, Tronc F, Cottin V, et al. FDG-PET improves tumor detection in patients with paraneoplastic neurological syndromes. Brain. 2004;127:2331–8. doi: 10.1093/brain/awh247. [DOI] [PubMed] [Google Scholar]


