Abstract
Although mumps-containing vaccines were introduced in China in 1990s, mumps continues to be a public health concern due to the lack of decline in reported mumps cases. To assess the mumps vaccine effectiveness (VE) in Guangzhou, China, we performed a 1:1 matched case-control study. Among children in Guangzhou aged 8 mo to 12 y during 2006 to 2012, we matched one healthy child to each child with clinically diagnosed mumps. Cases with clinically diagnosed mumps were identified from surveillance sites system and healthy controls were randomly sampled from the Children’s Expanded Programmed Immunization Administrative Computerized System in Guangzhou. Conditional logistic regression was used to calculate VE. We analyzed the vaccination information for 1983 mumps case subjects and 1983 matched controls and found that the overall VE for 1 dose of mumps vaccine, irrespective of the manufacture, was 53.6% (95% confidence interval [CI], 41.0–63.5%) to children aged 8 mo to 12 y. This post-marketing mumps VE study found that immunization with one dose of the mumps vaccine confers partial protection against mumps disease. Evaluation of the VE for the current mumps vaccines, introduction of a second dose of mumps vaccine, and assessment of modifications to childhood immunization schedules is essential.
Keywords: mumps, vaccine effectiveness, matched case-control studies, children, China
Introduction
Mumps virus causes an acute febrile illness with a nonspecific prodrome followed by painful swelling of the parotid and less commonly, other salivary glands. Complications of mumps include deafness, mastitis, aseptic meningitis, encephalitis, and, in postpubertal age groups, oophoritis and orchitis.1 The incubation period of mumps ranges from 12–25 d but parotitis may develop 16–18 d post exposure.
Although mumps-containing vaccines were introduced in China in 1990s, mumps continues to be a public health concern due to the lack of decline in reported mumps cases. A total of 909 087 cases of mumps were reported accumulatively in China during 2008–2010 with annual average incidence of 22.8 per 100 000. Up to 81.8% of the cases occurred in children aged 3–14 y and 97.0% of the outbreaks occurred in child care settings and schools, especially in the primary schools.2,3 Reported mumps incidence is not declining in China, but the increase or lack of decrease may be due to improved surveillance in the country.
Located on the Tropic of Cancer, with a subtropical climate with an average temperature of 22.9 °C, Guangzhou is the largest metropolis in Southern China with a population over 12.75 million in 2012. Mumps has been a notifiable disease since 1990 in Guangzhou; 18 072 mumps cases were reported during 2004 to 2012 (Fig. 1), with the peak period of May to August each year. The international measles-mumps-rubella (MMR) vaccine (Jeryl-Lynn and RIT4385) was introduced in 1990 in Guangzhou. Since 1995, domestic live attenuated mumps vaccine (S79 and Wm84 strain, derived from Jery1-Lynn) has been in use in Guangzhou and is provided to children more than 8 mo of age.4 Previous evaluations of the mumps vaccine effectiveness (VE) in Guangzhou during 2004–2005 showed a moderate protection of 86.0% (95% CI, 77.2–91.5%) to children aged 8 mo to 12 y.4,5 Since 2008, MMR or MM (measles-mumps) vaccine has been administered to children 18–24 mo of age as part of the national routine vaccination schedule. To continue to assess the mumps VE in Guangzhou, China during 2006 to 2012, we performed a 1:1 matched case-control study.
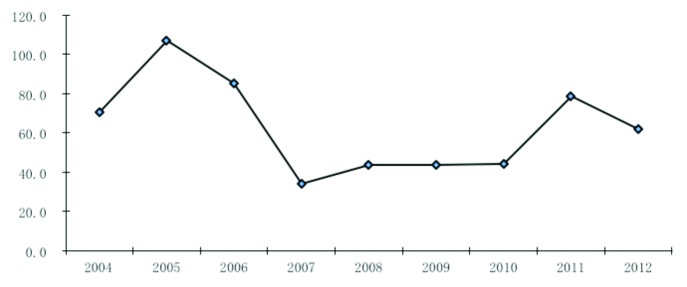
Figure 1. Mumps incidence in Guangzhou during 2004–2012 (per 100 000).
