Abstract
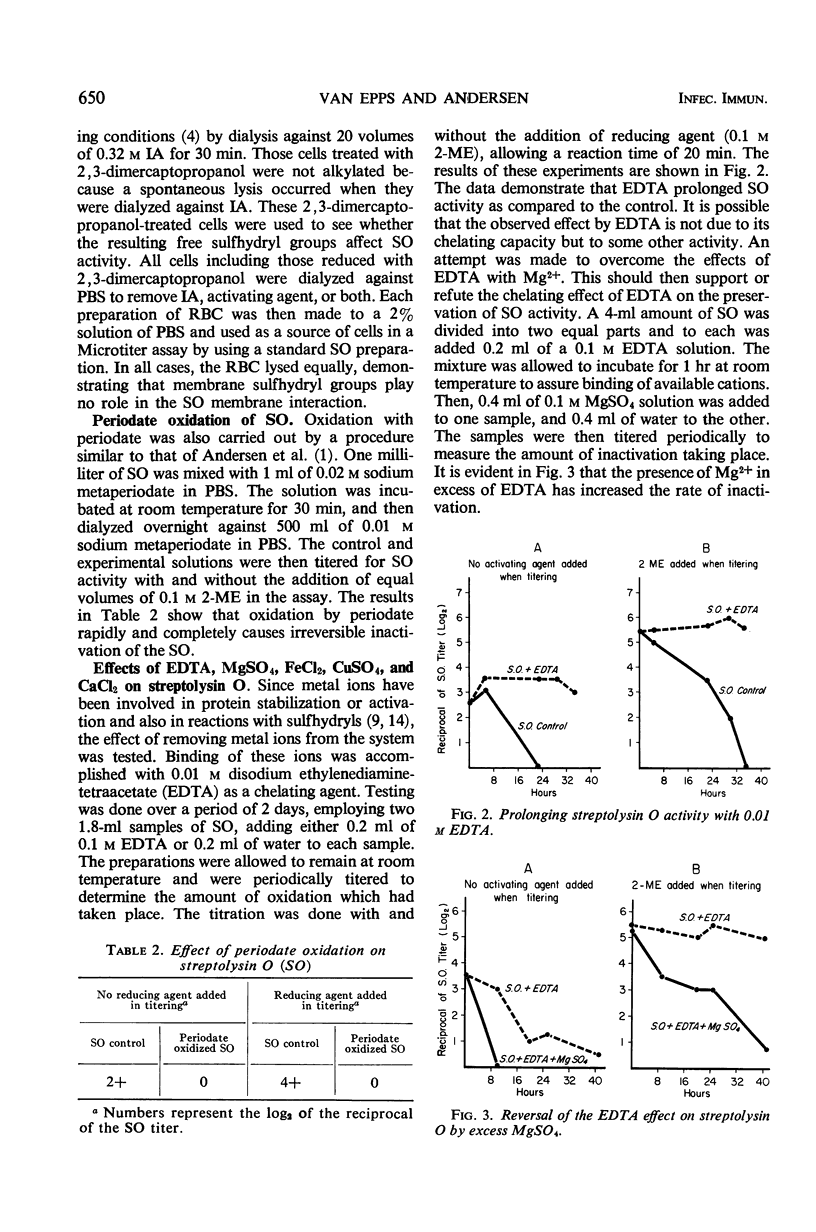
Streptolysin O (SO), a group A streptococcal toxin, exists in two forms, a reduced active state and an oxidized reversibly inactive state. Activity is measured by red blood cell hemolysis. SO is a labile toxin, and, with time, activity is irreversibly lost. The rate of activity loss is slowed by incubation with 0.1 m 2-mercaptoethanol or 0.01 m ethylenediaminetetraacetic acid (EDTA). The effect of EDTA can be reversed by excess MgSO4. Reversibly oxidized SO is activated by cleavage of disulfide bonds. When the free sulfhydryl groups of the active SO are alkylated with iodoacetamide, complete and irreversible loss of activity results. Periodate (0.01 m) oxidation also causes complete loss of activity which may be due to oxidation of sulfhydryl groups. SO in the active form reacts with Fe2+, Cu2+, Ca2+, and Mg2+, causing loss of activity in various degrees depending on the ions and the concentration used. Ferric and cupric ions are most effective and cause loss of activity at concentrations on the order of 10−4m. The reversibly oxidized form of SO is not influenced by exposure to cupric ions (0.01 m), indicating that the reaction is only with the active form of SO, probably involving the free sulfhydryl groups. These groups may be responsible for the direct binding of the toxin to the target membrane or for the maintenance of the proper conformation for activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. R., Abele D. C., Vannier W. E. Effects of mild eriodate oxidation on antibodies. J Immunol. 1966 Dec;97(6):913–924. [PubMed] [Google Scholar]
- BERNHEIMER A. W., DAVIDSON M. LYSIS OF PLEUROPNEUMONIA-LIKE ORGANISMS BY STAPHYLOCOCCAL AND STREPTOCOCCAL TOXINS. Science. 1965 May 28;148(3674):1229–1231. doi: 10.1126/science.148.3674.1229. [DOI] [PubMed] [Google Scholar]
- BIANCHI M. R., BOCCACCI M., MISITI-DORELLO P., QUINTILIANI M. FURTHER OBSERVATIONS ON IN VITRO RADIOSENSITIZATION OF RABBIT ERYTHROCYTES BY IODOACETIC ACID AND RELATED SUBSTANCES. Int J Radiat Biol Relat Stud Phys Chem Med. 1964;8:329–342. doi: 10.1080/09553006414550391. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W. Cytolytic toxins of bacterial origin. The nature and properties of cytolytic proteins are discussed with emphasis on staphylococcal alpha-toxin. Science. 1968 Feb 23;159(3817):847–851. doi: 10.1126/science.159.3817.847. [DOI] [PubMed] [Google Scholar]
