Abstract
Background:
Atherosclerosis, with its major manifestation, coronary artery disease (CAD) is a chronic inflammatory disease. Dietary fatty acids intakes favorably effect on inflammatory responses. This study was conducted to examine the association between dietary fatty acid intakes and inflammatory markers, interleukin 6 (IL-6) and high sensitivity C-reactive protein (hs-CRP), in CAD patients among Iranian population.
Materials and Methods:
This hospital-based, cross-sectional study was conducted in Chamran Heart Hospital, Isfahan, Iran in 2012. Patients aged ≥45 years with first ever symptomatic CAD confirmed by angiography were included. A semi-quantitative food frequency questionnaire (FFQ) was used to assess the usual intakes of dietary fatty acids.
Results:
The energy-adjusted daily intakes (mean ± SD) of saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), linoleic acid, α-linolenic acid, and eicosapentaenoic acid and docosahexaenoic acid (EPA + DHA) were 27 ± 9, 22 ± 6, 21 ± 5, 0.4 ± 0.32, and 0.85 ± 0.82 g/d; respectively. After adjustment for potential confounders, SFA was directly related to hs-CRP (P = 0.01) and IL-6 (P < 0.001) concentrations. Intakes of EPA + DHA and MUFA, were significantly adversely related to plasma hs-CRP concentration (P = 0.002 and 0.001, respectively) but not IL-6, albeit MUFA was modestly inversely related to IL-6 (P = 0.08). No significant relationships were observed for other fatty acids, α-linolenic acid, and linoleic acid.
Conclusions:
These findings suggest that saturated fatty acids, EPA + DHA and MUFA were significantly related to plasma inflammatory markers in CAD patients.
Keywords: Coronary artery disease, fatty acids, high sensitivity C-reactive protein, interleukin-6
INTRODUCTION
Inflammation plays an essential role in development of insulin resistance and type 2 diabetes, initiation and progression of atherosclerotic lesions, and plaque disruption.[1,2] Interleukin-6 (IL-6), is an inflammatory cytokine and one of the main inducers of C-reactive protein (CRP) secretion in the liver.[3,4] Atherosclerosis, with its major manifestation, coronary artery disease (CAD) is the major cause of morbidity and mortality in the world.[5] Reports have documented CAD incidence rates to be as high as 166 per 100,000 in Middle Eastern countries.[6] CAD is also the most important cause of morbidity and mortality in Iran and includes about 50% of all the deaths.[7]
It is being recognized that traditional risk factors; such as dietary fats, smoking, dyslipidemia, hypertension, and diabetes; do not explain the presence of coronary atherosclerosis in a large proportion of patients.[8] During the past decade, with the recognition that atherosclerosis is an inflammatory process, several inflammatory markers have also been considered as a potential tool for prediction of coronary events and the observations taken together suggest that atherosclerosis is a chronic inflammatory disease.[9]
The results of experimental and epidemiological studies have shown that habitual diet is the most important modifiable risk factor for prevention and control of CAD.[10,11,12] Dietary fat intake has long been implicated in the etiology of cardiovascular disease (CVD) and study of lipids and their major structural elements, the fatty acids, remains one of the most enigmatic research fields in nutrition and biology.[13]
The results of experimental studies have shown that the quantity and quality of dietary fat intake influence on intensity and rate of inflammatory responses.[14] High intakes of trans fatty acids (TFAs) were associated with increased tumor necrosis factor (TNF) system activity not only among healthy women,[15] but also among patients with established heart disease.[16] A study in Iran revealed that healthy women with higher intakes of partially hydrogenated vegetable oils (PHVOs), a rich source of TFAs, had elevated concentrations of inflammatory biomarkers, whereas higher intakes of non-hydrogenated vegetable oils (non-HVOs) are associated with lower plasma concentrations of these biomarkers.[15] Polyunsaturated fatty acids (PUFA) including n-3 and n-6 fatty acids are substrates for human eicosanoids production. Eicosanoids derived from n-3 fatty acids have fewer inflammatory properties than those derived from n-6 fatty acids.[17] In Nurses’ Health Study (NHS) and Health Professional Follow-up Study (HPFS), two prospective cohort studies, intakes of n-3 fatty acids were modestly inversely related to CRP levels.[18] n-6 PUFA consumption shows variable effects on inflammation, both anti-inflammatory and pro-inflammatory effects.[18,19] Monounsaturated fatty acids (MUFAs) are predominant in olive oil, and have received increased attention as being potentially beneficial because of their association with low rates of coronary heart disease (CHD) in olive oil consuming populations of the Mediterranean basin.[20] Recommendations to decrease the intake of saturated fatty acids (SFAs) have been as a goal in prevention of CAD. In PIVUS study, a Swedish cross-sectional population based study of healthy elderly men and women, failed to identify a relationship between levels of SFAs in cholesteryl esters and high sensitivity-CRP (hs-CRP) concentration.[21] It is expected that CAD will be the leading cause of death in developing countries by the year 2020.[22] Limited data are available from Asian countries linking dietary fatty acids intake to plasma inflammatory markers, especially in patient with CAD. The present study was conducted to examine the relationship between dietary fatty acids and inflammatory markers, IL-6 and hs-CRP, in angiographically defined CAD among Iranian population.