Results
Of the 11 579 reported mumps cases in Guangzhou between May 1 and August 31, during 2006 to 2012, 4629 (40.0%) mumps case subjects were randomly sampled. A group of 1983 case subjects (42.8%) and 1983 controls were finally included in our analysis. The remaining 57.2% of 4629 cases were not enrolled because they could not be identified in the Children EPI Administrative Computerized System. The sampled cases did not differ from the included cases in respect to gender (3078 males vs. 1551 females 1.98:1, 1294 males vs. 689 females 1.88; χ2 = 0.952, p = 0.329), but the sampled cases were older than included cases (5.36 ± 2.55 y old, 4.53 ± 2.42 y old; t = 12.233, p = 0.000).
The median age of case subjects at time of diagnosis was 4.53 y (range: 8 mo to 11.83 y). Among the 3966 study participants, 2217 (55.9%) had received 1 dose of mumps vaccine. The percentage of vaccinated controls was higher than the percentage of vaccinated cases (62.7% vs. 49.1%)(Table 1).
Table 1. Demographic characteristics of study subjects.
| Number of mumps cases/total (%) | Number of controls/total (%) | P value | |
|---|---|---|---|
| Gender | 1.000 | ||
| Male | 1294(65.3) | 1294(65.3) | |
| Area | 1.000 | ||
| Urban | 479(24.2) | 479(24.2) | |
| Rural | 732(36.9) | 732(36.9) | |
| Age of onseta | 4.53 ± 2.42 | 4.54 ± 2.43 | 0.943 |
| Vaccinatedb | 974(49.1) | 1243(62.7) | 0.000 |
| Age of vaccinationa,c | 1.60 ± 0.76 | 1.63 ± 0.74 | 0.262 |
aTwo-sample t-test was used to analyze group difference. Mean ± standard deviation. bVaccination vs. No vaccination. χ2 test was used to analyze group differences. cOnly included children received mumps vaccines.
After adjusting for vaccination age, the overall VE of one dose of the mumps vaccine relative to no vaccination was 53.6% (95% CI, 41.0–63.5%) for the prevention of clinical mumps among children aged 8 mo to 12 y (Table 2). Mumps vaccination provided a moderate protection for children aged 19 mo to 12 y old. And no protective effect was observed for children aged 8 to 18 mo. The VE reached statistical significance during the first 8 y. The international vaccine provided 51.3% (95%CI, 7.2–74.4%) protection for children, while no protective effect was found for domestic vaccine.
Table 2. Estimates of the effectiveness of one dose mumps vaccination in children 8 mo to 12 y old, multivariate conditional logistic regression analysis.
| Vaccinated case (%) | Vaccianted control (%) | Vaccine effectiveness (95%CI),% | P | |
|---|---|---|---|---|
| Vaccination age (months) | ||||
| 8–11 | 95(75.4) | 96(76.2) | 98.5(-14734584.4,100.0) | 0.610 |
| 12–18 | 443(80.8) | 538(83.7) | 50.0(-451.4,95.5) | 0.571 |
| 19–24 | 315(76.3) | 447(86.0) | 75.0(33.4,90.6) | 0.006 |
| ≥ 25 | 121(13.5) | 162(23.3) | 64.6(46.0,76.8) | < 0.0001 |
| Time since vaccination (year) | ||||
| < 1 | 202(10.2) | 269(13.6) | 53.6 (37.8,65.4) | < 0.0001 |
| 1 | 221(11.1) | 260(13.1) | 49.0(28.7,63.5) | < 0.0001 |
| 2 | 150(7.6) | 196(9.9) | 54.5(34.0,68.6) | < 0.0001 |
| 3 | 101(5.1) | 132(6.7) | 52.0(27.0, 68.4) | 0.001 |
| 4 | 73(3.7) | 110(5.5) | 62.8(40.3,76.9) | < 0.0001 |
| 5 | 80(4.0) | 93(4.7) | 56.0(25.0,74.2) | 0.003 |
| 6 | 58(2.9) | 76(3.8) | 57.4(25.9,75.5) | 0.002 |
| 7 | 57(2.9) | 66(3.3) | 52.4(12.8,74.0) | 0.016 |
| 8 | 30(1.5) | 36(1.8) | 52.9(1.2,77.6) | 0.046 |
| ≥ 9 | 2(0.1) | 5(0.3) | 87.7(-18.6,98.7) | 0.07 |
| Manufacturer | ||||
| International | 112(5.6) | 145(7.3) | 51.3(7.2,74.4) | 0.029 |
| Domestic | 242(12.2) | 261(7.3) | 21.4(-54.2,59.9) | 0.484 |
| Missing | 620(31.3) | 837(42.2) | 57.8(42.1,69.3) | < 0.0001 |
| Total | 974(49.1) | 1243(62.7) | 53.6(41.0,63.5) | < 0.0001 |
Discussion
We analyzed the vaccination information for 1983 mumps case subjects and 1983 matched controls aged 8 mo to 12 y from 2006 to 2012 in Guangzhou, China. The overall VE for 1 dose of mumps vaccine, irrespective of the manufacture, was 53.6% (95% CI, 41.0–63.5%). This post-marketing mumps VE study found that immunization with one dose of the mumps vaccine confers partial protection against mumps disease. VE since nine years after vaccination was lack of statistical power and the small sample size (2 vaccinated cases) might be a contributing factor.
