Abstract
Background:
Statins are one of the most frequently prescribed medications to reduce the risk of cardiovascular events. Statins appear to be safe however, there are contradictory data regarding their adverse effects, which might be due to genetic variation in their metabolism. Hence, this prospective study was aimed to evaluate the effects of atorvastatin on liver transaminase changes in a clinical setting, in north Iran.
Materials and Methods:
This prospective semi-experimental study was performed on hyperlipidemic adults in 2010-2011. Patients received atorvastatin (5-40 mg/d) based on the American National Cholesterol Education Program guidelines. Liver aminotransferases were measured in three occasions of baseline, 8 and 16 weeks period.
Results:
A total of 206 patients were included in the study. Of which 178 were female and 30 were male. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were in normal range before intervention in the majority, except in 25 (12.1%) and 16 (7.8%) of patients, respectively. In general, ALT and AST remained in normal range over the study period (23.3 IU/L and 21.8 IU/L, respectively). There was found no relationship between different doses of atorvastatin prescribed and ALT/AST changes in the patients. The males’ ALT means at baseline (26.9 IU/L), 8 weeks (30 IU/L) and 16 weeks (28.8 IU/L) after statin therapy were significantly higher than females (22 IU/L, 22.2 IU/L and 22.1 IU/L, respectively; P < 0.05 for all).
Conclusion:
The absence of any hepatic adverse effect in the present study supports safety of atorvastatin and emerging opinion that routine screening of liver function tests is not necessary in patients on statins.
Keywords: Atorvastatin, hepatotoxicity, hyperlipidemia, Iran, liver transaminase, statins
INTRODUCTION
Statins are one of the most frequently prescribed classes of medications, since their launch as the first 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, in 1987. Although, the effect of statins in reducing the risk of cardiovascular disease is well-established, less is known regarding their possible side-effects. In general, statins appear to be safe, but several clinical studies are demonstrated evidences of liver toxicity for up to 3% of patients receiving statins, mostly manifesting as minor and persistent elevations in liver aminotransferases.[1] A meta-analysis of 35 randomized trials reports an excess risk of aminotransferase elevation with statin therapy.[2] Other studies display that hepatotoxicity primarily occurs during the first 3 months of therapy and usually is dose dependent.[3,4,5] Rare episodes of severe liver injury are also reported 3-4 months after initiation of statin therapy.[6] On the other hand, other studies find no significant difference in the incidence of persistently elevated liver enzymes between statin and placebo therapy.[7,8,9] There are contradictory data regarding the role of genetic in development of statin side-effects and the incidence of liver transaminases elevation.[2,5,10] Some studies suggest that part of the variability in response to and side-effects with statins may be related to genetic differences in the rate of drug metabolism.[11,12] For instance, Asians might have greater responses to low doses of statins than Caucasians. The potential mechanisms of this heightened response are related to genetically based differences in the metabolism of statins at the level of hepatic enzymes and drug transporters.[13] Among statins, atorvastatin typically is the most common statin reported to cause drug induced liver injury.[6,14,15,16] However, a recent large cohort study from England and Wales has reported similar risks of hepatic dysfunction with different statins, with the exception of a higher rids with Fluvastatin.[17] Our study, therefore, was conducted to evaluate the effects of atorvastatin, the most commonly used medication in this class, on liver transaminase changes in a clinical setting, in north Iran.