MATERIALS AND METHODS
Participant population
The present hospital-based cross-sectional study was conducted in Chamran Heart Hospital, Isfahan, Iran in 2012. This study included 150 CAD male patients undergoing diagnostic coronary angiography at the Angiography Department of this hospital. Patients aged ≥45 years with first ever symptomatic CAD were included. The patients with prior history of CAD, any systemic inflammation, recent trauma, kidney disease, liver disease, cancer, and autoimmune disease were excluded from the study. We selected patients through consecutive sampling procedure. Participants were conscious and able to answer the questions. Written informed consent was obtained from each participant.
Angiographic estimation of CAD
The coronary angiogram is a two dimensional picture of inner lumen of the artery. Coronary angiography was performed by femoral approach and included at least four views of the left coronary artery and two views of right coronary artery. Each of eight vessel segments indicates the status of coronary stenosis. CAD was defined when a person had at least one epicardial vessel with more than 50% stenosis. The study protocol was approved by local Ethics Review Committee of Isfahan University of Medical Sciences.
Assessment of dietary intake
Usual dietary intake was assessed using a validated 168-item semi-quantitative food frequency questionnaire (FFQ).[23] A trained dietitian administered all the questionnaires. The FFQ consisted of a list of foods with standard serving sizes commonly consumed by Iranians. Participants were asked to report their frequency of consumption of a given serving of each food item during the previous year on a daily (e.g., bread), weekly (e.g., rice, meat), or monthly (e.g., fish) basis. The reported frequency for each food item was then converted to a daily intake. Portion sizes of consumed foods were converted to gram (g) using household measures.[24] Total energy intake was calculated by summing energy intakes from all item foods. N4 nutritional software was used to determine nutrient compositions of all foods. Total fat and total dietary fatty acids consumption was calculated by summing the consumption of fish (65-190 g/serving), tuna (75 g/serving), butter, margarine, hydrogenated vegetable oil, non-hydrogenated vegetable oil (olive oil, sunflower oil, soybean oil, corn oil, and canola oil), nuts (almond, walnut, hazelnut, pistachio, and peanut), animal oil, dairy fat, red meat fat, and mayonnaise. We also inquired about types of fats or oils used by each subject for frying, cooking, and baking.
Laboratory measurement
The 12-h fasting blood samples were collected into tubes containing 0.1% Ethylenediaminetetraacetic acid (EDTA) and were centrifuged at 4°C and 500 g for 10 min to separate plasma. Serum samples were promptly frozen (−20°C). Serum total cholesterol, low density lipoprotein (LDL), and triglyceride (TG) concentrations were measured by commercially available enzymatic reagents (Pars Azmoon, Iran) on a BT-3000 (Biotechnica) auto analyzer. High density lipoprotein (HDL) was also measured using a photometric enzyme assay (Pars Azmoon, Iran). Hs-CRP was measured by immunoturbidometry (Pars Azmoon, Iran) and IL-6 enzyme-linked immunosorbent assay (ELISA) was performed on serum (BBT International, England).