MMR or MM vaccine has been included in the National Immunization Program in China in 2008. That is to say, eligible children aged 8–24 mo are able to mainly receive free domestic MMR or MM vaccine for preventing mumps. However, the vaccinations among children older than 2 y have to be paid by their guardians. Consequently, the mumps vaccination coverage may increase dramatically since 2008 among children 8–24 mo. The mumps incidence rate among children of 3–12 y old may be higher than other age groups, since this cohort was not “eligible” for vaccination under the prior National Immunization Program. Some parents may choose international vaccines for their children (8–24 mo) even if the extra charges, because they would believe that international vaccines may provide better protection than the domestic vaccines. As a result, vaccine type is highly correlated with age at vaccination, the younger children may be likely to receive domestic vaccines rather than international vaccines, and the older children would receive both kind of vaccines. Due to the 63.7% missing of the manufacture information in the electronic system, the small sample size in the study made it hard to conduct separately by vaccine type to address whether VE wanes over time, we only addressed it in general.
The 1 dose VE for Jeryl Lynn-containing mumps vaccine ranges from 49–88% (median: 77%).6,7 There is dearth of studies that have examined vaccine effectiveness exclusively for mumps vaccines containing the RIT 4385 strain although there are several published studies that include individuals who received the RIT 4385 strain mumps vaccine.8,9 The VE for the RIT 4385 strain is expected to be similar to the Jeryl-Lynn strain as it is a derivative of the Jery1-Lynn strain.
Our VE estimate for 1 dose of mumps vaccine for the international mumps vaccine is similar to lower range Jery1 Lynn-containing vaccine, ie, 49–66%6,8,10–13 and VE for outbreaks in Zhongshan, China in 2008 (65%).14 Different study designs, including case definition, and availability of vaccination information, may lead to the variable findings. Other possible factors include the response to a mumps virus genotype. The distribution of mumps virus genotypes (especially the small hydrophobic protein gene) varies extensively both temporally and geographically.15-17 However, we cannot compare the prevalent serotype with other areas because there is no such data in Guangzhou, even little is known on the presence of antibody to mumps virus in Guangzhou. Future work should be taken to reveal the circulation of mumps virus in Guangzhou.
The difference between the VE for the domestic vaccine in our study and that from the controlled random clinical trials may be related to the cold chain. Conditions in the cold chain may not be as adequate as required.18 In addition, with a wide spectrum of vaccine recipients, it is possible that the observed impact of vaccination is lower than results from the controlled random clinical trials. Another possible reason is that this study has longer follow-up than most clinical trials, which typically have very short-term follow-up.19 Although there are no established immunologic correlates of mumps protection in China, mumps VE estimates are usually based on serological studies, which have shown to provide moderate protection to children. A meta-analysis based on 23 studies on domestic or international mumps vaccines conducted from 1979 to 2011 showed that the hemagglutination inhibition antibody positive conversion rate was 23.2–93.2%.20
There are several strengths to this study. Above all, by using information about cases’ clinical diagnoses from the surveillance system and vaccination records from the children’s EPI Administrative Computerized System, we eliminated the recall bias that is a common problem in traditional case-control studies. A second strength is that controls matched cases with respect to the street where cases lived, thus cases and controls may be comparable to exposure to mumps, regardless of underlying medical condition and family financial status, which improve the validity of the results.
