Abstract
There is a need for both new and improved vaccination formulations for a range of diseases for which current vaccines are either inadequate or non-existent. Biodegradable polymer-based vaccines fulfill many of the desired properties in achieving effective long-term protection in a manner that is safe, economical, and potentially more practicable on a global scale. Here we discuss some of the work performed with micro/nanoparticles made from either synthetic (poly[lactic-co-glycolic acid] [PLGA] and polyanhydrides) or natural (chitosan) biodegradable polymers. Our attention is focused on, but not limited to, the generation of antitumor immunity where we stress the importance of particle size and co-delivery of antigen and adjuvant.
Keywords: biodegradable polymer, cancer vaccine, adjuvant, PLGA, polyanhydride, chitosan
Introduction
Vaccines have been responsible for the eradication and almost complete elimination of smallpox and poliomyelitis respectively as well as the largely effective control, in first world countries, of a number of other potentially fatal diseases including tetanus, rabies, and rubella.1 However, there are a number of diseases, such as cancers and those caused by intracellular pathogens, for which there are no effective prophylactic or therapeutic vaccines. These diseases require vaccine formulations that promote strong cellular, or Th1-biased, immune responses as opposed to humoral, or Th2-biased, immune responses only. In addition, in developing countries there is a need for vaccines formulations with long shelf lives that require minimal, or ideally no, follow-up boosts. With an increasing understanding of the immune system much research has been focused on improving the current approach toward vaccine development in these areas. Particle-based antigen (Ag) delivery can potentially provide many levels of adjuvancy that include: prolonging Ag presence (depot formation); enhancing dendritic cell (DC)-mediated Ag uptake; direct stimulation of DC and promotion of cross-presentation.2 In this commentary we discuss three biodegradable polymers that are strong candidates for future use as vaccine carriers and focus particularly on formulations designed to promote cellular immune responses.
Biodegradable Polymer Particles
Biodegradable polymers have a number of desirable qualities which have led to their use as vectors for drugs and proteins over the past 20 years. Such qualities include biocompatibility, sustained release, and tunable release kinetics.3 Particle-based vaccine delivery vehicles formulated from synthetic or natural polymers offer attractive advantages over current formulations, not least of which is their high degree of adaptability.4-6 Structural modifications to these polymers can affect a range of important parameters such as: release kinetics of loaded molecules, targeting to a specific population of cells, shielding properties, and particle size, all of which, along with the encapsulate characteristics, can significantly influence the nature of the immune response generated. Highly desirable goals for new vaccine formulations are that they are safe, inexpensive, stable, and mimic pathogenic infection.7 Polymer-based vaccines can mimic infection in a number of ways. First, they can act as a depot thereby persisting long enough to generate adaptive immune responses. Second, they can ensure efficient co-delivery of Ag and adjuvant to DCs resulting in effective priming of naïve T-cell responses.8-11 Third, the size of micro- and nano-particles is often similar to the size of various bacterial and viral pathogens respectively.12
Co-delivery of antigen and adjuvant
Possibly one of the most salient properties possessed by polymeric particles is their capacity to co-deliver Ag and adjuvant to the same DC.11 In order to generate effective immune responses against intracellular pathogens and malignant disease a vaccine must ensure both efficient uptake of Ag by immature DCs, and concomitant stimulation with an adjuvant that drives DC maturation. The importance of co-delivery of Ag and adjuvant to the same DC has been elegantly demonstrated to be not only dependent on simultaneous uptake but also on both components being delivered in an associated form.11,13 Until recently, the only adjuvant approved for use by the US Food and Drug Administration (FDA) has been alum which is not effective at generating Th1-biased immune responses.14 The only exception to this is the recently approved adjuvant, ASO4 (a combination of monophosphoryl lipid A and alum), which is used in the human papillomavirus vaccine, Cervarix.15 The most potent and promising adjuvants for generating Th1-biased immune responses are agonists to Toll-like receptors (TLRs), often referred to as pathogen-associated molecular patterns (PAMPs), examples of which include lipid A and CpG oligodeoxynucleotides (CpG ODN).6,11,15,16 Co-encapsulation of Ag and adjuvant within the one particle also avoids uptake of Ag independently, which under certain conditions may lead to induction of tolerance.17 Aside from co-encapsulation of PAMPs it has been shown that polymeric particles per se are capable of exerting a mild to strong adjuvant effect.9,11,18
Poly(lactic-co-glycolic acid) (PLGA)
