Abstract
Background:
Several markers have been used to make a distinction between metastatic adenocarcinoma and reactive mesothelial cells in the body cavity effusions. This study aimed to evaluate the diagnostic value of claudin-4 marker in making such a distinction.
Materials and Methods:
In this cross-sectional study, a total of 92 pleural/peritoneol effusions have been studied, including 47 cases of definite metastatic carcinoma and 45 cases of reactive mesothelium, and definitely negative for malignancy. Specimens were collected from patients; cell block samples were derived and used for immunohistochemical staining. The antibody used for immunohistochemical labeling was monoclonal anti-claudin-4. In the evaluation, membrane-bound reactivity was considered as significant and positive cases were defined when at least more than 10% of tumor cells were distinctly labeled.
Results:
Claudin-4 protein was positive in 40 specimens of metastatic carcinoma, while none of the cases of reactive mesothelium stained with the marker. This was not detected in the mesothelial cells, though. Positive staining for claudin-4 was significantly more frequent in metastatic carcinoma than in the reactive mesothelium (P > 0.0001). The sensitivity and specificity of claudin-4 to distinguish reactive mesothelium from metastatic carcinoma were 85% (95% confidence interval [CI], 71.1-93.8%) and 100% (95% CI, 91.1-100%), respectively. Furthermore, negative likelihood ratio was 0.15 (95% CI, 0.08-0.29).
Conclusion:
The results of this study demonstrated that claudin-4 is less frequently expressed in reactive mesothelium. Thus, this claudin may be helpful in differentiating metastatic carcinoma from reactive mesothelial cells in pleural and peritoneal fluid cytology specimen.
Keywords: Body cavity effusion, claudin-4, malignancy, metastatic carcinoma, reactive mesothelium
INTRODUCTION
A variety of benign and malignant disorders can present with serous effusion. Diagnostic difficulties can be marked by the difference between metastatic carcinoma and atypical reactive mesothelium. Hence, in serous effusion smears, the morphologic criteria used in cytology have not always ensured diagnostic accuracy and determination of source and cell behavior has always been a matter of diagnostic confusion among investigators all over the world.[1] A common diagnostic problem in serous effusions is diagnosing cells as being either malignant or reactive mesothelium correctly.[2]
The presence of antigen selectively on metastatic carcinoma, which is absent in reactive mesothelium or vice versa, is the possible way to identify malignant cells in the body cavity effusions.[3] Cytokeratins and some epithelial membrane antigens are antigens which are characteristically expressed by metastatic carcinoma, but have limited usefulness in the differential diagnosis, because these are also expressed on mesothelial cells.[4,5,6] For cancer diagnosis, claudins have been shown to represent new interesting targets.[7]
The claudin family, the main essential membrane proteins forming the backbone of tight junctions, consists of 23 transmembrane proteins exhibiting distinct tissue- and development-specific distribution patterns.[8,9] Hetero- or homo-dimers can be formed by claudins to produce paired strands between adjacent cells determining, the characteristic permeability properties of different epithelial tissues.[10] Claudin-4, a major modulator of tight junctions consists of 209 amino acids and contains four putative transmembrane segments.[11,12] Claudin-4 identifies neoplasms potentially metastasizing to serosal surfaces, while it is usually not expressed in nonmetastatic carcinoma tumors, as claudin-4 is negative in normal mesothelium.[13] Furthermore, claudin-4 in pancreatic cancer in various expression profiling approaches identified; however, the physiological relevance of this finding remains unknown.[14,15]
The inability to separate without dispute the exfoliated atypical benign reactive mesothelium in effusions is the most common difficulty encountered by cytopathologists worldwide, like the lack of a standard, accurate panel of immunomarkers as a diagnostic aid in solving the problem.[16] Furthermore, very few studies have evaluated the combined predictive values of the metastatic carcinoma and mesothelial markers. Therefore, this study was designed to evaluate the diagnostic role of claudin-4 marker to differentiate metastatic carcinoma from reactive mesothelium in pleural and peritoneal fluid cytology specimen.