Our study has several limitations. First, approximately 60% of the case subjects were missing from the computerized system EPI system. It is possible that the exclusion of these case subjects may reduce the generalizability of our findings. This may be due to children from neighboring cities who seek medical treatment in Guangzhou, or the floating children lived in Guangzhou. Missing vaccination records may be more frequent among unvaccinated children, which can result in a negative bias to the results. However, according to sample size calculations, based on Schlesselman’s equation for matched case-control studies, the large sample size (especially in the 2011–12 season) could be sufficient to provide insightful evidence on the mumps VE, our findings could provide insightful evidence on the mumps VE. Second, the sample may not be representative of all mumps patients in that the cases in our study were not laboratory confirmed and some of the controls may have been latent or subclinical cases. Third, we did not collect and estimate other potential confounders, e.g., co-morbidity, socio-economic status, physical health status, and the severity of mumps cases. The collection of these factors may have been important because they may indicate children with underlying health problems and/or children who may be more likely to present to the hospital, which could result in positive or negative biases. Fourth, mumps complications such as deafness and orchitis were not investigated among the cases, which is also a shortcoming and thus we failed to calculate the VE against mumps complications. Finally, compared with the prospective VE studies conducted in special hospitals (outpatient or inpatient), the retrospective case-control study may be less comparable in every subject (cases vs. controls) within the same age category equally exposed to mumps virus.
Our study indicates that one dose of mumps vaccine administered between 19 mo to 12 y of age confers partial protection against mumps but with the possibility of waning immunity. With the increasing number of mumps cases in China, we believe that mumps-containing vaccination should be continued. What’s more, the mumps VE against complications in theory could be higher than against parotitis. Although the VE of longer time-periods since vaccination are mostly not statistically significant, this study also suggests that mumps VE may decline nine years after vaccination, and previous studies also documented increased risk of developing mumps with increasing time after vaccination.9,21 The second dose of mumps-containing vaccination campaign should be added in the national schedule, may be performed in ~11 y old. Otherwise, providing catch-up vaccination opportunities for older children who were not eligible for the national vaccination schedule also would increase vaccination coverage in the age groups most affected by mumps. Efforts to explore modifications in the immunization schedule to optimize the performance of the current vaccine for mumps control should be encouraged.
Methods
Case subjects, 8 mo to 12 y of age, were randomly selected from two electronic databases in Guangzhou: the Notifiable Disease Reporting System and the Children’s Expanded Programmed Immunization (EPI) Administrative Computerized System, which have been described in detail elsewhere.22 A mumps case was defined as having acute onset of unilateral or bilateral tender swelling of the parotid of salivary gland lasting two or more days without any other apparent cause.4 Bacterial infection was excluded by the absence of an increase in the white blood cell count. Because of the large number of cases reported each year, the case subjects were selected by the simple random sampling method from May to August, the peak mumps reporting periods, during 2006 to 2012.
Controls were randomly selected among children, aged 8 mo to 12 y, listed in the Children’s EPI Administrative Computerized System, which was designed to manage the immunization records of children less than 7 y of age in Guangzhou in 1997. The EPI system allows health care workers to easily record, retrieve and analyze all children’s vaccination information; registration of vaccination information in the system is required.23,24 Controls were accepted if they did not have prior history of mumps, as confirmed by a phone call by physicians from the Guangzhou Center for Disease Control and Prevention (GZCDC). A list of potential controls with sequence number for each case subject was then created and matched by birth date, gender, and residence (living area, in the same community or village, and residence was categorized into urban, rural, and rural-urban continuum area). A random number was used to select the potential control. If the potential control declined to participate and/or had prior history of mumps disease, a control candidate with the next closet date of birth to the case subject was enrolled to participate.
VE was calculated as one minus the odds ratio (OR) × 100%, where the odds of mumps among the vaccinated subjects was compared with the odds of mumps among the unvaccinated subjects as before.25 For cases, only mumps vaccinations received at least 30 d before the onset of mumps disease were considered valid. For controls, we considered only doses administered up to 30 d before the date of symptom onset in the corresponding case subject. The vaccines were categorized either as domestic or international based on (1) manufacture information in the database and (2) the age of receipt of the vaccine. For example: If the information of the manufacture was missing but the subject did receive a vaccine when less than 12 mo of age then ‘domestic’ was assigned to the manufacture.
