SUMMARY
A number of genetic syndromes are known to convey a high risk of colorectal cancer. Current standards of medical practice for these patients involve genetic testing followed by screening and surgical procedures. Pharmaceutical therapies for any of these syndromes are limited in number and are generally not approved by any regulatory body for applications in these genetic groups. This review discusses advances in mechanistic understanding of the disease processes leading to the development of promising pharmaceutical therapies. Clinical trials of potential chemotherapeutic agents must focus on the reduction of disease-related events, including cancer and cancer-related mortality, in patients with genetic syndromes.
Introduction to chemoprevention
Chemoprevention is the use of pharmaceutical drugs or other agents, such as vitamins or other dietary supplements, to reduce the incidence or onset of disease. Chemoprevention was proposed as a method of preventing cancer over three decades ago [1,2]. Levin suggested that patients with certain genetic risk factors for colorectal cancer (CRC) might be appropriate candidates for chemoprevention, and discussed the importance of prostaglandin metabolism in cancer development and as a potential target for chemopreventive agents [3]. Agents that target COX, especially COX-2, involved in prostaglandin synthesis have been shown to reduce colorectal adenomas (CRAs), which are precursors of CRC, in patients with prior sporadic (nongenetic) CRAs [4–6]. In spite of these positive findings, the use of COX-2-selective agents to manage patients with average risk of CRC is discouraged [7]. Intake of aspirin, which also inhibits COX activities, has been associated with a reduced risk of CRA in prospective clinical trials [8,9] and CRC in population studies [10]. In spite of this benefit, concerns over toxicities have prevented clinicians from broadly prescribing aspirin to manage CRC risk, although some authors have suggested application in patients with a higher-than-average risk of CRC [11]. While the syndromes targeted for chemoprevention carry high cancer risks, the patients receiving chemopreventive medications are currently healthy, limiting tolerance for toxicity [12]. Because of this, and because of difficulty in determining what are clinically meaningful end points for studies, no current agent has regulatory approval by either the US FDA or EMA for use in the usual management of patients with either genetic or sporadic risk of CRC (Table 1). This challenge may be overcome through the use of combinations of agents [13,14] and, perhaps, in ‘personalizing’ chemoprevention strategies based on individual patient’s pharmacogenomic characteristics.
Table 1.
The regulatory status of chemopreventive agents being studied for management of colorectal cancer risk patients with genetic risk factors.
| Agent(s) | Efficacy | Regulatory status |
|---|---|---|
| Celebrex® | Reduction in colonic polyp load (FAP) Trial results pending (Lynch syndrome) |
US FDA approved for use as an anti-inflammatory; FDA approval for use in FAP withdrawn in 2011 |
| EPA | Reduction in colonic polyp load (FAP) Trend toward reduced polyp load (FAP) Reduced cancer (Lynch syndrome) Efficacy in nonrandomized trials (FAP) Preclinical models suggest lack of efficacy (Lynch syndrome) |
Not FDA-approved for use in chemoprevention |
| Aspirin | Nonprescription medication in many countries; no regulatory approval for cancer prevention | |
| Sulindac | FDA approved for use as an anti-inflammatory; not FDA approved for use in chemoprevention | |
| Black raspberry extract | Trial results pending (FAP) | – |
| Curcumin | Possible efficacy, trial in progress (FAP) | – |
| Celebrex + ursodiol | Trial results pending (FAP) | – |
| Celebrex + DFMO Aspirin + DFMO Sulindac + erlotinib |
Trial results pending (FAP) | – |
| Trial in progress (FAP) | – | |
| Trial in progress (FAP) | – | |
| Sulindac + DFMO | Trial start pending (FAP) |
DFMO: Difluoromethylornithine; EPA: Eicosapentaenoic acid; FAP: Familial adenomatous polyposis.
Familial adenomatous polyposis
Familial adenomatous polyposis (FAP) is caused by mutations in the APC gene (the rodent gene is known as Apc). FAP affects approximately three in 100,000 people [15–17]. People with FAP develop CRC at an average age of 39 years [18], although there is variability within and between families, some of which correlates with the specific causative mutation [19]. FAP patients are also at risk for extracolonic manifestations including gastric and duodenual polyps, osteomas and dental anomalies, congenital hypertrophy of the retinal pigment epithelium, desmoids and other soft tissue tumors. The current standard of treatment includes intensive monitoring with prophylactic surgery (either restorative proctocolectomy or ileorectal anastomosis [IRA]) when the adenoma burden becomes unmanageable by endoscopy, often by 20 years of age [20,21]. While surgery is life-saving [22], reported postoperative quality-of-life outcomes are highly variable [23–26] and suggest that surgery may increase the risk of infertility [27,28]. The very high risk of CRC, and the young age at which FAP patients undergo preventive surgery make chemoprevention attractive. Significant preclinical work has led to the development of agents whose efficacy has been tested in clinical trials (Table 2).
Table 2.
Studies of agents for chemoprevention in hereditary cancer syndromes.
| Syndrome and trial title | Study design (start/end year) |
Enrollment target |
Agent(s) | End point(s) & clinical relevancy |
Major finding/trial status | Ref. |
|---|---|---|---|---|---|---|
| FAP: colonic end points | ||||||
| The effect of celecoxib, a COX-2 inhibitor, in FAP | Randomized, double-blind safety/efficacy study; Phase II/III (1996/2000) | 77 | Celebrex®, placebo | Mean number of polyps and colorectal polyp burden | Treatment with 400 mg of celecoxib twice daily for 6 months was associated with a significant reduction from baseline in the number of colorectal polyps compared with the placebo group (28.0 vs 4.5%, p = 0.003) | [45] |
| A two-arm chemoprevention trial in familial APC patients using the purified FFA, eicosapentaenoic acid | Randomized, double-blind safety/efficacy study; Phase II/III (1996/2000) | 63 | EPA | Absolute change in the number of polyps measured in a defined focal area of the rectum | EPA–FFA has chemopreventive efficacy in FAP, to a degree similar to that previously observed with selective COX-2 inhibitors | [58,105] |
| A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with FAP (CAPP1) | Randomized, efficacy study (1993/2010) | 208 | Aspirin, resistant starch, placebo | Proportion of patients with an increased polyp count in the rectum and sigmoid colon after intervention | Trend of reduced polyp load (number and size) with 600 mg of aspirin daily. Resistant starch had no clinical effect on adenomas | [32] |
| A pilot study to investigate the biological modulation of FAP by lyophilized black raspberries | Randomized, double-blind safety/efficacy study; Phase I (2005/2008) | 14 | Lyophilized black raspberries, placebo | Number of rectal polyps | This study has been completed | [106] |
| A registry-based observational study assessing clinical outcomes in FAP in patients receiving celecoxib (Celebrex, Onsenal®) compared with control patients | Observational cohort study; Phase IV (2004/2008) | 68 | Celebrex | Time from IRA or IPAA to time of first excisional polypectomy of a rectal polyp post-surgery | The study prematurely discontinued on 11 April 2008 due to slow enrollment. It should be noted that safety concerns have not been seen in this study and have not factored into this decision | [45,92,107] |
| A randomized, double-blind, placebo-controlled study to assess the safety and efficacy of rofecoxib in FAP | Randomized, double-blind safety/efficacy study; Phase IV (2003/2006) | 62 | Rofecoxib, placebo | Number and average size of rectal polyps Safety and tolerability | This study has been terminated | [108] |
| Use of curcumin in the lower GI tract in FAP patients | Nonrandomized, open-label crossover study; Phase II (2005/2008) | 5 | Curcumin | Use of curcumin in the lower GI tract in FAP patients | This study has been terminated. (subsequent data generated by our collaborators have shown efficacy with curcumin and quercetin in five patients in a nonplacebo controlled trial) | [109] |
| A two-arm chemoprevention trial in adenomatous polyposis coli patients | Randomized, double-blind safety/efficacy study; Phase II (2001) | 120 | Celebrex, eflornithine (DFMO), placebo | Percentage change of polyps in a focal area of the colorectum | This study is ongoing, but not recruiting participants | [110] |
| A placebo-controlled trial of celecoxib in genotype positive subjects with FAP | Randomized, double-blind safety/efficacy study; Phase III (2006/2019) | 200 | Celebrex, placebo | To compare the time from randomization to treatment failure over a 5-year period in subjects treated with celecoxib vs placebo | Currently recruiting participants | [111] |
| Curcumin for treatment of intestinal adenomas in FAP | Randomized, double-blind; Phase II (2010/2018) | 50 | Curcumin, placebo | Polyp number and size | Currently recruiting participants | [112] |