Assessment of other variables
Weight was measured using digital scales and recorded to the nearest 100 g, while the participants were minimally clothed without shoes. Height was measured in a standing position, without shoes, using a tape measure; while the shoulders were in a normal position. Body mass index (BMI) was calculated as weight in kg divided by height in m2. Waist circumference (WC) was measured at the narrowest level and that of the hip at maximum level over light clothing using an tape measure, without any pressure to body surface; measurements were recorded to the nearest 0.1 cm. Data on physical activity were obtained using participants’ oral responses to an International Physical Activity Questionnaire and expressed as metabolic equivalent h/day (MET-h/day).[25] Additional covariate information regarding age, smoking status, medical history and current use of medications, and family history of diabetes mellitus and cardiovascular disease was obtained using questionnaires. Blood pressure was measured by an expert using a mercury sphygmomanometer and with the participant resting in a seated position for 10 min.
Statistical analysis
Statistical analyses were performed by using Statistical Package for Social Science (SPSS Inc, Chicago, IL, Version 18.0). General characteristics, biomarkers levels, and dietary intakes of participants across tertiles of total dietary fat intake were compared by the use of analysis of variance (ANOVA) and Chi-square where appropriate. Partial correlation analysis was performed to evaluate the association between fatty acid intakes and inflammatory markers. General linear model was used to examine the effect of fatty acid intakes including saturated fat, monounsaturated fat, linoleic acid (LA), α-linolenic acid (ALA), and eicosapentaenoic acid and docosahexaenoic acid (EPA + DHA) on plasma levels of inflammatory markers. We adjusted for age, waist circumference, BMI, physical activity, total energy intake, dietary cholesterol, protein, HDL, LDL, TG, serum total cholesterol, smoking status, hypertension, diabetes mellitus, family history of CVD, aspirin, and statins use. All probability values presented are two-tailed, and probability values below 0.05 were considered statistically significant. Values reported in the text are mean ± SD.
RESULTS
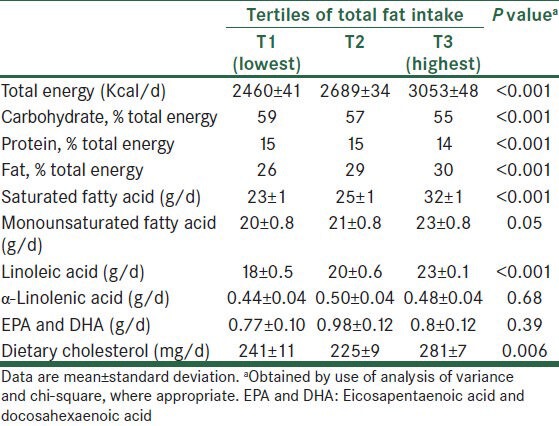
The energy-adjusted daily intakes (mean ± SD) of SFA, MUFA, linoleic acid, α-linolenic acid, and DHA + EPA were 27 ± 9, 22 ± 6, 21 ± 5, 0.48 ± 0.32, and 0.85 ± 0.82 g/d, respectively. General characteristics and laboratory features of study participants according to tertiles of total fat intake are presented in Tables 1 and 2. Compared with patients in the lowest tertile, those in the upper teritle of fat intake had higher levels of IL-6 and lower levels of TG. Dietary intakes of study participants across tertiles of total fat intake are shown in Table 3. Participants in the top tertile of fat intake had higher intakes of total energy, saturated fat, monounsaturated fat, linoleic acid, and cholesterol. Furthermore, CAD patients in the highest tertile of fat intake had lower intakes of carbohydrate and protein compared with those in the lowest tertile. No significant difference was found for other characteristics and dietary variables.
Table 1.
General characteristics of patients across tertiles of total fat intake
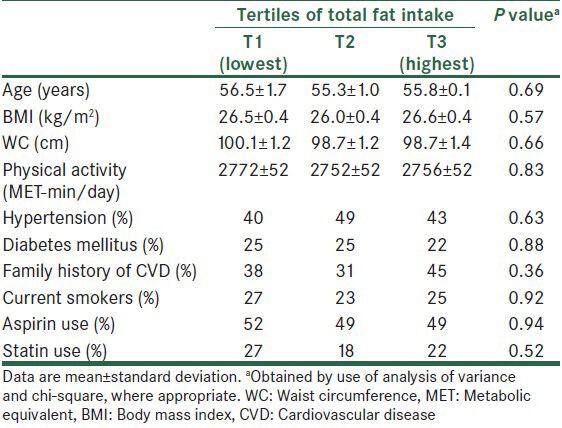
Table 2.
