Abstract
Background:
Poor vitamin D status and low serum insulin-like growth factor-1(IGF-1) are associated with metabolic syndrome (MetS) and its components. But, there is no adequate evidence about this. The aim of this study was to examine relationship of factors with MetS features.
Materials and Methods:
In this cross-sectional study, 156 women aged 28-76 years with MetS were recruited by consecutive random sampling. Dietary vitamin D, serum 25-hydroxyvitamin D (25(OH) D) and IGF-1 levels and also MetS components were determined.
Result:
The mean of serum 25(OH) D and IGF-1 concentrations were 20.5 ± 10.8, 194 ± 47 ng/mL, respectively. Overall, near 54.5% and 24% of subjects were vitamin D deficienct and insufficienct respectively. Univariate regression analysis showed that 25(OH) D concentration was negatively correlated with fasting blood sugar (P < 0.001) and no significant relation was observed between vitamin D status and serum IGF-1 with blood pressure and waist circumference. Multivariate regression analysis showed positive relation of 25(OH) D concentration with HDL-cholesterol (P = 0.031) and also dietary vitamin D is positively correlated with triglyceride (P = 0.026). IGF-1 as a predictor was not related to any of the MetS components.
Conclusion:
Our findings show that vitamin D status was related to FBS, HDL-C and triglyceride concentration; hence, with regard to findings of previous studies it seems that vitamin D is related to components of MetS. However, to determine the role of vitamin D status and IGF-1 in the development MetS and related components, further longitudinal studies and randomized clinical trials should be prescribed.
Keywords: IGF-1, metabolic syndrome, nutrition, vitamin D
INTRODUCTION
According to guidelines of National Cholesterol Education Program/Adult Treatment Panel III (NCEP/ATP III) Syndrome X or Metabolic syndrome (MetS) included having at least three of five following criteria: fasting blood sugar ≥110 mg/dl, TG ≥ 150 mg/dl, HDL-C <40 mg/dl (males) or <50 mg/dl (females), waist circumference >102 cm (males) or >88 cm (females), blood pressure ≥130/85 mmHg or using blood pressure-lowering medication.[1,2,3]
The prevalence of MetS varies widely in different studies, and it is increasing in the United States. The Third National Health and Nutrition Examination Survey (NHANES III) estimated the prevalence of MetS in United States as 22% to 24%.[4] However, Tehran glucose, lipid study reported the prevalence of MetS 30.1% among a population aged >20 years[5] Therefore, based on the diagnostic criteria ATP III the prevalence of MetS among adult Iranian population aged ≥20 years is more than most developed countries.[6,7]
MetS significantly predicts type 2 diabetes and cardiovascular disease.[8] However, the most people with this syndrome have insulin resistance, type 2 diabetes, polycystic ovary syndrome, fatty liver, cholesterol gallstones, asthma, autoimmune diseases, sleep disturbances and some types of cancers.[9,10]
It is thought that various factors such as physical inactivity, aging, obesity especially (central), inappropriate diet influence the development of MetS and related diseases. Diet is an important determinant in developing of MetS.[11,12,13,14] But, dietary components are briefly studied and limited to few observational studies.[7,9,15,16]
Vitamin D deficiency is one of the most common health problems worldwide. Food sources of vitamin D are naturally limited. However, most of vitamin D which is needed comes from sunlight exposure.[17] Serum 25-hydroxy vitamin D (25(OH) D), not 1,25 dihydroxy vitamin D {1, 25(OH) 2D)} is considered as a predictor determinant of sun exposure and dietary vitamin D.[17,18] It has been shown that sun exposure is a better predictor of 25(OH) D levels than dietary vitamin D.[19]
Most studies have used serum 25(OH) D concentration as a marker of human vitamin D status.[20,21] There is no consensus on optimal levels of serum 25(OH) D measurement. A serum concentration of 25(OH) D <20 ng/ml is considered as vitamin D deficiency and 20-30 ng/ml as vitamin D insufficiency and ≥30 ng/ml as vitamin D sufficiency status.[22,23]
Evidence about the relationship between vitamin D metabolism and incidence of diabetes is increasing. Vitamin D has a role in the secretion and action of insulin and likely modulates lipolysis and adiposity. Vitamin D might contribute to the development of the MetS.[6]