MATERIALS AND METHODS
This cross-sectional study was conducted between November, 2012 and March, 2013 to assess the diagnostic role of claudin-4 marker to differentiate metastatic carcinoma from reactive mesothelial cells in pleural and peritoneal fluid cytology specimen. A total of 92 inpatient subjects with pleural or peritoneal effusion in “Alzahra” hospital in Isfahan, Iran, were included in the present investigation. Patients of any age in both sexes were eligible if they had neoplastic cells and cellular elements with no equivocal results in primary malignant effusions confirmed by histological examination, recently had no history of chemotherapy and radiation prior to study. Also, exclusion criteria included the lack of agreement between positive and negative controls in immunohistochemical staining with the samples and nonmetastatic carcinoma tumor such as lymphomas, leukemia, and sarcomas. Written informed consent was obtained from all participants and the Institutional Review Board at the Isfahan University of Medical Sciences approved the study.
Specimens were collected from patients and cell block samples were derived as follow: The specimen was centrifuged, to form a pellet, suspended in agar, fixed in neutral buffered formalin and processed as a cell block from which H and E stained sections were cut. At first, H and E stained slides of pleural and peritoneal cytology cell block samples are examined to differentiate carcinoma from reactive mesothelium. Then cytology specimens by histologic confirmation of carcinoma and reactive mesothelial it has been re-cut 3 μm slide and stained for immunohistochemical with claudin-4 marker as follow: Slides were deparaffinized in xylene and graded ethanol, then antigen retrieval was performed in a citrate buffer and endogenous peroxidase activity was blocked with hydrogen peroxide. After added the primary antibody of claudin-4, all sections were incubated with primary antibody at refrigerator for 24 h. After washing, the sections were incubated with biotinylated secondary antibody. Staining were developed using diaminobenzidine “Sigma” and slides were counterstained with modified hematoxylin and blue in 0.3% ammonia water, followed by a tap water rinse. Finally, slides were mounted using an aqueous medium and viewed under a light microscope. The antibody used for immunohistochemical labeling was monoclonal anticlaudin-4 (clone 3E2C1, Zymed Laboratories, San Francisco, CA). Furthermore, the envision plus detection system (DAKO, Carpinteria, CA) was used for claudin-4 antibody, and appropriate positive and negative control samples were used throughout. In the evaluation, membrane-bound reactivity was considered significant, and positive cases were defined when at least >10% of tumor cells were distinctly labeled [Figure 1].
Figure 1.
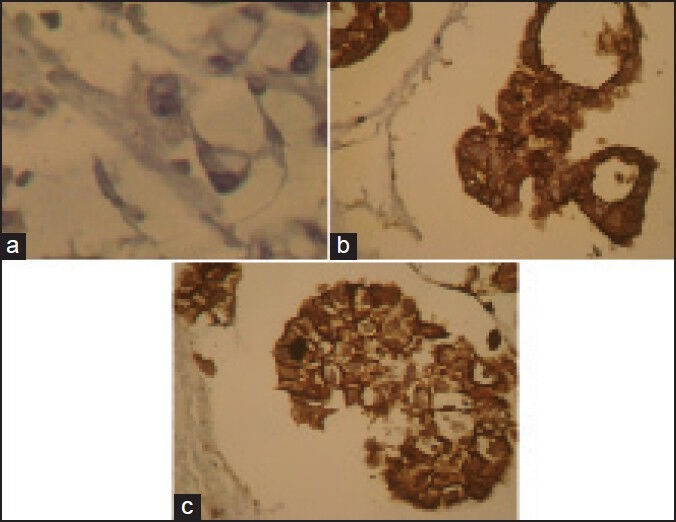
Expression of claudin-4. (a) Negative result of claudin-4 in reactive mesothelial cell (b and c) claudin-4 positivity is obvious in the metastatic carcinoma tumor cells
The sample size was calculated using the comparison of two proportions formula with two-sided log-rank test, α = 0.05, and 90% power (β = 0.10). All were analyzed descriptively using means ± standard deviation for continuous variables and number (%) for categorical variables. Significant differences between metastatic carcinoma tumor cells and reactive mesothelium were measured using the sample t-test and Chi-square test appropriately. Sensitivity, specificity, positive and negative predictive values and negative likelihood ratio were calculated for detecting diagnostic value of claudin-4 in metastatic carcinoma tumor cells and reactive mesothelium. Statistical analysis was carried out with the SPSS, version 20 (SPSS IBM, New York, U.S.A), and differences were considered significant if the P < 0.05.
