Abstract
Background:
Surfactant administration together with nasal Continuous Positive Airway Pressure (nCPAP) administration is considered to be the basis for Newborn's Respiratory Distress Syndrome (RDS) management. This study evaluated the method of directing the surfactant to the lungs in newborns affiliated with RDS through i-gel (i-gel surfactant administration/i-gelSA) compared to the standard care INSURE method, in a clinical trial.
Materials and Methods:
This randomized control trial (RCT) was done on newborns weighing ≥2000 g, with RDS, while being supported with Bubble-CPAP. Newborns, which required FiO2 ≥0.3 under Continuous Distending Pressure (CDP) ≥5 cm H2O for more than 30 minutes to maintain SpO2 in the range of 89 - 95%, were given 100 mg/kg of Survanta. In the interventional group or the i-gelSA (i-gel Surfactant Administration) group, 35 newborns experienced surfactant administration with i-gel and 35 newborns in the control or INSURE group. The average a/APO2 before and after surfactant administration, repeated need for surfactant administration, average nCPAP duration, need for invasive mechanical ventilation, pneumothorax, and the average duration of hospitalization in the Neonatal Intensive Care Unit (NICU) were compared.
Results:
Although the average a/APO2 showed no significant difference before the procedure; in the i-gelSA group, this average was meaningfully higher after the administration of the surfactant (P = 0.001). The other factors showed no significant difference.
Conclusion:
According to the results of this study, the surfactant administration using i-gel was more successful in oxygenation improvement than the INSURE method, and the i-gel method could even be promoted to the standard care position. However, more research is needed in this area.
Keywords: i-gel, INSURE, nCPAP, newborns respiratory distress syndrome
INTRODUCTION
A newborn's RDS is one of the most common reasons for morbidity in premature newborns. Prevalence of this disease decreases as the gestational age increases. In most cases, diagnosis occurs based on the findings from clinical and radiographic trials. Classic clinical demonstrations of the disease include grunting, intercostal and subcostal retractions, nasal flaring, cyanosis, and increase in the need for oxygenation. These symptoms occur shortly after birth. None of the interventions done to manage newborn RDS in the last 20 years has influenced this disease more than surfactant administration. Surfactant administration is undeniably followed by an increase in lung volume, functional residual capacity (FRC) stability, improved ventilation to perfusion ratio, improved oxygenation, and less air leak prevalence.[1]
At the moment nCPAP and surfactant administration are considered to be the basis and the first level of intervention for newborns affiliated with RDS. However, for surfactant administration we need to intubate the newborn and place an ET-tube.[2,3,4]
Undoubtedly, laryngoscope ET-tube placement is one of the most common methods used in the NICU.[5,6,7] Considering the pain and the stress imposed on the newborn by ET-tube placement, and also the point that laryngoscope usage may cause dangerous side effects, such as, severe trauma caused by hypopharyngeal or tracheal perforation, psudodiverticulum, bleeding, mucosa necrosis, vocal cord trauma, laryngeal edema or arytenoid cartilage dislocation, which are intensified if the newborn is awake, alternative solutions are more preferred.[8,9,10,11]
Excessive physical stimulation in the larynx, such as using a laryngoscope, is accompanied with pain and stress in the newborn (after 24 weeks of gestation the newborn feels pain). On the other hand, in newborns less than six month the experience of pain is more severe due to the absence of the pain-reduction nervous pathway.[3,9,12]
Hemodynamic effects caused by the pain during intubation increase the average blood pressure by 33 mmHg and the heart beat rate to 30 beats more than the basic rate. These effects are caused by the release of catecholamine and cortisol, which also induce changes in the Cerebral Blood Flow Velocity (CBFV). These physio-hormonal changes may also cause a sudden decrease of blood pressure and heart beat rate, even as they are accompanied by vagus nerve stimulation during the intubation. We should note that for a newborn that is awake, who can challenge intubation by resistance, these cardiovascular instabilities increase. Nevertheless, these abrupt changes in the heart rate, newborn's blood pressure, and increase in the need for oxygenation (due to the sudden decrease of the functional residual caused by vocal cord impairment) may bring about hypoxic-asphyxia, intraventricular hemorrhage (IVH), and intracranial hemorrhage (ICH).[5,6,9,13] During ET-tube placement, the pressure increase in the anterior fontanel causing Intracranial Pressure (ICP) is observed.[14]
