Abstract
Background:
The aim of this study was to evaluate the analgesic efficacy of pre-operative single dose of intravenous (I.V.) magnesium sulfate infusion in patients undergoing elective Cesarean section.
Materials and Methods:
Seventy pregnant women who underwent elective Cesarean section were randomly divided into two groups. Before induction of anesthesia, the magnesium group (Group A) received magnesium sulfate 50 mg/kg I.V. in bolus dose. The control group (Group B) received the same volume of isotonic saline. The pain scores at rest and also upon movement were evaluated up to 24 h post-operatively and analgesic requirement was recorded during the first 24 h after operation.
Results:
Cumulative analgesic consumption (24 h after operation was 11.2 ± 6.3 mg in group A vs. 13.9 ± 3.9 mg in group B). Post-operative pain scores (24 h after operation was 1.8 ± 2.1 in group A vs. 2.9 ± 1.2 in group B) and shivering incidents (8.57 in group A vs. 14.28 in group B) were significantly lower in Group A (P < 0.05). Mean arterial pressure just after intubation and during the immediate post-operative period was significantly lower in Group A (P < 0.05).
Conclusion:
Pre-operative intravenous magnesium sulfate infusion decrease post-operation pain and requirement of analgesia in Cesarean section.
Keywords: Cesarean section, magnesium sulfate, post-operative pain
INTRODUCTION
One of the most post-operative complications is pain. Pain is an unlike feel that due to tissue damage and there is usually after all surgeries. Post-operative respiratory complications associated with pain may delay recovery. Effective post-operative analgesia may facilitate recovery and decrease morbidity in surgical patients.[1] Pre-emptive analgesia has been defined as an anti-nociceptive treatment that prevents establishment of altered central processing of afferent input from injuries.[2] Therapies that have been tested in pre-emptive trials include NSAIDS, intravenous (I.V.) opioids, I.V. ketamine, peripheral local anesthetic, caudal and epidural analgesia, dextromethorphan and gabapentin and one I.V. adjuvant medication that has shown potential in pre-emptive analgesia is magnesium.[2] The goals of pre-emptive analgesia are to decrease acute pain after tissue injury and to inhibit the persistence of post-operative pain and the development of chronic pain.[3] One I.V. adjuvant medication that has shown potential in pre-emptive analgesia is magnesium sulfate. Magnesium sulfate has been previously investigated as a possible adjuvant for post-operative analgesia.[4,5,6] This studies suggest that peri-operative magnesium sulfate improves post-operative analgesia. However, some studies do not support that.[7,8] In this study, we investigated the effects of magnesium sulfate administration on post-operative pain relief and analgesic requirement in patients undergoing Cesarean section.
MATERIALS AND METHODS
In this randomized (computerized), double-blind, placebo-controlled, clinical trial study that was done in Alzahra University Hospital, in Isfahan from January to July 2012, after hospital ethics committee approval and written informed consent from each patient, 70 pregnant women aged between 20 and 35 years undergoing elective Cesarean section were enrolled into the study. Patients with a history of allergy to magnesium sulfate or any other study drug, hepatic, renal or cardiovascular dysfunction, neurological disorders, and opioid or analgesic abuse were not included. Patients receiving chronic treatment with magnesium or calcium-channel blockers were not included also. The patients with any unpredictable condition in surgery or any complication such as severe hypotension (whenever SBP was reduced more than 25% of baseline) or more intra-operative analgesic requirement were excluded.
Power analysis (α = 0.01 and β = 0.05) suggested that a sample size of 35 patients per group was needed to detect a 20% reduction in post-operative pain score and also post-operative analgesic requirement. Seventy women, ASA I or II aged 18-35 years, undergoing elective Cesarean section were studied.
Patients were randomly assigned to one of the two groups; the magnesium group (Group A, n = 35) received 50 mg/kg of magnesium sulfate in 100 mL of isotonic saline over 10 min in 30 min before anesthesia induction, whereas patients in the saline group (Group B, n = 35) received the same volume of isotonic saline over the same period.
The baseline heart rate (HR), systolic, diastolic and mean arterial pressure, and also SaO2 were recorded every 15 min during the entire anesthesia period and also recorded every 15 min during the recovery period. No pre-medication was given to the patients.
In all groups, induction of anesthesia was carried out with Na thiopental 6 mg/kg, Fentanyl 2 mic/kg, succynilcholine 1.5 mg/kg, and then trachea was intubated.
Maintenance of anesthesia was performed with 50% N2O and O2 and also 1 MAC of isoflurane with controlled ventilation. After child delivery, morphine 0.15 mg/kg and atracurium 0.6 mg/kg were injected I.V.