| Randomized trial of aspirin and DFMO in patients at high risk of colorectal cancer | Randomized, double-blind efficacy study; Phase II (2009/2015) | 52 | Eflornithine (DFMO), acetylsalicylic acid (aspirin), placebo | Adenoma recurrence rate for the treatment arm relative to placebo | Currently recruiting participants | [101] |
| Adenoma-carcinoma sequence in the IPAA in patients with FAP: studies on luminal and mucosal risk factors and chemoprevention | Randomized open-label, crossover efficacy study; Phase II (2006/2006) | 30 | Sulindac, VSL#3 (probiotic), inulin (probiotic) | Pouch mucosal proliferation index at 0, 1 and 2 months; Pouch mucosal apoptosis index at 0, 1 and 2 months | Information has not been verified recently. Recruitment status was: recruiting | [102] |
| Use of curcumin for treatment of intestinal adenomas in FAP | Randomized, doubleblind, safety/efficacy study (2007/2016) | 50 | Curcumin, placebo | Duodenal and colorectal/ileal polyps | This study is currently recruiting participants. | [113] |
| FAP: both colonic & extracolonic | ||||||
| Trial of the safety and efficacy of eflornithine combined with sulindac compared with eflornithine and sulindac as single agents in patients with FAP and attenuated FAP | Randomized, double-blind safety/efficacy study; Phase III (2012/2015) | 150 | Sulindac, eflornithine (DFMO), placebo | Delaying time to the first occurrence of any FAP-related event. FAP-related events include excisional intervention involving the colon, rectum, pouch, duodenum and/or clinically important events of progression of duodenal polyposis, cancer or death | Not yet open for participant recruitment | [104] |
| Genetic events leading to APC-dependent colon cancer in high-risk families; a clinical trial of COX and EGFR inhibition in familial polyposis patients | Randomized, double-blind efficacy study; Phase II (2010/2015) | 100 | Sulindac, erlotinib, placebo | Compare the change in total duodenal and colorectal polyp burden at 6 months | Currently recruiting participants | [114] |
| Recurrent desmoids determine outcome in patients with Gardner syndrome: a cohort study of three generations of an APC mutation-positive family across 30 years | Retrospective, observational cohort study (1978/2010) | 105 | Brachytherapy, radiotherapy | Mortality | Following brachytherapy or radiotherapy, all patients showed full or partial remission | [115] |
| FAP: extracolonic | ||||||
| Coxib inhibition of duodenal polyp growth in FAP | Randomized, double-blind study; Phase II/III (2003/2004) | 38 | Rofecoxib, placebo | Premalignant adenomatous lesions in the duodenal mucosa | This study has been terminated (drug withdrawal) | [116] |
| Prevention of progression of duodenal adenomas to cancer in patients with FAP | Randomized, double-blind efficacy study; Phase II/III (2009/2012) | 80 | Celebrex, UDCA, placebo | Change in number and size of duodenal adenomas | This study is ongoing, but not recruiting participants | [117] |
| Efficiency of ursodeoxycholic acid in the treatment of duodenal adenomas in FAP patients (URSOPAF) | Randomized, double-blind efficacy study; Phase II/III (2004/2009) | 90 | UDCA, placebo | SPIGELMAN severity score of duodenal lesion after 2 years of follow-up | Information has not been verified recently; it was: active, not recruiting | [118] |
| Colorectal adenoma/carcinoma prevention program (CAPP-2) | Randomized, double-blind study (1999/2011) | 861 | Aspirin, resistant starch, placebo | Development of colorectal cancer | 600 mg aspirin per day for a mean of 25 months substantially reduced cancer incidence after 55.7 months in carriers of hereditary colorectal cancer | [33,81,119] |
| Lynch syndrome: colonic | ||||||
| Sulindac treatment in hereditary nonpolyposis colorectal cancer | Randomized, double-blind surrogate end point study (2006) | 22 | Sulindac, placebo | Cell proliferation | Sulindac induces an increase in epithelial cell proliferation in the proximal colon of subjects with HNPCC | [84] |
| Multiple-dose safety and efficacy study of a selective inhibitor of COX-2 (SC-58635) in hereditary HNPCC patients and carriers | Safety study; Phase I/II (1998/2002) | 20 | Celecoxib, placebo | Safety monitoring | This study has been completed | [120] |
| Prevention of endometrial tumors | Randomized, open label study; Phase III (2007/2008) | 600 | Levonorgestrel-releasing intrauterine system | Rate of atypical endometrial hyperplasia or endometrial cancer during the active follow-up period of the study | This study has been terminated (withdrawn due to poor accrual) | [121] |
| Lynch syndrome: extracolonic | ||||||
| Modulation of putative surrogate end point biomarkers in endometrial biopsies from women with HNPCC | Randomized, open-label efficacy study; Phase II (2002/2013) | 52 | Ethinyl estradiol, medroxy-progesterone norgestrel | Changes in endometrial abnormalities, histology and ultrasound appearance at 3 months | This study is ongoing, but not recruiting participants | [122] |
APC: Adenomatous polyposis coli; DFMO: Difluoromethylornithine; EPA: Eicosapentaenoic acid; FAP: Familial adenomatous polyposis; FFA: Free fatty acid; HNPCC: Hereditary nonpolyposis colorectal cancer; IPAA: Ileopouch anal anastomosis; IRA: Ileorectal anastomosis; UDCA: Ursodeoxycholic acid.
Clinical trials of chemopreventive agents in FAP used CRA and/or duodenal polyp number or burden as primary end points. The clinical significance of these end points is questionable, given the current standard of care by screening and surgical treatment with colectomy or proctocolectomy. The current most clinically relevant disease sites in FAP are the rectum, ileal pouch, duodenum and abdominal desmoids [29]. End points with clinical meaning relevant to pharmacologic adjunct to standard of care include increasing the time to FAP-related events that influence morbidity and mortality from this genetic disease. Current medical practice utilizes diagnostic and surgical methods to monitor and treat FAP patients. While surgical procedures can effectively eliminate the risk of colorectal cancer, patients must deal with subsequent sequelae involving surgical repairs, duodenal polyposis, development of desmoid tumors in a subset of patients and subsequent cancers leading to death.
Chemoprevention may have the consequence of reducing disease incidence or onset or suppressing the course and/or severity of disease and/or disease-related sequelae. Chemoprevention would not have to prevent cancer; even postponing uncontrollable polyp development by 5–10 years would delay surgery long enough for patients to develop a stable identity and to reproduce [30].
For chemoprevention strategies to be implemented in clinical practice, strategies must address current unmet medical needs and/or improve the patient’s quality of life. Table 1 lists a number of past and current efforts to develop pharmaceutical agents for use in the management of patients with FAP, many of which target the prostaglandin–COX pathway.
Nonselective inhibition of prostaglandin synthesis
NSAIDs including aspirin, sulindac and indomethacin, inhibit COX-1 and COX-2 and signaling through WNT and other pathways, and have been studied extensively in chemoprevention of CRAs and in FAP [31]. The CAPP1 trial examined whether aspirin (600 mg/day) and/or resistant starch (RS; 30 g/day) reduced polyp number in the rectum and sigmoid colon of young patients with FAP [32]. The largest polyp size in each individual was a secondary end point. This study relied on participants having intact colons, so patients aged 10–21 years were recruited. The average age of enrollment was 18 years and approximately half of the participants were female. The median time to endoscopic polyp assessment was 17 months, so more than half of patients remained on treatment for over a year. A total of 206 patients were randomized to receive RS alone, RS plus aspirin, aspirin alone or placebo, and 133 underwent follow-up endoscopy. Neither aspirin nor RS significantly reduced polyp number or size, although there was a trend towards fewer polyps and smaller largest polyp size in the patients treated with aspirin. Secondary analyses found a significant reduction in polyp size for patients who received aspirin and remained on the study for more than a year. RS had no effect. This is similar to CAPP2 findings with aspirin in Lynch syndrome [33]. An ongoing trial is studying whether aspirin and difluoromethylornithine (DFMO) are synergistic [101].
Giardiello and colleagues studied the nonselective COX inhibitor sulindac (150 mg twice daily) in patients with polyposis, most of whom had intact colons [34]. At 9 months, there was a significant reduction in polyp number and size, although these increased after stopping the medication. A 48-month trial in FAP patients without polyposis found no reduction in number or size of polyps [35]. Postsurgical patients treated with sulindac at 300–400 mg daily showed significant reduction in rectal polyp number and size [36,37]. The second showed a nonsignificant improvement in duodenal polyps [37]. These are similar to eight nonrandomized trials showing reduction in polyp load (reviewed in [38]). Several current trials are studying sulindac in FAP patients [39], including one in conjunction with probiotics [102].
The NSAID indomethacin has been studied in short-term (1–11 months) nonrandomized trials for patients with previous IRA, with rectal polyp number as the end point [40,41]. Polyp numbers decreased after indomethacin given either by suppository or orally, and reached statistical significance in the oral trial.
