Background: Intracellular carbonic anhydrase (CAi) activity, commonly detected in cancer, accelerates CO2/HCO3− equilibration.
Results: In cells with high CAi activity, fluctuations in pCO2 (which can arise from intermittent blood flow) evoke substantial intracellular pH oscillations that modulate downstream pathways (e.g., calcium, mTOR).
Conclusions: CAi transduces the state of perfusion to intracellular signaling.
Significance: Coupling between environment and cell behavior may influence cancer progression.
Keywords: Calcium, Cancer Biology, Metabolism, Perfusion, pH Regulation, Proton Transport, Signal Transduction, Signaling
Abstract
Carbonic anhydrase (CA) enzymes catalyze the chemical equilibration among CO2, HCO3− and H+. Intracellular CA (CAi) isoforms are present in certain types of cancer, and growing evidence suggests that low levels correlate with disease severity. However, their physiological role remains unclear. Cancer cell CAi activity, measured as cytoplasmic CO2 hydration rate (kf), ranged from high in colorectal HCT116 (∼2 s−1), bladder RT112 and colorectal HT29, moderate in fibrosarcoma HT1080 to negligible (i.e. spontaneous kf = 0.18 s−1) in cervical HeLa and breast MDA-MB-468 cells. CAi activity in cells correlated with CAII immunoreactivity and enzymatic activity in membrane-free lysates, suggesting that soluble CAII is an important intracellular isoform. CAi catalysis was not obligatory for supporting acid extrusion by H+ efflux or HCO3− influx, nor for maintaining intracellular pH (pHi) uniformity. However, in the absence of CAi activity, acid loading from a highly alkaline pHi was rate-limited by HCO3− supply from spontaneous CO2 hydration. In solid tumors, time-dependence of blood flow can result in fluctuations of CO2 partial pressure (pCO2) that disturb cytoplasmic CO2-HCO3−-H+ equilibrium. In cancer cells with high CAi activity, extracellular pCO2 fluctuations evoked faster and larger pHi oscillations. Functionally, these resulted in larger pH-dependent intracellular [Ca2+] oscillations and stronger inhibition of the mTORC1 pathway reported by S6 kinase phosphorylation. In contrast, the pHi of cells with low CAi activity was less responsive to pCO2 fluctuations. Such low pass filtering would “buffer” cancer cell pHi from non-steady-state extracellular pCO2. Thus, CAi activity determines the coupling between pCO2 (a function of tumor perfusion) and pHi (a potent modulator of cancer cell physiology).
Introduction
The chemical components of carbonic buffer (CO2/HCO3−) participate in metabolic reactions and in pH homeostasis (1–3). Due to rapid permeation of CO2 gas across cell membranes (4), carbonic buffer is present in both intra- and extracellular tissue compartments. On the time scale of cellular physiology, the spontaneous equilibration of carbonic buffer is relatively slow, as exemplified by a CO2 hydration time constant of several seconds (rate constant kf = 0.18 s−1) (5). Consequently, biological processes that involve a change in the concentration of CO2, HCO3− or H+ can become rate-limited by carbonic buffer re-equilibration. This limitation has presumably driven the evolution of at least a dozen mammalian carbonic anhydrase (CA)2 isozymes that accelerate CO2/HCO3− equilibration (6, 7).
The CAs are grouped as intra- (CAi) or extracellular (CAe) depending on the orientation of the catalytic site (5–8). Activity assays and immunotechniques have identified CAi and CAe isoforms in cancer cells (9–17). Physiologically, CAe isoforms, such as CAIX and CAXII, facilitate CO2 and H+ diffusion across the continuous and tortuous interstitial space (18, 19). Thus, CAe activity can improve the venting of acidic products of metabolism over the long diffusion distances found in inadequately perfused solid tumors, allowing their faster growth (20, 21). The role of CAi isoforms in cancer physiology is still debated. Down-regulation of gap junctions in cancer cells prevents the intracellular compartment from becoming syncytial (22), and this restricts the spatial range over which CAi activity could facilitate CO2 or H+ diffusion. Previously, it has been suggested that CAi activity facilitates the transport of HCO3− or H+ ions across membranes by reducing the extent to which cytoplasmic reactions slow the delivery or removal of the transported ion (23–26). To benefit from CA activity, these transporter-evoked H+ or HCO3− fluxes would have to exceed the spontaneous chemical re-equilibration kinetics of carbonic buffer (27).
Another source of disturbance to carbonic buffer equilibrium is fluctuating CO2 partial pressure (pCO2). Cancer cells produce large quantities of CO2 from titration of acids (e.g. lactic) with HCO3− and decarboxylation by mitochondria and the pentose phosphate shunt (28). Ultimately, the excess CO2 must be removed with the blood flow. In tumors, vasomotion and hemodynamic factors can produce cycles of intermittent blood flow, commonly observed with periodicities of several minutes (29–33). Unstable perfusion is the basis for acute hypoxia (32, 34–40), characterized by oxygenation-reoxygenation cycles as fast as 2/min (30) and amplitudes of tens of mmHg O2 (30, 35, 41, 42). Episodes of inadequate blood flow produce “closed” pockets of blood, which become oxygen-depleted and accumulate CO2 (43–45). Periodic restoration of flow returns pCO2 and pO2 to normal. The resulting fluctuations in pCO2 (mirroring pO2) are transmitted across the intra- and extracellular compartments because CO2 gas (unlike H+ or HCO3− ions) crosses membranes rapidly (4). Due to reversible intracellular CO2 hydration, pHi will respond to pCO2 fluctuations, but the coupling between these depends critically on CAi activity. Because cell behavior is strongly modulated by H+ ions (46–48), CAi activity may act as an important transducer between blood flow and cell signaling.
The aim of the present study was to measure CAi activity in the native environment of intact cancer cells and to identify the physiological processes, relevant to cancer, that depend on CAi activity. We describe a novel role for CAi activity in sensitizing cancer cell pH to pCO2 changes, thereby linking metabolism and perfusion with pHi-responsive signaling cascades.
EXPERIMENTAL PROCEDURES
Cell Lines and Cell Culture
Cancer cell lines were obtained from ATCC (colorectal HCT116), Cancer Research UK (bladder RT112, colorectal HT29, fibrosarcomal HT1080, and breast MDA-MB-468) or were a gift from Professor Silvia Pastorekova, Bratislava, Slovakia (cervical HeLa). Fibroblast cell lines were obtained from Lonza (NHDF-Ad adult dermal fibroblasts and InMyoFib intestinal myofibroblasts) or ATCC (normal colon CCD-112-Con and carcinoma-derived Hs675.T fibroblasts). Cells were grown in HCO3−-containing DMEM in 5% CO2 and 21% O2 for 48 h until 80–100% confluent (Galaxy 48R, New Brunswick). For some experiments, cells were cultured in the presence of 1 mm dimethyl oxalylglycine (DMOG) dissolved in DMSO.
CAII Knockdown
CA2 gene expression in HCT116 cells was silenced using one of four shRNA constructs cloned into psi-LVRU6-GFP lentiviral vector targeting the 351st, 493rd, 597th, and 695th position of CA2 mRNA (NCBI Reference Sequence NM_000067). All constructs, including scrambled-eGFP, were purchased from Genecopoiea. Stable HCT116 cell clones with silenced CA2 gene expression were selected in the presence of 2 μg/ml puromycin for 2 weeks, and selected clones were pooled together for experiments.
Western Blotting
Cells were lysed at 4 °C (1% Triton X-100, 0.5% Nonidet P-40 substitute (Applichem), 150 mm NaCl, 50 mm NaF, 50 mm Tris-HCl (pH 7.5) plus Roche Applied Science protease inhibitor mixture) and pelleted at 13,000 rpm for 20 min at 4 °C. Proteins were resolved by SDS-PAGE, transferred to a PVDF membrane (Bio-Rad), and detected using mouse monoclonal M75 antibody against human CAIX (a gift from Prof. Pastorekova; Ref. 49); antibodies against CAI, -III (R&D Systems), -II (Novus), -VII (AbD Serotech), and -XIII (Abcam); and goat polyclonal antibody to actin (Santa Cruz Biotechnology). Kinase phosphorylation in cells cultured to high density was measured with the phospho-MAPK array profiler (R&D Systems) and mechanistic target of rapamycin (mTOR) signaling kit (Cell Signaling Technology).