Mechanically controlled ventilation was adjusted to maintain an end-tidal CO2 concentration of between 35 and 45 mm Hg throughout the surgery.
Injected drugs were prepared in identical syringes by a researcher not otherwise involved in this study.
At the end of the surgery, the residual of neuromuscular block was reversed with a mixture of 0.02 mg/kg atropine and 0.04 mg/kg of neostigmine.
After the operation, the patients were transferred to the recovery room and the consciousness score was evaluated every 5 min until ready for discharge from the recovery room. Analgesia was administered in the form of morphine 5 mg I.V. and repeated on demand. Pain scores at rest were evaluated using a 0-10 cm visual analogue scale (VAS, starting from 0; no pain, to 10; worst pain imaginable). The VAS score was recorded at emergence from anesthesia and at 2, 12, and 24 h after the surgery. The dosage and timing of analgesia was recorded immediately after consciousness, 2, 12, and 24 h after operation. In addition, episodes of shivering and of post-operative nausea and vomiting (PONV) were monitored and recorded.
All the data were compiled and continuous variables were analyzed using t-test. Repeated measures ANOVA was used to compare measurements over time (hemodynamic variables and post-operative VAS). P values of < 0.05 were considered statistically significant.
RESULTS
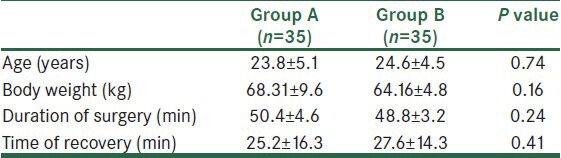
The patient characteristics are described in Table 1. There was no statistical difference in age, body weight, duration of surgery, and time of recovery between the two groups.
Table 1.
Patients’ characteristics (group A; magnesium group, group B; control group). Values are expressed as means
Hypotension (systolic arterial pressure <90 mmHg) or bradycardia (HR < 60 beat/min) did not occur during bolus injection in either group. Mean arterial pressures before (P = 0.003), immediately after intubation (P = 0.015) and 5 min after intubation (P = 0.005) and 30 min after operation (P = 0.007) were significantly lower in Group A [Figure 1].
Figure 1.
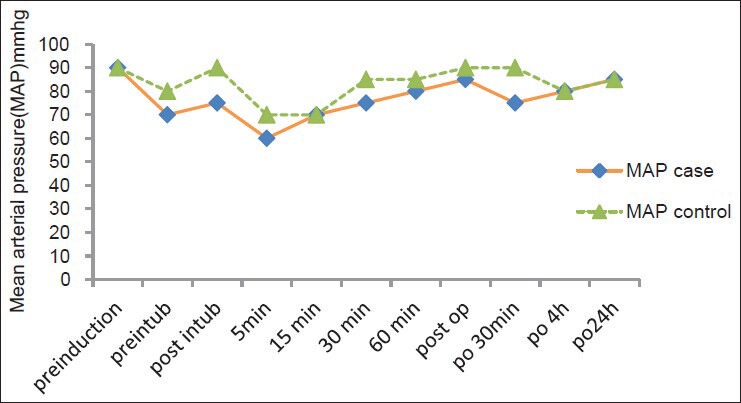
Pre-operative changes in mean arterial pressure
There was no statistical difference in HR between the two groups before anesthetic induction to discharge of recovery room (P = 0.99) [Figure 2].
Figure 2.
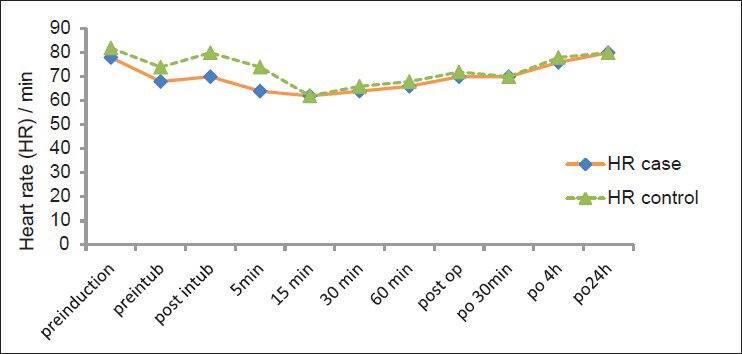
Pre-operative changes in heart rate
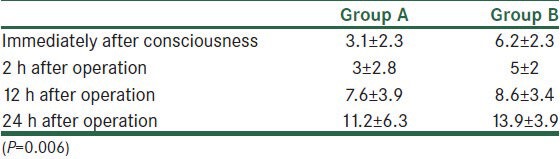
Cumulative postoperative analgesic consumption was less in group A (P = 0.006, 24 h after operation) [Table 2].