Exisulind (sulindac sulfone) has limited activity against COX enzymes, but retains WNT inhibition. Efficacy in stopping polyp formation or causing polyp regression has been reported in one randomized, double-blind placebo-controlled trial and two nonrandomized trials [42–44]. Preliminary work showed that treatment with exisulind 600 mg daily for 6–24 months caused significant polyp regression (>50%) and inhibition of new polyp formation (25–50%) [43,44]. The randomized trial studied exisulind at a dose of 100 or 200 mg twice daily (200–400 mg total) in 155 patients with FAP and adenomatous polyps. In the higher-dose group, they reported a similar reduction in polyp size (50%; p = 0.03 in the efficacy evaluable population, but not significant for the intention-to-treat group) as well as a significant reduction in ‘disease progression’ for the efficacy evaluable population. However, exisulind use is limited by toxicity including abdominal pain, elevated transaminase levels, cholecystitis and pancreatitis [42]. The occurrence of all of these effects was significantly higher in the 400-mg arm than the placebo or 200-mg cohorts and 38% of people in the exisulind 400-mg group discontinued the study prematurely [42]. A clinical trial to study the efficacy of exisulind in preventing polyp formation in FAP patients was withdrawn [103].
Selective inhibition of COX-2
Agents targeting COX-2 have been a major focus of attention, in part due to preclinical studies in mouse models of FAP that identified COX-2 as a mediator of tumorigenesis resulting from loss of function of the APC tumor-suppressor gene. In addition to their inhibition of COX activity, these agents inhibit β-catenin activity. Celecoxib (Celebrex®, Pfizer, KS, USA) has been evaluated alone and in combination with other agents. Celecoxib was approved over 10 years ago by both the US FDA and by the EMA for use in the treatment of FAP, based on statistically significant 28% reductions in total colorectal polyps and 30% reduction in polyp burden [45]. Furthermore, management of FAP evolved in such a way that most FAP patients received colectomy/proctocolectomy, and thus colorectal adenomas were not a significant clinical problem in most patients. These clinically significant issues, along with agent toxicity limiting recruitment to a postmarketing registry, resulted in withdrawal of regulatory approval for the FAP indication for celecoxib in 2011. Current clinical trials are evaluating the potential of celecoxib alone in pediatric FAP patients or in combination with other agents (Table 2).
Other COX-2 inhibitors have been studied in randomized and nonrandomized trials. Rofecoxib at 25 mg daily was studied in patients with previous IRA for 9–30 months. It caused a significant decrease in both polyp number and size [46,47]. Surprisingly, a randomized trial of tiracoxib at 150–200 mg daily for 6 months failed to show a change in polyp size or number [48]. Neither is available commercially.
COX-2-selective inhibitors as primary colorectal cancer prevention have fallen out of favor due to unexpected cardiovascular events [4,49–51] and a recent pooled analysis of cardiovascular events in six clinical trials of nonarthritis patients demonstrates that celecoxib is indeed associated with a dose-dependent increased risk of cardiovascular events [52].
Polyamine inhibition
Preclinical studies have identified the rationale for combinations of sulindac with other agents, including inhibitors of EGF signaling pathways [53] and polyamine metabolism [32]. These studies have also identified downstream genes regulated by the APC tumor-suppressor gene using genetically modified human colon tumor-derived cells. The MYC oncogene is one of these APC-regulated genes [54]. MYC regulates a number of genes and pathways, including the polyamine pathway [55]. Agents that suppress intestinal and colonic polyamine contents, including the ornithine decarboxylase inhibitor DFMO and several NSAIDs, inhibit intestinal and colonic tumorigenesis in the ApcMin/+ mouse model of FAP [55,56]. Results from these preclinical human cell and rodent models were subsequently translated into a series of Phase II/III studies in patients with prior sporadic colorectal polyps. After dose-finding, safety and biochemical efficacy studies were completed. The combination of DFMO and sulindac reduced total and advance/multiple metachronous colorectal adenomas by 70% and greater than 90%, respectively, in patients with prior sporadic colorectal polyps [53]. These preclinical and clinical studies are the rationale for a new clinical trial to study the efficacy of DFMO and sulindac, alone or in combination, in adult FAP patients [104].
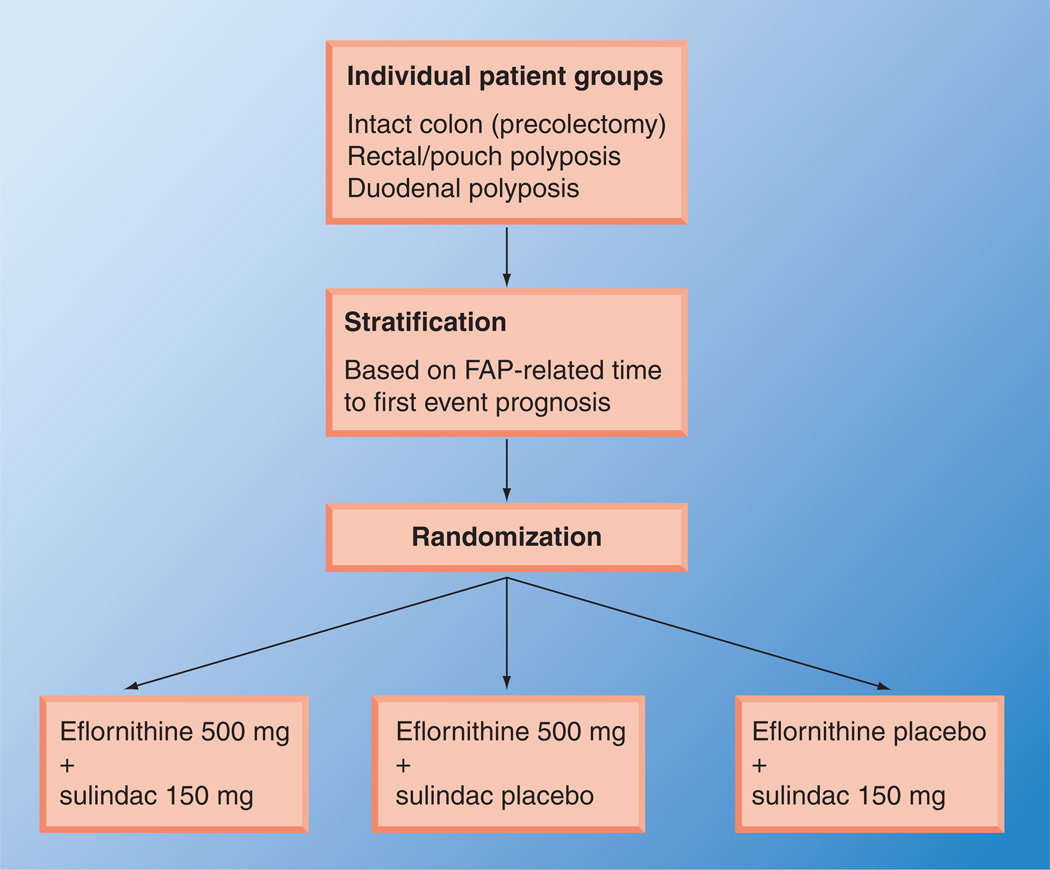
CPP-FAP-310 is a trial planned to study the efficacy of DFMO and sulindac in adult FAP patients [104]. It is unique in that the outcomes studied will be timed to a FAP-related event (polypectomy, surgery or bleeding) (Table 2). This new trial will focus on clinically significant events involving intestinal polyposis. Major eligibility criteria for this trial will include diagnosis of genotypic and/or phenotypic FAP or attenuated FAP (AFAP), and an age of 18 years or over; and in the case of prior colorectal surgery, at least 3 years since colectomy/proctocolectomy with IRA or pouch. Patients will be randomized to one of three arms, as shown in the schema presented in Figure 1. The trial is designed to measure the time to clinically significant events and is based on an estimated total event number. The treatment period is expected to last 2 years.
Figure 1. Schema for a trial to evaluate combination therapy for patients with familial adenomatous polyposis.
FAP: Familial adenomatous polyposis.
Data taken from [104].
Other agents
Eicosapentaenoic acid (EPA) is an omega-3 polyunsaturated fatty acid that is found naturally in cold-water fish and has been suggested to have protective effects against CRC. Its mechanism is not clear, but EPA may act through inhibition of COX-2 enzyme activity and WNT signaling [57]. Using a protocol similar to that used for celecoxib [45], West and colleagues conducted a double-blind, randomized, placebo-controlled trial to ask whether EPA reduced polyp load in the rectum of people with FAP treated with IRA [58,105]. Participants were over 18 years of age and had undergone a colectomy with IRA for FAP risk reduction at least 1 year prior to enrollment. A total of 58 participants were randomized to either 2 g daily of an enteric-coated EPA or placebo, and rectal endoscopy was performed to assess rectal polyp burden after 6 months of medication. Individuals taking EPA had a ~12% reduction in both polyp number and size after 6 months, compared with a 10% (number) and 30% (size) increase in people from the placebo arm. The differences in polyp number, change in polyp number and change in polyp diameter were statistically significant between treatment and placebo groups, as was the 42% reduction in global rectal polyp burden between the groups (p = 0.011). The direction and magnitude of polyp reduction is similar between EPA and celecoxib [58]. It is hoped that the improved side-effect profile of EPA (e.g., fish oil) compared with celecoxib, with its increased cardiovascular risk, will make this a more attractive chemopreventive agent.