Obesity is one of the predictors of MetS.[24] Because of the increasing prevalence of obesity, MetS is common.[8] Indicators of adiposity are important determinant of serum 25(OH) D level.[25]
The significant link between hypovitaminosis D and adiposity has been widely reported.[26,27,28,29,30,31]
Increase in insulin concentration of the plasma suppresses the production of insulin-like growth factor binding protein-1 (IGFBP-1) which increases plasma-free insulin like growth factor-1 (IGF-1) concentration.[32] Decreasing IGFBP-1 has been associated with insulin resistance, the MetS, and also several other risk factors.[33] A number of cross-sectional studies have reported a strong and independent inverse relation between circulating IGF-1 concentrations and markers of MetS.[34,35] Low levels of IGFBPS have been associated with insulin resistance, the MetS, obesity, and with several cardiovascular risk factors.[33,36,37,38]
One study showed that, administration of recombinant human IGF-1 for diabetics by increasing insulin sensitivity could reduce insulin dose requirement by 50% and serum glucose levels by 23% while improves MetS components.[39,40,41]
IGF-1 is a small peptide that structurally and functionally is similar to insulin and is an active form of IGFs. However, distinct roles of insulin and IGF-1 are difficult. Circulating IGF-I plays an important role in metabolic actions.[42] It is thought that IGF-1 is a potent stimulant of glucose transport into cells.[40,43] Most of plasma IGFs are secreted from liver and are bound to specific Six Proteins, which regulate availability of free IGFs for uptaking by target tissues.[44] IGFBPS modulates IGF-I activity.[45] Some proteins, such as IGFBP-3 and IGFBP-2, are reservoir and may decrease the free form of IGF-1 concentration.[46]
In spite of the increasing prevalence of MetS and vitamin D deficiency, in Iranian population especially in women, few studies have been undertaken. Therefore, it is important to clarify this relation and distinguish appropriate methods for we can remove problems. Assessment of relation between vitamin D status and IGF-1 with components of MetS in Western countries has been limited and in Iran no undertaken and findings are conflicting. On the other hand, studies of the association between vitamin D status and IGF-1 simultaneously with components MetS have not been examined.
Our hypothesis is that IGF-1 and vitamin D are related to components of MetS. Thus, it is important to find relations to clarify of the possible mechanisms that IGF-1 and vitamin D may act on components of MetS. Therefore, we assessed association of vitamin D status and IGF-1 concentrations with components of MetS among Iranian Women.
MATERIALS AND METHODS
In this cross-sectional study, 156 women of 28-76 years with MetS were enrolled by using consecutive random sampling. We used NCEP-ATP III criteria for MetS diagnosis. We excluded women whom consumed vitamin D and or calcium supplements, specific medications during past 3 to 4 months history of renal or hepatic disease, overt diabetes and malignancies. After filling demographic questionnaire and taking consent, fasting blood samples were collected for determining of serum fasting glucose (FBS), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and 25(OH) D, IGF-1 concentrations.
Laboratory measurement
After a 12-h overnight fasting, blood samples were collected and serum was promptly separated by centrifugation and frozen (–20°C). FBS and TG concentrations were measured using the enzymatic colorimetric method. HDL-C was also measured after precipitation of the apolipoprotein B-containing lipoproteins with phosphotungstic acid. Commercial reagents used for the above-mentioned factors blood was Pars Azmoon, Iran.
Serum 25(OH) D and IGF-I concentrations were determined by radioimmunoassay ELISA (Euroimmune, Germany).
Assessment of dietary intake
Usual dietary intake was assessed using a validated 168-item semiquantitative food-frequency questionnaire (FFQ).[47] FFQ evaluated dietary intake during a past year according to frequency of consumption daily, weekly and monthly. This questionnaire included a list of foods with standard exchanges. Portion sizes of consumed foods were converted to gram using household measures.[48] Each food and beverage was then coded according to the prescribed protocol and analyzed for content of energy and the other nutrients using N4 nutritional software, which was designed for Iranian foods. All FFQs were administered by a trained dietitian.
