Abstract
Purpose
The attentional visual field (AVF), which describes a person’s ability to divide attention and extract visual information from the visual field (VF) within a glance, has been shown to be a good predictor of driving performance. Despite this, very little is known about the shape of the AVF and the factors that affect it. The aims of this study were to describe the AVF in a large sample of older drivers and identify demographic, cognitive, and vision factors associated with AVF performance and shape.
Methods
Registered drivers aged between 67 – 87 years, residing in Greater Salisbury, MD, were recruited to participate in the study. Participants underwent a battery of visual and cognitive assessments and completed various questionnaires for demographic, medical history and depression information. The AVF was assessed using a divided attention protocol within the central 20° radius along the four principal meridians. The shape of the AVF was classified as either symmetric or one of two asymmetric shape profiles.
Results
Symmetrically shaped AVFs were found in just 34% of participants. AVF performance was significantly better along the horizontal (15.3°) than vertical (11.3°) meridian (p<0.05). After adjusting for AVF area, we found that poorer cognitive and vision performance was associated with a symmetric AVF shape. Overall AVF extent was predicted from vision and cognitive measures as well as with various demographic factors.
Conclusions
Good vision and cognitive abilities appear to be associated with having an asymmetric as opposed to a symmetric AVF shape profile.
INTRODUCTION
The attentional visual field (AVF) refers to the size of the visual field (VF) over which a person can effectively divide their attention and extract visual information within a glance. The AVF is typically estimated by measuring the most eccentric position or the processing speed at a set eccentric position, that a person can simultaneously process visual information from both central and peripheral VF locations. Previous studies have linked the AVF with performance on a variety of everyday activities such as mobility 1, 2, driving performance 3–6 including crash rate 7, 8 and other general activities of daily living such as reading food cans and using a screwdriver and touch-tone telephone 9.
The size or extent of the AVF however is dependent on the test parameters or display factors used to measure it. Specifically, the size of the AVF has been shown to decrease with an increase in the level of difficulty of the central task 10, with the presence of peripheral distractors 10–13, with the presence of backward masking 14 and when peripheral distractors are flashed briefly as opposed to a constant display 14. Additionally, the size of the AVF decreases with age 10, 11, 13–16, eccentricity 10–14, 16 but can be improved with training 10, 11, 16.
While the measurement parameters affecting the size of the AVF have been well documented, characteristics about the shape of the AVF are less known. For example, is the AVF symmetrical about fixation or does AVF extent differ with meridian? How do losses in either vision and/or cognitive function affect the shape of the AVF? Knowing the shape of a person’s AVF may provide useful information for predicting how well a person is able to perform a certain task. Given that losses in the inferior VF have been linked to deficits in mobility performance 17 and driving cessation 18, one may expect that constriction of the AVF in the inferior hemi-field will also have a significant impact on driving and mobility performance since both these tasks also utilize the inferior AVF.
In the majority of previous AVF studies, performance at each eccentricity has simply been averaged across the different meridians (typically the 8 principal meridians). Sekuler and Ball 11 and Ball et al. 10 reported that performance did not vary from one meridian to the next, although they did not provide any supporting data or statistical analyses. Seiple et al. 14 did not find a significant interaction between meridian and eccentricity. The findings from these earlier studies however differ from those of more recent studies which have reported differences in the extent of the AVF between the horizontal and vertical meridians 19–21.
Given the inconsistent findings of previous research about the shape of the AVF, the aims of this study were to: (1) describe the AVF along the four principal meridians in a large population of older drivers; (2) identify demographic, cognitive, and vision factors associated with AVF performance; and (3) explore factors associated with anisotropy seen in AVF extent.
METHODS
Population
The Salisbury Eye Evaluation Driving Study (SEEDS) is a longitudinal study of vision, cognition and driving behavior of drivers aged between 67and 87 years living in the Greater Salisbury Metropolitan Area. Using the Maryland Department of Motor Vehicles roster of registered drivers aged between 67 and 87 years as of June 1 2005, postcards were mailed to all eligible drivers residing in Wicomico County, Maryland. From the 1425 participants enrolled in the study, reliable AVF test results were obtained in 1386 participants (97%) and their results are included in this report (Table 1). AVF results deemed as unreliable or inaccurate (ie. incomplete AVF results from participants due to illness, refusal to participate or inability to follow instructions) were excluded from all data analyses.
Table 1.