Measuring Carbonic Anhydrase Activity in Lysates
Cells were lysed by repeated freeze-thaw cycles in buffer containing 140 mm potassium gluconate, 0.5 mm EGTA, 1 mm MgCl2, 15 mm Hepes, 15 mm Mes at pH 7.8 (4 °C), and protease inhibitor. Membranes were removed by centrifugation (20 min at 15,000 rpm at 4 °C), and the supernatant was diluted to a total protein concentration between 1 and 10 mg/ml (Bradford assay). The CA-catalyzed reaction was triggered by adding 0.33 ml CO2-saturated water to 0.67 ml of lysate in a stirred chamber at 4 °C. The time course of pH (Hamilton Biotrode) was fitted with a kinetic model (19) to obtain the CO2 hydration rate constant kf.
Superfusion
CO2/HCO3−-free solution contained 125 mm NaCl, 20 mm Hepes (pH to 7.4 with NaOH), 4.5 mm KCl, 11 mm glucose, 1 mm CaCl2, and 1 mm MgCl2. For CO2/HCO3−-buffered solution, Hepes was replaced with 22 mm NaHCO3, and the solution was bubbled with either 5% CO2 (pH 7.4) or 20% (pH 6.8). For Cl−-free solutions, Cl− was replaced with gluconate. For acetate-containing solution, 80 mm NaCl was replaced with 80 mm sodium acetate. For NH4+-containing solution, 20 mm NaCl was replaced with 20 mm NH4Cl. Cells were superfused (37 °C at 4 ml/min) in a poly-l-lysine-treated Perspex chamber (solution exchange time constant <3 s) except for fluctuating pCO2 protocols, which were performed in 2-ml Lab-Tek chambers (Nunc) that allow a more gradual solution exchange.
Fluorescence Measurements of Intracellular pH and Calcium
Cells were imaged confocally using a Zeiss LSM 700 confocal system on an Axiovert inverted microscope. To measure pHi, cells were AM-loaded for 10 min with 10 μm cSNARF1 (excitation, 555 nm; emission, 580 and 640 nm). The fluorescence ratio was converted to pHi using a calibration curve obtained by the nigericin method (50). Calibration was performed twice a year for each cell line, and the stability of the curve was tested by the null point method (51). Membrane H+ (or H+-equivalent) flux was calculated as the rate of pHi change × buffering capacity (3). Intrinsic buffering capacity was measured in separate experiments using the graded ammonium removal technique (3). CO2/HCO3−-dependent buffering was estimated using the equation for an open buffer system: 2.303 × [HCO3−]i. Intracellular [HCO3−] was calculated by best fitting a mathematical model of pHi regulation to the time course of pHi recovery from an acid or base load (see below). This approach derives instantaneous carbonic buffering capacity (rather than assuming that the buffer is distributed at equilibrium across the cell membrane at all times). To measure [Ca2+]i, cells were AM-loaded for 20 min with 50 μm Fluo3 (excitation, 488 nm; emission, >510 nm). For co-cultures, regions with distinct areas of cancer cells and fibroblasts (distinguished by cell morphology and size) were selected for imaging.
Mathematical Modeling
A mathematical model of pHi control was parameterized using data for buffering capacity (β) and membrane transport fluxes of H+ and HCO3− ions (JH and JHCO3) (3). The carbonic buffer equilibrium constant, KCO2, was 10−6.1 m, and the spontaneous CO2 hydration rate constant (kf) was 0.18 s−1 (the reverse rate constant, kr, was kf/KCO2). CAi activity was represented as a dimensionless scalar (ca). Surface area/volume ratio was 1/h where h is the monolayer height (10 μm). CO2 permeability, p, was 104 μm/s (4). pCO2 was a sinusoidal function, f, of amplitude A, frequency φ, and baseline of 5% CO2. The equations are as follows.
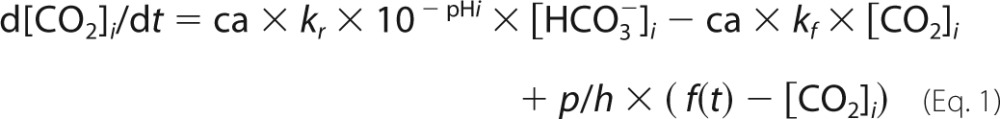 |
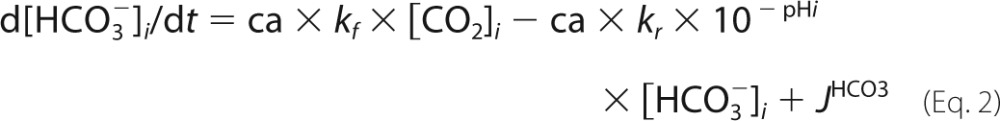 |
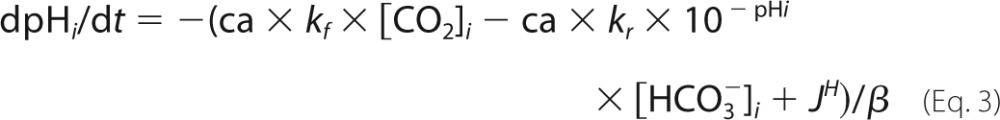 |
RESULTS
CAi Activity in Cancer and Fibroblast Cell Lines
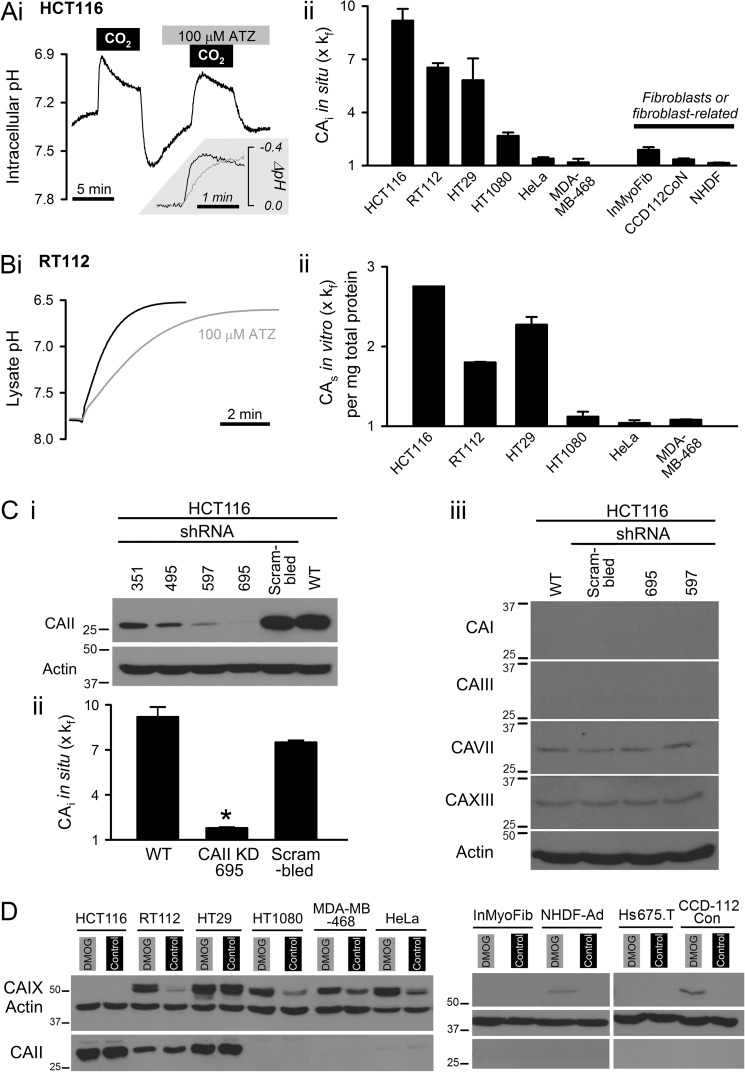
Total CAi activity was measured in intact cells superfused continuously with physiological salt solution at body temperature. The CAi-catalyzed reaction was triggered by switching between CO2/HCO3−-free and 5% CO2/22 mm HCO3−-buffered superfusates. The time constant of solution exchange (2.7 s; determined by fluorescently labeling one solution with 30 μm fluorescein) was adequately fast to ensure that pCO2 was manipulated rapidly. CAi-catalyzed reaction kinetics were determined from the initial rate of pHi change (reported using the fluorescent pH indicator cSNARF1 AM-loaded into cells) and intrinsic buffering capacity (52). Fig. 1A, panel i, shows an example pHi time course recorded from colorectal HCT116 cells paired with an experiment performed in the presence of the broad spectrum CA inhibitor acetazolamide (ATZ; 100 μm) to determine spontaneous kinetics over a matching pHi range. CAi activity was expressed relative to spontaneous reaction kinetics (Fig. 1A, panel ii). Experiments were also performed on bladder RT112, colorectal HT29, fibrosarcoma HT1080, cervical HeLa, and breast MDA-MB-468 cells as well as dermal NHDF-Ad, colonic CCD-112-CoN fibroblasts, and intestinal InMyoFib myofibroblasts. CAi activity ranged from ∼9-fold above the spontaneous rate in HCT116 cells to very low in HeLa, MDA-MB-468, and fibroblast/fibroblast-related cells. Thus, the CO2 hydration capacity of cancer cell cytoplasm spanned over an order of magnitude in range and was cell line-dependent.