SPSS statistical software (version 16.0, SPSS, Inc.) was used for data validation and statistical analysis. Vaccination age and vaccination status were included in the model. Using the methods by Niccolai LM to calculate VE for time since vaccination and age at the time of vaccination.26 p ≤ 0.05 was regarded as a statistically significant difference. Study approval was obtained from the GZCDC ethics committee.
Disclosure of Potential Conflicts of Interest
The authors have no other conflicts of interest or funding to disclose.
Funding
This work was supported by grants from the National Science and Technology Major Projects of China (No. 2012ZX10004213-005) , the Guangdong Provincial Department of Science and Technology (2011B061300044 and 2012B091100045) and the Department of Guangzhou Science and Information Technology (2012J5100005). The funders had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We would like to thank the healthcare personnel and staff who collaborated in this study. We wish to also give thanks to Dr Preeta K Kutty, who provided her helpful comments during the manuscript preparation process.
References
- 1.Hviid A, Rubin S, Mühlemann K. Mumps. Lancet. 2008;371:932–44. doi: 10.1016/S0140-6736(08)60419-5. [DOI] [PubMed] [Google Scholar]
- 2.Fei F, Feng L, Xu Z, Feng Z. Epidemiology of mumps in China, 2008-2010. Dis Surveill. 2011;26:691–3. [Google Scholar]
- 3.Yin D, Fan C, Cao L, Wang H, Zhou Y, Liang X. Epidemiological analysis of epidemic parotitis in China from 2004 to 2006. Dis Surveill. 2007;22:310–1. [Google Scholar]
- 4.Fu C, Liang J, Wang M. Matched case-control study of effectiveness of live, attenuated S79 mumps virus vaccine against clinical mumps. Clin Vaccine Immunol. 2008;15:1425–8. doi: 10.1128/CVI.00122-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu CX, Nie J, Liang JH, Wang M. Evaluation of live attenuated S79 mumps vaccine effectiveness in mumps outbreaks: a matched case-control study. Chin Med J (Engl) 2009;122:307–10. [PubMed] [Google Scholar]
- 6.Deeks SL, Lim GH, Simpson MA, Gagné L, Gubbay J, Kristjanson E, Fung C, Crowcroft NS. An assessment of mumps vaccine effectiveness by dose during an outbreak in Canada. CMAJ. 2011;183:1014–20. doi: 10.1503/cmaj.101371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Immunological basis for immunization: Mumps. 24 November 2010.http://whqlibdoc.who.int/publications/2010/9789241500661_eng.pdf
- 8.Harling R, White JM, Ramsay ME, Macsween KF, van den Bosch C. The effectiveness of the mumps component of the MMR vaccine: a case control study. Vaccine. 2005;23:4070–4. doi: 10.1016/j.vaccine.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Cohen C, White JM, Savage EJ, Glynn JR, Choi Y, Andrews N, Brown D, Ramsay ME. Vaccine effectiveness estimates, 2004-2005 mumps outbreak, England. Emerg Infect Dis. 2007;13:12–7. doi: 10.3201/eid1301.060649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castilla J, García Cenoz M, Arriazu M, Fernández-Alonso M, Martínez-Artola V, Etxeberria J, Irisarri F, Barricarte A. Effectiveness of Jeryl Lynn-containing vaccine in Spanish children. Vaccine. 2009;27:2089–93. doi: 10.1016/j.vaccine.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Sartorius B, Penttinen P, Nilsson J, Johansen K, Jönsson K, Arneborn M, Löfdahl M, Giesecke J. An outbreak of mumps in Sweden, February-April 2004. Euro Surveill. 2005;10:191–3. [PubMed] [Google Scholar]
- 12.Chamot E, Toscani L, Egger P, Germann D, Bourquin C. [Estimation of the efficacy of three strains of mumps vaccines during an epidemic of mumps in the Geneva canton (Switzerland)] Rev Epidemiol Sante Publique. 1998;46:100–7. [PubMed] [Google Scholar]