Characteristics of the Population Completing the Attentional VF Assessment
| Characteristic | N | Statistic |
|---|---|---|
| Demographics | ||
| Age in years (mean (SD), IQR*)) | 1386 | 76.0 (5.2), 9.0 |
| Race | ||
| Blacks | 173 | 12.5% |
| Whites | 1206 | 87.0% |
| Other | 7 | 0.5% |
| Gender | ||
| Males | 686 | 49.5% |
| Females | 700 | 50.5% |
| Years of education (mean (SD), IQR)) | 1386 | 13.5 (2.6), 4.0 |
| Cognitive function | ||
| Minimental score (mean (SD), IQR)) | 1386 | 28.3 (1.8), 2.0 |
| Brief test of attention score (mean (SD), IQR)) | 1381 | 6.5 (2.5), 4.0 |
| Trails B (time to complete in seconds) (mean (SD), IQR)) | 1366 | 127.6 (73.6), 64.0 |
| Visual function | ||
| Binocular presenting acuity (mean (SD), IQR)) | 1386 | −0.01 (0.11), 0.16 |
| Contrast Sensitivity (better eye) (mean (SD), IQR)) | 1386 | 35.2 (2.3), 2.0 |
| Bilateral visual field (# of either missed or initially undetected points within central 22° VF) (mean (SD), IQR)) | 1376 | 0.33 (1.8), 0 |
SD=standard deviation, IQR = inter-quartile range
Data for this report were collected in round 1 of the study (July 2005 – June 2006). Basic demographic data such as age, gender, race, level of education and current medication use were obtained using structured questionnaires administered at the participant’s home. Information about current and past history of specific co-morbidities such as arthritis, stroke, heart disease, and depression were derived through structured medical history questionnaires and the Geriatric Depression Scale questionnaire.
The research followed the tenets of the Declaration of Helsinki, and informed consent was obtained from subjects following explanation of the nature and possible consequences of the study. The Johns Hopkins Medical Institutions’ review board approved the research.
Procedure
Following the home interview, subjects underwent cognitive and visual assessments at a central examination site by trained technicians. The cognitive and visual assessments used in this study were selected because of their association to driving performance in other studies 22–25.
Cognitive Measures
General cognitive status was assessed using the standardized Mini-Mental State Examination (MMSE)26 which was administered in the usual manner. Research by Marottoli et al. 25 has shown that the MMSE is related to self-reported driving performance.
The Brief Test of Attention (BTA)27, which has been shown to be linked to motor vehicle crashes among older individuals 24, was used to assess the cognitive domain of auditory divided attention. In this task, subjects were required to focus their attention on a specified stimulus set and use their working memory to maintain it while withstanding distraction. Subjects were instructed to listen to a tape-recorded list of 20 strings of inter-mixed numbers and letters, (eg. “T-6-1-A-6-T”), that contained between 4 and 18 items. After listening to each string, subjects reported the number of letters (part 1) and numbers (part 2) that they heard and scored based on the number of correct responses.
The Trail Making Test, Part B (Trails B), which has been shown to predict both motor vehicle collisions and on-road driving performance 22, 23, was used to assess executive cognitive function requiring psychomotor speed, visual search and attention. In this paper-based task, subjects were required to consecutively connect labeled circles that alternated between numbers (1 – 13) and letters (A – L), as quickly as possible. The number of seconds participants took to complete this task was recorded, with a maximum timeout score of 480 seconds.
Vision Measures
All vision assessments were done with the participant’s habitual correction if normally worn for driving although for the VF and AVF assessments, participants were optically corrected for the shorter test distances. Binocular visual acuity (VA) was measured using a high contrast ETDRS acuity chart 28 that was trans-illuminated, using a Lighthouse Light box, to the recommended chart luminance of 85 – 120 cdm−2. A strict forced–choice procedure was used by forcing participants to guess letters until they missed at least 4 out of 5 letters in a row. Binocular threshold-VA was determined by assigning each correctly identified letter with a value of −0.02 log MAR 29.
Binocular and monocular contrast sensitivity (CS) were assessed using the Pelli-Robson letter contrast sensitivity chart 30 which was luminated to the recommended chart luminance of 85 cdm−2 31. A forced-choice procedure was used to determine the threshold CS score by forcing participants to guess letters until 2 out of 3 letters within a triplet were read incorrectly. Threshold CS was scored by assigning each correctly identified letter a value of 0.05 log CS. A call of “O” for “C” was accepted as correct 32, 33. Monocular CS measurements always preceded binocular CS assessment and the order of the charts and eyes assessed were counter-balanced across subjects.
Monocular VFs in both eyes were measured in each participant using the 81-point, quantify defect screening test strategy on the Humphrey Field Analyzer (HFA). This automated VF test assesses the static VF over a 60° radius VF using a Goldmann III white target against a background luminance of 31.5 apostilb or 10 cdm−2. The quantify defect screening strategy initially presents light stimuli that are 6 dB brighter than the expected hill of vision based on the subject’s age. If the subject does not detect this light, a 4–2 staircase procedure is used to determine a VF threshold value. As is standard procedure, participants were optically corrected during the central (30°) VF testing, but not for the peripheral (from 30° to 60°) VF assessment 34.
For this report, the VF results of the two eyes were combined using the algorithm of Nelson-Quigg et al. 35 to create a binocular VF plot consisting of 96 test points. The number of missed points (defects) in the binocular field within the central 20° VF was tallied for each participant and used for analyses.
The AVF was assessed binocularly using a custom-written program that was modeled after the work of Sekuler et al 15. The commercially available Vision Attention Analyzer (Visual Resources) was not utilized in this study because it assesses processing speed and only measures divided attention out to a maximum eccentricity of 15° radius. The custom program developed for this study assessed the AVF extent out to 20° radius in a divided attention protocol.