FIGURE 1.
Intracellular carbonic anhydrase activity. A, panel i, determination of cytoplasmic CO2 hydration rate from pHi dynamics in intact HCT116 cells (AM-loaded with pH dye cSNARF1). Switching between CO2/HCO3−-free and 5% CO2, 22 mm HCO3−-containing superfusate triggers intracellular CO2 hydration. The experiment was repeated with 100 μm ATZ to inhibit CA. Inset, pHi response to CO2 addition. Panel ii, CAi activity in cancer and fibroblast/fibroblast-related cells (n > 20 each), normalized to measurements in the presence of ATZ (kf = 0.18 s−1). B, panel i, measuring CA activity in membrane-free RT112 lysates. Panel ii, CAs activity normalized to spontaneous rate determined in ATZ (expressed per mg/ml of total protein). C, panel i, knockdown of CAII in HCT116 cells using one of four shRNA constructs, scrambled shRNA (sham) and non-transfected wild type (WT). *, p < 0.05, relative to WT. Panel ii, total CAi activity in knockdown, sham, and WT HCT116 cells (n > 20 each). Panel iii, Western blot for soluble CA isoforms other than CAII in HCT116 cells treated with shRNA. D, Western blot for CAII and CAIX under control (normoxic) incubation or 48 h in 1 mm dimethyl oxalylglycine (DMOG). Error bars represent S.E.
Soluble cytoplasmic CA (CAs) isoforms (e.g. CAI, -II, -III, -VII, and -XIII) are plausible contributors to CAi activity. This was tested in cell lysates after removing membranes by centrifugation. Lysates were injected with CO2-saturated water, and the CO2 hydration constant was estimated from the time course of medium pH at 4 °C (Fig. 1B, panel i). CAs activity was quantified in terms of the CO2 hydration rate constant relative to the measurement in the presence of ATZ (100 μm). Kinetic data were normalized to total protein concentration (19). Substantial CAs catalysis was detected in lysates of cancer cells with high total CAi activity determined in intact cells. This argues that soluble isoforms underlie at least part of intracellular CO2 hydration catalysis (Fig. 1B, panel ii). The contribution of CAII to total CAi activity was tested in HCT116 cells by knockdown using shRNA constructs 351, 495, 597 and 695 and compared with scrambled shRNA and wild-type cells. Construct 695 resulted in the most substantial decrease in CAII immunoreactivity (Fig. 1C, panel i), and it reduced total CAi activity by ∼90% relative to wild-type (Fig. 1C, panel ii). To test whether the decrease in total CAi activity is attributable to disruption of CAII alone, the specificity of shRNA constructs 695 and 597 was investigated. Constructs 695 and 597 have very low sequence homology (16–42%) with membrane-associated CAs (IV, IX, XII, and XIV) and low homology (53–68%) with soluble isoforms other than CAII (I, III, VII, and XIII). Of the soluble isoforms, HCT116 expressed only low levels of CAVII and -XIII, and immunoreactivity for CAI and -III was absent (Fig. 1C, panel iii). Constructs 695 and 597 did not alter this expression pattern, indicating that the knockdown experiment reduced CAi activity by targeting CAII selectively. The findings indicate that CAII is a principal contributor to CAi activity in HCT116 cells.
Across the tested cancer, fibroblast, and fibroblast-related cells, CAII immunoreactivity (Fig. 1D) correlated with in situ CAi activity (Pearson's correlation coefficient r2 = 0.83). CAII expression was notably absent in fibroblast and fibroblast-related cells, including cancer-derived Hs675.T cells. In cancer cells, CAII expression correlated strongly (r2 = 0.97) with CAs activity measured in membrane-free lysates. As expected from active site topology, the expression of membrane-tethered CAIX did not correlate with CAi activity. Hypoxia plays an important role in cancer biology by regulating gene expression through mechanisms that include hypoxia-inducible factor. To investigate the effect of long term hypoxia on CAII expression, Western blot analysis was performed on cells incubated for 48 h with the hypoxia-inducible factor-stabilizing drug dimethyl oxalylglycine (3, 53). This treatment did not affect CAII expression but produced the expected induction of CAIX in RT112, HT1080, MDA-MB-468, and HeLa (54) and to a lesser degree in NHDF-Ad and CCD-112-CoN fibroblasts.
Role of CAi Activity in pHi Regulation
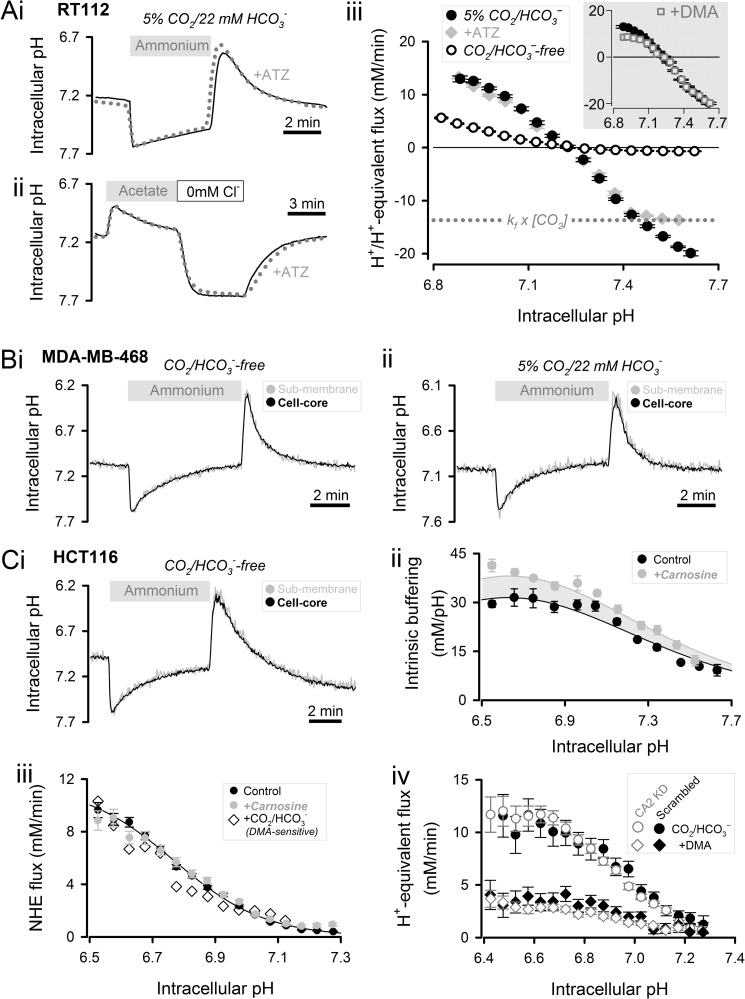
The supply of H+ or HCO3− ions for pHi-regulatory transport across membranes may be rate-limited by the CAi-catalyzed reaction. This was investigated in RT112, MDA-MB-468, and HCT116 cells.