- 13.Toscani L, Batou M, Bouvier P, Schlaepfer A. [Comparison of the efficacy of various strains of mumps vaccine: a school survey] Soz Praventivmed. 1996;41:341–7. doi: 10.1007/BF01324283. [DOI] [PubMed] [Google Scholar]
- 14.Man W, Jin-Kou Z, Tao W, Li-Xin H, Chao M, Qi-Ru S, Hui-Ming L. Mumps-containing vaccine effectiveness during outbreaks in two schools in Guangdong, China, 2012. Western Pac Surveill Response J. 2012;3:29–32. doi: 10.5365/wpsar.2012.3.4.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Ma Y, Han Y, Guo JQ. [SH protein genetic characterization analysis of wild-type mumps virus isolated in Liaoning province from 2008 to 2011] Bing Du Xue Bao. 2012;28:506–10. [PubMed] [Google Scholar]
- 16.Zhang DY, Feng Y, Zhong SL, Lu YY, Zhuang FC, Xu CP. [Comparative analysis on the complete genome sequence of mumps epidemic strain and mumps vaccine strain S79 isolated in Zhejiang province, China between year 2005 and 2010] Zhonghua Yu Fang Yi Xue Za Zhi. 2012;46:252–7. [PubMed] [Google Scholar]
- 17.Carr MJ, Moss E, Waters A, Dean J, Jin L, Coughlan S, Connell J, Hall WW, Hassan J. Molecular epidemiological evaluation of the recent resurgence in mumps virus infections in Ireland. J Clin Microbiol. 2010;48:3288–94. doi: 10.1128/JCM.00434-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu C, Wang M, Liang J, He T, Wang D, Xu J. Effectiveness of Lanzhou lamb rotavirus vaccine against rotavirus gastroenteritis requiring hospitalization: a matched case-control study. Vaccine. 2007;25:8756–61. doi: 10.1016/j.vaccine.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 19.Clemens J, Brenner R, Rao M, Tafari N, Lowe C. Evaluating new vaccines for developing countries. Efficacy or effectiveness? JAMA. 1996;275:390–7. doi: 10.1001/jama.1996.03530290060038. [DOI] [PubMed] [Google Scholar]
- 20.Xu W, Zhang G, Liang S, Wang Y, Shang Q, Teng X, Li D. Systematic review on immunology effect and protective efficacy of mumps attenuated live vaccine. Chinese Journal of Vaccines and Immunization. 2011;17:426–50. [Google Scholar]
- 21.Cortese MM, Jordan HT, Curns AT, Quinlan PA, Ens KA, Denning PM, Dayan GH. Mumps vaccine performance among university students during a mumps outbreak. Clin Infect Dis. 2008;46:1172–80. doi: 10.1086/529141. [DOI] [PubMed] [Google Scholar]
- 22.Fu C, He Q, Xu J, Xie H, Ding P, Hu W, Dong Z, Liu X, Wang M. Effectiveness of the Lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine. 2012;31:154–8. doi: 10.1016/j.vaccine.2012.10.078. [DOI] [PubMed] [Google Scholar]
- 23.Fu C, Xu J, He Q, Li Z, Liu F. Seasonal influenza vaccine effectiveness in children during 2010-2011 season: A case-cohort study. Hum Vaccin Immunother. 9(5) doi: 10.4161/hv.23457. Forthcoming 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Q, Xu J, Chen X, Lu J, Li K, Li Z, Wang M, Yang Q, Dong Z, Liu X, et al. Effectiveness of seasonal influenza vaccine against clinically diagnosed influenza over two consecutive seasons in children in Guangzhou, China: A matched case-control study. Hum Vaccin Immunother. 9(5) doi: 10.4161/hv.24980. Forthcoming 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu C, Wang M, Liang J, Xu J, Wang C, Bialek S. The effectiveness of varicella vaccine in China. Pediatr Infect Dis J. 2010;29:690–3. doi: 10.1097/INF.0b013e3181d7380e. [DOI] [PubMed] [Google Scholar]
- 26.Niccolai LM, Ogden LG, Muehlenbein CE, Dziura JD, Vázquez M, Shapiro ED. Methodological issues in design and analysis of a matched case-control study of a vaccine’s effectiveness. J Clin Epidemiol. 2007;60:1127–31. doi: 10.1016/j.jclinepi.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