PLGA is a FDA approved biodegradable and biocompatible synthetic polymer which has been extensively studied as a vaccine delivery system in preclinical settings as well as having been used as sutures and for controlled drug delivery in the biomedical arena.4,19-28 PLGA particles as vaccine carriers offer multiple advantages over many other vaccine delivery systems. For example, PLGA is a synthetic polymer which offers high reproducibility during the fabrication process. Ag can be encapsulated in PLGA particles or attached to the particle surface using covalent or ionic bonding.29 Surface modifications of PLGA particles by conjugating targeting antibodies, biotin, polyethylene glycol (PEG), polyethylenimine (PEI),30 and mannose have shown to improve the efficacy of vaccines by increasing bioavailability of particles, improving systemic circulation, or by targeting these particles to DCs.29,31-33
Our laboratory has performed many studies using PLGA formulations fabricated using a double emulsion solvent evaporation technique.34,35 As mentioned above, co-delivery of Ag and adjuvant to DCs is important for optimal immune responses, and we emphasized this through murine vaccination studies revealing PLGA co-encapsulating Ag and CpG ODN could invoke stronger Ag-specific IgG (IgG1 and IgG2a), and IFN-γ responses than soluble Ag and CpG ODN.36 Improved immune responses at later time-points using PLGA particles co-encapsulating Ag and CpG ODN were also observed over a covalently fused product of Ag and CpG ODN, thereby demonstrating the sustained release benefits of the particle based system. There is nevertheless the problematic issue of immunosuppressive elements that may curtail any potential beneficial outcome on host survival derived from a cancer vaccine that co-delivers Ag and adjuvant. We have shown that when Ag, in the form of tumor lysate, and adjuvant, CpG ODN, are co-delivered in PLGA microparticles as a prophylactic vaccine, protection against subsequent tumor (melanoma) challenge was only effective when regulatory T cells (Tregs) were diminished.37 Certain chemotherapeutic agents, aside from imparting direct cytotoxic effects on tumor cells, are capable of diminishing immunosuppressive populations such as Tregs and/or myeloid derived suppressor cells.38,39 Our work with two of these agents, cyclophosphamide and 5-fluorouracil, in conjunction with PLGA (encapsulating Ag and CpG ODN) or a model adenoviral cancer vaccine respectively, independently suggest that such combinations may ultimately have synergistic therapeutic effects for cancer patients while reducing the severity of side effects often associated with high dose chemotherapy.37,40
The size of biodegradable micro/nano-particle-based vaccines is likely to be of significant immune consequence both qualitatively and quantitatively for reasons which include impact on efficiency of uptake by DCs vs. other cell types (e.g., somatic cells or macrophages) along with the influence on release kinetics of the antigenic load.12 We and others have established the optimal size(s) required for PLGA based vaccines (encapsulating Ag) to generate appropriate or effective immune responses. Early studies in mice showed that Ag encapsulated in PLGA particles of approximately 500 nm (in diameter) were superior at generating Ag-specific cytotoxic T-lymphocyte responses over 2 µm and 7 µm particles after multiple intraperitoneal (IP) vaccinations.41 The subsequent discovery of various adjuvants enhancing the immunogenicity of particle-based vaccines, led our laboratory to investigate the optimal size required for PLGA-based vaccine formulations, co-encapsulating Ag and adjuvant, to generate effective Th1-type immune responses after IP administrations. Our in vivo murine vaccination studies revealed that 300 nm PLGA particles generated greater Ag-specific immune responses (IgG2a and CD8+ T lymphocyte) compared with particles of sizes 1 μm, 7 μm, or 17 μm. We also found that particles of 300 nm co-encapsulating Ag and CpG ODN were more readily taken up by DCs and more efficient at stimulating DC maturation in vitro than the larger sized particles.35 That particle size and DC uptake are inversely proportional parameters has been previously demonstrated using variously sized latex particles and human DCs, suggesting the generality of such a finding.42 Based on the results from the few studies performed comparing differently sized PLGA or poly(lactic acid) (PLA) particles encapsulating Ag (with or without adjuvant) there is a recurring theme of smaller particles (300–600 nm) promoting Th1-biased immune responses while larger particles (2–8 μm) tending to promote Th2-biased responses (see Table 1).
Table 1. Summary of murine vaccination experiments comparing immunogenicity of PLGA/PLA particles encapsulating Ag (+/−adjuvant).
| Compared sizes | Polymer | Vaccination route | Cargo | Results summary | References | |
|---|---|---|---|---|---|---|
| Output | Optimum size(s) | |||||
| 500 nm, 2 μm, and 7 μm | PLGA 50:50 | IP | OVA | Th1 + CTL responses | 500 nm | 41 |
| 300 nm, 1 μm, 7 μm, and 17 μm | PLGA 50:50 | IP | OVA and CpG | IgG2a + CTL responses | 300 nm | 35 |
| 200 nm, 500 nm, and 1 μm | PLGA 50:50 | subQ or Oral | BSA | IgG responses | 1 μm | 43 |
| 200–600 nm vs 2–8 μm | PLA | IM | HBsAg | Th1 responses | 200–600 nm | 44 |
| Th2 responses | 2–8 μm | |||||
| 50–150 μm, 10–70 μm, 2–8 μm and < 2 μm | PLA | IM | Tetanus Toxoid | IgG responses | 2–8 μm | 45 |
| 1 μm and 5 μm | PLGA 50:50 | Oral | BSA | IgG responses | 1 μm | 46 |
IP, intraperitoneal; subQ, subcutaneous; IM, intramuscular; HBsAg, hepatitis B surface antigen; CTL, cytotoxic T lymphocyte.