Treatment of 19 FAP patients with 3 g of vitamin C daily for 9 months showed a significant reduction in polyp area compared with 17 controls, although polyp number was never significantly reduced. A later trial combining vitamins C and E, with or without grain fiber, showed a trend toward reduction in polyp number by fiber, but not by vitamin treatment [59]. Treating 25 post-IRA FAP patients with oral calcium carbonate 1500 mg for 6 months showed no difference in polyp number or size, although there were lower crypt cell production rates in rectal biopsies from treated patients [60]. Curcumin and quercetin reduced polyp number and size in four patients with previous surgery [61]. Other trials of natural agents, including black raspberry extract and probiotics, have yet to be reported or completed.
Lynch syndrome
Hereditary nonpolyposis colorectal cancer (HNPCC) kindreds were initially identified by use of the Amsterdam Criteria based on familial and clinical features [62]. A later revised criteria included extraintestinal cancers as well as tumor histology (Bethesda Criteria) [63,64]. Approximately half of these families have a disease-causing mutation in one of four DNA mismatch repair (MMR) genes MSH2, MLH1, MSH6 and PMS2, providing a genetic explanation and allowing this latter group to be further defined as Lynch syndrome (LS), an autosomal dominant disorder of variable penetrance. Individuals with LS have up to an 80% lifetime risk of developing CRC as well as extraintestinal cancers, including endometrial, ovarian, urinary, brain and others. Inactivation of the MMR genes can occur in sporadic CRC where gene silencing takes place due to hypermethylation of the promoter region [65]. Somatic loss of the second MMR allele inactivates the mismatch repair system, leading to genome-wide mutagenesis resulting in the early development of colon adenomas that rapidly progress through the adenoma–carcinoma sequence. As the most common inherited colorectal cancer syndrome in western countries, LS accounts for 3–5% of all CRCs with MMR gene mutation carrier rate of approximately one in 3000 [66]. Simultaneous cancers (synchronous malignancy) as well as recurrent or later cancers in both colon and other organs (metachronous malignancy) are a hallmark of LS. This is the basis for aggressive therapeutic and prophylactic surgical management in the setting of CRC in LS patients [67,68]. Published surveillance guidelines have been shown to improve survival in patients with LS [69]; however, there is continued interest in methods to reduce cancer risk. Chemoprevention may prove effective both clinically and from a cost perspective, although it is not as well studied in LS as in FAP. As a heterogeneous disease, no single common mechanism or pattern of gene mutations are common to all sporadic or LS CRCs. Genes such as COX-2 are more commonly involved with elevated mRNA and protein levels in up to 50% of adenomas and 80% of sporadic CRCs. In studies comparing COX-2 overexpression with MMR status, COX-2 overexpression was observed in 79% of tumors with intact MMR repair compared with 48% with deficient; MMR system (p < 0.001). COX-2 overexpression decreases in the setting of defective MMR [70,71]. Nonetheless, chemopreventive agents targeting COX overexpression have received the greatest attention although these drugs are also likely to have other COX-independent mechanisms [72]. Here, we review the preclinical studies since they highlight agents that may be promising for future study, as well as the few reported clinical trials.
Rodent models
Mouse models carrying germline mutations of the homologous MMR genes have been present for over two decades. Unlike human disease, mice with heterogeneous mutation in the MMR genes do not display tumor development, rendering these models of limited value in chemoprevention studies. This lack of tumor development is thought to be secondary to the shorter lifespan of mice, significantly limiting the time available to silence the normal wild-type (WT) allele and fewer cell numbers due to body size. Mice possessing germline mutation of both alleles display a cancer phenotype differing from that of LS with lymphoma and skin tumors along with GI tract tumors that are predominantly small intestinal. This phenotype is nearly identical to that seen in children and adolescents that carry biallelic germline mutations of the MMR genes [73,74].
Utilizing a Cre-LoxP-mediated inactivation of Msh2, a conditional Msh2-knockout mouse model has been created (VC-Msh2-/LoxP) that develops intestinal adenomas and adenocarcinomas without the lymphomas or skin tumors noted in other animal models [75]. This model more closely approximates LS colon cancer and would appear to be an ideal model for the testing of chemopreventive agents. This model was recently used to assess the effect of aspirin and low-dose nitric oxide donating aspirin (NO-ASA) [76]. Treatment with either drug at lower doses increased lifespan by 18–21% when compared with controls; however, drug treatment had minimal effect on eventual tumor numbers, with aspirin treatment tending to reduce microsatellite instability, which was not seen in the NO-ASA treatment group. By contrast, high-dose NO-ASA treatment increased microsatellite instability and tumor burden with a decreased lifespan [77].
The nonselective NSAID sulindac was studied in a Mlh1+/− mouse model as part of a group study including an Apc1638N/+ mouse model with contrasting results. After 6 months of treatment by addition to the diet, small intestinal tumor number and mucosal inflammation was decreased, as expected, in the Apc model but small intestinal inflammation and tumor numbers were increased in the Mlh1+/− animals. Sulindac treatment increased cecal tumor number and inflammation in all animals regardless of genetic background, suggesting a connection between inflammation and tumor formation [78]. Another study in a mouse model with both Apc and Msh2 mutations (Apcmin/−Msh2−/−) found that the specific COX-2 inhibitor MF-tricyclic had a greater effect on suppressing small bowel tumors than sulindac when compared with controls. Neither treatment affected tumor number or aberrant crypt foci in the colon [79]. The effects of sulindac on tumor prevention in Msh2 and p53 mouse models with a WT control using azozymethane to drive tumor formation has also been reported. Sulindac blocked azozymethane-associated tumor formation in the distal colon of all animals. Sulindac treatment was associated with proximal colonic inflammation that progressed to adenocarcinoma in up to 25% of either the p53- or Msh2-deficient mice but not in the WT controls [80]. Collectively, these three animal studies show that sulindac can influence tumor development in genetically predisposed mouse models on a regional basis. However, they also argue for regional drug-associated changes in mucosal inflammation that appear to be associated with increased tumor formation suggesting a secondary, dose-dependent proinflammatory effect that may also play a role in carcinogenesis.
Human studies
Aspirin
The largest clinical trial of LS to date (the CAPP2 trial) was a randomized placebo-controlled trial using a 2 × 2 factorial design in genotype-positive patients (n = 746 included in final analysis) that received either aspirin at a dose of 600 mg per day, resistant starch (Novelose®, National Starch Food Innovation, NJ, USA) at a dose of 30 g per day, both or placebo for up to 4 years. Initial data analysis at 2.5 years of follow-up showed no statistical significant reduction for adenoma incidence with either treatment separately or combined (relative risk [RR]: 1.03; 95% CI: 0.75–1.41) or colorectal cancer incidence (RR: 0.87; 95% CI: 0.39–1.96) [81]. However, additional follow-up at four years found a significant reduction in time to first colon and other LS cancers between aspirin and placebo groups (HR: 0.62; 95% CI: 0.41–0.96) [32]. This suppressive effect of aspirin was seen long after the medication was discontinued, arguing for a delayed chemopreventive effect. A similar therapeutic deferral has been documented in observational studies of regular aspirin users where a reduction in cancer was seen after 10 years [82,83]. Due to the rapid progression from adenoma to carcinoma known to occur in LS, a significant number of the cancers identified at follow-up surveillance developed after discontinuation of treatment. The authors comment on a possible mechanism of enhanced apoptosis possibly affecting aberrant stem cells that are destined to progress quickly to cancer. Cell culture work in MMR-deficient cells treated with aspirin show increased apoptosis and reduced microsatellite instability [77].
Sulindac
The nonselective COX inhibitor sulindac has been studied in an HNPCC patient population (defined MMR family mutation with a previously diagnosed HNPCC-related cancer or polyp) to assess the effect of treatment on proliferation and apoptosis, as assessed in colon biopsies on and off treatment. Using a randomized, double-blinded, placebo-controlled cross-over study design, 22 subjects were treated with sulindac 150 mg twice daily or 4 weeks of placebo separated by a 4-week washout period. This short duration study found increased markers of proliferation in the right colon, but not in the left, when comparing treatment with placebo. No change was noted in markers of apoptosis (staining of cytokeratin 18 cleavage products) or levels of Cyclins B1, D3 and E and p21, p27, BAX and BCL by immunohistochemistry [84].
Other agents
Hormonal agents are being formally studied for prevention of uterine cancer (Table 2). These agents are attractive in light of the possibility that they might also protect against colorectal cancer [85].