MATERIALS AND METHODS
This prospective quasi-experimental study was conducted on hyperlipidemic patients, who presented to a single endocrinology out-patient department in the city of Rasht, Center of Guilan Province, North Iran. Hyperlipidemic adult patients were consecutively selected by convenience sampling method over a period of 2 years, between 2010 and 2011. The exclusion criteria of the study were positive history of taking oral contraceptives, alcoholism and having liver function tests more than three times normal. Furthermore, patients taking drugs that potentially could affect liver function or medications known to interact with statins-particularly attention given to agents metabolized through the same metabolic pathway as atorvastatin (i.e., Cytochrome P4503a4)-or having diseases that might change liver tests such as chronic liver diseases, hepatitis, biliary disease, hypothyroidism and congestive heart failure, were excluded from the study. Aminotransferases elevation preceded statin initiation was considered unrelated to statin therapy. The selected patients, who fulfilled the inclusion criteria, received atorvastatin therapy based on the American National Cholesterol Education Program guidelines.[18] Liver enzymes, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), were measured in three occasions; before starting atorvastatin therapy as a baseline, in 8 weeks and in 16 weeks interval during therapy. The enzymes were tested using biochemical methods (normal range 0-35 IU/L). The patients’ demographic data, underlying diseases, medical history and the results of liver enzymes tests were recorded in a structured questionnaire. The patients based on their baseline liver enzymes tests were subsequently categorized into two groups; patients with normal test and cases with elevated ALT/AST up to 3 times of normal level. Epi Info [public domain statistical software for epidemiology developed by Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia (USA)] version 7, was used for descriptive (frequencies and cross tabulation) and bivariate statistics analysis (means, t-test and ANOVA). Approval for the study was obtained from the Research Committee of Medical School, Guilan University of Medical Sciences, Rasht, Iran. Informed consent to participate was obtained from all the patients. The participants were free to decline or withdraw at any time without suffering any disadvantage or prejudice.
RESULTS
A total number of 206 patients who fulfilled the inclusion criteria were consecutively selected over the study period. The patients’ mean (standard deviation) of age was 50.5 (11.5) years, ranging from 19 to 73 years old. One hundred and seventy six (85.4%) patients were female and 30 (14.6%) were male. Diabetes mellitus was the most frequent underlying disease, detected in 24 (11.7%) patients. The other underling disease of the patients was goiter, hypertension and some irrelevant complaints which do not interfere with our results. Baseline liver enzyme tests before intervention were in normal range in the majority of the patients, though ALT and AST were between 35 IU/L and 100 IU/L in 25 (12.1%) and 16 (7.8%) cases, respectively. As shown in Table 1, the mean levels of ALT and AST in those with elevated transaminases before treatment were 46.6 IU/L and 44.4 IU/L respectively, which were decreased in 8 weeks and 16 weeks of therapy to 39.1 IU/L and 42.9 IU/L for ALT and 34.4 IU/L and 35.6 IU/L for AST, accordingly. In general, the mean levels of ALT and AST did not change significantly over the study period and remained in normal range during the intervention, as shown in Table 1. The effect of gender on liver enzymes is shown in the Figure 1. The males’ ALT means at baseline (26.9 IU/L), 8 weeks (30 IU/L) and 16 weeks (28.8 IU/L) after statin therapy were significantly higher than in females (22 IU/L, 22.2 IU/L and 22.1 IU/L, respectively; P < 0.05 for all). The mean of AST in men (24.7 IU/L) also was significantly higher than in women (21.5 IU/L) in 8 weeks after therapy (P = 0.04). Atorvastatin was prescribed in different dosages of 5 mg in 17 (8.3%), 10 mg in 171 (83%), 20 mg in 14 (6.8%) and 40 mg in 4 (1.9%) patients respectively. As shown in Table 2, there was found no significant relation between different dosages of statin and ALT/AST changes over the study period; and the enzymes fluctuations in all age groups remained in normal range. The effect of age on the enzymes changes is shown in Table 3. Although, there was a non-significant steadily increase in ALT and AST levels in patients older than 70 years old over the study period, as a whole ALT/AST variation did not differ significantly during treatment and was not related to age in our study.
Table 1.
Liver enzyme changes in patients with normal or increased baseline values during the 16 weeks of follow-up
Figure 1.