Polyanhydrides
Biodegradable amphiphilic polyanhydrides (PAs) represent a class of synthetic polymers that have attractive properties when considering them for use as vaccine vectors. They can be easily manufactured by double emulsion techniques and the degraded by-products are readily metabolized and non-toxic.47 The properties of particles made from PAs, such as release kinetics, Ag retention and adjuvant effect, can be tailored based on polymer composition.48 Of particular importance when using PA formulations, is to limit the degree of hydrophobicity which, if too high, can negatively affect antigenicity of the protein cargo through the induction of protein aggregation and/or the creation of an overly acidic microenvironment during polymer degradation.47,49
Amphiphilic PA microparticles based on 1,8-bis(p-carboxyphenoxy)-3,6-dioxaoctane (CPTEG) and 1,6-bis(p-carboxyphenoxy) hexane (CPH) copolymers have been shown capable of stabilizing the structural integrity of encapsulated Ag and providing sustained protein release.47,50 The observed sustained Ag release derives from the general property of PAs to degrade through surface erosion thus ensuring zero order release of encapsulated molecules.51,52 This is in contrast to PLGA particles which degrade through bulk erosion, a process that may lead to unwanted aggregation of encapsulated proteins as well as accumulation of acidic byproducts in the depot of PLGA, resulting in undesired inflammatory reactions.53 It has been reported that PA particles can act as strong TLR agonists and that 50:50 CPTEG:CPH particles in particular can promote DC maturation in a manner similar to LPS.18,54,55 In a recent study, involving informatics analysis, it was shown that 50:50 CPTEG:CPH nanoparticles possessed characteristics that resembled those of intracellular pathogens such as E. coli and Y. pestis.8 To elaborate, 50:50 CPTEG:CPH nanoparticles could persist in late endosomes in a similar fashion to many microbial pathogens thereby suggesting that the particles were being channeled through the exogenous pathway for antigen processing and presentation. In addition, it was observed that 50:50 CPTEG:CPH possessed both structural and functional similarities with lipopolysaccharide (LPS). These particles promoted upregulated surface expression of MHC class I, MHC class II, CD86, and CD40 on DCs, paralleling results obtained with LPS. On the other hand, 50:50 CPTEG:CPH nanoparticles did not induce an inflammatory cytokine response often associated with LPS and in vivo administration of PA consequently led to minimal damage at the site of injection.56 In a separate murine study it was shown that a single intranasal vaccination of 50:50 CPTEG:CPH particles encapsulating a Y. pestis fusion protein/Ag (delivered with soluble Ag [40 μg]) was capable of generating long-term protection against lethal Y. pestis challenge as well as inducing high pathogen-specific antibody titers with high avidity.57
Many of the traits exhibited by 50:50 CPTEG:CPH particles implicate them as strong candidates for cancer vaccine formulations. We therefore recently explored this possibility in the context of a well established tumor model system in mice.58 It was found that vaccination (prime plus boost) with 50:50 CPTEG:CPH particles encapsulating a model tumor Ag, ovalbumin (OVA), provided enhanced protection from subsequent OVA-expressing tumor challenge over other formulations which included: (1) particles encapsulating both OVA and CpG ODN, (2) solution of OVA and CpG ODN, and (3) particles encapsulating OVA plus soluble CpG ODN. Unlike the situation with PLGA particles, co-encapsulation of CpG-ODN reduced rather than enhanced the immunogenicity of the encapsulated Ag (Fig. 1). Since it has been reported that LPS can abrogate the effects of CpG ODN it is conceivable that 50:50 CPTEG:CPH may have a phenotypic dominance over CpG ODN.59
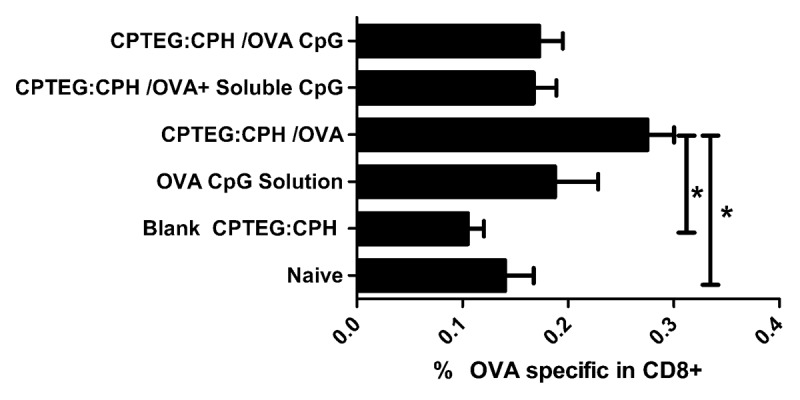
Figure 1. OVA-specific CD8+ T-cell frequency in mice vaccinated with polyanhydride microparticles prepared from 50:50 ratio of CPTEG:CPH. Mice were vaccinated twice at 7 d intervals with 100 µg of ovalbumin (OVA) and 50 µg of CpG ODN. Peripheral blood lymphocytes were co-stained using a fluorescently tagged tetramer (binding to OVA/MHC-specific T-cell receptor) and fluorescently labeled anti-CD3 and CD8 antibodies. All groups were statistically compared using ANOVA followed by tukey post-test (*p < 0.05). Adapted from Joshi et al. (2013).58
Chitosan