Assessment of other variables
Body weight was measured by Seca digital scale with light clothes and without shoes to the nearest 100 gram. Height was measured by nonstretch tape to the nearest 0.1 cm while not wearing shoes and the shoulders were in a normal position. Waist circumference was measured at the narrowest level between the lowest rib and the iliac crest using nonstretch tape while were at the end of a normal expiration. Body mass index (BMI) was calculated as weight (in kilograms) divided on the square of height (in meters).
Data on physical activity were obtained by an interview based on International Physical Activity Questionnaire and expressed as metabolic equivalent h/day (MET-h/day).[49]
Duration exposure to sunlight in previous month was asked according to the demographic questionnaire that included: Less than 30 min/day, between 30 and 60 min/day, between 60 and 120 min/day and more than 120 min/day.
Blood pressure was measured two times using a mercury sphygmomanometer on the right hand in a sitting position with 15 minutes intervals. The mean of two measurements was recorded as blood pressure.
At the end, we calculated mean arterial blood pressure (MAP) is equal to  Hypertension (HTN) was defined as systolic blood pressure ≥140 mmHg and diastolic blood pressure ≥90 mmHg.[50]
Hypertension (HTN) was defined as systolic blood pressure ≥140 mmHg and diastolic blood pressure ≥90 mmHg.[50]
Statistical analysis
We analyzed all data by SPSS software version 16. To assess the relationship between 25(OH) D, IGF-1, dietary vitamin D with the MetS components we used univariate regression analyses. Partial correlation analysis was performed to evaluate the association between 25(OH) D, IGF-1, dietary vitamin D with the MetS components. All variables reported as Means ± SD and probability values are two-tailed and values below 0.05 were considered statistically significant.
RESULTS
Total 156 women who participated in this study, had at least three of five specific MetS based on (NCEP/ATP III) definition. General characteristics, dietary intakes and laboratory features of study subjects are shown in Table 1.
Table 1.
Anthropometric, biochemical and diet characteristics of subjects
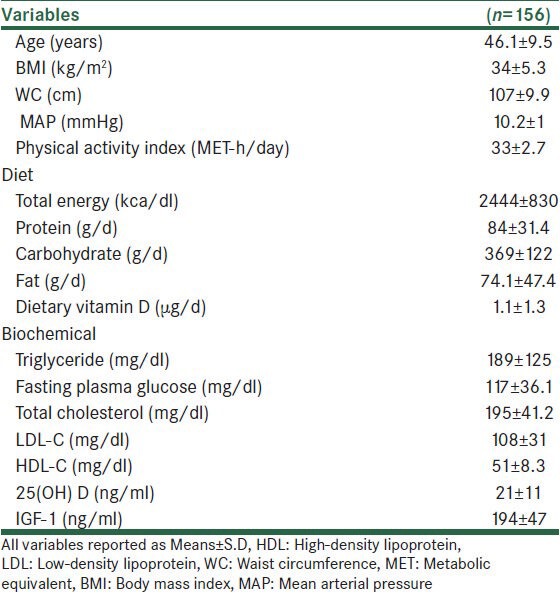
The mean of age was 46.1 ± 9.5 years and it was for serum 25(OH) D and IGF-1 concentrations 20.5 ± 10.8, 194 ± 47 ng/ml, respectively. Overall, %54.5 of women were vitamin D deficient and 23.7% of women were vitamin D insufficienct based on serum concentration of 25(OH) D.
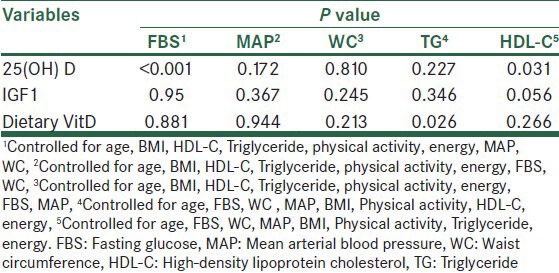
Univariated-adjusted General Linear Model was used to assess the relation between predictor factors to MAP, FBS, WC, HDL-C and TG.