MUTYH-associated polyposis
In addition to FAP and LS, a third adenomatous polyposis syndrome was identified in 2002 and found to be due to biallelic mutation in the base excision repair gene MUTYH [86]. The three disorders share a similar phenotype of variable colorectal adenomas and increased the risk of CRC; however, MUTYH-associated polyposis (MAP) is inherited as an autosomal recessive disorder with typical onset in the fourth or fifth decade with limited numbers of adenomas prominently in the right colon. Duodenal adenomas and extraintestinal malignancies of the bladder, ovary and skin are part of the MAP phenotype, which again overlaps with both features of attenuated FAP and LS [87]. Chemoprevention in MAP remains to be addressed.
Chemoprevention in the hamartomatous polyp syndromes
Several of the less-common polyp syndromes are characterized by development of hamartomatous polyps throughout the GI tract. These are also associated with significant cancer risk and, as such, people with these syndromes could benefit from effective chemoprevention strategies. Most are due to specific gene mutations that affect signaling pathways that regulate cell growth, maturation and adhesion with dysregulation of COX-2 expression a common finding (Table 3). Neoplasia is thought to develop through either a hamartoma-adenoma-carcinoma sequence or through a ‘landscaper mechanism’ that postulates that the abnormal stromal environment of the hamartoma enables carcinogenic transformation in the adjacent epithelium [88]. These autosomal dominant syndromes show great variation in expression and convey both gastrointestinal and extra-intestinal cancer risk as shown in Table 3. Optimal chemoprevention for these syndromes should be both safe, effective and target other potential cancer sites in addition to the colon.
Table 3.
Potential treatments for polyposis syndromes.
| Disorder | Affected gene(s) | Pathway | Sites at risk of cancer | Possible targets for chemoprevention |
|---|---|---|---|---|
| Juvenile polyposis syndrome | SMAD4, BMPR1A | BMP–TGF-β signaling pathway | GI tract and pancreas | COX-2 [93,94] |
| Peutz–Jeghers syndrome | LKB1, STK11 | PI3K signaling transduction pathway | GI tract, breast, ovarian, uterine, pancreas, lung, biliary tree and testis | mTOR and COX-2 [39,95,96] |
| PTEN hamartoma tumor syndrome | PTEN | PI3K–AKT–mTOR pathway | Thyroid, breast, endometrium, colorectum, kidney and melanocytes | P13K, AKT1, mTOR or PDK1 [97–100] |
Future perspective
Chemoprevention of cancer in carriers of genetic traits that predispose to cancer is not implemented in the usual clinical management of these patients for several reasons. A major reason has been ineffectiveness of drug therapy. An example of this lack of efficacy is the treatment of FAP with celecoxib, which was referred to earlier. This agent administered as a single agent produced statistically significant, but clinically insignificant, responses [45]. In spite of regulatory body approvals, the agent did not become a standard feature of management of patients with FAP and the sponsor subsequently withdrew the agent from this market. Successful implementation of chemoprevention in the management of these patients will require that agents successfully address unmet clinical needs, be effective in improving clinical outcomes and display safety profiles that do not exceed benefits and are acceptable to patients. For example, toxicities of agents such as tamoxifen remain problematic and negatively impact patient acceptance [12]. Solutions to these dilemmas may be found in the use of combinations of agents [13], a strategy that has already shown positive results in patients with sporadic risk of CRC [53]. Whether chemoprevention strategies can be prescribed in a ‘personalized medicine’ manner in the future remains to be determined. Information regarding rare alleles (i.e., <1% in the general population) is already attainable and is beginning to be used in decisions for chemoprevention of genetic risk groups. An example is the use of aspirin in patients with hereditary nonpolyposis colon cancer [33]. Information regarding more common alleles (i.e., >5% in the general population) may also be useful in the future. Studies in sporadic patients indicate that polymorphisms in specific genes may identify the responsiveness of patients to the colorectal adenoma preventive activity of aspirin in patients with sporadic risk of colorectal cancer [89]. Some of these common polymorphisms may be useful in predicting responses to combinations of agents [90]. Measures of certain tissue properties may also be helpful in prediction of agent utility. For example, measures of rectal prostaglandin and polyamine contents appear to be associated with responses in patients with sporadic risk of CRC [91]. Future studies will determine if any of these genetic, biochemical or other types of measures can be used to ‘personalize’ chemoprevention for patients with genetic risk of cancer.
There is a significant need for new treatments to complement existing diagnostic and surgical methods for patients with genetic risk of colorectal and other syndrome-associated cancers. Over the next 5 years, studies will evaluate effects of pharmaceutical strategies on unmet medical needs in these patients, in order to assess therapeutic risks and benefits. Agents will be selected for efficacy to convey a specific treatment benefit, but these benefits may not be limited to reducing the risk of CRC death. Other clinically significant end points include reduction in disease-associated sequelae (e.g., subsequent surgeries and related treatment complications). Studies focusing more broadly on current unmet medical needs will allow for more rapid determination of clinical benefit of new interventions than those that only consider cancer or mortality end points.
Clinical studies need to address questions of agent dose and duration. Combinations of agents have the potential to reduce individual agent dose prescriptions as a way of reducing treatment toxicities. Investigators need to determine if pharmaceutical therapies need to be taken continuously over long periods by at-risk patients in order to convey benefit, and document the toxicity of these treatments. Studies must determine if stopping use of a drug or drug combination is associated with a ‘rebound’ or increased risk of CRC. New pharmaceutical therapies should be evaluated in the framework of existing medical practice in the management of patients with genetic risk of CRC. In this context, approaches in these patient groups may be different for pediatric and adult patients.
Development of prognostic and predictive markers will aid in the development and evaluation of pharmaceutical agents for these patients. Prognostic markers will provide useful methods of risk-stratifying patients. Predictive markers will provide clinicians indications of treatment efficacy that can complement longer-term end points such as cancer development.
Implementation into clinical practice will also require that any new therapies are cost effective, in comparison to existing methodologies.
Practice points.
-
■
The prevention of colorectal cancer remains an attractive and important goal, especially for patient populations known to be at increased risk due to inherited genetic predisposition.
-
■
Several drugs, common and investigational, as well as naturally occurring substances have been, or are currently, undergoing study.
-
■
Although some of these substances show promise in altering cancer risk, much remains to be learned about real benefits, dosing and potential toxicities. This can only be acquired through human trials.
-
■
Currently, there are no approved chemopreventive agents available in the USA or Europe, illustrating the need for further appropriately controlled human studies.
-
■
Clinicians can contribute to this important goal by educating their patients regarding ongoing clinical trials that are currently recruiting patients.
Acknowledgements
The authors would like to thank C Mauss for assistance with manuscript submission, and A Cohen and K Grenier for their work on the FAP protocol.
EW Gerner has ownership interest in Cancer Prevention Pharmaceuticals, Tucson, AZ, USA. S Erdman has research grant funding from Cancer Prevention Pharmaceuticals. Work described in this review was supported by grants from the NIH to EW Gerner and colleagues, including CA047396, CA059024, CN075019, CA072008, CA088078, CA095960 and CA123065. C Laukaitis was also supported by CA023074.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
- 1.Sporn MB. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 1976;36(7 Pt 2):2699–2702. [PubMed] [Google Scholar]
- 2.Sporn MB. Combination chemoprevention of cancer. Nature. 1980;287(5778):107–108. doi: 10.1038/287107a0. [DOI] [PubMed] [Google Scholar]
- 3.Levin B. Genetic syndromes as potential targets for chemoprevention of colorectal neoplasia. J. Cell Biochem. Suppl. 2000;34:19–22. doi: 10.1002/(sici)1097-4644(2000)77:34+<19::aid-jcb5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N. Engl. J. Med. 2006;355(9):873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 5.Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N. Engl. J. Med. 2006;355(9):885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 6.Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131(6):1674–1682. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 7.Psaty BM, Potter JD. Risks and benefits of celecoxib to prevent recurrent adenomas. N. Engl. J. Med. 2006;355(9):950–952. doi: 10.1056/NEJMe068158. [DOI] [PubMed] [Google Scholar]
- 8.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N. Engl. J. Med. 2003;348(10):891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 9.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N. Engl. J. Med. 2003;348(10):883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 10.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369(9573):1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 11.Langley RE, Burdett S, Tierney JF, Cafferty F, Parmar MK, Venning G. Aspirin and cancer: has aspirin been overlooked as an adjuvant therapy? Br. J. Cancer. 2011;105(8):1107–1113. doi: 10.1038/bjc.2011.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lippman SM, Lee JJ. Reducing the “risk” of chemoprevention: defining and targeting high risk – 2005 AACR Cancer Research and Prevention Foundation Award Lecture. Cancer Res. 2006;66(6):2893–2903. doi: 10.1158/0008-5472.CAN-05-4573. [DOI] [PubMed] [Google Scholar]
- 13.Sporn MB, Hong WK. Concomitant DFMO and sulindac chemoprevention of colorectal adenomas: a major clinical advance. Nat. Clin. Pract. Oncol. 2008;5(11):628–629. doi: 10.1038/ncponc1221. [DOI] [PubMed] [Google Scholar]
- 14. Sporn MB, Hong WK. Clinical prevention of recurrence of colorectal adenomas by the combination of difluoromethylornithine and sulindac: an important milestone. Cancer Prev. Res. (Phila.) 2008;1(1):9–11. doi: 10.1158/1940-6207.CAPR-08-0049. ■ Seminal paper describing the concept of combinations of pharmaceutical agents for cancer prevention; outlined the rationale for this approach, including the reduction of agent toxicities and the ability to target multiple features of cancer development.