RT112 cells have naturally high CAi activity and can produce large HCO3− fluxes at both low and high pHi (3). Acid extrusion was evoked at low pHi by means of an “ammonium prepulse” solution maneuver performed on cSNARF1-loaded cells (Fig. 2A, panel i). An “acetate prepulse” was performed to raise pHi. Because abrupt base loading of cytoplasm upon acetate removal drives carbonic buffer out-of-equilibrium, cells were superfused briefly in Cl−-free medium to allow buffer re-equilibration (55). Subsequent re-exposure to Cl−-containing superfusates activated acid loading transporters (Fig. 2A, panel ii). H+/H+-equivalent flux was calculated as the product of the rate of pHi change and buffering capacity (the carbonic buffer component was estimated using a mathematical model to predict out-of-equilibrium behavior during dynamic pHi regulation). pHi-regulatory fluxes, shown in Fig. 2A, panel iii, were symmetrical around the resting pHi. To establish the contribution of Na+/H+ exchange (NHE) to pHi regulation, measurements were repeated in the presence of 30 μm 5-(N,N-dimethyl)amiloride (DMA). Acid loading was DMA-insensitive, whereas acid extrusion flux was reduced only modestly, confirming a minor role for NHE in pHi regulation in RT112 cells (Fig. 2A, panel iii, inset). Replacing superfusate carbonic buffer with Hepes reduced flux substantially, indicating that pHi regulation relies principally on HCO3− transport. The effect of CAi activity on acid/base membrane transport was inferred from the effect of 100 μm ATZ (in the presence of carbonic buffer). Although ATZ targets both intra- and extracellular CAs, the latter isoforms would not be exerting a net catalytic effect because superfusion presents cells with pre-equilibrated buffer at all times (19). ATZ did not affect resting pHi or acid extrusion flux, but it limited acid-loading fluxes to no greater than ∼14 mm/min. This flux is equal to the maximal rate of HCO3− delivery by CO2 hydration under spontaneous reaction kinetics (kf × [CO2] = 14 mm/min). Thus, acid loading by means of HCO3− export can be rate-limited by CAi activity, but this requires large fluxes evoked by substantially raised pHi.
FIGURE 2.
Role of CAi activity in membrane H+/HCO3− transport and cytoplasmic H+ diffusion. A, 20 mm ammonium (panel i) and 80 mm acetate (panel ii) prepulse performed on RT112 cells (5% CO2/22 mm HCO3− superfusate). Experiment repeated in 100 μm ATZ (dotted line). Following acetate removal, cells were stabilized in Cl−-free superfusate to allow for CO2/HCO3− re-equilibration. Panel iii, acid-extrusion and acid-loading fluxes (n > 20). Dashed line, limit of spontaneous production of HCO3−. Inset, effect of 30 μm DMA. B, 20 mm ammonium prepulse performed on MDA-MB-468 cells in the absence (panel i) or presence (panel ii) of 5% CO2/22 mm HCO3−. Cytoplasmic pH was measured in concentric layers of thickness ∼1.5 μm near surface membrane (black trace) and at cell core (gray trace). Mean of 10 cells (error bars not shown). C, panel i, 20 mm ammonium prepulse performed on HCT116 cells in the absence of CO2/HCO3−. Panel ii, intrinsic buffering capacity (measured by graded ammonium removal) in control HCT116 cells and cells cultured with 6 mm carnosine for 48 h (n > 20). Panel iii, NHE flux (n > 20) in control (CO2/HCO3−-free), in carnosine-loaded cells (CO2/HCO3−-free), and in the presence of 5% CO2/22 mm HCO3− (determined by subtracting the DMA-sensitive component of flux). Panel iv, H+-equivalent flux in CO2/HCO3− buffer measured in CAII knockdown HCT116 cells (construct 695) and compared with scrambled (sham). Experiments were repeated in DMA to inhibit the NHE component of acid extrusion (n > 15). Error bars represent S.E.
Intracellular H+ ions diffuse considerably slower than expected from their low atomic weight because of extensive binding to larger buffer molecules, including immobile proteins (56, 57). NHE activity could plausibly be rate-limited by a slow diffusive supply of H+ ions and therefore require CAi activity for delivering H+ ions from CO2 hydration. As shown in non-cancerous cells, CAi activity can facilitate H+ diffusion by improving the reaction kinetics of CO2/HCO3−, a mobile buffer (58, 59). If the intrinsic H+ ion diffusivity in cytoplasm were sufficiently restricted, a large acid/base flux at the surface membrane would be expected to produce measurable pHi non-uniformity in the absence of carbonic buffer. This was tested in MDA-MB-468 cells, which produce very high NHE fluxes at low pHi (3). Cells were trypsinized to produce spherical cells with the smallest possible surface area-to-volume ratio, i.e. longest average surface-to-core diffusion distance. The mean radius of MDA-MB-468 cells was 7.11 ± 0.12 μm, which is typical of most cancer cells. NHE is functional in the presence and absence of carbonic buffer; therefore it is possible to investigate the effect of removing CO2/HCO3− on spatial H+ dynamics in cytoplasm. pHi uniformity during rapid acid extrusion was assessed by imaging pHi confocally near the surface membrane and at the core of the cell. The pHi time courses shown in Fig. 2B, panel i, indicate that even at maximal acid extrusion rates, pHi remained uniform in the absence of carbonic buffer. The addition of carbonic buffer did not affect the degree of pHi uniformity (Fig. 2B, panel ii). Thus, carbonic buffer and hence CAi activity are not required for ensuring adequate H+ diffusion in MDA-MB-468 cells. These findings are indicative of good diffusive coupling by intrinsic H+ buffers.