When predictor factors were entered together in this model, none of the predictors was significantly related to MAP and WC. However, levels of serum 25(OH) D was signifcantly related to FBS (P < 0.001). We observed that 25(OH) D concentration was related to HDL-C (P = 0.03) and dietary vitamin D was related to triglyceride (P = 0.02).
NO significant relationship were observed between IGF1, 25(OH) D, dietary vitamin D. Also IGF1 was not associated with none of components of MetS [Table 2].
Table 2.
Results of fitting univariate models to predictor factors on FBS, MAP, WC, HDL-C and TG
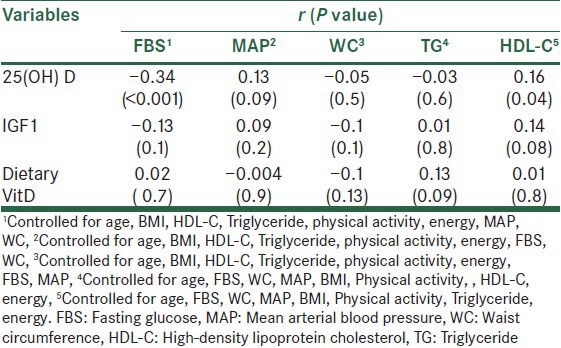
Partial correlation analysis were used to evaluate the relationship of predictive factors with components of metabolic syndrome.
After adjusting for age, BMI, HDL-C, TG, physical activity, energy, FBS, MAP, The correlation (r) between WC and 25(OH) D was −0.05; P = 0.5 and IGF1 was −0.1; P = 0.1 and dietary vitamin D was −0.1; P = 0.1.
Correlation of 25(OH) D and TG was −0.03; P = 0.6 when we adjusted for age, FBS, WC, MAP, BMI, Physical activity, energy [Table 3].
Table 3.
Results of partial correlation to predictor factors on FBS and MAP, WC, TG and HDL-C
DISCUSSION
In this study, our findings showed that levels of serum 25(OH) D and dietary vitamin D were directly related to HDL-C and TG levels, respectively. We also observed statically significant inverse relation between FBS and 25(OH) D levels. These relations were independent of confounders. We found no significant association between IGF-1 with components of MetS and vitamin D status. This study is the first report on the relationship between vitamin D status and IGF-1 with components of the MetS that were simultaneously examined in an Iranian population with MetS.
Growing evidence have shown that vitamin D likely plays a role in the development of MetS and DM2.[6,34] Similarly, serum levels of IGF-1 are also predictive of MetS. But, these associations are somewhat weaker than those observed for 25(OH) D.[34]
In our study, we found a significant inverse association between FBS with 25(OH) D levels. Similar studies have shown an inverse association between serum vitamin D with levels of FBS. NHANES, the largest cross-sectional study up to now, show that hyperglycemia is independently associated with weak vitamin D status.[6] A cross-sectional study in the 1958 British Birth Cohort showed that the association of 25(OH) D with serum glucose levels did not vary in obese compared with normal-weight individuals.[18,34,51] One prospective study of 524 nondiabetic individuals reported inverse associations between baseline serum 25(OH) D and the incidence of hyperglycemia after 10 years of follow up.[16] In a randomized placebo-controlled study, supplementation vitamin D and calcium led to a lower rise in glucose levels.[52] However, no effect on FBS was observed in another randomized study.[53] Inconsistent findings in different trials could be related to small sample size and short duration and lack of control for cofounding variables.
Our results are in agreement with observational evidence, primarily from cross sectional. It seems hypovitaminosis D through reduction insulin secretion, regulation of intracellular calcium and change in IGF-1 regulation is associated with higher fasting glucose levels.[34,53]
Several studies have examined the association of vitamin D status with lipid profile. With conflicting results[34,54,55] we found statistically significant association between dietary vitamin D and serum TG levels.
In the current study, HDL-C levels after adjusting for age, FBS, WC, MAP, BMI, physical activity and total energy were directly associated with 25(OH) D. A cross-sectional study, in adult men and women, found that for each 10-ng/ml increment in 25(OH) D, HDL-C was increased 3.8 to 4.2 mg/dl.[22] Also, Dobnig et al. reported similar results.[22] However, there is little evidence for suggesting likely mechanism which vitamin D status could affect in development of dyslipidemia.