Biomarkers levels of patients across tertiles of total fat intake
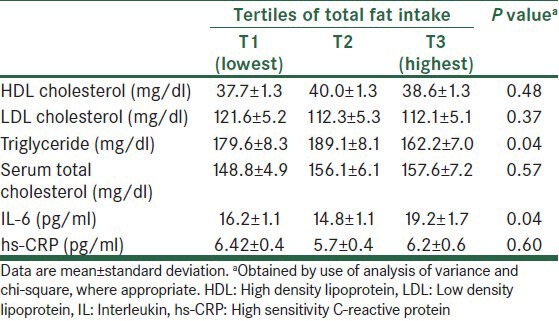
Table 3.
Dietary intakes of patients across tertiles of total fat intake
Partial correlation analysis was performed to evaluate association of fatty acid intakes with plasma IL-6 and hs-CRP concentrations. We adjusted for total energy, age, BMI, waist circumference, smoking status, hypertension, diabetes mellitus, family history of CVD, aspirin use, statin use, HDL, LDL, TG, serum total cholesterol, protein, dietary cholesterol, and physical activity. The correlation (r) between EPA + DHA and IL-6 was −0.25; P = 0.003 and hs-CRP was −0.38; P < 0.001. Monounsaturated fat was significantly inversely associated with plasma levels of IL-6 (r = −0.18; P = 0.03) and hs-CRP (r = −0.35; P < 0.001). SFA was directly related to IL-6 (r = 0.33; P = < 0.001) and hs-CRP (r = 0.22; P = < 0.001). The correlation of α-linolenic acid and IL-6 was −0.02; P = 0.81 and hs-CRP was −0.03; P = 0.69. No significant association was found for linoleic acid and IL-6 (r = −0.01; P = 0.91) and hs-CRP (r = −0.10; P = 0.22).
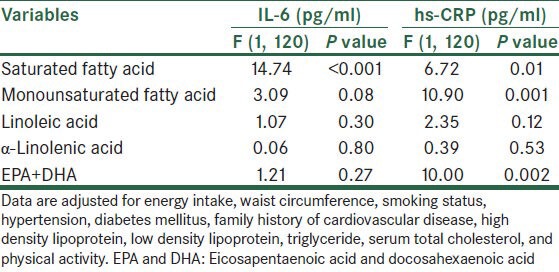
Multivariate-adjusted general linear model was used to examine effect of different fatty acids intakes on plasma inflammatory makers [Table 4]. For two reasons, better fitting and no significane between BMI, age, protein, dietary cholesterol, aspirin and statin use with IL-6 and hs-CRP, these variables were not taken into account. The overall results of IL-6 changed slightly after use of this model. SFA was statistically significantly related to hs-CRP (P = 0.011) and IL-6 (P < 0.001) concentrations. MUFA and EPA + DHA were significantly related to plasma hs-CRP concentration, but not IL-6; albeit MUFA was modestly inversely related to IL-6 (P = 0.08). No significant relationships were observed for other fatty acids, α-linolenic acid, and linoleic acid.
Table 4.
Multivariate-adjusted general linear model estimates for the association between fatty acids intakes and inflammatory markers
DISCUSSION
In this cross-sectional study of dietary fatty acids and inflammatory markers, SFA was directly related to plasma IL-6 and hs-CRP levels. We observed statistically significant inverse association between n-3 fatty acids and MUFA intake with plasma levels of hs-CRP. The associations were restricted to long-chain PUFAs, EPA, and DHA and not ALA. In this study, we did not find statistically significant association between other fatty acids and inflammatory markers. To our knowledge, this study is the first to report association between dietary fat intake and inflammatory markers in patients with CAD from an Iranian population.