Measurements of pHi gradients in bulk cytoplasm may not identify diffusion barriers in the immediate environment of the transport protein. An example of such a barrier is slow H+ transfer from immobile buffers to the transporter proteins (60, 61). Given that CO2 is a highly penetrating source of H+ ions (26), a role for CAi activity in overcoming these localized H+ barriers is conceivable. To test for possible submembrane barriers, acid extrusion was measured in HCT116 cells, which have naturally high CAi activity and the capacity to produce large NHE fluxes (3). If CAi activity were important for delivering H+ ions to NHE protein, then removal of carbonic buffer would slow overall flux to a baseline set by the intrinsic capacity of the cytoplasm to supply the transporter with H+ ions. Loading cytoplasm with exogenous mobile buffers, such as imidazoles, would then be expected to rescue acid extrusion (60). Fig. 2C, panel i, shows the time course of pHi recovery from low pHi in the absence of CO2/HCO3−. This eliminates CO2-sourced delivery of H+ ions and would expose any submembrane diffusional barriers. Experiments were repeated on cells loaded with the imidazole-containing mobile buffer carnosine (6 mm for 48 h). Intrinsic buffering capacity, measured by the “graded ammonium removal” technique (62), was higher in carnosine-incubated cells, consistent with the loading of cytoplasm with an exogenous buffer of pK 6.7 (similar to that of carnosine) and concentration of 12 mm (Fig. 2C, panel ii). After accounting for the additional buffering capacity, flux in carnosine-loaded cells was no different from control (Fig. 2C, panel iii). Further experiments were performed on control HCT116 cells superfused with carbonic buffer. Because HCO3−-dependent mechanisms (e.g. Na+-HCO3− co-transport) are activated in the presence of carbonic buffer, NHE-mediated flux was calculated by subtracting the DMA-sensitive component from the total flux. Neither CO2/HCO3− nor carnosine increased NHE flux (Fig. 2C, panel iii), arguing that these additional mobile buffers are not required for delivering H+ ions to NHE. Thus, CAi catalysis is unlikely to accelerate transport by providing an additional route of H+ delivery. An earlier study had suggested that imidazole groups projecting from the CAII molecule deliver H+ ions to H+ transporters without involving the catalytic process (60). Because large acid extrusion fluxes could be produced by CAII-negative MDA-MB-468 cells, an acatalytic effect of CAII is unlikely to be an absolute requirement for fast NHE activity. This was explored further in CAII knockdown HCT116 cells (construct 695; Fig. 1C, panel i). Acid extrusion in carbonic buffer, which includes NHE and HCO3−-dependent components, was not different in CAII-negative cells compared with scrambled controls (Fig. 2C, panel iv). The HCO3−-dependent flux component, measured by inhibiting NHE with DMA, was also unaffected by CAII knockdown (Fig. 2C, panel iv).
Role of CAi Activity during Fluctuations in pCO2
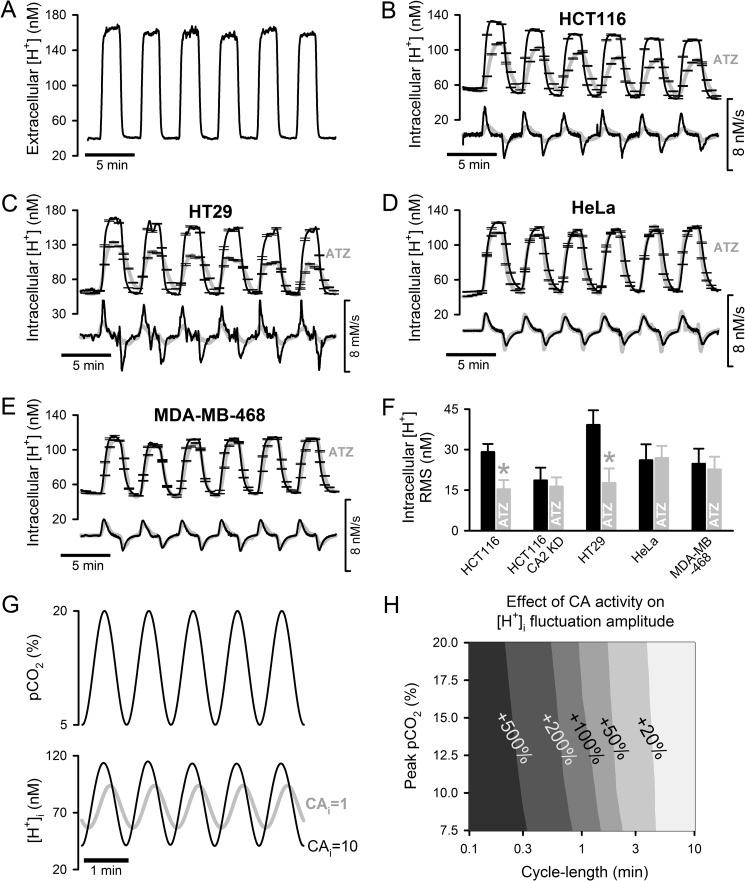
To explore the effects of CAi activity during pCO2 fluctuations associated with intermittent blood flow, superfusion of confluent monolayers (grown in slow exchange chambers) was alternated between 5% CO2/22 mm HCO3− (pH 7.4; representing normal arterial blood plasma) and 20% CO2/22 mm HCO3− (pH 6.8; representing respiratory acidosis) with a periodicity of 4 min. By labeling one solution with fluorescein (30 μm) in separate experiments, the solution mixing interval was estimated to be ∼15 s, which allows for a relatively slow transition between 5 and 20% CO2. Fig. 3A shows the time course of extracellular [H+] in the superfusion chamber reported by fluorescein (an indicator of solution pH) added to both solutions at 30 μm. The response of intracellular [H+] ([H+]i) to pCO2 fluctuations was recorded in cSNARF1-loaded monolayers of cells with high CAi activity (HCT116 and HT29; Fig. 3, B and C), low CAi activity (HeLa and MDA-MB-468; Fig. 3, D and E), and CAII knockdown HCT116 (CAII-KD). The measured [H+]i fluctuations were quantified in terms of root mean square in Fig. 3F. Experiments were repeated in the presence of ATZ (100 μm) to inhibit CA activity. The temporal behavior of [H+]i was ATZ-sensitive in HCT116 and HT29 cells but not in HeLa, MDA-MB-468, or CAII knockdown HCT116 cells (Fig. 3F). These responses did not correlate with normoxic CAIX expression (high in MDA-MB-468 and HT29 and low in HeLa and HCT116), and therefore cannot be attributed to the inhibition of exofacial CA isoforms. Thus, in cells with high CAi activity, [H+]i fluctuated faster and over a larger range, demonstrating a tighter coupling between pCO2 and cytoplasmic [H+].
FIGURE 3.
Effect of CAi activity on cytoplasmic pH dynamics during fluctuating pCO2. pCO2 was changed between 5 and 20%. A, extracellular pH reported using fluorescein (30 μm). B, intracellular [H+] measured in cSNARF1-loaded HCT116 monolayers with naturally high CAi activity. The protocol was repeated in 100 μm ATZ. The rate of change, d[H+]/dt, is plotted below the [H+] time course. An average of >20 cells was analyzed. Experiments were also performed on HT29 monolayers with naturally high CAi activity (C), HeLa (D), and MDA-MB-468 monolayers with naturally low CAi activity (E). F, intracellular [H+] changes quantified as root mean square (RMS). *, p < 0.05, relative to control. G, simulated sinusoidal waveform of pCO2 and its effect on intracellular [H+]. H, model simulation of the effect of CA activity (10-fold acceleration of hydration) on peak-to-nadir [H+]i amplitude (percentage increase). Error bars represent S.E.
The relationship between pCO2 and [H+]i was explored further using a mathematical model parameterized using data for CAi activity and buffering capacity in HCT116 cells (3). Smooth transitions between normal and raised pCO2 were simulated with a sinusoidal wave (Fig. 3G) over a range of periodicity and amplitude. Fig. 3H shows the predicted effect of 10-fold CAi activity on the size of [H+]i fluctuations. [H+]i fluctuations were amplified 30% for periodicities of 4 min and at least doubled for periodicities of <2 min. The relative effect of CAi activity on [H+]i dynamics was essentially independent of pCO2 amplitude. pHi-regulatory H+ and HCO3− membrane transport is expected to curtail the extent of [H+]i changes, but mathematical modeling (not shown) predicts this to be a minor effect because the magnitude of membrane H+/HCO3− transport is considerably smaller than that of CO2 flux, particularly for short pCO2 wave periodicities.
In summary, CAi activity sensitizes cancer cell pH to fluctuations in pCO2. In contrast, cytoplasm with low CAi activity behaves as a low pass filter that dampens [H+]i changes. Essentially all biological processes are pH-sensitive; therefore the effect that CAi activity has on [H+]i dynamics during pCO2 fluctuations is potentially of major functional importance. This was explored by measuring Ca2+ dynamics and kinase activity as examples of intracellular signaling.