In this study, we found nonsignificant negative relationship between 25(OH) D and WC. Our result had similar trend to numerous studies although their findings showed significant relation. The difference about significance between our finding and similar studies may be attributed to our smaller sample size. According to studies, potential mechanisms included solubility of vitamin D in adipose tissue and slow bioavailability of vitamin D into the circulation, inadequate intake of vitamin D due to inactivity of obese subjects and less sun exposure.[17]
Several studies have shown that vitamin D may regulate blood pressure by regulating the renin-angiotensin system and inhibits the renin mRNA expression.[56,57] But, most of results in this area are conflicted.
We found no significant relation between serum 25(OH) D and blood pressure. Similarly, Forouhi et al. in ELY Prospective study did not find association after 10 year follow up[16] and pointed out that the association of blood markers of vitamin D with blood pressure is complex.
Our results are in contrast with some studies. The KEEP study in Korea found statistically significant inverse association between 25(OH) D and blood pressure.[58] Also, Burgaz et al. in a meta-analysis reported the same.[59] Scragg et al. in another study reported inverse association 25(OH) D with blood pressure.[60] In these studies, significant relationships were attributed to using predicted vitamin D levels, self-reported hypertension and using repeated measures analysis. Thus, relationship between 25(OH) D and blood pressure without these cannot be significant.
The key role of calcium in the regulation of 25(OH) D for hypertension is advocated.[61] As, a meta-analysis of 40 randomized controlled trials, calcium supplementation equal to 1 g/day significantly reduce blood pressure.[40] Regard to in our study, mean intake of dietary calcium is much less than dietary recommendation intake (DRI). Therefore, it seems determination of serum calcium could help us for further explaining likely association of 25(OH) D with blood pressure.
With the exception of triglyceride, we no found relation between dietary vitamin D to markers of the MetS.[56] Also, 25(OH) D is not related to dietary vitamin D. There is several explanations to these null results. One possible explanation is that there is no relation. Other explanations may be limited food sources of vitamin D, measurement error for dietary assessment of vitamin D by FFQ. However, it should be noted that compared with sunlight, dietary vitamin D is not a strong predictor of body vitamin D status.
In the present study, sun exposure was also not associated with levels of 25(OH) D. Similar finding by Sima Hashemipour et al. in a population of Tehran was reported.[62] One hypothesis might be low precision of oral answers subjects about sun exposure period. Another hypothesis is type of clothing in Iranian women. Since, changes in the amount of sunlight in our study was limited thus as a related factor could not to be considered.
We did not find any association of serum IGF-I with vitamin D status and every component of the MetS. But we observed nonsignificant negative and positive relationship about IGF-1 with FBS and 25(OH) D, respectively, although their relation was not significant. According to a cross-sectional study[34] and a prospective study[16] IGF-1 is directly related to 25(OH) D until when 25(OH) D reached ~ 75-85 nmol/L. It was suggested that IGF-1 may change activation of vitamin D. Determination of effective factors of IGF-I levels such as circulating GH, insulin, IGFBPS, parathyroid hormone, calcium levels would be required for accurate assessing of IGF-I with 25(OH) D and component of the MetS. Also, identification of genetic polymorphisms could help to characterize IGF-I long-term production in tissues.
It should be noted that our study has the following limitations: Small sample size, only looking at females, no direct method of measuring sun exposure and therefore, random error due to design study, measurement errors associated with the assessment questionnaires and lack of control confounding despite adjustments in statistical methods. All of the above limitations may be effective to find associations between vitamin D status and IGF-1 with components of the MetS.
In conclusion, the findings of this study support relation of 25(OH) D to HDL-C and FBS. These associations are independent of confounding. But no relation was observed between IGF-I to 25(OH) D and components of the MetS. Therefore, to clarify and quantify the associations, further cohort investigations are warranted.