In fact, in order to avoid intubation in the process of surfactant administration in the INSURE method, the development of other methods of surfactant administration such as intra-amniotic surfactant administration for women in danger of pre-term newborn birth, nasopharyngeal surfactant administration for newborns at birth, before the birth of the shoulders, administering surfactant using nebulizers, surfactant administration through a catheter into the trachea in spontaneous breathing or administration by laryngeal mask airway (LMA) is highlighted more.[15]
The laryngeal mask airway provides not only the capability of ventilation, but also a reservoir for gradual administration of the drugs, which can be absorbed through the lungs, by providing a space between the larynx and the mask, which is completely leak-proof through the cuff.[16]
I-gel, is modeled after LMA, with the exception of not having a system to distend the cuff pneumatically, due to the use of a silicon gel combination in its structure, which has the capability of distention and seal with heat (body temperature). This design made the application of i-gel easier and avoided the problem of leakage due to less than desired distention and the excessive distention of the cuff, which could cause compression and ischemia in the tissues and damage in the larynx.[17]
Considering the point that i-gel is categorized as a supraglottic airway device among the respiratory management devices, it meanwhile can direct liquids to the trachea. Therefore, we decided to study the method of directing the surfactant to the lungs in newborns affiliated with RDS through i-gel (i-gel surfactant administration/i-gelSA) compared to the standard care INSURE method, in a clinical trial.
MATERIALS AND METHODS
This study is an RCT done on newborns weighing 2000 g or more, affiliated with RDS, in the neonatal care units of the Shahid Beheshti and Al-Zahra Hospitals, relevant to the Isfahan University of Medical Sciences, from September 2012 to April 2013, after acquiring the Ethics Committee approval (number 391345).
Newborns with RDS symptoms (tachypnea, intercostal retraction, nasal flaring, and grunting) at birth or within 48 hours of birth, who were treated with Bubble CPAP with CDP equal to 5 cm H2O and still required FiO2 ≥0.3 under a CDP ≥5 cm H2O for more than 30 minutes, to maintain SpO2 in the range of 89 - 95% in the right hand, were included in the study. These newborns were given 100 mg/kg of Survanta.[18,19,20]
Newborns with airway abnormalities, cardiothoracic or craniofacial malformations, perinatal asphyxia (five minute Apgar 0 to 3; umbilical cord pH less than 7, and bicarbonate level less than 12 mEq/L), and air-leak syndromes, were excluded from the study.[18]
In the control (INSURE) group, newborns were intubated with the Dual-Lumen endotracheal tube (Portex, Smiths Medical, UK) after discontinuation of the nCPAP, and once their vital signs were stable (auscultation for adequate breathing sounds in both lungs, SpO2 in the range of 89 - 95%), Survanta was administered to them in four divided doses. After each administration, Positive Pressure Ventilation (PPV) was done for at least one minute, to finally finish the intervention in less than 10 minutes[19,21]
In the i-gelSA (intervention) group, the newborns were set in sniffing position after separation from the nCPAP and supraglottic airway (i-gel, Intersurgical, UK) size No. 1 was placed in them. After stabilizing the vital signs, Survanta was administered to them in four divided doses through a 5 Fr catheter into the laryngeal mask space. After each administration, PPV was administered for the newborn for at least one minute, to finally finish the intervention in less than 10 minutes.[19]
If the newborns in both groups needed persistent oxygen concentration of more than 0.4 in order to maintain the oxygen saturation in the desired range, they would receive another dose of Survanta after six hours from the previous administration until the total four administrations were completed for the newborn.[20] Arterial blood gas (ABG) was done on the newborns before and three hours after surfactant administration, to identify the a/APO2.[22]
Occurrence of each of the following criterion meant that the non-invasive respiratory support was discontinued and invasive ventilation was administered:
Need for FiO2 ≥0.7 to maintain oxygen saturation from 89 to 95%[23]
Apnea more than thrice, which needed stimulation and a bag and mask ventilation[24]
Inability to maintain the acceptable ventilation and respiratory failure, which was identified by pH <7.2 and PCO2 >65 mmHg.[25]
During respiratory management, if the newborn needed an FiO2 of less than 50% to maintain the O2 S at in the desired range for more than four hours, CDP decreased in each turn and gradually to 1 to 2 cm H2O and once CDP = 4 cm H2O and FiO2 <30%, the newborn was separated from the nCPAP.[24]
RESULTS
In Table 1, the demographic characteristics of the both the groups are given. According to the findings, the only meaningful difference was related to the five-minute Apgar in both groups (P = 0.004).