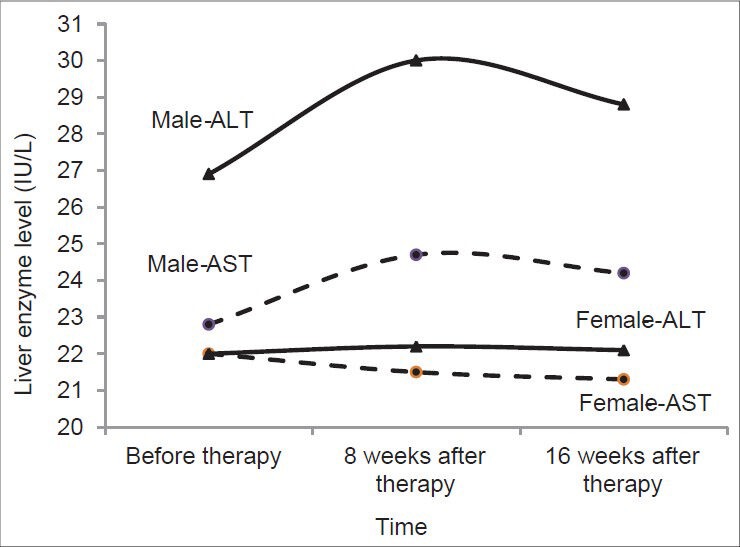
Liver enzyme changes in male and female patients, before and for up to 16 weeks after treatment
Table 2.
Liver enzyme changes related to different doses of atorvastatin during the 16 weeks of follow-up
Table 3.
Liver enzyme changes in different age groups, before and for up to 16 weeks after treatment
DISCUSSION
Statins are among the most commonly used medications world-wide. They are the drug of choice for patients with hypercholesterolemia and other risk factors for cardiovascular disease and are proven to be a live-reserving therapy in many of these patients.[19,20,21,22] Although statins appear safe, animal and pre-marketing clinical trials are shown signs of liver toxicity, mostly manifesting as minor elevations in aminotransferase concentrations.[1] In patients on statins, mild elevation of liver enzymes are observed in 1-3% of cases, but in the vast majority of patents this is not clinically significant and there is no need for discontinuation of therapy.[23,24] In clinical trials, a similar proportion of patients randomized to treatment with stains and placebo are depicted elevations in aminotransferases levels.[23,24] Several other studies also report no significant difference in the incidence of persistently elevated aminotransferases between statin and placebo therapy.[7,8,9] There is also no idiosyncratic liver injury and cholestatic liver damage with atorvastatin during 12-16 weeks after therapy, according to the report of the Swedish Adverse Drug Reaction Advisory Committee.[6] The results of our study also indicated that prescription of atorvastatin was safe in terms of liver dysfunction or hepatotoxicity in Iranian patients. This finding was compatible with results of other studies from Iran and abroad. In a similar study from Shiraz, south Iran, the means of liver enzymes show no significant difference before and after atorvastatin therapy. More than 90% of patients, who receive atorvastatin show no change in their liver enzymes, however ALT and AST elevate for up to 3 times of normal level, in approximately 6% of the cases.[25] In contrast, our findings were different from the results of other studies and clinical trials, reporting that up to 2% of their patients had transaminase elevations greater than three times of normal level.[26,27,28] Our results also differ from another large study, showing a transaminase level more than 10 times normal in 0.1% of 23,000 patients[29] and from a meta-analysis of 35 randomized controlled trials, enrolling more than 74,000 subjects with a follow-up between 1 and 65 months, which find an excess risk of aminotransferase elevation in 4.2 case/1000 patients with statin therapy.[2] Patients who simply have baseline elevations in aminotransferases do not appear to be at increased risk when prescribed a statin.[30,31] It is convincingly shown in a study by Chalasani et al.[31] that the risk of developing statin induced liver damage is not related to the presence of pre-existing liver abnormalities; and most of the non-alcoholic fatty liver disease and individuals with elevated baseline liver enzymes are not at increased risk of hepatotoxicity from statin therapy. Similarly, a post-hoc analysis of a randomized clinical trial find no evidence of increased hepatic risk in patients with moderately abnormal liver function test at baseline, who were treated with statins, mainly atorvastatin.[32] Even the use of statins is shown to be associated with improvement in liver function tests in patients with fatty liver.[32,33,34,35] The present study complies with the above findings. The aminotransferase levels did not increased during 8 weeks and 16 weeks of treatment with atorvastatin, but mostly were regressed to lower levels in this period, as shown in Table 1. In our survey, there was not a significant difference between ALT and AST changes; and because ALT is a more specific indicator of liver injury than AST, the measurement of ALT appear to be enough for evaluation of liver function, when indicated.[3] In contrast to some studies,[4,5] we did not find any significant relation between different atorvastatin dosage and transaminase changes in our patients, as shown in Table 2. Our findings, however, accord with a meta-analysis indicating that the difference between liver enzyme changes is not significant in statin and placebo groups with different doses, up to 40 mg daily.