Chitosan is a biodegradable, non-toxic natural polysaccharide which has been approved by the FDA for wound healing applications.60 Chitosan has been studied extensively as an orally or nasally delivered vaccine carrier owing to its excellent mucoadhesive properties.61,62 It is prepared by deacetylation of N-acetyl-β-D-glucosamine units of chitin. The degree of deacetylation and length of the polymer chain can be varied to change its physiochemical properties.63 Chitosan particles can be prepared by an ionic gelation process, spray drying, complex coacervation, reverse micellar or an emulsion-droplet coalescence method.64,65 Chitosan can also be chemically modified at primary amine groups, which facilitates the covalent attachment of Ag (or other molecules) to the chitosan backbone. Surface modified chitosan particles with trans-activated transcription (TAT) peptide, RGD sequence (Arg-Gly-Asp), alginate or PEG sequences have been shown to enhance stability and immunogenicity of vaccines.66-69 Due to its hydrophilicity and positive charge, chitosan possesses a higher protein loading capacity compared with inert polymer delivery systems. Ag or DNA loaded chitosan particles have been shown to induce DC maturation and cytokine production which consequently facilitates T-cell priming, making it a promising vaccine delivery system.70,71
There has been increasing interest in using chitosan in association with viruses primarily for oral/nasal delivery systems because of the mucoadhesive properties of chitosan.72 Simply by mixing virus (usually attenuated or inactivated) with the desired chitosan solution results in viral units at least partially coated with chitosan. In a clinical trial it was shown that intranasal administration of norwalk virus-like particles and monophosphoryl lipid A mixed with a proprietary chitosan formulation (ChiSys®, Archimedes Pharma LTD) induced norwalk virus-specific serum IgA antibody responses in 70% of vaccinated human volunteers with a marginal increase in IgG antibodies. The vaccine provided protection against both viral gastroenteritis and norwalk virus associated infection.73 In an immunization study in calves using attenuated human adenovirus encoding a bovine herpes Ag it was shown that addition of glycol chitosan improved virological protection over vaccination with adenovirus alone.74 Enigmatically, it was reproducibly observed that the degree of protection did not correlate with the magnitude of the neutralizing antibody responses.74,75 One possible explanation is that chitosan may mask the adenoviral surface Ag thus reducing their antigenicity and consequently allowing more time for the adenovirus to persist and allow for increased expression of the encoded herpes Ag. In a separate murine study, N,N,N,-trimethyl chitosan was used to coat whole inactivated influenza virus prior to intranasal vaccination.76 The presence of chitosan resulted in enhanced viral Ag-specific IgG1 and IgG2a/c responses as well as increased protection against challenges with live influenza. In a separate murine study of influenza vaccinations, delivered intramuscularly, it was shown that chitosan dramatically improved protection from influenza challenge when mixed with inactivated influenza strains.77 In a tumor protection study performed in our laboratory we observed that, in contrast to expectations, low molecular weight chitosan complexed with adenovirus encoding a model tumor Ag actually reduced Ag-specific CD8+ T-cell levels and consequently mice vaccinated subcutaneously with these complexes were more susceptible to subsequent tumor challenge than mice vaccinated with adenovirus alone.78 We also discovered that chitosan when complexed with adenovirus interfered with transduction of bone marrow derived dendritic cells (but not other cell types) in vitro. It is possible therefore that chitosan may not be an ideal adjuvant for adenoviral vaccines delivered via non mucosal routes, particularly if strong cytotoxic T-cell responses are desired over humoral responses.
Conclusions
In this article we have stressed that co-delivery of Ag and adjuvant is important in the generation of immune responses in order to ensure that maturation and Ag presentation occurs within the same DC. Co-delivery by micro/nanoparticles, in particular, also promotes cross-presentation of antigen and stimulation of Th1-biased/CTL-mediated immune responses that can be further enhanced by diminishing immunosuppressive cells in conjunction with vaccination delivery (Fig. 2). This commentary describes three polymer types with distinct and adjustable physiochemical properties that may be variably exploited to improve current vaccination strategies or establish new ones. PLGA polymers possibly offer the greater diversity of applications and have shown potential as cancer vaccines when combined with agents that deplete Treg function. Polyanhydrides have the advantage of possessing strong TLR agonist properties obviating the requirement for co-encapsulation of an adjuvant with Ag, however, their versatility requires further investigation. Variously modified forms of chitosan have shown great promise as oral/nasal influenza and cancer vaccine carriers, however, the route of administration and formulation characteristics must be carefully considered prior to testing and use. All three polymers have shown enough promise as vaccine vectors to warrant further preclinical and translational studies with a particular focus on diseases requiring Th1-biased immune responses.