RESULTS
The mean age of the patients was 59.1 ± 18.5 years old. Forty-one patients (45%) were male and 51 patients (55%) were female. Table 1 shows the comparison of sample characteristics, and the concentration of the site of the tumor and claudin-4 results between patients with metastatic carcinoma tumor cells and patients with reactive mesothelium. As shown, the mean age in patients with metastatic carcinoma tumor cells and patients with reactive mesothelium was not significantly different. The frequency of metastatic carcinoma was higher than the frequency of reactive mesothelium in female patients, while in male patients reactive mesothelium was more frequent than metastatic carcinoma. The difference between the two groups concerning this issue was significant (P = 0.038). Analysis of collected data showed that of 92 studied cases, 53 (58%) were pleural effusion and 39 (42%) were peritoneal effusion. Reactive mesothelium are also much more frequently encountered in the peritoneum than in the pleura, but this difference was not statistically significant (P = 0.38). Claudin-4 protein was detected in 40 metastatic carcinoma cells. This was not detected in the reactive mesothelium, though. Positive staining for claudin-4 was significantly more frequent in metastatic carcinoma than in the reactive mesothelium (P > 0.0001).
Table 1.
Comparison of characteristics between study groups
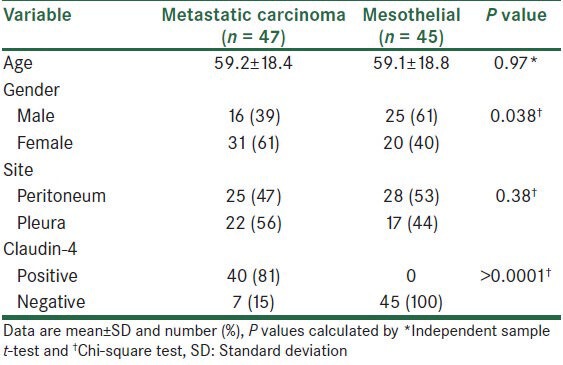
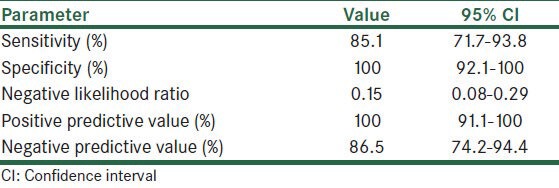
Table 2 shows sensitivity, specificity, negative likelihood ratio, positive and negative predictive value of claudin-4 in making a distinction between metastatic carcinoma and reactive mesothelium. The sensitivity and specificity of claudin-4 to distinguish reactive mesothelium from metastatic carcinoma were 85% (95% confidence interval [CI], 71.1-93.8%) and 100% (95% CI, 91.1-100%), respectively. Furthermore, negative likelihood ratio was 0.15 (95% CI, 0.08-0.29).
Table 2.
Sensitivity, specificity, negative likelihood ratio, and predictive values of claudin-4 in prognostic metastatic carcinoma and reactive mesothelium
DISCUSSION
When a patient is diagnosed with the presence of malignant cells in serous effusions, the irresistible prognostic implications and therapeutic challenges need for refinement of the existing diagnostic procedures.[16] Immunocytochemical techniques have now become widely used in cytopathology for the demonstration of a large number of various antigens in effusion smears.[17] In this study, we assessed the presence of claudin-4 in pleural and peritoneal fluid cytology specimen to differentiate metastatic carcinoma from reactive mesothelial cells. The results have shown that claudin-4 can be found in most of metastatic carcinoma, but this was not observed in none of reactive mesothelium. The sensitivity and specificity of claudin-4 were 85% and 100%, respectively with negative likelihood ratio of 0.15 which showed that claudin-4 can be a good marker to distinguish reactive mesothelium from metastatic carcinoma.
The location and histologic pattern of the tumor and the sex of the patient are among factors that are effective on the selection of the markers to be used in the diagnosis of reactive mesothelium. The pathologist can establish differential diagnosis based on the information.[18]
Evidence in support of a role for claudins as diagnostic markers in neoplastic diseases is rising in recent years. The expression of claudins 1, 2, 3, 4, 5, and 7 were analyzed in 35 reactive mesothelium and 24 metastatic adenocarcinomas of different origins by Soini et al.[19] Authors in this study reported that reactive mesothelium have a lower expression of claudins 1, 3, 4, 5, and 7 than adenocarcinomas, and their expression could thus be used as an adjunct in the differential diagnosis between the two. In another study, Kleinberg et al.[20] analyzed the diagnostic role of claudins in effusion cytology in 325 effusions, including 218 ovarian, 49 breast, 15 cervical or endometrial, 10 gastrointestinal, and 8 lung adenocarcinomas and 25 malignant mesotheliomas, and suggested that claudins can be included in a panel of diagnostic markers for the differential diagnosis of effusion samples, whereas the expression of claudin-3 or claudin-7 is specific for adenocarcinoma and rules out the diagnosis of cells as mesothelial. They also recommended that the absence of claudin-1 expression essentially excludes ovarian carcinoma as the possible origin in metastatic adenocarcinoma. Significantly higher expression of claudins 3, 4, and 6 in ovarian carcinoma compared with diffuse malignant peritoneal mesothelioma effusions was find in Davidson et al.'s study,[21] and confirmed this finding by quantitative real-time polymerase chain reaction for claudin-3 and claudin-4 and immunohistochemical analysis for claudin-3 in a limited set of cases. Also, in Hough et al.'s study[22] claudins 3 and 4 were identified as ovarian cancer — associated molecules based on serial analysis of gene expression technology. Furthermore, Lódi et al.'s study[23] reported that claudin-4 expression was seen in biliary tract adenocarcinomas, but not in hepatocellular carcinoma. Results of these studies reported the role of kinds of claudins in detection of metastatic tumor cells, which were similar to our results about claudin-4.