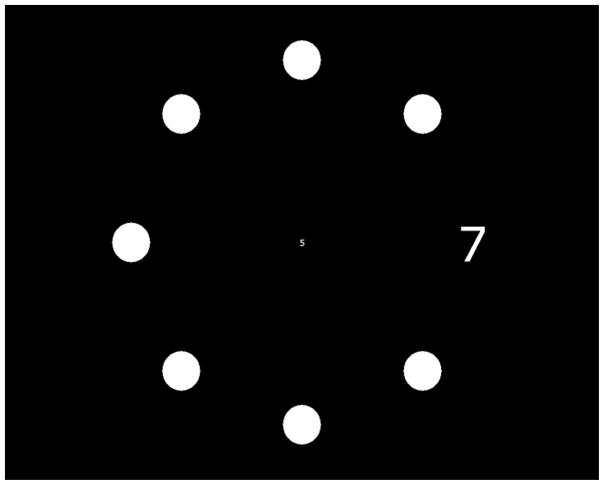
For this test, participants were seated 35cm in front of a touch-screen monitor and optically corrected for the test distance. Participants were instructed to fixate on a circular target positioned in the center of the monitor. Two numbers were simultaneously presented for a brief period of time (250 ms) (Figure 1). One number was located at the center of the screen and subtended a visual angle of 0.8°. The other (peripheral target) was located along one of four possible meridians (0°, 90°, 180° and 270°) eccentric to the central target (Figure 1). The visual angle subtended by the eccentric number was scaled in size using the equation of Anstis 36 to ensure equal legibility of numbers with increasing eccentricity. At the same time the targets were presented, seven filled circles (distractors) were presented at the same eccentricity and same size as the peripheral target (Figure 1). The distractors and peripheral target were arranged into 8 evenly spaced radial spokes. Following the presentation of the targets and distractors, a masking pattern appeared. The numbers presented were chosen randomly between 0 and 9 and both the targets and the distracters were white presented against a black background. The short display duration of the test stimuli minimized the likelihood of subjects making eye movements during the presentation. Subjects were required to report out loud the central and peripheral target numbers (which were entered into a computer by the experimenter) and to indicate the location of the peripheral target by touching the touch-screen monitor at its location.
Figure 1.
Sample Screen Display of a Single Trial on the Attentional Visual Field Task
We used a Parameter Estimation by Sequential Testing (PEST) procedure 37 to determine the eccentricity of the peripheral target on each trial for each meridian. Throughout the test session, four PEST algorithms, one for each meridian, were randomly interleaved. For a response to be correct, the numbers for both the central and peripheral targets and the location of the peripheral target had to be correctly identified. To reduce participant burden, the PEST was limited to a total of 24 trials; 6 trials in each of the 4 meridians. The last value calculated by the PEST procedure was used as the final estimate of the subject’s AVF for that meridian. There is a trade-off in using a fixed number of trials versus a fixed variance level. We chose to fix the number of trials to minimize the participant’s testing time and level of fatigue. It is acknowledged that by fixing the number of trials there may be increased uncertainty among the participants’ AVF thresholds compared to allowing the PEST to run until a fixed estimate of the final AVF variance had been attained.
Data Analysis
To identify demographic, cognitive, and vision factors associated with AVF, a linear regression model was created. The model included pre-selected demographic (age, race, gender education, depression), cognitive (MMSE, BTA, Trails B), and visual function (VA, CS in the better eye, and bilateral VF) measures. Variance Inflation Factors (VIF) for this model were also calculated to assess for collinearity between the vision and cognitive measures.
To assess the shape of each subject’s AVF, the ratio between the horizontal and vertical AVF extent was computed for each participant. With this method of scoring, a ratio of 1.0 represented a symmetric AVF in which the extents of the AVF along the horizontal and vertical meridians were the same. Shape ratios between 0.8 and 1.2 were considered to represent a ‘symmetric’ AVF while shape ratios outside of this range were considered to be ‘asymmetric’. The cut-off values used to classify the AVF shape profiles represented a 20% cut-off on either side of the symmetrical shape ratio of 1.0. This cut-off value was selected to ensure that each AVF shape category had sufficient participant numbers. Inferences however did not change when a 25% cut-off value was used.
To explore factors associated with anisotropy of the AVF, each subject’s AVF was classified into one of three AVF shape categories: (i) symmetric AVF; (ii) vertical AVF extent > horizontal AVF extent (ie. shape ratio < 0.8), and (iii) vertical AVF extent < horizontal AVF extent (ie. shape ratio > 1.2). Univariate polytomous regressions were performed (the CATMOD procedure SAS, version 9.0; SAS, Cary, NC) to determine the log odds of asymmetry in either direction compared with the symmetric category for demographic, cognitive and vision measures while adjusting for AVF area. Given that similar results were obtained when a multi-variate analysis was performed, only the results of the univariate polytomous regressions for AVF asymmetry are reported in this paper.