Ca2+ Signaling
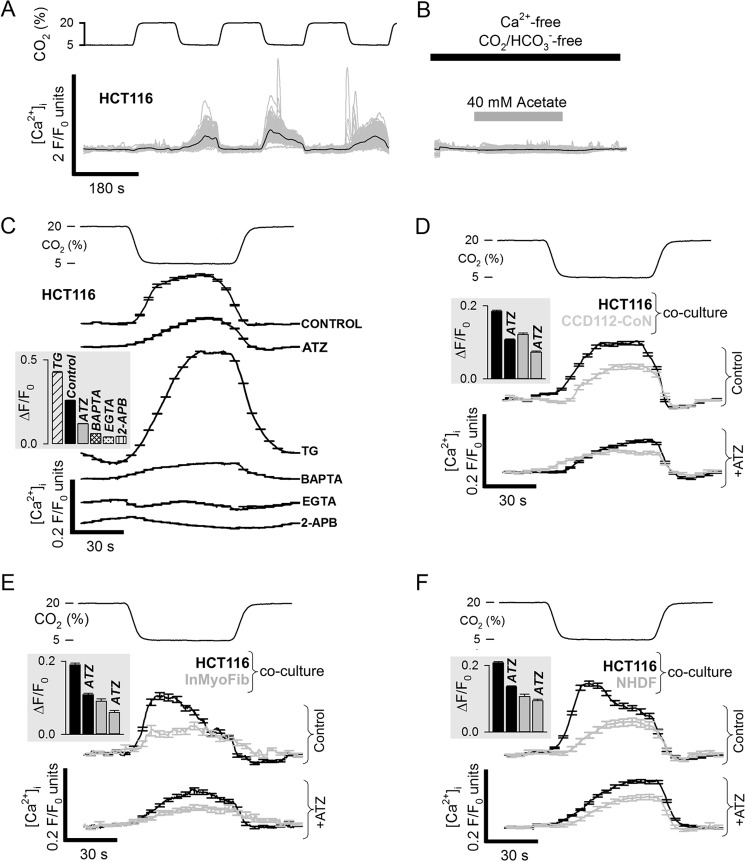
The behavior of the signaling ion Ca2+ was measured in Fluo3-loaded HCT116 cells during oscillations of pCO2 between 5 and 20% (4-min periodicity). A significant increase in Fluo3 fluorescence was observed upon returning pCO2 from 20 to 5% (Fig. 4A). Fluo3 fluorescence is modestly pH-sensitive, and to assess the magnitude of this artifact, HCT116 cells superfused in Ca2+-free and CO2/HCO3−-free media were exposed to 40 mm acetate for 4 min to evoke a pHi change comparable to that caused by the pCO2 fluctuations. Fluo3 fluorescence did not change substantially during this protocol (Fig. 4B), arguing that the responses shown in Fig. 4A report a genuine rise of cytoplasmic [Ca2+] ([Ca2+]i). The mechanism of this response was explored using inhibitor drugs and Ca2+ buffers (Fig. 4C). The Ca2+ response was dampened in cells AM-loaded with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA; 100 μm), adding to the evidence that Fluo3 reports [Ca2+]i changes rather than pHi. Responses were considerably greater when intracellular Ca2+ stores were depleted by pretreatment with 10 μm thapsigargin (sarco/endoplasmic reticulum Ca2+-ATPase inhibitor). In the absence of extracellular Ca2+ (nominally Ca2+-free; residual Ca2+ buffered with 1 mm EGTA), the Fluo3 response was abolished, arguing that Ca2+ influx is required. Collectively, these data suggest that cytoplasmic alkalinization evokes Ca2+ influx through store-operated channels, such as Orai (64). In support of this, 2-aminoethoxydiphenyl borate (100 μm) blocked the Ca2+ response. The CO2-evoked [Ca2+]i response was dampened in the presence of 100 μm ATZ, illustrating a role for CAi activity in accentuating a cellular response to changing pCO2. Experiments on co-cultures of HCT116 cells with fibroblasts of low CAi activity (NHDF-Ad, CCD-112-CoN, InMyoFib; Fig. 4, D–F) confirmed that CAi activity can influence the Ca2+ response to pCO2 fluctuations in a cell-dependent manner.
FIGURE 4.
Effect of CAi-mediated CO2-pH coupling on Ca2+ signaling. A, intracellular Ca2+ (Fluo3 F/F0 ratio) during 5–20% CO2 oscillations (cycle length of 4 min). B, transient pHi change evoked by acetate (in the absence of extracellular Ca2+ and absence of CO2/HCO3−) did not evoke a Fluo3 response. C, Fluo3 response recorded once consistent cyclic behavior had been attained. The response was reduced in 100 μm ATZ or in cells AM-loaded with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) (Ca2+ buffer; 100 μm), abolished by removing extracellular Ca2+ (replaced with EGTA Ca2+ buffer) or in 100 μm 2-aminoethoxydiphenyl borate (2-APB), and augmented in 10 μm thapsigargin (TG)-pretreated cells (endoplasmic reticulum depletion). Data are the means of >50 cells. HCT116 cancer cells co-cultured with either CCD112-CoN fibroblasts (D), InMyoFib myofibroblasts (E), or NHDF-Ad fibroblasts (F). The Ca2+ response was larger and ATZ-sensitive in HCT116 cancer cells. Data are the means of >50 cells. Error bars represent S.E.
Kinase Activity
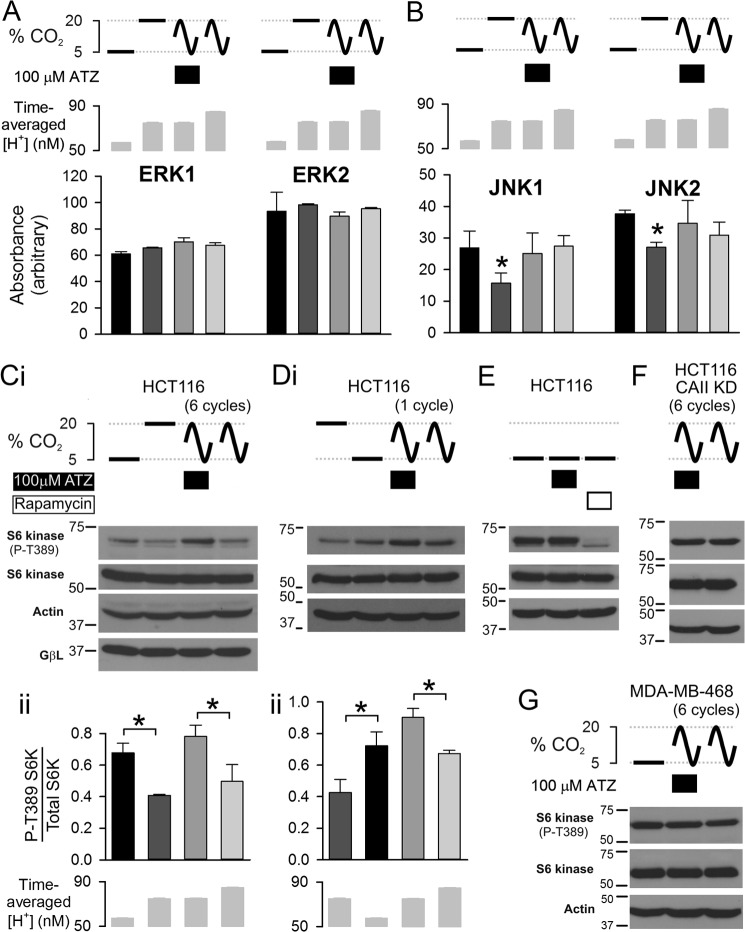
Many kinase-operated signaling pathways are modulated by pH (65) and are expected to respond to changes in pCO2. This was tested in HCT116 cells by measuring the phosphorylation state of the major mitogen-activated kinases, extracellular signal-regulated kinases, c-Jun N-terminal kinases, and p38 isoforms. pCO2 was held constant at 5 or 20% or oscillated between 5 and 20% CO2 for six cycles (4-min periodicity) in the presence or absence of ATZ to manipulate the dynamics of the pHi response (note that at the end of pCO2 oscillations cells were returned to 5% CO2 for 2 min). After 26 min of superfusion, lysates were tested for kinase activity readouts to identify pH-sensitive and pH-insensitive kinases over the pH range studied. Among the kinases investigated, phosphorylation of ERK1 (Thr-202/Tyr-204) and ERK2 (Thr-185/Tyr-187) was unaffected by pCO2 changes (Fig. 5A). In contrast, kinases, such as JNK1 and JNK2, were dephosphorylated (Thr-183/Tyr-185) by stably raised pCO2. Transient exposure to 20% CO2 (i.e. oscillating pCO2) was inadequate to reduce phosphorylation, indicating that a sustained fall in pHi is necessary for eliciting a change in JNK1/2 activity (Fig. 5B). Stably decreased pHi also reduced the activity of mTOR complex 1 (mTORC1), reported as S6 kinase (S6K) phosphorylation at Thr-389 (65–67). Unlike the other kinases tested, mTORC1 signaling was dependent on CAi activity under fluctuating pCO2 protocols (higher S6K phosphorylation in the presence of ATZ; Fig. 5C; note that expression of the mTOR regulator GβL did not underlie differences in S6K phosphorylation). For the four pCO2 protocols, S6K phosphorylation did not correlate with the time-averaged [H+]i (Fig. 5C, panel ii), arguing that the dynamics of pHi must be influencing the mTORC1 pathway. A single, transient (2-min) exposure to 20% CO2 was sufficient for observing an effect of CAi activity on mTORC1 (higher S6K phosphorylation in the presence of ATZ; Fig. 5D). The mTOR inhibitor rapamycin (10 μm) produced a substantial decrease in S6K phosphorylation (Fig. 5E), confirming the validity of using S6K as an mTORC1 readout. At constant (5%) pCO2, ATZ did not affect mTORC1 activity (Fig. 5E), indicating that the ATZ sensitivity of S6K phosphorylation measured under oscillating pCO2 is not an off-target effect of the CA inhibitor. Consistent with this finding, ATZ had no effect on S6K phosphorylation in CAII knockdown HCT116 (Fig. 5F) and wild-type MDA-MB-468 (Fig. 5G), i.e. cells with low CAi activity. In summary, mTORC1 signaling responds to sharp pCO2 changes that are attainable with high CAi activity. These proof-of-principle experiments demonstrate that CAi activity is able to modulate the coupling between extracellular pCO2 (a function of blood flow) and intracellular signaling.
FIGURE 5.