ACKNOWLEDGMENT
Our greatest thanks go to subjects who participated in this study. This study was financially supported by School of Nutrition and Food Sciences, Isfahan University of Medical Sciences (code: 391096).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Gulseth HL, Gjelstad IM, Tierney AC, Lovegrove JA, Defoort C, Blaak EE, et al. Serum vitamin D concentration does not predict insulin action or secretion in European subjects with the metabolic syndrome. Diabetes Care. 2010;33:923–5. doi: 10.2337/dc09-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereira MA, Jacobs DR, Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: The CARDIA study. JAMA. 2002;287:2081–9. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 4.Mauras N, Haymond MW. Are the metabolic effects of GH and IGF-I separable? Growth Horm IGF Res. 2005;15:19–27. doi: 10.1016/j.ghir.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Azizi F, Salehi P, Etemadi A, Zahedi-Asl S. Prevalence of metabolic syndrome in an urban population: Tehran Lipid and Glucose Study. Diabetes Res Clin Pract. 2003;61:29–37. doi: 10.1016/s0168-8227(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 6.Hossein-Nezhad A, Khoshniat NM, Maghbooli Z, Karimi F, Mirzaei K, Hosseini A, et al. Relationship between serum vitamin D concentration and metabolic syndrome among Iranian adults population. Daru. 2009;17(Suppl 1):1–5. [Google Scholar]
- 7.Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28:2926–32. doi: 10.2337/diacare.28.12.2926. [DOI] [PubMed] [Google Scholar]
- 8.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham risk score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–50. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 9.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi F. Dairy consumption is inversely associated with the prevalence of the metabolic syndrome in Tehranian adults. Am J Clin Nutr. 2005;82:523–30. doi: 10.1093/ajcn.82.3.523. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C American Heart Association; National Heart Lung and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol. 1999;83:25F–9F. doi: 10.1016/s0002-9149(99)00211-8. [DOI] [PubMed] [Google Scholar]
- 12.Bartke A. Insulin and aging. Cell Cycle. 2008;7:3338–43. doi: 10.4161/cc.7.21.7012. [DOI] [PubMed] [Google Scholar]
- 13.Straznicky NE, Lambert EA, Lambert GW, Masuo K, Esler MD, Nestel PJ. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90:5998–6005. doi: 10.1210/jc.2005-0961. [DOI] [PubMed] [Google Scholar]
- 14.Anderssen SA, Carroll S, Urdal P, Holme I. Combined diet and exercise intervention reverses the metabolic syndrome in middle-aged males: Results from the Oslo diet and exercise study. Scand J Med Sci Sports. 2007;17:687–95. doi: 10.1111/j.1600-0838.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 15.Wirfält E, Hedblad B, Gullberg B, Mattisson I, Andrén C, Rosander U, et al. Food patterns and components of the metabolic syndrome in men and women: A cross-sectional study within the Malmö diet and cancer cohort. Am J Epidemiol. 2001;154:1150–9. doi: 10.1093/aje/154.12.1150. [DOI] [PubMed] [Google Scholar]
- 16.Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: The medical research council Ely prospective study 1990-2000. Diabetes. 2008;57:2619–25. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martini LA, Wood RJ. Vitamin D status and the metabolic syndrome. Nutr Rev. 2006;64:479–86. doi: 10.1111/j.1753-4887.2006.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 18.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–5. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 19.Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89:1196–9. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 21.Kulie T, Groff A, Redmer J, Hounshell J, Schrager S. Vitamin D: An evidence-based review. J Am Board Fam Med. 2009;22:698–706. doi: 10.3122/jabfm.2009.06.090037. [DOI] [PubMed] [Google Scholar]