RESULTS
Of the 1425 participants in the study, the results of 39 participants (~3%) were not used for analyses due to unreliable AVF results because of illness, refusal to participate, or inability to follow instructions.
AVF Extent
The median (and interquartile range, IQR) AVF extent across the four principal meridians in this cohort was 12.4° (7.8°) while the median (IQR) AVF extent in the horizontal and vertical meridians were 15.3° (9.3°) and 11.3° (8.3°) respectively. AVF extent along the horizontal meridian was significantly better than AVF extent along the vertical meridian (paired t test, p<0.0001). The AVF extent along the superior (90°) meridian (11.3°) was significantly better than AVF extent along the inferior (270°) meridian (10.8°) (paired t test, p=0.004); no significant difference in AVF extents was found between the 0° (14.1°) and 180° (14.0°) meridians (p>0.05).
The factors associated with overall AVF extent consisted of demographic, cognitive and vision variables (Table 2) and these variables collectively accounted for approximately 32% of the total variance in overall AVF extent. AVF extent of the male participants was significantly better than that of the female participants (p=0.0012).
Table 2.
Multivariate Model describing Associations between-Average Attentional VF Extent and Demographic, Cognitive and Visual Factors
| Characteristic | Parameter Estimate | Standard Error | p-value |
|---|---|---|---|
| Demographics | |||
| Age (per year increase) | −0.15 | 0.02 | <0.0001 |
| Male | 0.60 | 0.25 | 0.013 |
| Blacks/White | −1.66 | 0.37 | <0.0001 |
| Years of Education (per year increase) | 0.14 | 0.05 | 0.003 |
| Depression score (per point) | −0.03 | 0.03 | 0.27 |
| Cognitive function | |||
| Minimental/per 1 point increase | 0.09 | 0.08 | 0.25 |
| Brief test of attention/per 1 point increase | 0.34 | 0.06 | <0.0001 |
| Trails B/per 5 seconds | −0.09 | 0.01 | <0.0001 |
| Visual Function | |||
| Visual acuity/per loss of 1 line | −0.37 | 0.11 | 0.0012 |
| Better eye contrast/per increase of 3 letters | 0.58 | 0.18 | 0.0009 |
| Central visual fields/per 5 points either missed or initially undetected | −1.05 | 0.33 | 0.0017 |
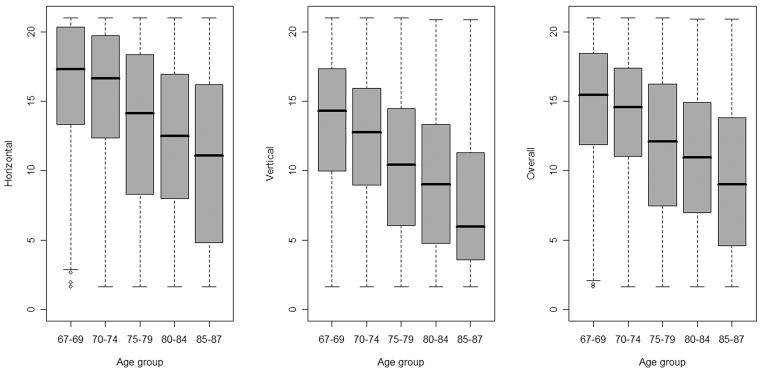
As illustrated in Figure 2 AVF extent decreased with increasing age. This was true for both the horizontal and vertical meridians as well as for overall AVF extent.
Figure 2. Average Attentional VF Extent along Horizontal Meridian (left panel), Vertical Meridian (middle panel) and Overall Extent across all Four Principal Meridians (right panel).
*
*The whiskers extend to the most extreme data point.
Each box contains 50% of the observations, and the median value is indicated as the line within the box.
Older participants tended to have lower AVF extent; for every year increase in age, the overall AVF extent decreased on average by 0.15°. On average, the overall AVF extent was reduced by 1.66° in blacks and by 0.03° for every point increase in the depression score. On average, being male increased the overall AVF extent by 0.6° and there was a 0.14° increase in overall AVF extent for every year increase in years of education.
Independent of the demographic factors detailed above, overall AVF extent was also associated with various cognitive functions. As listed in Table 2, on average, for every point increase in the MMSE and the BTA, the overall AVF extent increased by 0.09° and 0.34° respectively. AVF extent decreased by 0.09° for every 5 second increase in performance time in Trials B.
Visual function also had an independent and significant contribution to overall AVF performance (Table 2). The overall extent of the AVF decreased on average by 0.37° for every line of VA lost and 1.05° per 5 points not detected in the central 20° radius VF. Overall AVF extent increased by 0.58° for every 3 letters correctly identified on the Pelli-Robson Contrast Sensitivity Letter Chart in the better seeing eye.