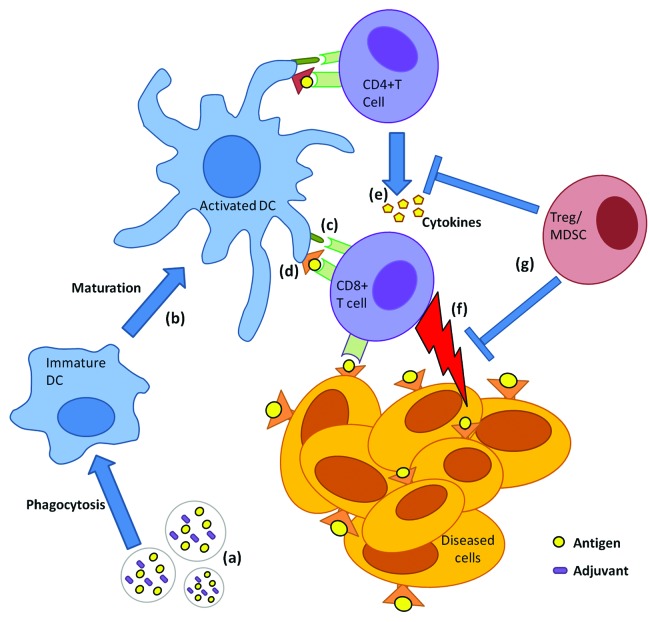
Figure 2. General mechanism of induction of antigen-specific CD8+ T-cell immunity by biodegradable particles co-encapsulating Ag and adjuvant. Particles co-encapsulating Ag and an adjuvant (A) are phagocytosed by dendritic cells (DCs). DCs undergo maturation (B), upregulating costimulatory molecules (C) and simultaneously cross-presenting Ag in context of MHC class I (i.e., presentation to CD8+ T cells) (D). In the draining lymph node CD8+ T cells become activated (i.e., cytotoxic T cells) and concomitantly activated CD4+ T cells secrete cytokine help (E) to activated CD8+ T cells. Cytotoxic T cells travel via peripheral blood to tumor sites and eradicate Ag expressing cancerous cells or those infected with intracellular pathogens (F). Suppressor cells such as Tregs secrete inhibitory cytokines that prevent T-cell effector activation/function (G) leading to dampening of immune response.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Plotkin SA, Offit PA, eds. A short history of vaccination, 1-16 (Elsevier, New York, 2008). [Google Scholar]
- 2.Rice-Ficht AC, Arenas-Gamboa AM, Kahl-McDonagh MM, Ficht TA. Polymeric particles in vaccine delivery. Curr Opin Microbiol. 2010;13:106–12. doi: 10.1016/j.mib.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Sinha VR, Trehan A. Biodegradable microspheres for parenteral delivery. Crit Rev Ther Drug Carrier Syst. 2005;22:535–602. doi: 10.1615/CritRevTherDrugCarrierSyst.v22.i6.20. [DOI] [PubMed] [Google Scholar]
- 4.Abbas AO, Donovan MD, Salem AK. Formulating poly(lactide-co-glycolide) particles for plasmid DNA delivery. J Pharm Sci. 2008;97:2448–61. doi: 10.1002/jps.21215. [DOI] [PubMed] [Google Scholar]
- 5.Krishnamachari Y, Geary SM, Lemke CD, Salem AK. Nanoparticle delivery systems in cancer vaccines. Pharm Res. 2011;28:215–36. doi: 10.1007/s11095-010-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnamachari Y, Salem AK. Innovative strategies for co-delivering antigens and CpG oligonucleotides. Adv Drug Deliv Rev. 2009;61:205–17. doi: 10.1016/j.addr.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zepp F. Principles of vaccine design-Lessons from nature. Vaccine. 2010;28(Suppl 3):C14–24. doi: 10.1016/j.vaccine.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Ulery BD, Petersen LK, Phanse Y, Kong CS, Broderick SR, Kumar D, Ramer-Tait AE, Carrillo-Conde B, Rajan K, Wannemuehler MJ, et al. Rational design of pathogen-mimicking amphiphilic materials as nanoadjuvants. Sci Rep. 2011;1:198. doi: 10.1038/srep00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida M, Babensee JE. Poly(lactic-co-glycolic acid) enhances maturation of human monocyte-derived dendritic cells. J Biomed Mater Res A. 2004;71:45–54. doi: 10.1002/jbm.a.30131. [DOI] [PubMed] [Google Scholar]
- 10.Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, Elhasi S, Samuel J, Lavasanifar A. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine. 2008;26:5046–57. doi: 10.1016/j.vaccine.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Zhang XQ, Dahle CE, Baman NK, Rich N, Weiner GJ, Salem AK. Potent antigen-specific immune responses stimulated by codelivery of CpG ODN and antigens in degradable microparticles. J Immunother. 2007;30:469–78. doi: 10.1097/CJI.0b013e31802fd8c6. [DOI] [PubMed] [Google Scholar]
- 12.Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, Plebanski M. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006;40:1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–12. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 14.Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82:488–96. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 15.Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. 2011;239:178–96. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geary SM, Lemke CD, Lubaroff DM, Salem AK. Tumor immunotherapy using adenovirus vaccines in combination with intratumoral doses of CpG ODN. Cancer Immunol Immunother. 2011;60:1309–17. doi: 10.1007/s00262-011-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keijzer C, Slütter B, van der Zee R, Jiskoot W, van Eden W, Broere F. PLGA, PLGA-TMC and TMC-TPP nanoparticles differentially modulate the outcome of nasal vaccination by inducing tolerance or enhancing humoral immunity. PLoS One. 2011;6:e26684. doi: 10.1371/journal.pone.0026684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen LK, Ramer-Tait AE, Broderick SR, Kong CS, Ulery BD, Rajan K, Wannemuehler MJ, Narasimhan B. Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants. Biomaterials. 2011;32:6815–22. doi: 10.1016/j.biomaterials.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 19.Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005;57:391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Le Corre P, Rytting JH, Gajan V, Chevanne F, Le Verge R. In vitro controlled release kinetics of local anaesthetics from poly(D,L-lactide) and poly(lactide-co-glycolide) microspheres. J Microencapsul. 1997;14:243–55. doi: 10.3109/02652049709015336. [DOI] [PubMed] [Google Scholar]