- 22.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 23.Maki KC, Rubin MR, Wong LG, McManus JF, Jensen CD, Marshall JW, et al. Serum 25-hydroxyvitamin D is independently associated with high-density lipoprotein cholesterol and the metabolic syndrome in men and women. J Clin Lipidol. 2009;3:289–96. doi: 10.1016/j.jacl.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Utzschneider KM, Van de Lagemaat A, Faulenbach MV, Goedecke JH, Carr DB, Boyko EJ, et al. Insulin resistance is the best predictor of the metabolic syndrome in subjects with a first-degree relative with type 2 diabetes. Obesity (Silver Spring) 2010;18:1781–7. doi: 10.1038/oby.2010.77. [DOI] [PubMed] [Google Scholar]
- 25.Kim MK, Il Kang M, Won Oh K, Kwon HS, Lee JH, Lee WC, et al. The association of serum vitamin D level with presence of metabolic syndrome and hypertension in middle-aged Korean subjects. Clin Endocrinol (Oxf) 2010;73:330–8. doi: 10.1111/j.1365-2265.2010.03798.x. [DOI] [PubMed] [Google Scholar]
- 26.Kamycheva E, Sundsfjord J, Jorde R. Serum parathyroid hormone level is associated with body mass index. The 5th Tromsø study. Eur J Endocrinol. 2004;151:167–72. doi: 10.1530/eje.0.1510167. [DOI] [PubMed] [Google Scholar]
- 27.Compston JE, Vedi S, Ledger JE, Webb A, Gazet JC, Pilkington TR. Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr. 1981;34:2359–63. doi: 10.1093/ajcn/34.11.2359. [DOI] [PubMed] [Google Scholar]
- 28.Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29:3713–20. [PubMed] [Google Scholar]
- 29.Major GC, Alarie F, Doré J, Phouttama S, Tremblay A. Supplementation with calcium+vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am J Clin Nutr. 2007;85:54–9. doi: 10.1093/ajcn/85.1.54. [DOI] [PubMed] [Google Scholar]
- 30.Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, et al. Adiposity in relation to vitamin D status and parathyroid hormone levels: A population-based study in older men and women. J Clin Endocrinol Metab. 2005;90:4119–23. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- 31.Liu E, Meigs JB, Pittas AG, McKeown NM, Economos CD, Booth SL, et al. Plasma 25-hydroxyvitamin D is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr. 2009;139:329–34. doi: 10.3945/jn.108.093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attia N, Tamborlane WV, Heptulla R, Maggs D, Grozman A, Sherwin RS, et al. The metabolic syndrome and insulin-like growth factor I regulation in adolescent obesity. J Clin Endocrinol Metab. 1998;83:1467–71. doi: 10.1210/jcem.83.5.4827. [DOI] [PubMed] [Google Scholar]
- 33.Wolk K, Larsson SC, Vessby B, Wolk A, Brismar K. Metabolic, anthropometric, and nutritional factors as predictors of circulating insulin-like growth factor binding protein-1 levels in middle-aged and elderly men. J Clin Endocrinol Metab. 2004;89:1879–84. doi: 10.1210/jc.2003-031349. [DOI] [PubMed] [Google Scholar]
- 34.Hyppönen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: A cross-sectional study in the 1958 British Birth Cohort. Diabetes. 2008;57:298–305. doi: 10.2337/db07-1122. [DOI] [PubMed] [Google Scholar]
- 35.Gómez JM, Maravall FJ, Gómez N, Navarro MA, Casamitjana R, Soler J. Interactions between serum leptin, the insulin-like growth factor-1 system, and sex, age, anthropometric and body composition variables in a healthy population randomly selected. Clin Endocrinol (Oxf) 2003;58:213–9. doi: 10.1046/j.1365-2265.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- 36.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: The role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17:328–36. doi: 10.1016/j.tem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Rasmussen SK, Lautier C, Hansen L, Echwald SM, Hansen T, Ekstrøm CT, et al. Studies of the variability of the genes encoding the insulin-like growth factor 1 receptor and its ligand in relation to type 2 diabetes mellitus. J Clin Endocrinol Metab. 2000;85:1606–10. doi: 10.1210/jcem.85.4.6494. [DOI] [PubMed] [Google Scholar]
- 38.Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ. Circulating concentrations of insulin-like growth factor-1 and development of glucose intolerance: A prospective observational study. Lancet. 2002;359:1740–5. doi: 10.1016/S0140-6736(02)08655-5. [DOI] [PubMed] [Google Scholar]
- 39.Clemmons DR, Moses AC, McKay MJ, Sommer A, Rosen DM, Ruckle J. The combination of insulin-like growth factor 1 and insulin-like growth factor-binding protein-3 reduces insulin requirements in insulin-dependent type 1 diabetes: Evidence for in vivo biological activity. J Clin Endocrinol Metab. 2000;85:1518–24. doi: 10.1210/jcem.85.4.6559. [DOI] [PubMed] [Google Scholar]