[10] In our study, we did not find any significant relation between age of the patients on atorvastatin and liver enzyme variations; however, the mean of ALT was significantly higher in males than in females, as shown in the figure. The low ratio of males to females and significant difference of ALT before treatment between them might explain the relation between sex and transaminases changes in patients on atorvastatin in our study. The existence of statin induced hepatotoxicity has been put into question and called a myth.[36] A study by Smith et al. fail to find any efficacy in routine screening of serum creatine kinase and transaminase in patients on statins.[26] The non-significant changes of liver enzymes in our study support the current guidelines of the American College of Cardiology/American Heart Association/National Heart, Lung and Blood Institute, to recommend screening only after symptoms are reported by patients.[37] Our findings also are in line with the US Food and Drug Administration revision in 2012 on its labeling information on statins to recommend liver function testing only prior to initiation of statin therapy and to repeat such testing only in case of clinical indications.[38] Our results also comply with other studies supporting the safety of atorvastatin and argue against routine screening of liver function tests in patients on statins.[9,29,39,40]
CONCLUSION
In sum, prescription of atorvastatin find to be safe in terms of liver transaminases elevations and hepatotoxicity in hyperlipidemic patients in north Iran; and routine checks of liver function tests seem unnecessary in patients on statins in this setting, except in the case of symptomatic patients. The absence of any hepatic adverse effect in the present study supports safety of atorvastatin and current opinion that routine screening of liver function tests is not necessary in patients on statins.
ACKNOWLEDGMENTS
Hereby we are very grateful to the sincere co-operation of Mrs. Soheila Mohamad Pur and Arezu Niaz bakhsh for fulfilling this work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Tolman KG. The liver and lovastatin. Am J Cardiol. 2002;89:1374–80. doi: 10.1016/s0002-9149(02)02355-x. [DOI] [PubMed] [Google Scholar]
- 2.Kashani A, Phillips CO, Foody JM, Wang Y, Mangalmurti S, Ko DT, et al. Risks associated with statin therapy: A systematic overview of randomized clinical trials. Circulation. 2006;114:2788–97. doi: 10.1161/CIRCULATIONAHA.106.624890. [DOI] [PubMed] [Google Scholar]
- 3.Seehusen DA, Asplund chad A, Johnson DR, Kevin A. Primary Evaluation and Management of Statin Therapy Complication. South Med Assoc. 2006;99:250–6. doi: 10.1097/01.smj.0000202691.52352.55. [DOI] [PubMed] [Google Scholar]
- 4.Clarke AT, Mills PR. Atorvastatin associated liver disease. Dig Liver Dis. 2006;38:772–7. doi: 10.1016/j.dld.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Kinnman N, Hultcrantz R. Lipid lowering medication and hepatotoxicity. J Intern Med. 2001;250:183–5. doi: 10.1046/j.1365-2796.2001.00887.x. [DOI] [PubMed] [Google Scholar]
- 6.Björnsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: Reports of idiosyncratic liver injury post-marketing. J Hepatol. 2012;56:374–80. doi: 10.1016/j.jhep.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 8.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 9.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 10.de Denus S, Spinler SA, Miller K, Peterson AM. Statins and liver toxicity: A meta-analysis. Pharmacotherapy. 2004;24:584–91. doi: 10.1592/phco.24.6.584.34738. [DOI] [PubMed] [Google Scholar]
- 11.Mulder AB, van Lijf HJ, Bon MA, van den Bergh FA, Touw DJ, Neef C, et al. Association of polymorphism in the cytochrome CYP2D6 and the efficacy and tolerability of simvastatin. Clin Pharmacol Ther. 2001;70:546–51. doi: 10.1067/mcp.2001.120251. [DOI] [PubMed] [Google Scholar]
- 12.Chasman DI, Posada D, Subrahmanyan L, Cook NR, Stanton VP, Jr, Ridker PM. Pharmacogenetic study of statin therapy and cholesterol reduction. JAMA. 2004;291:2821–7. doi: 10.1001/jama.291.23.2821. [DOI] [PubMed] [Google Scholar]