CAD has been among the leading causes of death in Asian countries.[6] Inflammation plays a central role in development of atherosclerosis, and elevated CRP levels are an important risk factor for cardiovascular disease.[26] Similarly, plasma levels of IL-6 are also predictive of CVD; however, this relationship is less strong than for CRP, which may be due in part to higher variability or lower specificity of this cytokine.[27]
Several studies have investigated the effects of different fatty acids on cytokines secretion, with conflicting results. A recent multicenter clinical trial performed on end-stage renal disease patients undergoing dialysis in Iran revealed that after supplementation with n-3 fatty acids, there was no statistically significant difference in the levels of hs-CRP (P = 0.44).[28] The ATTICA study in Greece found that total plasma n-6 fatty acids were inversely associated with hs-CRP and IL-6.[17] For Japanese men, but not women, there is an inverse relationship between dietary intake of both n-3 and n-6 PUFA and hs-CRP.[29] In a randomized crossover study, IL-6 concentrations were lower after consumption of oleic acid than after consumption of the SFAs;[30] however, no effects on CRP and IL-6 were observed in another randomized crossover study.[31] In LIPGENE study, a European multicenter dietary intervention study in subjects with metabolic syndrome, comparing high fat diets (MUFA or SFA) vs low fat diets, did not significantly alter the concentrations of CRP.[32] In contrast, a study of Indian adolescents and young adults found that for every 1% decrease in energy intake from SFAs, hs-CRP was decreased by 0.14 mg/L.[33]
According to experimental studies[34,35] and clinical trials[36] strong association has been described between cardiovascular disease and SFAs. Varied mechanisms have been proposed to account for pro-inflammatory effect of dietary SFA. Mechanisms responsible for these unhealthy effects of SFAs include: (1) Accumulation of diacylglycerol and ceramide; (2) activation of nuclear factor-KB and subsequent induction of inflammatory genes in white adipose tissue, immune cells, and myotubes; (3) decreased peroxisome proliferator-activated receptor gamma (PPAR-γ) activation and adiponectin production, which decreases the oxidation of glucose and fatty acids; and (4) recruitment of immune cells like macrophages, neutrophils, and bone marrow-derived dendritic cells to white adipose tissue and muscle.[22] Our results are in agreement with known inflammatory action of SFA. A few studies suggest that dietary consumption of MUFA, oleic acid in particular, may have anti-inflammatory effects.[37,38] In our study we found a statistically inverse association between MUFA with hs-CRP. Inconsistent findings in different studies might be dose related and might also be explained by lack of control for confounding variables in some studies and subject baseline levels of inflammatory markers. Fish and n-3 fatty acid intakes have received considerable attention in the recent past as potential dietary factors to reduce the risk of developing CAD.[39,40] The association between n-3 fatty acids and reduced CVD risk has been attributed to a number of potential mechanisms, including effects on platelet function, plasma TG concentrations, inflammatory factors, and arrhythmia.[41,42] In the current study, we failed to find a significant association between ALA intake and inflammatory markers. This might be attributed to more effective role of EPA and DHA in altering membrane composition and eicosanoid production. It was recently estimated that in humans, only 0.2% of plasma ALA is converted to EPA.[43,44] The conversion rate of LA to arachidonic acid is also very low[45]; however, because of the much higher intake, we speculate that LA still affects arachidonic acid concentrations; whereas ALA, because of its low intake and low conversion rate, has no substantial effect on EPA levels, and thus on inflammation. n-6 PUFA has long been considered as pro-inflammatory molecules because they are the main precursors of eicosanoids.[46] It is also argued that higher dietary intake of n-6 PUFA may lead to a competition between n-6 and n-3 metabolism resulting in a reduced production of anti-inflammatory molecules from n-3 PUFA.[47] However, in human subjects, higher intakes of n-6 fatty acids do not appear to be associated with elevated levels of inflammatory markers and there are no data from human studies that support a detrimental effect of dietary n-6 fatty acids on coronary heart disease.[48,49] The present results are in contrast to customary assumption that high intake of n-6 fatty acids antagonizes the anti-inflammatory effects of n-3 fatty acids. One possible explanation for our observation is that the activities of n-6 fatty acids Δ6 and Δ5-desaturase (which are the rate-limiting steps for arachidonic acid and EPA synthesis in humans) and the activity of cyclooxygenase are inhibited by both n-3 and n-6 PUFAs. Through this mechanism, high intake of both types of fatty acids could reduce inflammatory mediators.[50,51]
Our study has several limitations. In this study we used N4 nutritional software to determine nutrient compositions of all food items, but this software did not have any information about trans-fatty acids. Our study has been done in the framework of cross-sectional study and there is no control group in this study. Also, the sample size of 150 individuals might be insufficient to detect possible associations between dietary fatty acids and inflammatory markers. Furthermore, cross-sectional design of the study would not allow us to infer causality. Future longitudinal studies are required to further explore for the possible association. The next limitation is the use of FFQ as the dietary assessment method. The use of FFQ would result in misclassification of participants and this is usual with all nutritional epidemiologic studies. Although we controlled the analysis for several confounding variables, the existence of residual confounding cannot be excluded. Furthermore, random errors as in all epidemiologic studies might affect our results because diet and lifestyle information might be collected with some degree of errors. The existence of recall bias and selection bias in cross-sectional studies must also be taken into account. In the current study, it is possible that CAD patients might associate their disease to fat intake and therefore over-report consumption of fatty foods and oils. This is particularly relevant for our study because we enrolled newly diagnosed cases of CAD and as shown previously,[52] the interviewing close to the time of diagnosis may increase the potential for recall bias. However, some studies in the field of nutritional epidemiology that have assessed the effect of recall bias on the overall study results reported that this bias was minor and negligible.[53] Since all study participants were recruited from the province of Isfahan, Iran; the observed associations between dietary fatty acid and inflammatory markers may not generalize to other geographic areas.