- 21.Greenwald D, Shumway S, Albear P, Gottlieb L. Mechanical comparison of 10 suture materials before and after in vivo incubation. J Surg Res. 1994;56:372–7. doi: 10.1006/jsre.1994.1058. [DOI] [PubMed] [Google Scholar]
- 22.Blanco FC, Srinivasan P, Walk RM, Snyder JA, Behlke M, Salem AK, Vukmanovic S, Sandler AD. Development of an siRNA delivery system targeting macrophage function in-vivo. J Am Coll Surg. 2012;215:S74–74. doi: 10.1016/j.jamcollsurg.2012.06.204. [DOI] [Google Scholar]
- 23.Hong L, Krishnamachari Y, Seabold D, Joshi V, Schneider G, Salem AK. Intracellular release of 17-β estradiol from cationic polyamidoamine dendrimer surface-modified poly (lactic-co-glycolic acid) microparticles improves osteogenic differentiation of human mesenchymal stromal cells. Tissue Eng Part C Methods. 2011;17:319–25. doi: 10.1089/ten.tec.2010.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong L, Wei N, Joshi V, Yu Y, Kim N, Krishnamachari Y, Zhang Q, Salem AK. Effects of glucocorticoid receptor small interfering RNA delivered using poly lactic-co-glycolic acid microparticles on proliferation and differentiation capabilities of human mesenchymal stromal cells. Tissue Eng Part A. 2012;18:775–84. doi: 10.1089/ten.tea.2011.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Intra J, Salem AK. Fabrication, characterization and in vitro evaluation of poly(D,L-lactide-co-glycolide) microparticles loaded with polyamidoamine-plasmid DNA dendriplexes for applications in nonviral gene delivery. J Pharm Sci. 2010;99:368–84. doi: 10.1002/jps.21840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Intra J, Salem AK. Rational design, fabrication, characterization and in vitro testing of biodegradable microparticles that generate targeted and sustained transgene expression in HepG2 liver cells. J Drug Target. 2011;19:393–408. doi: 10.3109/1061186X.2010.504263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Intra J, Zhang XQ, Williams RL, Zhu X, Sandler AD, Salem AK. Immunostimulatory sutures that treat local disease recurrence following primary tumor resection. Biomed Mater. 2011;6:011001. doi: 10.1088/1748-6041/6/1/011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santillan DA, Rai KK, Santillan MK, Krishnamachari Y, Salem AK, Hunter SK. Efficacy of polymeric encapsulated C5a peptidase-based group B streptococcus vaccines in a murine model. Am J Obstet Gynecol. 2011;205:e1–8. doi: 10.1016/j.ajog.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon JJ, Suh H, Polhemus ME, Ockenhouse CF, Yadava A, Irvine DJ. Antigen-displaying lipid-enveloped PLGA nanoparticles as delivery agents for a Plasmodium vivax malaria vaccine. PLoS One. 2012;7:e31472. doi: 10.1371/journal.pone.0031472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang XQ, Intra J, Salem AK. Comparative study of poly (lactic-co-glycolic acid)-poly ethyleneimine-plasmid DNA microparticles prepared using double emulsion methods. J Microencapsul. 2008;25:1–12. doi: 10.1080/02652040701659347. [DOI] [PubMed] [Google Scholar]
- 31.Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, Mark Saltzman W, Mellman I, Ledizet M, Fikrig E, Flavell RA, et al. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27:3013–21. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raghuwanshi D, Mishra V, Suresh MR, Kaur K. A simple approach for enhanced immune response using engineered dendritic cell targeted nanoparticles. Vaccine. 2012;30:7292–9. doi: 10.1016/j.vaccine.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 33.Diesner SC, Wang XY, Jensen-Jarolim E, Untersmayr E, Gabor F. Use of lectin-functionalized particles for oral immunotherapy. Ther Deliv. 2012;3:277–90. doi: 10.4155/tde.11.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lassalle V, Ferreira ML. PLA nano- and microparticles for drug delivery: an overview of the methods of preparation. Macromol Biosci. 2007;7:767–83. doi: 10.1002/mabi.200700022. [DOI] [PubMed] [Google Scholar]
- 35.Joshi VB, Geary SM, Salem AK. Biodegradable particles as vaccine delivery systems: size matters. AAPS J. 2013;15:85–94. doi: 10.1208/s12248-012-9418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X-Q, Dahle CE, Weiner GJ, Salem AK. A comparative study of the antigen-specific immune response induced by co-delivery of CpG ODN and antigen using fusion molecules or biodegradable microparticles. J Pharm Sci. 2007;96:3283–92. doi: 10.1002/jps.20978. [DOI] [PubMed] [Google Scholar]
- 37.Goforth R, Salem AK, Zhu X, Miles S, Zhang XQ, Lee JH, Sandler AD. Immune stimulatory antigen loaded particles combined with depletion of regulatory T-cells induce potent tumor specific immunity in a mouse model of melanoma. Cancer Immunol Immunother. 2009;58:517–30. doi: 10.1007/s00262-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18 doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 39.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 40.Geary SM, Lemke CD, Lubaroff DM, Salem AK. The combination of a low-dose chemotherapeutic agent, 5-Fluorouracil, and an adenoviral tumor vaccine has a synergistic benefit on survival in a tumor model system. PLoS One. 2013;8:e67904. doi: 10.1371/journal.pone.0067904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nixon DF, Hioe C, Chen PD, Bian Z, Kuebler P, Li ML, Qiu H, Li XM, Singh M, Richardson J, et al. Synthetic peptides entrapped in microparticles can elicit cytotoxic T cell activity. Vaccine. 1996;14:1523–30. doi: 10.1016/S0264-410X(96)00099-0. [DOI] [PubMed] [Google Scholar]