AVF Shape
Symmetrically shaped AVFs were found in 476 (34%) of the 1386 participants. The remaining 910 participants (66%) had an asymmetric AVF shape profile. Of the 910 participants that had an asymmetric AVF shape profile, 751 participants (~83%) had a shape ratio greater than 1.2. Thus their horizontal AVF extent was greater than their vertical AVF extent, or “H-AVF > V-AVF”. The remaining 159 participants (17%) had a shape ratio less than 0.8 where the vertical AVF extent was greater than the horizontal AVF extent (“V-AVF > H-AVF”).
Table 3 lists the percentage of participants in each of the AVF shape profiles within each 5 year age group, gender and race. Just over half of all participants, regardless of race, gender and 5 year age group category had an asymmetric AVF shape of H-AVF > V-AVF. A third of all participants had a symmetrically shaped AVF while the remaining ~10 – 15% of participants had an asymmetric AVF shape of V-AVF > H-AVF. With each 5 year increase in age, there was an increase in the percentage of participants who had an asymmetric AVF shape of H-AVF > V-AVF. A greater percentage of females and blacks had an AVF shape of H-AVF > V-AVF compared to males and whites. After adjusting for AVF area, the effects of age, gender and race on the percentage of participants in each of the AVF shape profiles were less evident.
Table 3.
Percentage of Participants in each of the different AVF Shape Profiles broken down by Age, Gender and Race
| Characteristic | N | Percentage of participants in each AVF Shape Category (%) | ||
|---|---|---|---|---|
| Horizontal/Vertical ratio < 0.8 | Horizontal/vertical ratio between 0.8 –1.2 | Horizontal/Vertical ratio > 1.2 | ||
| Age | ||||
| <75 | 631 | 9.7 | 38.7 | 51.7 |
| 75–79 | 388 | 12.1 | 32.5 | 55.4 |
| 80–84 | 307 | 15.3 | 27.4 | 57.3 |
| 85+ | 57 | 7.0 | 33.2 | 59.7 |
| Gender | ||||
| Females | 698 | 12.2 | 31.8 | 56.0 |
| Males | 685 | 10.8 | 36.6 | 52.6 |
| Race | ||||
| Blacks | 173 | 13.3 | 27.8 | 59.0 |
| Whites | 1210 | 11.2 | 35.1 | 53.6 |
The results of regressions examining the vision and cognitive factors associated with the two different AVF shape asymmetry groups are listed in Table 4. We adjusted for AVF area, and because of the association of area with demographic factors, we did not include the demographic factors in the models. When including the demographic factors, the inferences did not change (data not shown). Using the symmetric shape category as reference, we found that participants with worse performance on Trials B were less likely to have an H-AVF > V-AVF asymmetric AVF shape profile compared to participants with better Trial B scores. We also found, that participants with better VA were more likely to have a V-AVF > H-AVF asymmetric AVF shape profile compared to participants with poorer VA. Participants with greater VF loss within the central 20° radius were less likely to have an H-AVF > V-AVF asymmetric AVF shape profile compared to participants with less VF loss in the central 20° radius.
Table 4.
| Characteristic | Horizontal/Vertical ratio <
0.8 N=159 |
Horizontal/Vertical ratio >
1.2 N=751 |
|---|---|---|
| Age (per yr/increment) | 1.00 (0.96–1.04) | 1.00 (0.98–1.02) |
| Females/Males | 0.85 (0.58–1.25) | 0.89 (0.70–1.13) |
| Blacks/Whites | 0.80 (0.45–1.42) | 0.96 (0.65–142) |
| Years of education/yr | 1.02 (0.95–1.10) | 1.03 (0.98–1.08) |
| Brief test of attention | 1.02 (0.94–1.11) | 1.04 (0.99–1.10) |
| Time trails B (per add 10 sec) | 0.98 (0.95–1.00) | 0.98 (0.96–0.99) |
| Visual acuity (per line gain) | 1.23 (1.03–1.47) | 1.09 (0.97–1.22) |
| Binocular Contrast (inc 3 letters) | 1.12 (0.9–1.38) | 1.06 (0.93–1.20) |
| VF points missing (inc 5) | 0.91 (0.63–1.32) | 0.63 (0.43–0.93) |
Adjusted for AVF area
Results listed as Odds Ratio (95% CI). Results in bold are statistically significant at p ≤ 0.05
DISCUSSION
One of the primary aims of this study was to describe AVF in a large population of elderly drivers. Using our method of AVF assessment, we found that active, elderly drivers were able to successfully divide their attention on average out to an extent of 12.4°. Participants however did not divide their attention evenly across the different meridians. The average horizontal and vertical AVF extents were 15.3° and 11.3° respectively. This difference was statistically significant and remained regardless of age and gender, thus revealing a larger AVF along the horizontal meridian.