Data regarding the potential role of claudin-4 is limited; one study by Lonardi et al.[24] was done to recognize the role of junction-associated protein claudin-4 in detection of metastatic tumor cells and the differential with reactive and neoplastic mesothelium. Neoplastic serous effusions obtained from pleura, peritoneum, and pericardium in 345 cases and authors concluded that claudin-4 with high sensitivity (99.1%) and specificity (100%), might be used as an ideal “single-shot” marker for the identification of metastatic carcinoma in serous effusions. In another study, biopsies from 454 tumors were analyzed to evaluate the usefulness of claudin-4 in the diagnosis of mesothelioma and results in this study indicated that claudin-4 reacts with the majority of metastatic carcinoma neoplasms that often metastasize to serous membranes, representing a pancarcinoma marker with extremely high sensitivity and specificity. Furthermore, they suggested that claudin-4 may be considered a primary immunohistochemical reagent to rule out the diagnosis of mesothelioma.[25] In agreement with these studies, results of this study showed that claudin-4 with the sensitivity of 85% and specificity of 100% can be used as a diagnostic marker in metastatic carcinoma.
Our results show that claudin-4 is not observed in reactive mesothelium. Thus, claudin-4 seems to be helpful in making a distinction between metastatic carcinoma and reactive mesothelial cells. However, the low number of samples in this study is the main limitation, and further studies with good sample size are needed to be done to clarify more details in the role of claudin-4 to differentiate metastatic carcinoma from reactive mesothelial cells in pleural and peritoneal fluid cytology specimen.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ensani F, Nematizadeh F, Irvanlou G. Accuracy of immunohistochemistry in evaluation of malignant pleural and peritoneal effusions. Pol J Pathol. 2011;62:95–100. [PubMed] [Google Scholar]
- 2.Thapar M, Mishra RK, Sharma A, Goyal V, Goyal V. Critical analysis of cell block versus smear examination in effusions. J Cytol. 2009;26:60–4. doi: 10.4103/0970-9371.55223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magyarosy E, Martin WJ, Chu EW, Martin SE. Differential diagnostic significance of the paucity of HLA-I antigens on metastatic breast carcinoma cells in effusions. Pathol Oncol Res. 1999;5:32–5. doi: 10.1053/paor.1999.0032. [DOI] [PubMed] [Google Scholar]
- 4.Corson JM, Pinkus GS. Mesothelioma: Profile of keratin proteins and carcinoembryonic antigen: An immunoperoxidase study of 20 cases and comparison with pulmonary adenocarcinomas. Am J Pathol. 1982;108:80–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Said JW, Nash G, Banks-Schlegel S, Sassoon AF, Murakami S, Shintaku IP. Keratin in human lung tumors. Patterns of localization of different-molecular-weight keratin proteins. Am J Pathol. 1983;113:27–32. [PMC free article] [PubMed] [Google Scholar]
- 6.To A, Coleman DV, Dearnaley DP, Ormerod MG, Steele K, Neville AM. Use of antisera to epithelial membrane antigen for the cytodiagnosis of malignancy in serous effusions. J Clin Pathol. 1981;34:1326–32. doi: 10.1136/jcp.34.12.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morin PJ. Claudin proteins in human cancer: Promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–6. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 8.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–93. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 9.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–22. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki H, Matsui C, Furuse K, Mimori-Kiyosue Y, Furuse M, Tsukita S. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc Natl Acad Sci U S A. 2003;100:3971–6. doi: 10.1073/pnas.0630649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Moellic C, Boulkroun S, González-Nunez D, Dublineau I, Cluzeaud F, Fay M, et al. Aldosterone and tight junctions: Modulation of claudin-4 phosphorylation in renal collecting duct cells. Am J Physiol Cell Physiol. 2005;289:C1513–21. doi: 10.1152/ajpcell.00314.2005. [DOI] [PubMed] [Google Scholar]