- 42.Reece JC, Vardaxis NJ, Marshall JA, Crowe SM, Cameron PU. Uptake of HIV and latex particles by fresh and cultured dendritic cells and monocytes. Immunol Cell Biol. 2001;79:255–63. doi: 10.1046/j.1440-1711.2001.01011.x. [DOI] [PubMed] [Google Scholar]
- 43.Gutierro I, Hernández RM, Igartua M, Gascón AR, Pedraz JL. Size dependent immune response after subcutaneous, oral and intranasal administration of BSA loaded nanospheres. Vaccine. 2002;21:67–77. doi: 10.1016/S0264-410X(02)00435-8. [DOI] [PubMed] [Google Scholar]
- 44.Kanchan V, Panda AK. Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials. 2007;28:5344–57. doi: 10.1016/j.biomaterials.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 45.Katare YK, Muthukumaran T, Panda AK. Influence of particle size, antigen load, dose and additional adjuvant on the immune response from antigen loaded PLA microparticles. Int J Pharm. 2005;301:149–60. doi: 10.1016/j.ijpharm.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 46.Igartua M, Hernández RM, Esquisabel A, Gascón AR, Calvo MB, Pedraz JL. Enhanced immune response after subcutaneous and oral immunization with biodegradable PLGA microspheres. J Control Release. 1998;56:63–73. doi: 10.1016/S0168-3659(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 47.Lopac SK, Torres MP, Wilson-Welder JH, Wannemuehler MJ, Narasimhan B. Effect of polymer chemistry and fabrication method on protein release and stability from polyanhydride microspheres. J Biomed Mater Res B Appl Biomater. 2009;91:938–47. doi: 10.1002/jbm.b.31478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar N, Langer RS, Domb AJ. Polyanhydrides: an overview. Adv Drug Deliv Rev. 2002;54:889–910. doi: 10.1016/S0169-409X(02)00050-9. [DOI] [PubMed] [Google Scholar]
- 49.Torres MP, Determan AS, Anderson GL, Mallapragada SK, Narasimhan B. Amphiphilic polyanhydrides for protein stabilization and release. Biomaterials. 2007;28:108–16. doi: 10.1016/j.biomaterials.2006.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carrillo-Conde B, Schiltz E, Yu J, Chris Minion F, Phillips GJ, Wannemuehler MJ, Narasimhan B. Encapsulation into amphiphilic polyanhydride microparticles stabilizes Yersinia pestis antigens. Acta Biomater. 2010;6:3110–9. doi: 10.1016/j.actbio.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 51.Göpferich A, Tessmar J. Polyanhydride degradation and erosion. Adv Drug Deliv Rev. 2002;54:911–31. doi: 10.1016/S0169-409X(02)00051-0. [DOI] [PubMed] [Google Scholar]
- 52.Tamada JA, Langer R. Erosion kinetics of hydrolytically degradable polymers. Proc Natl Acad Sci U S A. 1993;90:552–6. doi: 10.1073/pnas.90.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Determan AS, Wilson JH, Kipper MJ, Wannemuehler MJ, Narasimhan B. Protein stability in the presence of polymer degradation products: consequences for controlled release formulations. Biomaterials. 2006;27:3312–20. doi: 10.1016/j.biomaterials.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 54.Tamayo I, Irache JM, Mansilla C, Ochoa-Repáraz J, Lasarte JJ, Gamazo C. Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin Vaccine Immunol. 2010;17:1356–62. doi: 10.1128/CVI.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mallapragada SK, Narasimhan B. Immunomodulatory biomaterials. Int J Pharm. 2008;364:265–71. doi: 10.1016/j.ijpharm.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 56.Huntimer L, Ramer-Tait AE, Petersen LK, Ross KA, Walz KA, Wang C, Hostetter J, Narasimhan B, Wannemuehler MJ. Evaluation of biocompatibility and administration site reactogenicity of polyanhydride-particle-based platform for vaccine delivery. Adv Healthc Mater. 2013;2:369–78. doi: 10.1002/adhm.201200181. [DOI] [PubMed] [Google Scholar]
- 57.Ulery BD, Kumar D, Ramer-Tait AE, Metzger DW, Wannemuehler MJ, Narasimhan B. Design of a protective single-dose intranasal nanoparticle-based vaccine platform for respiratory infectious diseases. PLoS One. 2011;6:e17642. doi: 10.1371/journal.pone.0017642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joshi VB, Geary SM, Carrillo-Conde BR, Narasimhan B, Salem AK. Characterizing the antitumor response in mice treated with antigen-loaded polyanhydride microparticles. Acta Biomater. 2013;9:5583–9. doi: 10.1016/j.actbio.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gould MP, Greene JA, Bhoj V, DeVecchio JL, Heinzel FP. Distinct modulatory effects of LPS and CpG on IL-18-dependent IFN-gamma synthesis. J Immunol. 2004;172:1754–62. doi: 10.4049/jimmunol.172.3.1754. [DOI] [PubMed] [Google Scholar]