- 15.Bulow S, Faurschou Nielsen T, Bulow C, Bisgaard Ml, Karlsen L, Moesgaard F. The incidence rate of familial adenomatous polyposis. Results from the Danish Polyposis Register. Int. J. Colorectal Dis. 1996;11(2):88–91. doi: 10.1007/BF00342466. [DOI] [PubMed] [Google Scholar]
- 16.Burn J, Chapman P, Delhanty J, et al. The UK northern region genetic register for familial adenomatous polyposis coli: use of age of onset, congenital hypertrophy of the retinal pigment epithelium, and DNA markers in risk calculations. J. Med. Genet. 1991;28(5):289–296. doi: 10.1136/jmg.28.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarvinen HJ. Epidemiology of familial adenomatous polyposis in Finland: impact of family screening on the colorectal cancer rate and survival. Gut. 1992;33(3):357–360. doi: 10.1136/gut.33.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulow S. Clinical features in familial polyposis coli. Results of the Danish Polyposis Register. Dis. Colon Rectum. 1986;29(2):102–107. doi: 10.1007/BF02555389. [DOI] [PubMed] [Google Scholar]
- 19.Newton K, Mallinson E, Bowen J, et al. Genotype–phenotype correlation in colorectal polyposis. Clin. Genet. 2011;81(6):521–531. doi: 10.1111/j.1399-0004.2011.01740.x. [DOI] [PubMed] [Google Scholar]
- 20.Banasiewicz T, Marciniak R, Kaczmarek E, et al. The prognosis of clinical course and the analysis of the frequency of the inflammation and dysplasia in the intestinal J-pouch at the patients after restorative proctocolectomy due to FAP. Int. J. Colorectal Dis. 2011;26(9):1197–1203. doi: 10.1007/s00384-011-1241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erkek AB, Church JM, Remzi FH. Age-related analysis of functional outcome and quality of life after restorative proctocolectomy and ileal pouch-anal anastomosis for familial adenomatous polyposis. J. Gastroenterol. Hepatol. 2007;22(5):710–714. doi: 10.1111/j.1440-1746.2007.04870.x. [DOI] [PubMed] [Google Scholar]
- 22.Bulow S. Results of national registration of familial adenomatous polyposis. Gut. 2003;52(5):742–746. doi: 10.1136/gut.52.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chew MH, Quah HM, Teh KL, Loi TT, Eu KW, Tang CL. Twenty years of familial adenomatosis polyposis syndromes in the Singapore Polyposis Registry: an analysis of outcomes. Singapore Med. J. 2011;52(4):246–251. [PubMed] [Google Scholar]
- 24.De Zeeuw S, Heikens JT, Gooszen HG, Van Laarhoven CJ. The ileo neo-rectal anastomosis in patients with familial adenomatous polyposis: a prospective case series with long-term follow-up. Colorectal Dis. 2011 doi: 10.1111/j.1463-1318.2011.02806.x. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 25.Fritzell K, Eriksson LE, Bjork J, Hultcrantz R, Wettergren L. Self-reported abdominal symptoms in relation to health status in adult patients with familial adenomatous polyposis. Dis. Colon Rectum. 2011;54(7):863–869. doi: 10.1007/DCR.0b013e3182147fbe. [DOI] [PubMed] [Google Scholar]
- 26.McNicol F, Kennedy R, Phillips R, Clark S. Laparoscopic total colectomy and ileorectal anastomosis (IRA), supported by an enhanced recovery programme in cases of familial adenomatous polyposis. Colorectal Dis. 2012;14(4):458–462. doi: 10.1111/j.1463-1318.2011.02683.x. [DOI] [PubMed] [Google Scholar]
- 27.Rajaratnam SG, Eglinton TW, Hider P, Fearnhead NS. Impact of ileal pouch-anal anastomosis on female fertility: meta-analysis and systematic review. Int. J. Colorectal Dis. 2011;26(11):1365–1374. doi: 10.1007/s00384-011-1274-9. [DOI] [PubMed] [Google Scholar]
- 28.Nieuwenhuis MH, Douma KF, Bleiker EM, Bemelman WA, Aaronson NK, Vasen HF. Female fertility after colorectal surgery for familial adenomatous polyposis: a nationwide cross-sectional study. Ann Surg. 2010;252(2):341–344. doi: 10.1097/SLA.0b013e3181e9829f. [DOI] [PubMed] [Google Scholar]
- 29.Al-Sukhni W, Aronson M, Gallinger S. Hereditary colorectal cancer syndromes: familial adenomatous polyposis and lynch syndrome. Surg. Clin. North Am. 2008;88(4):819–844. doi: 10.1016/j.suc.2008.04.012. vii. [DOI] [PubMed] [Google Scholar]
- 30.Anderson BJ, Wolpert HA. A developmental perspective on the challenges of diabetes education and care during the young adult period. Patient Educ. Couns. 2004;53(3):347–352. doi: 10.1016/j.pec.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 31. Keller JJ, Giardiello FM. Chemoprevention strategies using NSAIDs and COX-2 inhibitors. Cancer Biol. Ther. 2003;2(4) Suppl. 1:S140–S149. ■ Summary of benefits of NSAIDs in familial adenomatous polyposis, especially sulindac.
- 32.Burn J, Bishop DT, Chapman PD, et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev. Res. (Phila.) 2011;4(5):655–665. doi: 10.1158/1940-6207.CAPR-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer. an analysis from the CAPP2 randomised controlled trial. Lancet. 2012;378(9809):2081–2087. doi: 10.1016/S0140-6736(11)61049-0. ■ Showed benefit of aspirin in patients with hereditary nonpolyposis colorectal cancer; and underscored the problem of using cancer and death as end points.
- 34.Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N. Engl. J. Med. 1993;328(18):1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 35.Giardiello FM, Yang VW, Hylind LM, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N. Engl. J. Med. 2002;346(14):1054–1059. doi: 10.1056/NEJMoa012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labayle D, Fischer D, Vielh P, et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101(3):635–639. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- 37.Nugent KP, Farmer KC, Spigelman AD, Williams CB, Phillips RK. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br. J. Surg. 1993;80(12):1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 38. Kim B, Giardiello FM. Chemoprevention in familial adenomatous polyposis. Best Pract. Res. Clin. Gastroenterol. 2011;25(4–5):607–622. doi: 10.1016/j.bpg.2011.08.002. ■ Reviews all previous trials of familial adenomatous polyposis chemoprevention.