CONCLUSION
Given the above mentioned limitations, we found an inverse relationship between n-3 fatty acids, EPA and DHA, and MUFA with hs-CRP concentration in CAD patients. Our finding suggests that SFA was directly related to plasma IL-6 and hs-CRP levels. Further experimental and interventional studies under different conditions are needed to further explore for these associations.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 2.Willerson JT. Systemic and local inflammation in patients with unstable atherosclerotic plaques. Prog Cardiovasc Dis. 2002;44:469–78. doi: 10.1053/pcad.2002.123782. [DOI] [PubMed] [Google Scholar]
- 3.Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res. 2001;9:414–7. doi: 10.1038/oby.2001.54. [DOI] [PubMed] [Google Scholar]
- 4.Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: Prospective analysis from the Women's Health Initiative observational study. JAMA. 2002;288:980–7. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 5.Wilson PW. Evidence of systemic inflammation and estimation of coronary artery disease risk: A population perspective. Am J Med. 2008;121(10 Suppl 1):S15–20. doi: 10.1016/j.amjmed.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Okrainec K, Banerjee DK, Eisenberg MJ. Coronary artery disease in the developing world. Am Heart J. 2004;148:7–15. doi: 10.1016/j.ahj.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Azarpazhooh MR, Etemadi MM, Donnan GA, Mokhber N, Majdi MR, Ghayour-Mobarhan M, et al. Excessive incidence of stroke in Iran: Evidence from the Mashhad Stroke Incidence Study (MSIS), a population-based study of stroke in the Middle East. Stroke. 2010;41:e3–10. doi: 10.1161/STROKEAHA.109.559708. [DOI] [PubMed] [Google Scholar]
- 8.Mehta JL, Saldeen TG, Rand K. Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J Am Coll Cardiol. 1998;31:1217–25. doi: 10.1016/s0735-1097(98)00093-x. [DOI] [PubMed] [Google Scholar]
- 9.Alexander RW. Inflammation and coronary artery disease. N Engl J Med. 1994;331:468–9. doi: 10.1056/NEJM199408183310709. [DOI] [PubMed] [Google Scholar]
- 10.Buja LM. Does atherosclerosis have an infectious etiology? Circulation. 1996;94:872–3. doi: 10.1161/01.cir.94.5.872. [DOI] [PubMed] [Google Scholar]
- 11.Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285:304–12. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Manson JE, Stampfer MJ, Rexrode KM, Hu FB, Rimm EB, et al. Whole grain consumption and risk of ischemic stroke in women: A prospective study. JAMA. 2000;284:1534–40. doi: 10.1001/jama.284.12.1534. [DOI] [PubMed] [Google Scholar]
- 13.Hall WL. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr Res Rev. 2009;22:18–38. doi: 10.1017/S095442240925846X. [DOI] [PubMed] [Google Scholar]
- 14.Pischon T, Hankinson SE, Hotamisiligi GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–60. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 15.Esmaillzadeh A, Azadbakht L. Home use of vegetable oils, markers of systemic inflammation, and endothelial dysfunction among women. Am J Clin Nutr. 2008;88:913–21. doi: 10.1093/ajcn/88.4.913. [DOI] [PubMed] [Google Scholar]
- 16.Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC. Trans fatty acids and systemic inflammation in heart failure. Am J Clin Nutr. 2004;80:1521–5. doi: 10.1093/ajcn/80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalogeropoulos N, Panagiotakos DB, Pitsavos C, Chrysohoou C, Rousinou G, Toutouza M, et al. Unsaturated fatty acids are inversely associated and n-6/n-3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clin Chim Acta. 2010;411:584–91. doi: 10.1016/j.cca.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Margioris AN. Fatty acids and postprandial inflammation. Curr Opin Clin Nutr Metab Care. 2009;12:129–37. doi: 10.1097/MCO.0b013e3283232a11. [DOI] [PubMed] [Google Scholar]