- 40.Rozing MP, Westendorp RG, Frölich M, de Craen AJ, Beekman M, Heijmans BT, et al. Leiden Longevity Study (LLS) Group. Human insulin/IGF-1 and familial longevity at middle age. Aging (Albany NY) 2009;1:714–22. doi: 10.18632/aging.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cusi K, DeFronzo R. Treatment of NIDDM, IDDM, and other insulin-resistant states with IGF-I: Physiological and clinical considerations. Diabetes Rev. 1995;3:206–36. [Google Scholar]
- 42.Yakar S, Liu JL, Fernandez AM, Wu Y, Schally AV, Frystyk J, et al. Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes. 2001;50:1110–8. doi: 10.2337/diabetes.50.5.1110. [DOI] [PubMed] [Google Scholar]
- 43.Laron Z. Insulin-like growth factor 1 (IGF-1): A growth hormone. Mol Pathol. 2001;54:311–6. doi: 10.1136/mp.54.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–6. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 45.Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med. 1997;336:633–40. doi: 10.1056/NEJM199702273360907. [DOI] [PubMed] [Google Scholar]
- 46.Juul A, Main K, Blum WF, Lindholm J, Ranke MB, Skakkebaek NE. The ratio between serum levels of insulin-like growth factor (IGF)-I and the IGF binding proteins (IGFBP-1, 2 and 3) decreases with age in healthy adults and is increased in acromegalic patients. Clin Endocrinol (Oxf) 1994;41:85–93. doi: 10.1111/j.1365-2265.1994.tb03788.x. [DOI] [PubMed] [Google Scholar]
- 47.Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran lipid and glucose study. J Epidemiol. 2010;20:150–8. doi: 10.2188/jea.JE20090083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghafarpour M, Houshiar-Rad A, Kianfar H. Tehran: Nashre Olume Keshavarzy; 1999. The manual for household measures, cooking yields factors and edible portion of food; p. 49. [Google Scholar]
- 49.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(Suppl 9):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 50.Esmaillzadeh A, Mirmiran P, Azizi F. Evaluation of waist circumference to predict cardiova scular risk factors in an overweight Tehranian population: Findings from Tehran lipid and glucose study. Int J Vitam Nutr Res. 2005;75:347–56. doi: 10.1024/0300-9831.75.5.347. [DOI] [PubMed] [Google Scholar]
- 51.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care. 2007;30:1544–8. doi: 10.2337/dc06-1331. [DOI] [PubMed] [Google Scholar]
- 52.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–6. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 53.Song Y, Manson JE. Vitamin D, insulin resistance, and type 2 diabetes. Curr Cardiovasc Risk Rep. 2010;4:40–7. [Google Scholar]
- 54.Jorde R, Figenschau Y, Hutchinson M, Emaus N, Grimnes G. High serum 25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. Eur J Clin Nutr. 2010;64:1457–64. doi: 10.1038/ejcn.2010.176. [DOI] [PubMed] [Google Scholar]
- 55.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: Data from the third national health and nutrition examination survey. Arch Intern Med. 2007;167:1159–65. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 56.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28:1228–30. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 57.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–7. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 58.Park HY, Lim YH, Kim JH, Bae S, Oh SY, Hong YC. Association of Serum 25-Hydroxyvitamin D levels with markers for metabolic syndrome in the elderly: A repeated measure analysis. J Korean Med Sci. 2012;27:653–60. doi: 10.3346/jkms.2012.27.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: A meta-analysis. J Hypertens. 2011;29:636–45. doi: 10.1097/HJH.0b013e32834320f9. [DOI] [PubMed] [Google Scholar]
- 60.Scragg R. Calcium-Regulating Hormones and Cardiovascular Function. Boca Raton: CRC Press; 1995. Sunlight, vitamin D, and cardiovascular disease; pp. 213–37. [Google Scholar]
- 61.Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–56. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 62.Hashemipour S, Larijani B, Adibi H, Javadi E, Sedaghat M, Pajouhi M, et al. Vitamin D deficiency and causative factors in the population of Tehran. BMC Public Health. 2004;4:38. doi: 10.1186/1471-2458-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]