Effects of CAi-mediated CO2-pH coupling on kinase phosphorylation. A, Phosphorylation state of ERK1 (Thr-202/Tyr-204) and ERK2 (Thr-185/Tyr-187) was unaffected by changing pCO2 either tonically (5% or 20% for 30 min) or dynamically (six cycles of 5–20% fluctuations; cycle length of 4 min); densitometric analysis (n = 3). Time-averaged intracellular [H+] was measured in separate experiments. B, JNK1/2 were dephosphorylated (Thr-183/Tyr-185) by stably raised pCO2 but unaffected by oscillating pCO2 (n = 3). C, panel i, Western blot for S6 kinase phosphorylation (P) (readout of mTORC1 signaling) under 5% CO2, 20% CO2, and fluctuating pCO2 (six cycles; 5–20%) in the presence or absence of ATZ (100 μm). Panel ii, pCO2 fluctuations reduced S6 kinase phosphorylation but only under full CAi activity. S6K activity did not correlate with time-averaged [H+] (n = 3). D, panels i and ii, analysis for one cycle of pCO2 oscillation in HCT116 cells. E, at 5% CO2, mTOR activity was unaffected by ATZ but strongly inhibited by rapamycin (10 μm). F, six cycles of pCO2 fluctuations in CAII knockdown (KD) HCT116 cells (construct 695). G, six cycles of pCO2 fluctuations in MDA-MB-468 cells (low CAi activity). Error bars represent S.E. *, p > 0.05.
DISCUSSION
CAi Activity in Cancer Cells
In this study, we quantified CAi activity in a panel of cancer cells and compared these data with results from fibroblast and fibroblast-related cells. Activity measurements were performed under physiological conditions, in the native and undiluted cytoplasmic environment of intact cells, and in the presence of relevant regulatory cytoplasmic influences (Fig. 1A). CAi activity varied considerably among cancer cell lines from essentially absent in HeLa and MDA-MB-468 cells to high in HCT116, HT29, and RT112 cells. Low CAi activity was characteristic of fibroblasts/fibroblast-related cells, arguing that CAi catalysis in solid tumors is more likely to be associated with cancer cells rather than stromal fibroblasts. Soluble (but not secreted) and cytoplasm-facing membrane-tethered CA isoforms may contribute toward the CAi activity. Cancer cells with high total CAi activity were also positive for soluble CA activity in membrane-free cell lysates (Fig. 1B) and for CAII immunoreactivity (Fig. 1D). Genetic knockdown of CAII in HCT116 cells demonstrated that the majority of CAi activity was attributable to CAII (Fig. 1C). Additional isoforms may contribute to CAi activity in other cell lines, such as HT1080 cells that lack CAII expression and soluble CA activity, but have measurable overall CAi. Based on data from all cells investigated, CAII expression correlated strongly with soluble CA activity and was a good predictor of high total CAi activity.
CAi Activity Is Not Universally Rate-limiting for pHi Regulation
The physiological role for CAi activity in cancer is contentious although clearly distinct from that of exofacial CAs (6). Here, we explored the cellular processes that may depend on CAi activity. Previous studies have argued for a role of CAi activity in facilitating H+ or HCO3− transport across membranes (23–26) and H+ diffusion in cytoplasm (58, 59), but these interactions have not been tested robustly in cancer cells. CAi activity in the six cancer cell lines studied does not correlate with resting pHi, NHE flux, or HCO3− transporter flux measured previously in these cells (3). Our present data (Fig. 2) show that the H+ and HCO3− fluxes produced by cancer cells over the physiological pHi range are not of sufficient magnitude to require CAi catalysis, with the exception of acid loading by HCO3− export at high pHi. In the absence of CAi activity, the maximal capacity of CO2 hydration to generate HCO3− ions for extrusion is ∼14 mm/min at 5% CO2 (i.e. kf × [CO2]), and this can be rate-limiting for fast HCO3−-dependent acid-loading transporters as measured in RT112 cells (Fig. 2A, panel iii). However, the highly alkaline intracellular conditions that are required for producing this CAi dependence are unlikely to be typical of cancer cells. CAi-catalyzed hydration of CO2 has recently been proposed to facilitate Na+-HCO3− co-transport in cardiac myocytes by supplying the transport protein with H+ ions for titrating HCO3− (26). However, genetic CAII knockdown (HCT116; Fig. 2C, panel iv) or pharmacological inhibition with ATZ (RT112; Fig. 2A, panel iii) did not affect HCO3−-dependent acid extrusion. This difference may reflect contrasting structural and chemical properties of cardiac and cancer cytoplasm. Alternatively, CAi dependence may only be observed experimentally under high Na+-HCO3− co-transport fluxes, which are attainable in cardiac myocytes (under hyperkalemic stimulation) but not in cancer cells. NHE activity was able to produce rapid H+ extrusion in MDA-MB-468 cells that naturally have very low CAi activity. This argues that CAi-independent delivery of H+ ions does not limit the membrane transport process (Fig. 2B) in agreement with an earlier study on ventricular myocytes (26). In HCT116 cells (high CAi activity), intrinsic cytoplasmic buffers alone supported the same magnitude of NHE flux as measured in the presence of CO2/HCO3− or after loading cells with the highly mobile buffer carnosine (Fig. 2C). Even in the absence of CO2/HCO3− (hence no CAi catalysis), activation of NHE did not evoke measurable pHi non-uniformity. This supports the case that diffusive H+ coupling across cancer cytoplasm is normally adequate with intrinsic (non-carbonic) buffers. Knockdown of CAII did not affect NHE activity in HCT116 cells (measured as the DMA-inhibitable component of flux; Fig. 2C, panel iv), arguing that neither catalysis nor the presence of CAII protein is necessary for high NHE fluxes. Collectively, these observations argue for the absence of rate-limiting barriers to H+ diffusion over the relatively small dimensions of cancer cells (mean radii, <10 μm). Additionally, cancer cells typically down-regulate gap junctions (22), which in other tissues allow for greatly expanded diffusion lengths. The absence of electrical coupling explains why CAi catalysis cannot facilitate CO2 or H+ diffusion appreciably in tumors as this would require HCO3− ions to diffuse relatively freely between cells.