- 12.Katahira J, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Molecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J Cell Biol. 1997;136:1239–47. doi: 10.1083/jcb.136.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soini Y. Expression of claudins 1, 2, 3, 4, 5 and 7 in various types of tumours. Histopathology. 2005;46:551–60. doi: 10.1111/j.1365-2559.2005.02127.x. [DOI] [PubMed] [Google Scholar]
- 14.Gress TM, Müller-Pillasch F, Geng M, Zimmerhackl F, Zehetner G, Friess H, et al. A pancreatic cancer-specific expression profile. Oncogene. 1996;13:1819–30. [PubMed] [Google Scholar]
- 15.Geng MM, Ellenrieder V, Wallrapp C, Müller-Pillasch F, Sommer G, Adler G, et al. Use of representational difference analysis to study the effect of TGFB on the expression profile of a pancreatic cancer cell line. Genes Chromosomes Cancer. 1999;26:70–9. doi: 10.1002/(sici)1098-2264(199909)26:1<70::aid-gcc10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 16.Murugan P, Siddaraju N, Habeebullah S, Basu D. Immunohistochemical distinction between mesothelial and adenocarcinoma cells in serous effusions: A combination panel-based approach with a brief review of the literature. Indian J Pathol Microbiol. 2009;52:175–81. doi: 10.4103/0377-4929.48910. [DOI] [PubMed] [Google Scholar]
- 17.Shi SR, Shi Y, Taylor CR. Antigen retrieval immunohistochemistry: Review and future prospects in research and diagnosis over two decades. J Histochem Cytochem. 2011;59:13–32. doi: 10.1369/jhc.2010.957191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ordóñez NG. What are the current best immunohistochemical markers for the diagnosis of epithelioid mesothelioma? A review and update. Hum Pathol. 2007;38:1–16. doi: 10.1016/j.humpath.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Soini Y, Kinnula V, Kahlos K, Pääkkö P. Claudins in differential diagnosis between mesothelioma and metastatic adenocarcinoma of the pleura. J Clin Pathol. 2006;59:250–4. doi: 10.1136/jcp.2005.028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinberg L, Holth A, Trope CG, Reich R, Davidson B. Claudin upregulation in ovarian carcinoma effusions is associated with poor survival. Hum Pathol. 2008;39:747–57. doi: 10.1016/j.humpath.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Davidson B, Zhang Z, Kleinberg L, Li M, Flørenes VA, Wang TL, et al. Gene expression signatures differentiate ovarian/peritoneal serous carcinoma from diffuse malignant peritoneal mesothelioma. Clin Cancer Res. 2006;12:5944–50. doi: 10.1158/1078-0432.CCR-06-1059. [DOI] [PubMed] [Google Scholar]
- 22.Hough CD, Sherman-Baust CA, Pizer ES, Montz FJ, Im DD, Rosenshein NB, et al. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000;60:6281–7. [PubMed] [Google Scholar]
- 23.Lódi C, Szabó E, Holczbauer A, Batmunkh E, Szíjártó A, Kupcsulik P, et al. Claudin-4 differentiates biliary tract cancers from hepatocellular carcinomas. Mod Pathol. 2006;19:460–9. doi: 10.1038/modpathol.3800549. [DOI] [PubMed] [Google Scholar]
- 24.Lonardi S, Manera C, Marucci R, Santoro A, Lorenzi L, Facchetti F. Usefulness of Claudin 4 in the cytological diagnosis of serosal effusions. Diagn Cytopathol. 2011;39:313–7. doi: 10.1002/dc.21380. [DOI] [PubMed] [Google Scholar]
- 25.Facchetti F, Lonardi S, Gentili F, Bercich L, Falchetti M, Tardanico R, et al. Claudin 4 identifies a wide spectrum of epithelial neoplasms and represents a very useful marker for carcinoma versus mesothelioma diagnosis in pleural and peritoneal biopsies and effusions. Virchows Arch. 2007;451:669–80. doi: 10.1007/s00428-007-0448-x. [DOI] [PubMed] [Google Scholar]