Our finding of anisotropy of the AVF between the horizontal and vertical meridian is in agreement with previous research. Mackeben 19, Altpeter et al. 20 and Carrasco et al. 21 have all reported that the AVF was better along the horizontal meridian compared to the vertical meridian. Additionally, He et al 38, Carrasco et al 21 and Liu et al. 39 reported that performance in a discrimination task similar to a divided attention task with low foveal load was better along the inferior than superior portion of the vertical meridian. We did not find such a trend along the vertical meridian. The AVF extent along the inferior (270°) meridian was significantly worse compared to that along the superior (90°) meridian. Our result of decreased performance along the inferior meridian is in agreement with the results of Wood et al. 12 who found, averaging results across intermediate meridians, that their subjects had made a significantly greater percentage of errors in peripheral localization in the inferior hemi-field compared to the superior hemi-field.
A new finding of our study was the identification of different AVF shape profiles and the demographic, cognitive and visual factors associated with AVF shape. Our finding that most participants had an asymmetric AVF shape, especially one in which the horizontal meridian was greater than the vertical meridian, suggests that an asymmetrically-shaped AVF may be the “norm” AVF shape as opposed to a symmetric AVF shape for this age group. It may be that persons allocate their visual attention resources to areas of the visual field that maximize task performance.
The factors that were found to be significantly associated with AVF shape are listed in Table 4. For any two individuals with the same AVF area, the individual with the poorer Trials B score or worse vision, measured either as VA or VF, is more likely to have a symmetric AVF shape profile compared to an asymmetric AVF of either shape profile. This suggests that across individuals with the same overall AVF area, persons with failing resources, either visual and/or cognitive, lose the ability to re-distribute their visual attention resources to maximize task performance, thus resulting in a symmetric AVF shape.
The demographic, cognitive and visual factors measured in our study explained 32% of the variation in AVF extent. Specifically, we found that AVF performance decreased with increasing age, a finding that is consistent with several earlier studies 10, 11, 13–16, 40.
We also found that being female, being black and having less years of education were significantly associated with smaller AVF extent (Table 2). Another factor that was found to be predictive of overall AVF extent was depression. Participants with worse depression scores had worse AVF performance, perhaps because of the indirect effect that depression has on cognition. Previous research has shown that depressed patients exhibit lower cognitive abilities compared to people who are not depressed 41–43.
Cognitive and visual factors were also found to be significantly associated with overall AVF extent (Table 2). In general, we found that decreased cognitive ability on the MMSE, BTA and Trails B assessments were associated with smaller AVF extent. Similarly, vision loss, indexed in this study as decreased VA, CS in the better eye and VF loss in the central 20° radius VF, all resulted in decreased AVF extent. Given that the AVF relies both on visual and cognitive skills, it is not surprising that these measures were found to play a significant role in predicting overall AVF extent.
Very few studies have assessed the association between measures of cognitive ability and AVF extent. As found in our study, Edwards et al. 44 reported that those participants with poor MMSE scores had a significant decrease in AVF extent compared to participants with better cognitive status.
Our finding that measures of VF and CS were predictive of AVF performance is in agreement with previous studies 8, 13, 44, 45. The relationship between VA and AVF extent however is not as well understood. Like Edwards et al. 44, we found that binocular VA loss was related to smaller AVF extent. Studies by Leat et al. 13 and Owsley et al. 45 however have reported no associations between VA and AVF extent. A possible reason why some studies have found a significant association between VA and AVF extent and others have not, may relate to the size of the stimulus and distractors used in the divided attention task. In our study, the angular sub-tense of the central number (stimulus) was 0.8° and in Edwards et al’s 44 study was 1.4° x 1.88° (height x width). By comparison, the angular sub-tense of the central target used in Leat et al’s 13 study was 3.38° and in Owsley et al’s 45 study, 3° x 5° (height x width). The smaller central target used in ours and Edward et al’s study most likely made these AVF assessments more sensitive to the effects of VA loss since the smaller central target sizes would not be as robust against the detrimental effects of optical blur or distortion (ie. decreases in VA) compared to the larger central target sizes used in the other studies.
In conclusion, AVF extent was larger along the horizontal meridian compared to the vertical meridian and overall AVF extent was associated with various demographic, cognitive and vision measures. We also found that the majority of participants had an asymmetric AVF shape profile in which the horizontal AVF extent was greater than the vertical AVF extent. Finally, declines in cognitive and visual performance were associated with having a symmetric AVF shape profile.
Acknowledgments
This work was supported by a grant from the National Institute on Aging, AG16906. Dr West was awarded a Senior Scientific Investigator grant from Research to Prevent Blindness.
The authors wish to thank the Salisbury Eye Evaluation Study technicians and staff for collecting the data.