- 39.De Leng WW, Westerman AM, Weterman MA, et al. Cyclooxygenase 2 expression and molecular alterations in Peutz-Jeghers hamartomas and carcinomas. Clin. Cancer Res. 2003;9(8):3065–3072. [PubMed] [Google Scholar]
- 40.Akasu T, Yokoyama T, Sugihara K, Fujita S, Moriya Y, Kakizoe T. Peroral sustained-release indomethacin treatment for rectal adenomas in familial adenomatous polyposis: a pilot study. Hepatogastroenterology. 2002;49(47):1259–1261. [PubMed] [Google Scholar]
- 41.Hirota C, Iida M, Aoyagi K, et al. Effect of indomethacin suppositories on rectal polyposis in patients with familial adenomatous polyposis. Cancer. 1996;78(8):1660–1665. [PubMed] [Google Scholar]
- 42.Arber N, Kuwada S, Leshno M, Sjodahl R, Hultcrantz R, Rex D. Sporadic adenomatous polyp regression with exisulind is effective but toxic: a randomised, double blind, placebo controlled, dose-response study. Gut. 2006;55(3):367–373. doi: 10.1136/gut.2004.061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Stolk R, Stoner G, Hayton Wl, et al. Phase I trial of exisulind (sulindac sulfone, FGN-1) as a chemopreventive agent in patients with familial adenomatous polyposis. Clin. Cancer Res. 2000;6(1):78–89. [PubMed] [Google Scholar]
- 44.Stoner GD, Budd GT, Ganapathi R, et al. Sulindac sulfone induced regression of rectal polyps in patients with familial adenomatous polyposis. Adv. Exp. Med. Biol. 1999;470:45–53. doi: 10.1007/978-1-4615-4149-3_5. [DOI] [PubMed] [Google Scholar]
- 45.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 2000;342(26):1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 46.Hallak A, Alon-Baron L, Shamir R, et al. Rofecoxib reduces polyp recurrence in familial polyposis. Dig. Dis. Sci. 2003;48(10):1998–2002. doi: 10.1023/a:1026130623186. [DOI] [PubMed] [Google Scholar]
- 47.Higuchi T, Iwama T, Yoshinaga K, Toyooka M, Taketo MM, Sugihara K. A randomized, double-blind, placebo-controlled trial of the effects of rofecoxib, a selective cyclooxygenase-2 inhibitor, on rectal polyps in familial adenomatous polyposis patients. Clin. Cancer Res. 2003;9(13):4756–4760. [PubMed] [Google Scholar]
- 48.Iwama T, Akasu T, Utsunomiya J, Muto T. Does a selective cyclooxygenase-2 inhibitor (tiracoxib) induce clinically sufficient suppression of adenomas in patients with familial adenomatous polyposis? A randomized double-blind placebo-controlled clinical trial. Int. J. Clin. Oncol. 2006;11(2):133–139. doi: 10.1007/s10147-005-0548-z. [DOI] [PubMed] [Google Scholar]
- 49.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Five-year efficacy and safety analysis of the Adenoma Prevention with Celecoxib Trial. Cancer Prev. Res. (Phila.) 2009;2(4):310–321. doi: 10.1158/1940-6207.CAPR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solomon SD, Mcmurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 2005;352(11):1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 51.Solomon SD, Pfeffer MA, Mcmurray JJ, et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114(10):1028–1035. doi: 10.1161/CIRCULATIONAHA.106.636746. [DOI] [PubMed] [Google Scholar]
- 52.Solomon SD, Wittes J, Finn PV, et al. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials. The cross trial safety analysis. Circulation. 2008;117(16):2104–2113. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 54.Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer. 2004;4(10):781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 55.Ignatenko NA, Besselsen DG, Stringer DE, Blohm-Mangone KA, Cui H, Gerner EW. Combination chemoprevention of intestinal carcinogenesis in a murine model of familial adenomatous polyposis. Nutr. Cancer. 2008;60(Suppl. 1):30–35. doi: 10.1080/01635580802401317. [DOI] [PubMed] [Google Scholar]
- 56.Meyskens FL, Jr, Mclaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas. a randomized placebo-controlled, double-blind trial. Cancer Prev. Res. (Phila.) 2008;1(1):32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lynch PM. Eicosapentaenoic acid and chemoprevention of FAP. Gut. 2010;59(7):871–873. doi: 10.1136/gut.2009.204677. [DOI] [PubMed] [Google Scholar]
- 58.West NJ, Clark SK, Phillips RK, et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59(7):918–925. doi: 10.1136/gut.2009.200642. [DOI] [PubMed] [Google Scholar]
- 59.Decosse JJ, Miller HH, Lesser ML. Effect of wheat fiber and vitamins C and E on rectal polyps in patients with familial adenomatous polyposis. J. Natl Cancer Inst. 1989;81(17):1290–1297. doi: 10.1093/jnci/81.17.1290. [DOI] [PubMed] [Google Scholar]
- 60.Thomas MG, Thomson JP, Williamson RC. Oral calcium inhibits rectal epithelial proliferation in familial adenomatous polyposis. Br. J. Surg. 1993;80(4):499–501. doi: 10.1002/bjs.1800800432. [DOI] [PubMed] [Google Scholar]
- 61.Cruz-Correa M, Shoskes DA, Sanchez P, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006;4(8):1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 62.Vasen HFA, Mecklin J-P, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (IGC-HNPCC) Dis. Colon Rectum. 1991;34:424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 63.Vasen HFA, Watson P, Mecklin J-P, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez-Bigas MA, Boland CR, Hamilton SR, et al. National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J. Natl Cancer Inst. 1997;89:1758–1762. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 65.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 2003;349(21):2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 66.Dunlop MG, Farrington SM, Nicholl I, et al. Population carrier frequency of hMSH2 and hMLH1 mutations. Br. J. Cancer. 2000;83(12):1643–1645. doi: 10.1054/bjoc.2000.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers. The advantage of more extensive colon surgery. Gut. 2011;60(7):950–957. doi: 10.1136/gut.2010.228056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalady MF. Surgical management of hereditary nonpolyposis colorectal cancer. Adv. Surg. 2011;45:265–274. doi: 10.1016/j.yasu.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 69.Vasen HF, Abdirahman M, Brohet R, et al. One to 2-year surveillance intervals reduce risk of colorectal cancer in families with Lynch syndrome. Gastroenterology. 2010;138(7):2300–2306. doi: 10.1053/j.gastro.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 70.Sinicrope FA, Lemoine M, Xi L, et al. Reduced expression of cyclooxygenase 2 proteins in hereditary nonpolyposis colorectal cancers relative to sporadic cancers. Gastroenterology. 1999;117(2):350–358. doi: 10.1053/gast.1999.0029900350. [DOI] [PubMed] [Google Scholar]
- 71.Castells A, Paya A, Alenda C, et al. Cyclooxygenase 2 expression in colorectal cancer with DNA mismatch repair deficiency. Clin. Cancer Res. 2006;12(6):1686–1692. doi: 10.1158/1078-0432.CCR-05-1581. [DOI] [PubMed] [Google Scholar]
- 72.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer. 2001;1(1):11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 73.Poley JW, Wagner A, Hoogmans MM, et al. Biallelic germline mutations of mismatch-repair genes. a possible cause for multiple pediatric malignancies. Cancer. 2007;109(11):2349–2356. doi: 10.1002/cncr.22697. [DOI] [PubMed] [Google Scholar]
- 74.Herkert JC, Niessen RC, Olderode-Berends MJ, et al. Paediatric intestinal cancer and polyposis due to bi-allelic PMS2 mutations. case series, review and follow-up guidelines. Eur. J. Cancer. 2011;47(7):965–982. doi: 10.1016/j.ejca.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 75.Kucherlapati MH, Lee K, Nguyen AA, et al. An Msh2 conditional knockout mouse for studying intestinal cancer and testing anticancer agents. Gastroenterology. 2010;138(3):993.e1–1002.e1. doi: 10.1053/j.gastro.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mcilhatton MA, Tyler J, Kerepesi LA, et al. Aspirin and low-dose nitric oxide-donating aspirin increase life span in a Lynch syndrome mouse model. Cancer Prev. Res. (Phila.) 2011;4(5):684–693. doi: 10.1158/1940-6207.CAPR-10-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mcilhatton MA, Tyler J, Burkholder S, et al. Nitric oxide-donating aspirin derivatives suppress microsatellite instability in mismatch repair-deficient and hereditary nonpolyposis colorectal cancer cells. Cancer Res. 2007;67(22):10966–10975. doi: 10.1158/0008-5472.CAN-07-2562. [DOI] [PubMed] [Google Scholar]
- 78.Itano O, Yang K, Fan K, et al. Sulindac effects on inflammation and tumorigenesis in the intestine of mice with Apc and Mlh1 mutations. Carcinogenesis. 2009;30(11):1923–1926. doi: 10.1093/carcin/bgp200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lal G, Ash C, Hay K, et al. Suppression of intestinal polyps in Msh2-deficient and non-Msh2-deficient multiple intestinal neoplasia mice by a specific cyclooxygenase-2 inhibitor and by a dual cyclooxygenase-1/2 inhibitor. Cancer Res. 2001;61(16):6131–6136. [PubMed] [Google Scholar]