Table 2.
Mean of post-operative analgesic consumption (mg)
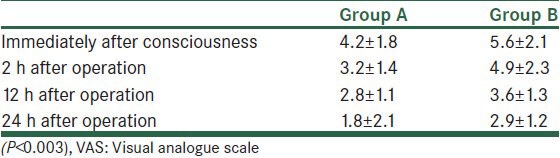
The postoperative VAS scores were less in group A (P < 0.003, 24 h after operation) [Table 3].
Table 3.
Mean VAS score (1-10 cm)
DISCUSSION
Previous studies investigating the analgesic efficacy of magnesium sulfate have shown conflicting results. Tramer and others observed that pre-treatment with IV magnesium sulfate had no impact on post-operative pain and analgesic consumption.[2] Our study has shown that infusion of magnesium sulfate (50 mg/kg) given before induction of anesthesia is associated with less post-operative pain in patients undergoing elective Cesarean section, which concurs with previous studies.[4,5,6] However, two reports have suggested that magnesium sulfate does not decrease the severity of pain after surgery.[7,8] The reasons for this discrepancy are unknown, although it is interesting to note that I.V. analgesia was used in the studies which found that magnesium potentiates analgesia, whereas epidural analgesia was used in the latter studies. Thus, it is possible that the superior analgesic efficacy of epidural analgesia might have masked the analgesia-potentiating effect of magnesium sulfate in these studies.
Magnesium has applications in anesthesia because of its actions as a calcium channel blocker and a non-competitive N-methyl-d-aspartate (NMDA) receptor antagonist. NMDA receptor antagonists are best administered before the generation of noxious stimuli in order to prevent central sensitization.[9,10]
Trends toward lower mean arterial pressure were observed in the magnesium group. These effects of magnesium might be explained by the vasodilation due to calcium channel blockade or by its analgesic effect and the consequent inhibition of catecholamine release.
The aim of this study was to describe a clinical effect of magnesium on post-operative analgesia and also analgesic requirement and we wished to administer a dose that was most likely to achieve an effect without adverse effects. Two major problems may arise with the use of magnesium in patients undergoing general anesthesia. First, magnesium enhances the action of non-depolarizing muscle relaxants. An increased serum concentration of magnesium per se may produce profound paralysis of skeletal muscles.[11,12] However, in the presence of normal renal function, renal elimination of magnesium is rapid. Second, magnesium may interact with calcium ions at vascular membranes and decrease peripheral vascular resistance[13] in our study, patients with major organ diseases (i.e., renal impairment) were excluded. In addition, we routinely monitored neuromuscular blockade. Post-operative peak-flow values as an indicator of muscle strength were similar in both groups. Hemodynamically, magnesium-treated patients did not show any difference compared to control patients, neither during nor after surgery. In the present study, patients in Group A showed less post-operative shivering and PONV. Wadhwa and colleagues suggested that magnesium sulfate infusion reduces the shivering threshold in humans, and I.V. magnesium sulfate has been reported previously to suppress post-anesthetic shivering.[10] Shivering causes discomfort and aggravates post-operative pain and the prevention of shivering may attenuate post-operative pain and enhance patients’ satisfaction.[14,15]
One limitation in our study was that we did not measure serum magnesium and cerebrospinal fluid magnesium concentration. However, it has been studied that most of total body magnesium (99%) is intracellular and estimation of plasma magnesium does not represent magnesium content of body tissues.[16] Therefore, there is lack of correlation between plasma magnesium concentration and total body magnesium content.