References
- 1.Owsley C, McGwin G. Association between visual attention and mobility in older adults. Journal of American Geriatrics Society. 2004;52:1901–1906. doi: 10.1111/j.1532-5415.2004.52516.x. [DOI] [PubMed] [Google Scholar]
- 2.Broman AT, West SK, Muñoz B, Bandeen-Roche K, Rubin GS, Turano KA. Divided visual attention as a predictor of bumping while walking: the salisbury eye evaluation. Investigative Ophthalmology & Visual Science. 2004;45:2955–2960. doi: 10.1167/iovs.04-0219. [DOI] [PubMed] [Google Scholar]
- 3.Clay OJ, Wadley VG, Edwards JD, Roth DL, Roenker DL, Ball KK. Cumulative meta-analysis of the relationship between usefull field of view and driving performance in older adults: current and future implications. Optometry and Vision Science. 2005;82:724–731. doi: 10.1097/01.opx.0000175009.08626.65. [DOI] [PubMed] [Google Scholar]
- 4.Bowers A, Peli E, Elgin J, McGwin G, Jr, Owsley C. On-road driving with moderate visual field loss. Optom Vis Sci. 2005;82:657–667. doi: 10.1097/01.opx.0000175558.33268.b5. [DOI] [PubMed] [Google Scholar]
- 5.Myers RS, Ball KK, Kalina TD, Roth DL, Goode KT. Relation of useful frield of view and other screening tests to on-road driving performance. Perceptual and Motor Skills. 2000;91:279–290. doi: 10.2466/pms.2000.91.1.279. [DOI] [PubMed] [Google Scholar]
- 6.Wood JM, Troutbeck R. Elderly drivers and simulated visual impairment. Optometry and Vision Science. 1995;72:115–124. doi: 10.1097/00006324-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Owsley C, Ball K, McGwin G, Jr, et al. Visual processing impairment and risk of motor vehicle crash among older adults. Jama. 1998;279:1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- 8.Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993;34:3110–3123. [PubMed] [Google Scholar]
- 9.Owsley C, McGwin G, Sloane ME, Stalvey BT, Wells J. Timed instrumental activities of daily living tasks: relationship to visual function in older adults. Optometry and Vision Science. 2001;78:350–359. doi: 10.1097/00006324-200105000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Ball KK, Beard BL, Roenker DL, Miller RL, Griggs DS. Age and visual search; expanding the useful field of view. Journal of Optical Society of America. 1988;5:2210–2219. doi: 10.1364/josaa.5.002210. [DOI] [PubMed] [Google Scholar]
- 11.Sekuler R, Ball K. Visual localization: age and practice. Journal of the Optical Society of America A, optics, image science and vision. 1986;3:864–867. doi: 10.1364/josaa.3.000864. [DOI] [PubMed] [Google Scholar]
- 12.Wood J, Chaparro A, Hickson L, et al. The effect of auditory and visual distracters on the useful field of view: implications for the driving task. Investigative Ophthalmology & Visual Science. 2006;47:4646–4650. doi: 10.1167/iovs.06-0306. [DOI] [PubMed] [Google Scholar]
- 13.Leat SJ, Lovie-Kitchin J. Visual impairment and the useful field of vision. Ophthalmic & Physiological Optics. 2006;26:392–403. doi: 10.1111/j.1475-1313.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 14.Seiple W, Szlyk JP, Yang S, Holopigian K. Age-related functional field losses are not eccentricity dependents. Vision Research. 1996;36:1859–1866. doi: 10.1016/0042-6989(95)00288-x. [DOI] [PubMed] [Google Scholar]
- 15.Sekuler AB, Bennett PJ. Effects of aging on the useful field of view. Experimental Aging Research. 2000;26:103–120. doi: 10.1080/036107300243588. [DOI] [PubMed] [Google Scholar]
- 16.Richards E, Bennett PJ, Sekuler AB. Age related differences in learning with the useful field of view. Vision Research. 2006;46:4217–4231. doi: 10.1016/j.visres.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Turano KA, Broman AT, Bandeen-Roche K, et al. Association of visual field loss and mobility performance in older adults: Salisbury eye evaluation study. Optometry and Vision Science. 2004;81:298–307. doi: 10.1097/01.opx.0000134903.13651.8e. [DOI] [PubMed] [Google Scholar]
- 18.Freeman EE, Munoz B, Turano KA, West SK. Measures of visual function and their association with driving modification in older adults. Investigative Ophthalmology & Visual Science. 2006;47:514–520. doi: 10.1167/iovs.05-0934. [DOI] [PubMed] [Google Scholar]
- 19.Mackeben M. Sustained focal attention and peripheral letter recognition. Spatial Vision. 1999;12:51–72. doi: 10.1163/156856899x00030. [DOI] [PubMed] [Google Scholar]
- 20.Altpeter E, Mackeben M, Trauzettel-Klosinski S. The importance of sustained attention for patients with maculopathies. Vision Research. 2000;40:1539–1547. doi: 10.1016/s0042-6989(00)00059-6. [DOI] [PubMed] [Google Scholar]
- 21.Carrasco M, Giordano AM, McElree B. Temporal performance fields: visual and attentional factors. Vision Research. 2004;44:1351–1365. doi: 10.1016/j.visres.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Ball KK, Roenker DL, Wadley VG, et al. Can high-risk older drivers be identified through performance-based measures in a department of motor vehicles setting? J Am Geriatr Soc. 2006;54:77–84. doi: 10.1111/j.1532-5415.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- 23.Kantor B, Mauger L, Richardson VE, Tschantz Unroe K. An analysis of an older driver evaluation program. The American Geriatrics Society. 2004;52:1326–1330. doi: 10.1111/j.1532-5415.2004.52363.x. [DOI] [PubMed] [Google Scholar]