- 80.Mladenova D, Daniel JJ, Dahlstrom JE, et al. The NSAID sulindac is chemopreventive in the mouse distal colon but carcinogenic in the proximal colon. Gut. 2011;60(3):350–360. doi: 10.1136/gut.2010.208314. [DOI] [PubMed] [Google Scholar]
- 81.Burn J, Bishop DT, Mecklin JP, et al. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N. Engl. J. Med. 2008;359(24):2567–2578. doi: 10.1056/NEJMoa0801297. [DOI] [PubMed] [Google Scholar]
- 82.Giovannucci E, Egan KM, Hunter DJ, et al. Aspirin and the risk of colorectal cancer in women. N. Engl. J. Med. 1995;333(10):609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 83.Liljegren A, Barker G, Elliott F, et al. Prevalence of adenomas and hyperplastic polyps in mismatch repair mutation carriers among CAPP2 participants. report by the colorectal adenoma/carcinoma prevention programme 2. J. Clin. Oncol. 2008;26(20):3434–3439. doi: 10.1200/JCO.2007.13.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rijcken FE, Hollema H, Van Der Zee AG, Van Der Sluis T, Boersma-Van EKW, Kleibeuker JH. Sulindac treatment in hereditary non-polyposis colorectal cancer. Eur. J. Cancer. 2007;43(8):1251–1256. doi: 10.1016/j.ejca.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 85.Tsilidis KK, Allen NE, Key TJ, et al. Oral contraceptives, reproductive history and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer. 2010;103(11):1755–1759. doi: 10.1038/sj.bjc.6605965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al-Tassan N, Chmiel NH, Maynard J, et al. Inherited variants of MYH associated with somatic G.C-->T:A mutations in colorectal tumors. Nat. Genet. 2002;30(2):227–232. doi: 10.1038/ng828. [DOI] [PubMed] [Google Scholar]
- 87.Vogt S, Jones N, Christian D, et al. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology. 2009;137(6):1976–1985. doi: 10.1053/j.gastro.2009.08.052. e1971–1910. [DOI] [PubMed] [Google Scholar]
- 88.Kinzler KW, Vogelstein B. Landscaping the cancer terrain. Science. 1998;280(5366):1036–1037. doi: 10.1126/science.280.5366.1036. [DOI] [PubMed] [Google Scholar]
- 89.Martinez ME, O’Brien TG, Fultz KE, et al. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc. Natl Acad. Sci. USA. 2003;100(13):7859–7864. doi: 10.1073/pnas.1332465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zell JA, Mclaren CE, Chen WP, Thompson PA, Gerner EW, Meyskens FL. Ornithine decarboxylase-1 polymorphism, chemoprevention with eflornithine and sulindac, and outcomes among colorectal adenoma patients. J. Natl Cancer Inst. 2010;102(19):1513–1516. doi: 10.1093/jnci/djq325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thompson PA, Wertheim BC, Zell JA, et al. Levels of rectal mucosal polyamines and prostaglandin E2 predict ability of DFMO and sulindac to prevent colorectal adenoma. Gastroenterology. 2010;139(3):797.e1–805.e1. doi: 10.1053/j.gastro.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.MHRA. Onsenal (Celecoxib) for familial adenomatous polyposis: withdrawal from EU market. MHRA Drug Safety Update. 2011;1(5) CON125965. [Google Scholar]
- 93.Brosens LA, Langeveld D, Van Hattem WA, Giardiello FM, Offerhaus GJ. Juvenile polyposis syndrome. World J. Gastroenterol. 2011;17(44):4839–4844. doi: 10.3748/wjg.v17.i44.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kurland JE, Beck SE, Solomon CJ, Brann OS, Carethers JM, Huang SC. Cyclooxygenase-2 expression in polyps from a patient with juvenile polyposis syndrome with mutant BMPR1A. J. Pediatr. Gastroenterol. Nutr. 2007;44(3):318–325. doi: 10.1097/MPG.0b013e31802e98e5. [DOI] [PubMed] [Google Scholar]
- 95.Kuwada SK, Burt R. A rationale for mTOR inhibitors as chemoprevention agents in Peutz-Jeghers syndrome. Fam. Cancer. 2011;10(3):469–472. doi: 10.1007/s10689-011-9471-9. [DOI] [PubMed] [Google Scholar]
- 96.Udd L, Katajisto P, Rossi DJ, et al. Suppression of Peutz-Jeghers polyposis by inhibition of cyclooxygenase-2. Gastroenterology. 2004;127(4):1030–1037. doi: 10.1053/j.gastro.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 97.Heald B, Mester J, Rybicki L, Orloff MS, Burke CA, Eng C. Frequent gastrointestinal polyps and colorectal adenocarcinomas in a prospective series of PTEN mutation carriers. Gastroenterology. 2010;139(6):1927–1933. doi: 10.1053/j.gastro.2010.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat. Rev. Cancer. 2011;11(4):289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lopiccolo J, Ballas MS, Dennis PA. PTEN hamartomatous tumor syndromes (PHTS): rare syndromes with great relevance to common cancers and targeted drug development. Crit. Rev. Oncol. Hematol. 2007;63(3):203–214. doi: 10.1016/j.critrevonc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 100.Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin. Cancer Res. 2012;18(2):400–407. doi: 10.1158/1078-0432.CCR-11-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.Aspirin and Eflornithine in Treating Patients at High Risk for Colorectal Cancer. http://clinicaltrials.gov/ct2/show/NCT00983580.
- 102.Influence of Sulindac and Probiotics on the Development of Pouch Adenomas in Patients With Familial Adenomatous Polyposis. http://clinicaltrials.gov/ct2/show/NCT00319 007?term=NCT00319007&rank=1.
- 103.Exisulind in Preventing Polyps in Patients With Familial Adenomatous Polyposis. http://clinicaltrials.gov/ct2/show/NCT0002 6468?term=exisulind+FAP&rank=1.
- 104.Trial of Eflornithine Plus Sulindac in Patients With Familial Adenomatous Polyposis (FAP) http://clinicaltrials.gov/ct2/show/NCT01483144.
- 105.Chemoprevention Trial in Familial Adenomatous Polyposis Coli Using EPA. http://clinicaltrials.gov/ct2/show/NCT00510692?term=NCT00510692&rank=1.
- 106.Lyophilized Black Raspberries in Adults With Familial Adenomatous Polyposis (FAP) http://clinicaltrials.gov/ct2/show/NCT00770991?term=NCT00770991&rank=1.
- 107.Observational Familial Adenomatous Polyposis Registry Study In Patients Receiving Celecoxib Compared to Control Patients. http://clinicaltrials.gov/ct2/show/NCT00151476?term=NCT00151476&rank=1.
- 108.A Study of Rofecoxib in Familial Adenomatous Polyposis (FAP) http://clinicaltrials.gov/ct2/show/NCT00140894?term=NCT00140894&rank=1.
- 109.Use of Curcumin in the Lower Gastrointestinal Tract in Familial Adenomatous Polyposis Patients. http://clinicaltrials.gov/ct2/show/NCT00248053?term=NCT00248053&rank=1.
- 110.Celecoxib With or Without Eflornithine in Preventing Colorectal Cancer in Patients With Familial Adenomatous Polyposis. http://clinicaltrials.gov/ct2/show/NCT00033371?term=NCT00033371&rank=1.
- 111.Trial In Pediatric Patients With Familial Adenomatous Polyposis (FAP) (CHIP) http://clinicaltrials.gov/ct2/show/NCT00585312?term=NCT00585312&rank=1.
- 112.Curcumin for Treatment of Intestinal Adenomas in Familial Adenomatous Polyposis (FAP) http://clinicaltrials.gov/ct2/show/NCT00641147?term=NCT00641147&rank=1.
- 113.Use of Curcumin for Treatment of Intestinal Adenomas in Familial Adenomatous Polyposis (FAP) http://clinicaltrials.gov/ct2/show/NCT00927485?term=NCT00927485&rank=1.
- 114.A Clinical Trial of COX and EGFR Inhibition in Familial Polyposis Patients. http://clinicaltrials.gov/ct2/show/NCT01187901?term=NCT01187901&rank=1.
- 115.A Cohort Study of Patients Treated With Brachytherapy for Selected Desmoid Patients in Gardner Syndrome. http://clinicaltrials.gov/ct2/show/NCT01286662?term=NCT01286662&rank=1.
- 116.Coxib-inhibition of Duodenal Polyp Growth in FAP. http://clinicaltrials.gov/ct2/show/NCT00844727?term=NCT00844727&rank=1.
- 117.Prevention of Progression of Duodenal Adenomas in Patients With Familial Adenomatous Polyposis (PreDuoFAP) http://clinicaltrials.gov/ct2/show/NCT00808743?term=NCT00808743&rank=1.
- 118.Ursodeoxycholic Acid in the Treatment of Duodenal Adenomas in Familial Adenomatous Polyposis (FAP) Patients. http://clinicaltrials.gov/ct2/show/NCT00134758?term=NCT00134758&rank=1.
- 119.A Randomised Controlled Trial of Colorectal Polyp and Cancer Prevention Using Aspirin and Resistant Starch in Carriers of Hereditary Nonpolyposis Colorectal Cancer. www.controlled-trials.com/ISRCTN59521990.
- 120.Phase I–II Multiple-Dose Safety and Efficacy Study of a Selective Inhibitor of Cyclooxygenase-2 (SC-58635) in Hereditary Non-Polyposis Colorectal Cancer (HNPCC) Patients and Carriers. http://clinicaltrials.gov/ct2/show/NCT00001693.
- 121.Intrauterine Levonorgestrel and Observation or Observation Alone in Preventing Atypical Endometrial Hyperplasia and Endometrial Cancer in Women With Hereditary Non-Polyposis Colorectal Cancer or Lynch Syndrome. http://clinicaltrials.gov/ct2/show/NCT00566644?term=NCT00566644&rank=1.
- 122.Hormone Therapy in Preventing Endometrial Carcinogenesis (Cancer) in Women With a Genetic Risk For Hereditary Nonpolyposis Colon Cancer. http://clinicaltrials.gov/ct2/show/NCT00033358?term=NCT00033358&rank=1.