- CLAMP J. R., HOUGH L. THE PERIODATE OXIDATION OF AMINO ACIDS WITH REFERENCE TO STUDIES ON GLYCOPROTEINS. Biochem J. 1965 Jan;94:17–24. doi: 10.1042/bj0940017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp J. R., Hough L. Some observations on the periodate oxidation of amino compounds. Biochem J. 1966 Oct;101(1):120–126. doi: 10.1042/bj1010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALBERT S. P., BIRCHER R., DAHLE E. The analysis of streptococcal infections. V. Cardiotoxicity of streptolysin O for rabbits in vivo. J Exp Med. 1961 Apr 1;113:759–784. doi: 10.1084/jem.113.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert D., Todd E. W. Purification and properties of a haemolysin produced by group A haemolytic streptococci (streptolysin O). Biochem J. 1941 Nov;35(10-11):1124–1139. doi: 10.1042/bj0351124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S., Rebeyrotte P., Halpern B., Besluau D. Kinetics of streptolysin O reactivation by different thiols. Biochim Biophys Acta. 1969 May 6;177(3):658–660. doi: 10.1016/0304-4165(69)90336-5. [DOI] [PubMed] [Google Scholar]
- Reitz B. A., Prager D. J., Feigen G. A. An analysis of the toxic actions of purified streptolysin O on the isolated heart and separate cardiac tissues of the guinea pig. J Exp Med. 1968 Dec 1;128(6):1401–1424. doi: 10.1084/jem.128.6.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWAB J. H., WATSON D. W., CROMARTIE W. J. Further studies of group A streptococcal factors with lethal and cardiotoxic properties. J Infect Dis. 1955 Jan-Feb;96(1):14–18. doi: 10.1093/infdis/96.1.14. [DOI] [PubMed] [Google Scholar]
- Thompson A., Halbert S. P., Smith U. The toxicity of streptolysin O for beating mammalian heart cells in tissue culture. J Exp Med. 1970 Apr 1;131(4):745–763. doi: 10.1084/jem.131.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Andersen B. R. Streptolysin O: sedimentation coefficient and molecular weight determinations. J Bacteriol. 1969 Oct;100(1):526–527. doi: 10.1128/jb.100.1.526-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