- 19.Yoneyama S, Miura K, Sasaki S, Yoshita K, Morikawa Y, Ishizaki M, et al. Dietary intake of fatty acids and serum C-reactive protein in Japanese. J Epidemiol. 2007;17:86–92. doi: 10.2188/jea.17.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Superko HR, Nejedly M, Garrett B. Small LDL and its clinical importance as a new CAD risk factor: A female case study. Prog Cardiovasc Nurs. 2002;17:167–73. doi: 10.1111/j.0889-7204.2002.01453.x. [DOI] [PubMed] [Google Scholar]
- 21.Petersson H, Lind L, Hulthe J, Elmgren A, Cederholm T, Risérus U. Relationships between serum fatty acid composition and multiple markers of inflammation and endothelial function in an elderly population. Atherosclerosis. 2009;203:298–303. doi: 10.1016/j.atherosclerosis.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy A, Martinez K, Chuang CC, LaPoint K, McIntosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: Mechanisms of action and implications. J Nutr. 2009;139:1–4. doi: 10.3945/jn.108.098269. [DOI] [PubMed] [Google Scholar]
- 23.Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J Epidemiol. 2010;20:150–8. doi: 10.2188/jea.JE20090083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghaffarpour M, Houshiar-Rad A, Kianfar H. Tehran: Keshaverzi Press; 1999. The manual for household measures, cooking yields factors and edible portion of foods; pp. 1–46. (in Farsi) [Google Scholar]
- 25.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2002;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 27.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: Induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–8. [PubMed] [Google Scholar]
- 28.Tayyebi-Khosroshahi H, Houshyar J, Deghan-Hesari R, Alikhah H, Vatankhah AM, Safaeian AR, et al. Effect of treatment with omega-3 Fatty acids on C-reactive protein and tumor necrosis facor-alfa in hemodialysis patients. Saudi J Kidney Dis Transpl. 2012;23:500–6. [PubMed] [Google Scholar]
- 29.Poudel-Tandukar K, Nanri A, Matsushita Y, Sasaki S, Ohta M, Sato M, et al. Dietary intakes of alpha-linolenic and linoleic acids are inversely associated with serum C-reactive protein levels among Japanese men. Nutr Res. 2009;29:363–70. doi: 10.1016/j.nutres.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 30.van Dijk SJ, Feskens EJ, Bos MB, Hoelen DW, Heijligenberg R, Bromhaar MG, et al. A saturated fatty acid-rich diet induces an obesity-linked proinflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. Am J Clin Nutr. 2009;90:1656–64. doi: 10.3945/ajcn.2009.27792. [DOI] [PubMed] [Google Scholar]
- 31.Gillingham LG, Gustafson JA, Han SY, Jassal DS, Jones PJ. High-oleic rapeseed (canola) and flaxseed oils modulate serum lipids and inflammatory biomarkers in hypercholesterolaemic subjects. Br J Nutr. 2011;105:417–27. doi: 10.1017/S0007114510003697. [DOI] [PubMed] [Google Scholar]
- 32.Petersson H, Riserus U, McMonnagle J, Gulseth HL, Tierney AC, Morange S, et al. Effect of dietary fat modification on oxidative stress and inflammatory markers in the LIPPGENE study. Br J Nutr. 2010;104:1357–62. doi: 10.1017/S000711451000228X. [DOI] [PubMed] [Google Scholar]
- 33.Arya S, Isharwal S, Misra A, Pandey RM, Rastogi K, Vikram NK, et al. C-reactive protein and dietary nutrients in urban Asian Indian adolescents and young adults. Nutrition. 2006;22:865–71. doi: 10.1016/j.nut.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Bassett CM, McCullough RS, Edel AL, Maddaford TG, Dibrov E, Blackwood DP, et al. Trans-fatty acids in the diet stimulates atherosclerosis. Metabolism. 2009;58:1802–8. doi: 10.1016/j.metabol.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Seo T, Qi K, Chang C, Liu Y, Worgall TS, Ramakrishnan R, et al. Saturated fat-rich diet enhances selective uptake of LDL cholesteryl esters in the arterial wall. J Clin Invest. 2005;115:2214–22. doi: 10.1172/JCI24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–55. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 37.Devaraj S, Kasim-Karakas S, Jialal I. The effect of weight loss and dietary fatty acids on inflammation. Curr Atheroscler Rep. 2006;8:477–86. doi: 10.1007/s11883-006-0023-y. [DOI] [PubMed] [Google Scholar]