Table 1.
Demographic variables distribution among two groups
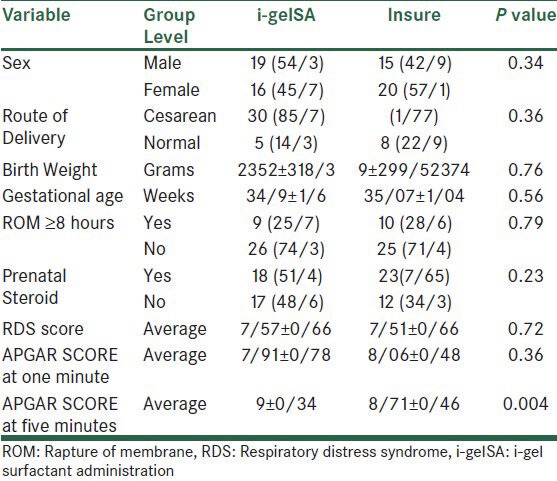
The average postnatal age for surfactant administration in the i-gelSA and INSURE groups was 5/23 ± 1/93 and 5/09 ± 1/92 per hour, respectively, which by using the t-test, showed no significant difference (P = 0.14). The duration of the surfactant administration was also 5/23 ± 0/65 minute and 5/17 ± 0/71 minute, respectively, in the two groups and showed no significant difference using the t-test (P = 0.73).
The mean blood pressure during the intervention in the i-gelSA and INSURE groups was 44/09 ± 4/4 and 43/2 ± 3/7 mmHg, respectively, which using the t-test, showed no significant difference (P = 0.98).
In Table 2, the mean and standard deviation for a/APO2, before and after the intervention, is given for both groups. The t-test showed that the mean a/APO2 before the intervention had no meaningful difference in the two groups (P = 0.39), while after the interventions the mean a/APO2 was higher in the i-gelSA group in such a way that it revealed a significant meaningful difference (P = 0.014). Figure 1 shows the difference of the levels.
Table 2.
The mean and the standard deviation of a/APO2 before and after treatment in both groups

Figure 1.
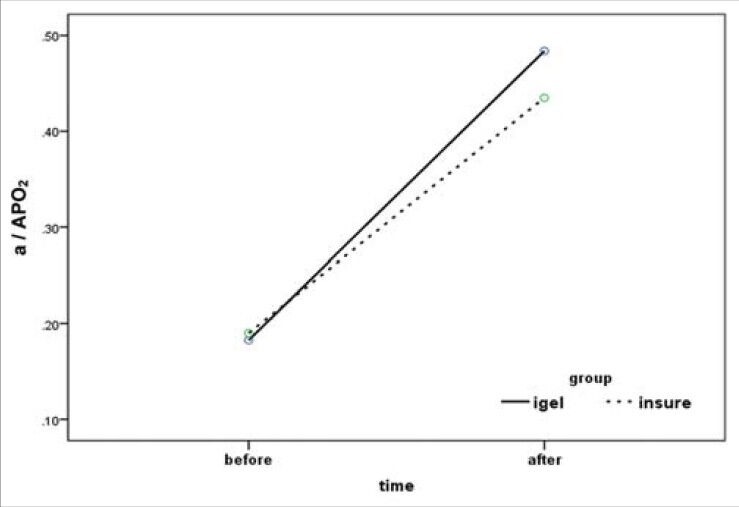
The mean gradient ratio for a / APO2 before and after surfactant administration in both groups
The average duration of non-invasive ventilation in the i-gelSA and INSURE groups was 40/57 ± 29/8 and 52/8 ± 35/4 hours, respectively, which showed no meaningful difference in the t-test (P = 0.12). There was a need for administering the second dose of surfactant in the i-gelSA group for two newborns, while the same need was for three newborns in the INSURE group, but according to the exact Fischer test, it showed no significant difference (P = 0.99). Moreover, the length of stay in the NICU for the i-gelSA and INSURE groups were 5/7 ± 3/5 and 6/3 ± 3/3 days, respectively, which using the exact Fischer test, showed no significant difference (P = 0.48). In Figures 2 and 3, the mean and confidence interval of the duration of non-invasive ventilation and the length of hospitalization are shown in both groups.