- 60.Wedmore I, McManus JG, Pusateri AE, Holcomb JB. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60:655–8. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 61.Saint-Lu N, Tourdot S, Razafindratsita A, Mascarell L, Berjont N, Chabre H, Louise A, Van Overtvelt L, Moingeon P. Targeting the allergen to oral dendritic cells with mucoadhesive chitosan particles enhances tolerance induction. Allergy. 2009;64:1003–13. doi: 10.1111/j.1398-9995.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- 62.Figueiredo L, Cadete A, Gonçalves LMD, Corvo ML, Almeida AJ. Intranasal immunisation of mice against Streptococcus equi using positively charged nanoparticulate carrier systems. Vaccine. 2012;30:6551–8. doi: 10.1016/j.vaccine.2012.08.050. [DOI] [PubMed] [Google Scholar]
- 63.Li XY, Li X, Kong XY, Shi S, Guo G, Zhang J, Luo F, Zhao X, Wei YQ, Qian ZY, et al. Preparation of N-trimethyl chitosan-protein nanoparticles intended for vaccine delivery. J Nanosci Nanotechnol. 2010;10:4850–8. doi: 10.1166/jnn.2010.2211. [DOI] [PubMed] [Google Scholar]
- 64.Fan W, Yan W, Xu ZS, Ni H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf B Biointerfaces. 2012;90:21–7. doi: 10.1016/j.colsurfb.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 65.Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release. 2004;100:5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 66.Malhotra M, Tomaro-Duchesneau C, Prakash S. Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases. Biomaterials. 2013;34:1270–80. doi: 10.1016/j.biomaterials.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 67.Oliveira CR, Rezende CM, Silva MR, Pêgo AP, Borges O, Goes AM. A new strategy based on SmRho protein loaded chitosan nanoparticles as a candidate oral vaccine against schistosomiasis. PLoS Negl Trop Dis. 2012;6:e1894. doi: 10.1371/journal.pntd.0001894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malik B, Goyal AK, Zakir F, Vyas SP. Surface engineered nanoparticles for oral immunization. J Biomed Nanotechnol. 2011;7:132–4. doi: 10.1166/jbn.2011.1236. [DOI] [PubMed] [Google Scholar]
- 69.Mangal S, Pawar D, Agrawal U, Jain AK, Vyas SP. Evaluation of mucoadhesive carrier adjuvant: Toward an oral anthrax vaccine. Artif Cells Nanomed Biotechnol. 2013 doi: 10.3109/21691401.2013.769447. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 70.Koppolu B, Zaharoff DA. The effect of antigen encapsulation in chitosan particles on uptake, activation and presentation by antigen presenting cells. Biomaterials. 2013;34:2359–69. doi: 10.1016/j.biomaterials.2012.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang L, Qian F, He X, Wang F, Ren D, He Y, Li K, Sun S, Yin C. Novel chitosan derivative nanoparticles enhance the immunogenicity of a DNA vaccine encoding hepatitis B virus core antigen in mice. J Gene Med. 2007;9:253–64. doi: 10.1002/jgm.1017. [DOI] [PubMed] [Google Scholar]
- 72.Jabbal-Gill I, Watts P, Smith A. Chitosan-based delivery systems for mucosal vaccines. Expert Opin Drug Deliv. 2012;9:1051–67. doi: 10.1517/17425247.2012.697455. [DOI] [PubMed] [Google Scholar]
- 73.Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, Estes MK, Graham DY, Opekun AR, Richardson C, et al. Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med. 2011;365:2178–87. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gogev S, de Fays K, Versali MF, Gautier S, Thiry E. Glycol chitosan improves the efficacy of intranasally administrated replication defective human adenovirus type 5 expressing glycoprotein D of bovine herpesvirus 1. Vaccine. 2004;22:1946–53. doi: 10.1016/j.vaccine.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 75.Gogev S, Vanderheijden N, Lemaire M, Schynts F, D’Offay J, Deprez I, Adam M, Eloit M, Thiry E. Induction of protective immunity to bovine herpesvirus type 1 in cattle by intranasal administration of replication-defective human adenovirus type 5 expressing glycoprotein gC or gD. Vaccine. 2002;20:1451–65. doi: 10.1016/S0264-410X(01)00458-3. [DOI] [PubMed] [Google Scholar]
- 76.Hagenaars N, Mastrobattista E, Verheul RJ, Mooren I, Glansbeek HL, Heldens JG, van den Bosch H, Jiskoot W. Physicochemical and immunological characterization of N,N,N-trimethyl chitosan-coated whole inactivated influenza virus vaccine for intranasal administration. Pharm Res. 2009;26:1353–64. doi: 10.1007/s11095-009-9845-y. [DOI] [PubMed] [Google Scholar]
- 77.Ghendon Y, Markushin S, Vasiliev Y, Akopova I, Koptiaeva I, Krivtsov G, Borisova O, Ahmatova N, Kurbatova E, Mazurina S, et al. Evaluation of properties of chitosan as an adjuvant for inactivated influenza vaccines administered parenterally. J Med Virol. 2009;81:494–506. doi: 10.1002/jmv.21415. [DOI] [PubMed] [Google Scholar]
- 78.Lemke CD, Graham JB, Geary SM, Zamba G, Lubaroff DM, Salem AK. Chitosan is a surprising negative modulator of cytotoxic CD8+ T cell responses elicited by adenovirus cancer vaccines. Mol Pharm. 2011;8:1652–61. doi: 10.1021/mp100464y. [DOI] [PMC free article] [PubMed] [Google Scholar]