CONCLUSIONS
I.V. magnesium has been reported to improve post-operative pain; however, the evidence is inconsistent.[17]
Eighteen published trials have examined the use of neuraxial magnesium as a peri-operative adjunctive analgesic since 2002, with encouraging results.[18] Our data showed that pre-operative administration of magnesium sulfate (50 mg/kg bolus) in patients significantly reduced post-operative pain score and analgesic consumption after operation. In addition, peri-operative magnesium sulfate administration attenuated the increase in arterial pressure after intubation and surgery, improved satisfaction scores, and reduced PONV and shivering. Thus, we conclude that I.V. magnesium sulfate pre-operative may be useful in pain relief after elective Cesarean section.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ryu JH, Kang MH, Park KS, Do SH. Effects of magnesium sulphate on intraoperative anaesthetic requirements and postoperative analgesia in gynaecology patients receiving total intravenous anaesthesia. Br J Anaesth. 2008;100:397–403. doi: 10.1093/bja/aem407. [DOI] [PubMed] [Google Scholar]
- 2.Kiran S, Gupta R, Verma D. Evaluation of a single-dose of intravenous magnesium sulphate for prevention of postoperative pain after inguinal surgery. Indian J Anaesth. 2011;55:31–3. doi: 10.4103/0019-5049.76605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levaux C, Bonhomme V, Dewandre PY, Brichant JF, Hans P. Effect of intra-operative magnesium sulphate on pain relief and patient comfort after major lumbar orthopaedic surgery. Anaesthesia. 2003;58:131–5. doi: 10.1046/j.1365-2044.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- 4.Ryu JH, Park KS, Kim KO, Do SH. The effects of magnesium sulfate infusion on tiva (propofol and remifentanil) in gynecologic operation. Reg Anesth Pain Med. 2006;31:87. [Google Scholar]
- 5.Tan TY, Hu XG, Xiao YF. The Effect of magnesium sulphate on postoperative pain after laparosopic cholecystectomy. J Clin Res. 2006;12:112–6. [Google Scholar]
- 6.Bhatia A, Kashyap L, Pawar DK, Trikha A. Effect of intraoperative magnesium infusion on perioperative analgesia in open cholecystectomy. J Clin Anesth. 2004;16:262–5. doi: 10.1016/j.jclinane.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Paech MJ, Magann EF, Doherty DA, Verity LJ, Newnham JP. Does magnesium sulfate reduce the short- and long-term requirements for pain relief after caesarean delivery? A double-blind placebo-controlled trial. Am J Obstet Gynecol. 2006;194:1596–602. doi: 10.1016/j.ajog.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Ko SH, Lim HR, Kim DC, Han YJ, Choe H, Song HS. Magnesium sulfate does not reduce postoperative analgesic requirements. Anesthesiology. 2001;95:640–6. doi: 10.1097/00000542-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Dube L, Granry JC. The therapeutic use of magnesium in anesthesiology, intensive care and emergency medicine: A review. Can J Anaesth. 2003;50:732–6. doi: 10.1007/BF03018719. [DOI] [PubMed] [Google Scholar]
- 10.Wadhwa A, Sengupta P, Durrani J, Akca O, Lenhardt R, Sessler DI, et al. Magnesium sulphate only slightly reduces the shivering threshold in humans. Br J Anaesth. 2005;94:756–62. doi: 10.1093/bja/aei105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James MF. Magnesium: An emerging drug in anaesthesia. Br J Anaesth. 2009;103:465–7. doi: 10.1093/bja/aep242. [DOI] [PubMed] [Google Scholar]
- 12.Dabbagh A, Elyasi H, Razavi SS, Fathi M, Rajaei S. Intravenous magnesium sulphate for post-operative pain in patients undergoing lower limb orthopedic surgery. Acta Anaesthesiol Scand. 2009;53:1088–91. doi: 10.1111/j.1399-6576.2009.02025.x. [DOI] [PubMed] [Google Scholar]
- 13.Farouk S. Pre-incisional epidural magnesium provides pre-emptive and preventive analgesia in patients undergoing abdominal hysterectomy. Br J Anaesth. 2008;101:694–9. doi: 10.1093/bja/aen274. [DOI] [PubMed] [Google Scholar]
- 14.Kaur S, Baghla N. Evaluation of intravenous magnesium sulphate for postoperative analgesia in upper limb orthopaedic surgery under general anaesthesia: A comparative study. Internet J Anesthesiol. 2012;30:742–4. [Google Scholar]
- 15.Banwait S, Sharma S, Pawar M, Garg R, Sood R. Evaluation of single epidural bolus dose of magnesium as an adjuvant to epidural fentanyl for postoperative analgesia: A prospective, randomized, double-blind study. Saudi J Anaesth. 2012;6:273–8. doi: 10.4103/1658-354X.101221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang JY, Na HS, Jeon YT, Ro YJ, Kim CS, Do SH. I.V. infusion of magnesium sulphate during spinal anaesthesia improves postoperative analgesia. Br J Anaesth. 2010;104:89–93. doi: 10.1093/bja/aep334. [DOI] [PubMed] [Google Scholar]
- 17.Albrecht E, Kirkham KR, Liu SS, Brull R. Peri-operative intravenous administration of magnesium sulphate and postoperative pain: A meta-analysis. Anaesthesia. 2013;68:79–90. doi: 10.1111/j.1365-2044.2012.07335.x. [DOI] [PubMed] [Google Scholar]
- 18.Albrecht E, Kirkham KR, Liu SS, Brull R. The analgesic efficacy and safety of neuraxial magnesium sulphate: A quantitative review. Anaesthesia. 2013;68:190–202. doi: 10.1111/j.1365-2044.2012.07337.x. [DOI] [PubMed] [Google Scholar]