Figure 2.
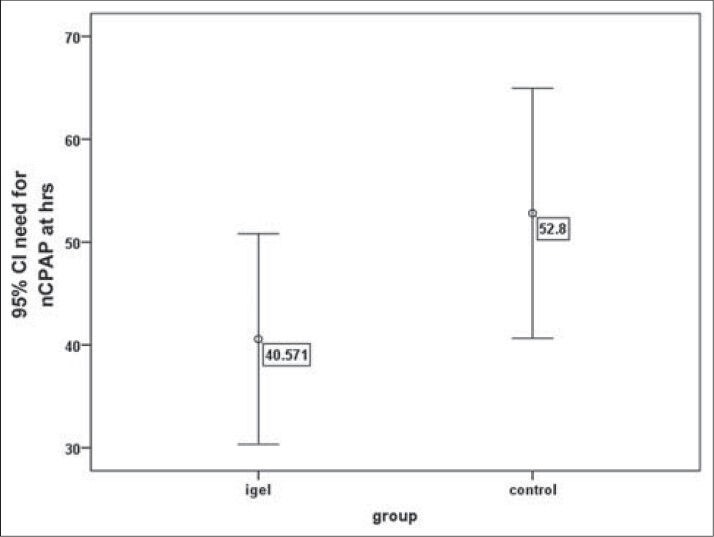
The mean and confidence interval for the duration of the need for non-invasive treatment in both groups
Figure 3.
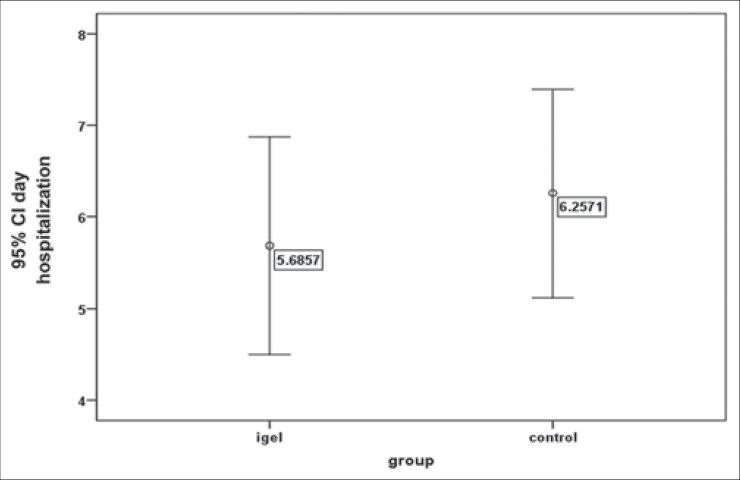
The mean and the confidence interval for the length of hospitalization in both groups
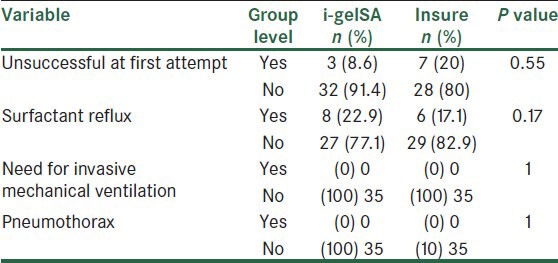
In Table 3, the frequency distribution of unsuccessful first attempts of intervention for i-gel and ET-tube placement are listed and compared, together with their side effects such as surfactant reflux, need for invasive ventilation, pneumothorax, which using the Chi-Square and exact Fischer tests showed no significant difference.
Table 3.