- 24.Keyl PM, Rebok GW, Gallo JJ. Screening elderly drivers in general medical settings: Toward the development of a valid and feasible assessment procedure (final report) In: Sbordone RJ, Saul RE, editors. Neurophsychology for Health Care Professionals and Attorneys. 2. Boca Raton, Florida, USA: CRC Press LLC; 1996. pp. 216–217. [Google Scholar]
- 25.Marottoli RA, Cooney LM, Jr, Wagner R, Doucette J, Tinetti ME. Predictors of automobile crashes and moving violations among elderly drivers. Ann Intern Med. 1994;121:842–846. doi: 10.7326/0003-4819-121-11-199412010-00003. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, RMP ‘Mini-mental state’. A practical method for grading cognitivie state of patients for the clinician. Journal of Pyschiatry Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Schretlen D, Brandt J, Bobholz JH. Validation of the Brief Test of Attention in patients with Huntington’s disease and amnesia. Clinical Neuropsychologist. 1996;10:10–95. [Google Scholar]
- 28.Ferris FL, Kassoff A, Bresnick G, Bailey I. New visual acuity charts for clinical research. American Journal of Ophthalmology. 1982;94:91–96. [PubMed] [Google Scholar]
- 29.Kitchin JE, Bailey IL. Task complexity and visual acuity in senile macular degeneration. Australian Journal of Optometry. 1981;64:235–242. [Google Scholar]
- 30.Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clinical Vision Science. 1988;2:187–199. [Google Scholar]
- 31.Clement Clarke Inc. Manufacturer’s specifications. London: 1989. [Google Scholar]
- 32.Elliott DB, Whitaker D, Bonette L. Differences in the legibility of letters at contrast threshold using the Pelli-Robson chart. Ophthalmic & Physiological Optics. 1990;10:323–326. doi: 10.1111/j.1475-1313.1990.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 33.Elliott DB, Yang KCH, Whitaker D. Visual acuity changes throughout adulthood in normal, healthy eyes: seeing beyond 6/6. Optometry and Vision Science. 1995;72:186–191. doi: 10.1097/00006324-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Anderson DR. Testing the Field of Vision. St Louis: The C. V. Mosby Company; 1982. [Google Scholar]
- 35.Nelson-Quigg JM, Cello K, Johnson AA. Predicting binocular visual field sensitivity from monocular visual field results. Investigative Ophthalmology & Visual Science. 2000;41:2212–2221. [PubMed] [Google Scholar]
- 36.Antis SM. Letter to the Editors: A chart demonstrating variations in acuity with retinal position. Vision Research. 1974;14:589–592. doi: 10.1016/0042-6989(74)90049-2. [DOI] [PubMed] [Google Scholar]
- 37.Lieberman HR, Pentland AP. Microcomputer-based estimation of psychophysical thresholds: the Best PEST. Behavioural Research Methods and Instruments. 1982;14:21–25. [Google Scholar]
- 38.He S, Cavanagh P, Intriligator J. Attentional resolution and the locus of visual awareness. Nature. 1996;383:334–337. doi: 10.1038/383334a0. [DOI] [PubMed] [Google Scholar]
- 39.Liu T, Heeger DJ, Carrasco M. Neural correlates of the visual vertical meridian asymmetry. Journal of Vision. 2006;6:1294–1306. doi: 10.1167/5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogé J, Pébayle T, Lambilliotte E, Spitzenstetter F, Giselbrecht D, Muzet A. Influence of age, speed and duration of monotonous driving task in traffic on the driver’s useful visual field. Vision Research. 2004;44:2737–2744. doi: 10.1016/j.visres.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 41.Godin O, Dufouil C, Ritchie K, et al. Depressive symptoms, major depressive episode and cognition in the elderly: the three-city study. Neuroepidemiology. 2007;28:101–108. doi: 10.1159/000101508. [DOI] [PubMed] [Google Scholar]
- 42.Ottowitz WE, Dougherty DD, Savage CR. The neural network basis for abnormalities of attention and executive function in major depressive disorder: implications for application of the medical disease model to psychiatric disorders. Harvard Review of Psychiatry. 2002;10:86–99. doi: 10.1080/10673220216210. [DOI] [PubMed] [Google Scholar]
- 43.Levin RL, Heller W, Mohanty A, Herrington JD, Miller GA. Cognitive deficits in depression and functional specificity of regional brain activity. Cognitive Therapy and Research. 2007;31:211–233. [Google Scholar]
- 44.Edwards JD, Ross LA, Wadley VG, et al. The useful field of view test: normative data for older adults. Archives of Clinical Neuropsychology. 2006;21:275–286. doi: 10.1016/j.acn.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Owsley C, Ball K, Keeton DM. Relationship between visual sensitivity and target localization in older adults. Vision Research. 1995;35:579–587. doi: 10.1016/0042-6989(94)00166-j. [DOI] [PubMed] [Google Scholar]