- 38.Basu A, Devaraj S, Jialal I. Dietary factors that promote or retard inflammation. Arterioscler Thromb Vasc Biol. 2006;26:995–1001. doi: 10.1161/01.ATV.0000214295.86079.d1. [DOI] [PubMed] [Google Scholar]
- 39.Daviglus ML, Stamler J, Orencia AJ, Dyer AR, Liu K, Greenland P, et al. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med. 1997;336:1046–53. doi: 10.1056/NEJM199704103361502. [DOI] [PubMed] [Google Scholar]
- 40.Albert CM, Hennekens CH, O’Donnell CJ, Ajani UA, Carey VJ, Willett WC, et al. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279:23–8. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 41.Harris WS. N-3 fatty acids and serum lipoproteins: Human studies. Am J Clin Nutr. 1997;65(Suppl 5):1645–54S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 42.Geelen A, Brouwer IA, Zock PL, Katan MB. Antiarrhythmic effects of n-3 fatty acids: Evidence from human studies. Curr Opin Lipidol. 2004;15:25–30. doi: 10.1097/00041433-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Whelan J, Broughton KS, Kinsella JE. The comparative effects of dietary alpha-linolenic acid and fish oil on 4- and 5-series leukotriene formation in vivo. Lipids. 1991;26:119–26. doi: 10.1007/BF02544005. [DOI] [PubMed] [Google Scholar]
- 44.Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N., Jr Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res. 2001;42:1257–65. [PubMed] [Google Scholar]
- 45.Emken EA, Adlof RO, Gulley RM. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim Biophys Acta. 1994;1213:277–88. doi: 10.1016/0005-2760(94)00054-9. [DOI] [PubMed] [Google Scholar]
- 46.Calder PC, Grimble RF. Polyunsaturated fatty acids, inflammation and immunity. Eur J Clin Nutr. 2002;56(Suppl 3):S14–9. doi: 10.1038/sj.ejcn.1601478. [DOI] [PubMed] [Google Scholar]
- 47.Simopoulos AP The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–88. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 48.Czernichow S, Thomas D, Bruckert E. n-6 Fatty acids and cardiovascular health: A review of the evidence for dietary intake recommendations. Br J Nut. 2010;104:788–96. doi: 10.1017/S0007114510002096. [DOI] [PubMed] [Google Scholar]
- 49.Horrobin DF. Commentary on workshop statement: Are we really sure that arachidonic acid and lonoleic acid are bad thing? Prostaglandings Leukot Essent Fatty Acids. 2000;63:145–7. doi: 10.1054/plef.2000.0183. [DOI] [PubMed] [Google Scholar]
- 50.Cho HP, Nakamura M, Clarke SD. Cloning, expression, and fatty acid regulation of the human delta-5 desaturase. J Biol Chem. 1999;274:37335–9. doi: 10.1074/jbc.274.52.37335. [DOI] [PubMed] [Google Scholar]
- 51.Ringbom T, Huss U, Stenholm A, Flock S, Skattebøl L, Perera P, et al. Cox-2 inhibitory effects of naturally occurring and modified fatty acids. J Nat Prod. 2001;64:745–9. doi: 10.1021/np000620d. [DOI] [PubMed] [Google Scholar]
- 52.Friedenreich CM, Howe GR, Miller AB. An investigation of recall bias in the reporting of past food intake among breast cancer cases and controls. Ann Epidemiol. 1991;1:439–53. doi: 10.1016/1047-2797(91)90013-3. [DOI] [PubMed] [Google Scholar]
- 53.Hislop TG, Lamb CW, Ng VT. Differential misclassification bias and dietary recall for the distant past using a food frequency questionnaire. Nutr Cancer. 1990;13:223–33. doi: 10.1080/01635589009514064. [DOI] [PubMed] [Google Scholar]