Complication distribution in both groups
DISCUSSION
In a study done in 2004, by Kattwinkel J et al., 23 newborns with gestational ages 23 to 27 weeks, weighing 560 to 1804 g at birth, were administered surfactant. For those born from the vagina, once the newborn's head appeared in the perinea the obstetrician, avoiding the birth of the shoulders, would provide the needed time for the neonatologist to administer 3 to 4.5 ml of surfactant (Infasurf) through a catheter in the posterior of the pharynx. For the newborns who were born via the Cesarean section, this process would start once the head of the newborn became visible in the cut area, and at the time that birth was allowed to be completed, CPAP with a pressure of 10 cm H2O was administered with a mask. Thirteen out of 15 vaginal-born newborns and three out of eight Cesarean born newborns needed no more respiratory support; however, other newborns were treated by nCPAP.[26]
In a study done in 2000, by Berggren E et al., 32 newborns affiliated with RDS, with gestational ages 27 to 34 weeks in both groups, with each group including 16 newborns, were treated with CPAP and CPAP with surfactant administration by nebulizer. This study could not achieve significant meaningful differences regarding the prevalence of invasive mechanical ventilation, patent ductus arteriosus (PDA), intra-ventricular hemorrhage (IVH), air leak or chronic lung disease (CLD) in both groups.[27]
For surfactant administration using the Aerosolized technique, a study was done by Finer NN et. al. in 2006 in which 17 newborns with gestational ages between 29 and 32 weeks were treated by CPAP and nebulizer surfactant, due to demonstration of RDS symptoms. In the mentioned study, the administration was based on the Aerosurf and Lucinactant systems. No mortality was reported for the newborns and the air leak syndrome and necrotizing enterocolitis were never reported. Thirteen newborns needed no more supplemental oxygen after 28 days and just four newborns showed the criterion of CLD.[28]
In a study done by Zhang JP in 2004, which aimed to study the intra-amniotic surfactant administration to prevent RDS syndromes, 15 out of 45 mothers exposed to pre-term delivery were treated with intra-amniotic surfactant and 30 cases of birth were considered as the control. RDS prevalence was statistically and meaningfully higher in the control group.[29]
In the case of surfactant administration through a catheter placed in the trachea, a study was done in 2007, by Kribs A et al., who supported 29 newborns in the Koln University Pediatric Hospital, aged between 23 and 27 gestational weeks, with nCPAP by the Infant Flow Driver (IFD) (EME, Brighton, UK), for a period of 13 months. If the newborns needed FiO2 ≥40% to maintain SpO2 in the range of 85 to 93%, they were treated with surfactant through a catheter in the trachea. To do this intervention, first 0.0025 mg/kg Atropin was administered IV and then they placed the head of the newborn in a intubation-like position, while a 4 F feeding tube with just a hole at the end was attached to a syringe containing 100 mg/kg Survanta, indicated at 1.5 cm from the end, and this was placed in the trachea using a Magill forceps through the laryngoscope in a way that the indicator was situated exactly at the vocal cords. Then the catheter was stabilized with the right hand fingers and the laryngoscope was extracted. Finally the surfactant was administered gradually, in one to three minutes These newborns were then statistically compared with the 34 newborns (control group) with a gestational age of 25 weeks, who were managed with the same care system, but the surfactant administration system was of the INSURE type, according to Necrotizing enterocolitis (NEC), pulmonary interstitial emphysema (PIE), CLD, periventricular leukomalacia (PVL), IVH, and retinopathy of prematurity (ROP). The Prevalence of IVH (Grade III and V) and PIE showed a meaningful increase in the control group.[18]
In a case report in 2004, by Brimacombe J et al., newborns weighing 1360 g and 3200 g, diagnosed as affiliated with RDS, were treated with surfactant administration through LMA while spontaneously breathing, and he reported that there were minimal fluctuations in cardiovascular and respiratory criteria in this treatment rather than INSURE, and concluded that this intervention can have less side-effects such as intracranial hemorrhage.[30]
In a study by Trevisanuto D et al., eight preterm newborns, with an average gestational age of 31 weeks, affiliated with RDS, were treated with surfactant through LMA. In this study, Trevisanuto D reported a significant difference, with an increase in the a/APO2, three hours after administration of the surfactant, rather than before the administration, in the newborns studied.[22]
Studies with a contrastive approach, done in the field of surfactant administration methods are limited; however, with this limited number of researches, both this study and the one by Brimacombe J show that surfactant administration through LMA is followed by minimal fluctuations in the cardiovascular criteria. Moreover, the increase in a/APO2 after surfactant administration through LMA, in this study and the one by Trevisanuto, is significant. On the other hand, even in this limited study, the prevalence of side effects compared between the two systems show the safety of this intervention, which can be challenged in further studies. Overall, considering the high efficacy of surfactant administration (which is shown in the changes of a/APO2 before and after the administration of the surfactant in the i-gel device), further studies to promote the standard care for the administration of the surfactant seems logical.