- 13.Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410–4. doi: 10.1016/j.amjcard.2006.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Björnsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481–9. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 15.Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, et al. Drug-induced liver injury: An analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–21. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. doi: 10.1053/j.gastro.2008.09.011. 19341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: Population based cohort study using the QResearch database. BMJ. 2010;340:c2197. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Third report of the National Cholestrol Education program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Circulation. 2002;106:3143. [PubMed] [Google Scholar]
- 19.Bradford RH, Shear CL, Chremos AN, Dujovne C, Downton M, Franklin FA, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study results. I. Efficacy in modifying plasma lipoproteins and adverse event profile in patients with moderate hypercholesterolemia. Arch Intern Med. 1991;151:43–9. doi: 10.1001/archinte.151.1.43. [DOI] [PubMed] [Google Scholar]
- 20.Wright RA, Flapan AD, McMurray J, Slattery J, White HD, Spaulding C, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 21.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: The MIRACL study: A randomized controlled trial. JAMA. 2001;285:1711–8. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 22.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 23.Chalasani N. Statins and hepatotoxicity: Focus on patients with fatty liver. Hepatology. 2005;41:690–5. doi: 10.1002/hep.20671. [DOI] [PubMed] [Google Scholar]
- 24.Bader T. Liver tests are irrelevant when prescribing statins. Lancet. 2010;376:1882–3. doi: 10.1016/S0140-6736(10)62142-3. [DOI] [PubMed] [Google Scholar]
- 25.Aghasadeghi K, Zare D. Efficancy of alternate day dosing of atorvastatin. Cent Eur J Med. 2008;3:163–6. [Google Scholar]
- 26.Smith CC, Bernstein LI, Davis RB, Rind DM, Shmerling RH. Screening for statin-related toxicity: The yield of transaminase and creatine kinase measurements in a primary care setting. Arch Intern Med. 2003;163:688–92. doi: 10.1001/archinte.163.6.688. [DOI] [PubMed] [Google Scholar]
- 27.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 28.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 29.Charles EC, Olson KL, Sandhoff BG, McClure DL, Merenich JA. Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization. Am J Med. 2005;118:618–24. doi: 10.1016/j.amjmed.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Cohen DE, Anania FA, Chalasani N National Lipid Association Statin Safety Task Force Liver Expert Panel. An assessment of statin safety by hepatologists. Am J Cardiol. 2006;97:77C–81C. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287–92. doi: 10.1053/j.gastro.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: A post-hoc analysis. Lancet. 2010;376:1916–22. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 33.Rallidis LS, Drakoulis CK, Parasi AS. Pravastatin in patients with nonalcoholic steatohepatitis: Results of a pilot study. Atherosclerosis. 2004;174:193–6. doi: 10.1016/j.atherosclerosis.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Hyogo H, Tazuma S, Arihiro K, Iwamoto K, Nabeshima Y, Inoue M, et al. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57:1711–8. doi: 10.1016/j.metabol.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 35.Ekstedt M, Franzén LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: A histopathological follow-up study. J Hepatol. 2007;47:135–41. doi: 10.1016/j.jhep.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Bader T. The myth of statin-induced hepatotoxicity. Am J Gastroenterol. 2010;105:978–80. doi: 10.1038/ajg.2010.102. [DOI] [PubMed] [Google Scholar]
- 37.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C, et al. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Circulation. 2002;106:1024–8. doi: 10.1161/01.cir.0000032466.44170.44. [DOI] [PubMed] [Google Scholar]
- 38. [Last accessed on 2013 Feb 28]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm .
- 39.Weismantel D, Danis P. Clinical inquiries. What laboratory monitoring is appropriate to detect adverse drug reactions in patients on cholesterol-lowering agents? J Fam Pract. 2001;50:927–8. [PubMed] [Google Scholar]
- 40.Gotto AM., Jr Safety and statin therapy: Reconsidering the risks and benefits. Arch Intern Med. 2003;163:657–9. doi: 10.1001/archinte.163.6.657. [DOI] [PubMed] [Google Scholar]