This research is only funded by the Isfahan University Vice-Chancellery for Research, and there were no conflict of interests throughout the research process.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hamvas A. Martin RJ, Fanaroff AA, Walsh MC. Fanaroff and Martin's Neonatal- Perinatal Medicine. 9th ed. St. Louis: Elsevier Mosby; 2011. Pathophysiology and management of respiratory distress syndrome; pp. 1106–11. [Google Scholar]
- 2.Soll RF, Morley CJ. Prophylactic versus selective use of surfsctant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2001;(2):CD000510. doi: 10.1002/14651858.CD000510. [DOI] [PubMed] [Google Scholar]
- 3.Dani C, Bertini G, Pezzati M, Cecchi A, Caviglioli C, Rubaltelli FF. Early extubation and nasal continuous positive airway pressure after surfactant treatment for respiratory distress syndrome among preterm infants <30 weeks gestation. Pediaterics. 2004;113:e560–3. doi: 10.1542/peds.113.6.e560. [DOI] [PubMed] [Google Scholar]
- 4.Booth C, Premkumar H, Yannoulis A, Thomson M, Harrison M, Edwards AD. Sustainable use of continuous positive airway pressure in extremely preterm infants during the first week after delivery. Arch Dis Child Fetal Neonatal Ed. 2006;91:F398–402. doi: 10.1136/adc.2005.092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan HP, Zurich NJ, Wolf AR. Should we reconsider awake neonatal intubation? Pediatr Anesth. 2001;11:135–45. doi: 10.1046/j.1460-9592.2001.00535.x. [DOI] [PubMed] [Google Scholar]
- 6.Vanlooy JW, Schumacher RE, Bhatt-Mehta V. Efficacy of premedication algorithm for nonemergent intubation in a neonatal intensive care unit. Ann Pharmacother. 2008;42:947–55. doi: 10.1345/aph.1K665. [DOI] [PubMed] [Google Scholar]
- 7.Kummar P, Denson SE, Mancuso TJ Committee on Fetus and Newborn, Section on Anesthesiology and Pain Medicine. Clinical report-premedication for nonemergency endotracheal intubation in the neonate. Pediatrics. 2010;125:608–15. doi: 10.1542/peds.2009-2863. [DOI] [PubMed] [Google Scholar]
- 8.Carbajal RI, Gall O, Annequin D. Pain management in neonates. Expert Rev Neurother. 2004;4:491–505. doi: 10.1586/14737175.4.3.491. [DOI] [PubMed] [Google Scholar]
- 9.Bartman T, Becker D. California, United States: UCSF (University of California, San Francisco); 2004. Intensive care nursery house staff manual; pp. 147–15. [Google Scholar]
- 10.Mhairi G, Jayashree R. 4th ed. Netherlands: Wolters Kluwer; 2007. Atlas of procedure in neonatology; pp. 251–2. [Google Scholar]
- 11.Crawford MW, Hayes J, Tan JM. Dose-response of remifentanil for tracheal intubation in infants. Anesth Analg. 2005;100:1599–604. doi: 10.1213/01.ANE.0000150940.57369.B5. [DOI] [PubMed] [Google Scholar]
- 12.Anand KJ. Pharamacological approaches to the management of pain in the neonatal intensive care unit. J Perinatol. 2007;27:S4–11. doi: 10.1038/sj.jp.7211712. [DOI] [PubMed] [Google Scholar]
- 13.Perlaman JM, Mcmenamin JB. Fluctuating cerebral blood flow velocity in respiratory distress synd. Relation to the developmental of IVH. N Engl J Med. 1983;309:204–9. doi: 10.1056/NEJM198307283090402. [DOI] [PubMed] [Google Scholar]
- 14.Stow PJ, Mcleod ME, Burrows FA, Creighton RE. Anterior fontanel pressure responses in the awake and anaesthetized infant. Br J Anaesth. 1988;60:167–70. doi: 10.1093/bja/60.2.167. [DOI] [PubMed] [Google Scholar]
- 15.Gizzi C, Papoff P, Barbara CS, Cangiano G, Midulla F, Moretti C. Old and new uses of surfactant. J Matern Fetal Neonatal Med. 2010;23:41–4. doi: 10.3109/14767058.2010.509912. [DOI] [PubMed] [Google Scholar]
- 16.Archibald IJ. Brain, Laryngeal mask airway device with drug delivery means. 2010. [Application 20100089393 Filed on 2007 Dec 14, published on 2010 Apr 15]. Available from: http://www.patentstorm.us . Available from: http: www.patentstorm.us/applications/20100089393/description.html [Last accessed on 2013 Jun. 15]
- 17.Intersurgical Ltd., i-gel supraglottic airway; 2007. [Last accessed on 2013 Jun. 15]. Available from: http://www.i-gel.com . [© 2013 Intersurgical Ltd. All Rights Reserved]. Available from: http://www.intersurgical. com/products/i-gel-supraglottic-airway, http://www.i-gel.com. [Last accessed on 2013 Jun 15] [Google Scholar]
- 18.Kribs A, Pillekamp F, Hunseler C, Vierzig A, Roth B. Early administration of surfactant in spontaneous breathing with nCPAP: Feasibility and outcome in extremely premature infants (postmenstrual age ≤27 weeks) Pediatr Anesth. 2007;17:364–9. doi: 10.1111/j.1460-9592.2006.02126.x. [DOI] [PubMed] [Google Scholar]
- 19.Albany Medical College: Randomized controlled trial of surfactant delivery via Laryngeal Mask Airway (LMA) Versus endotracheal intubation. 2010. [Last accessed on 2013 Jun. 15]. Available from: http://www.clinicaltrials.gov . Available from: http://www.clinicaltrials.gov/archive/NCT01042600 . [Last accessed on 2010 Jan 04, and updated on 2013 Apr 8]
- 20.Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev. 2007;(4):CD003063. doi: 10.1002/14651858.CD003063.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suresh GK, Soll RF. Pharmacologic adjunct II: Exogenous surfactants. In: Goldsmith JP, Karotkin EH, editors. Assisted ventilation of the neonate. St. Louis. Missouri: Elsevire; 2011. pp. 377–9. [Google Scholar]
- 22.Trevisanuto D, Grazzina N, Ferrarese P, Micaglio M, Vergehese C, Zanardo V. Laryngeal mask airway used as a delivery conduit for the administration of surfactant to preterm infants with respiratory distress syndrome. Biol Neonate. 2005;87:217–20. doi: 10.1159/000083370. [DOI] [PubMed] [Google Scholar]
- 23.Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA, et al. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: A Randomized Controlled Trial. Pediatrics. 2009;123:137–42. doi: 10.1542/peds.2007-3501. [DOI] [PubMed] [Google Scholar]
- 24.Mazella M, Bellini C, Calevo MG, Campone F, Massocco D, Mezzano P, et al. A randomized control study comparing the Infant Flow Driver with nasal continuous positive airway pressure in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2001;85:F86–90. doi: 10.1136/fn.85.2.F86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lista G, Castoldi F, Fontana P, Daniele I, Cavigioli F, Rossi S, et al. Nasal CPAP Vs Bi-level Nasal CPAP in preterms with RDS: A randomized control study. Arch Dis Child Fetal Neonatal Ed. 2010;95:F85–9. doi: 10.1136/adc.2009.169219. [DOI] [PubMed] [Google Scholar]
- 26.Kattwinkel J, Robinson M, Bloom BT, Delmore P, Ferguson JE. Technique for intrapartum administration of surfactant without requirement for an endotracheal tube. J Perinatol. 2004;24:360–5. doi: 10.1038/sj.jp.7211103. [DOI] [PubMed] [Google Scholar]
- 27.Berggren E, Liljedahl M, Winbladh B, Andreasson B, Curstedt T, Robertson B, et al. Pilot study of nebulized surfactant therapy for neonatal respiratory distress syndrome. Acta Paediatr. 2000;89:460–4. doi: 10.1080/080352500750028195. [DOI] [PubMed] [Google Scholar]
- 28.Finer NN, Merritt TA, Bernstein G, Job L, Mazela J, Liu G. A multicenter pilot study of Aerosurf delivered via nasal continuous positive airway pressure to prevent respiratory distress syndrome in preterm neonates. Pediatr Res. 2006;59:40–8. [Google Scholar]
- 29.Zhang JP, Wang YL, Wang YH, Zhang R, Chen H, Su HB. Prophylaxis of neonatal respiratory distress syndrome by intra-amniotic administration of pulmonary surfactant. Chin Med J (Engl) 2004;117:120–4. [PubMed] [Google Scholar]
- 30.Brimacombe J, Gandini D, Keller C. The laryngeal mask airway for administration of surfactant in two neonates with respiratory distress syndrome. Pediatr Anesth. 2004;14:188–90. doi: 10.1046/j.1460-9592.2003.01173.x. [DOI] [PubMed] [Google Scholar]