CAi Activity Influences the Degree of Coupling between pCO2 and pHi Dynamics
Most investigations of the role of CAi activity in pHi regulation have been performed under constant pCO2. This condition may be appropriate for well perfused tissues with stable CO2 production and venting but is not representative of metabolically active solid tumors with intermittent blood flow. Fluctuations in tumor blood flow are the basis for time-dependent changes in pO2 (also known as acute hypoxia) that influence tumor biology (34). During periods of inadequate capillary washout, pCO2 rises as O2 is depleted, and these changes reverse upon blood reperfusion. Although direct, high resolution measurements of pCO2 fluctuations are not available because of technical limitations, the inverse correlation between time-averaged pO2 and pCO2 (acidity) is well established (43–45). Our standard experimental protocol of varying pCO2 between 5 and 20% with 4-min periodicity is based on measurements of extracellular pH, blood flow, and pO2 in solid tumors in vivo. The pH of 20% CO2 superfusate is 6.8, which is considered to be typical of the mean extracellular pH of most solid tumors (63, 68); pH 7.4 of 5% CO2 superfusates is normal for blood plasma. Our choice of periodicity is in the range of fluctuation frequencies of blood flow and/or pO2 established by Fourier analysis in tumors in vivo (30, 33, 42).
Changes in pCO2 will have knock-on effects on [H+]i dynamics and hence the many pH-sensitive downstream processes. The coupling between pCO2 and pHi is strongly dependent on CAi as illustrated in Fig. 3 where the same experimental protocol of changing pCO2 between 5 and 20% produced different [H+]i responses depending on CAi activity. [H+]i fluctuations were slow and ATZ-insensitive in cells with very low CAi activity (MDA-MB-468 and HeLa). In contrast, high CAi activity in HCT116 and HT29 cells resulted in accentuated [H+]i fluctuations that were dampened in the presence of ATZ. The effect of ATZ was not due to inhibition of exofacial CAs because (i) superfusates were pre-equilibrated (obviating the need for CAe catalysis) and (ii) [H+]i dynamics were ATZ-insensitive in CAIX-expressing MDA-MB-468 cells.
Mathematical modeling demonstrates that the effect of CAi activity on [H+]i dynamics was important even with a smoother sinusoidal pCO2 waveform (Fig. 3G), which is more representative of undulating blood flow. The ability of CAi to influence [H+]i dynamics was predicted for a wide range of pCO2 waveform amplitudes but required a periodicity of 4 min or less for physiologically meaningful effects (Fig. 3H). This frequency dependence is expected because even at spontaneous reaction rates, [H+]i is able to track slow changes in pCO2. Given that fluctuations of blood flow (29–32) and pO2 (30, 32, 34–41) in solid tumors have been observed with a periodicity as short a 0.5 min (i.e. two cycles/min), a range of effects on [H+]i dynamics could be achieved by regulating CAi activity: from tight temporal pCO2-pHi coupling at high CAi activity to low pass filtering with negligible CAi activity. Cancer cells would be able to tune their pHi responsiveness to changes in pCO2 (blood flow) by regulating CAi activity, e.g. through CAII expression.
Our study explored the functional significance of the CAi dependence of [H+]i dynamics using Ca2+ signaling and kinase-operated cascades as proof-of-principle examples (Fig. 6). In HCT116 cells, a transient pHi rise, evoked by reducing pCO2, increased [Ca2+]i. Our evidence points to store-operated calcium entry as the mechanism triggering the [Ca2+]i response (Fig. 4C). Recent work has demonstrated that store-operated calcium entry is reduced at low pHi because of an uncoupling between the endoplasmic reticulum Ca2+ sensor STIM and the surface membrane Ca2+ channel Orai (64). Because [Ca2+]i responds dynamically to changes in the balance between influx and efflux pathways, pHi sensitivity of Ca2+ entry would produce the observed [Ca2+]i fluctuations. Under CAi inhibition with ATZ, the same pCO2 stimulus evoked a slower and smaller [Ca2+]i response.
FIGURE 6.
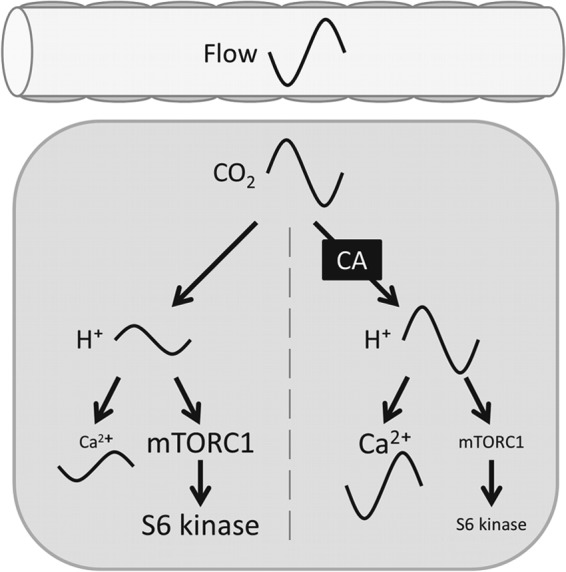
Effect of CAi activity on translating dynamic changes in pCO2 (e.g. due to intermittent blood flow) into pHi dynamics and thenceforth on signaling cascades (e.g. Ca2+ and mTOR signaling).
Previous studies have shown that the environmental sensor mTOR, which is strongly linked with cancer (66, 69, 70), is inhibited at low pHi (65). The present work shows that the degree of mTOR inhibition also depends on CAi activity when pCO2 is fluctuating. In HCT116 cells, a single cycle of pCO2 oscillation produced a lower readout of mTORC1 signaling (S6 kinase phosphorylation) when CAi activity was intact (Fig. 5D). This inhibitory effect of CAi catalysis on mTORC1 signaling was substantiated by expanding the protocol to six cycles of pCO2 fluctuations (Fig. 5C). mTORC1 signaling was generally higher when pCO2 oscillations were performed on HCT116 cells with pharmacologically (ATZ) or genetically (knockdown; Fig. 5F) reduced CAi activity or on cells with naturally low CAi activity, such as MDA-MB-468 (Fig. 5G). mTORC1 activity was not a unique function of time-averaged pHi. For example, holding pCO2 stably at 20% or fluctuating pCO2 between 5 and 20% in the presence of ATZ produced a similar time-averaged pHi, but S6 kinase phosphorylation differed by a factor of 2 (Fig. 5C, panel ii). We conclude that the dynamics of pHi changes must influence mTORC1. Akin to the notion that transient hypoxia (perfusion-limited) and stable hypoxia (diffusion-limited) have distinct biological consequences (71), there may be similar “frequency modulation” and “amplitude modulation” (72) aspects of H+ signaling in cancer. A possible explanation why some kinases (such as JNK1/2) do not respond to fluctuating pCO2 is a slow binding of H+ ions to modulatory sites that acts like a low pass filter. mTOR may be able to register rapid pHi fluctuations by faster H+ binding kinetics. Further studies are necessary to explore these possible mechanisms.
CAi-catalyzed CO2 hydration will produce fluctuations in [HCO3−] that parallel pHi changes, and it is plausible that HCO3− ions interact with targets in a pH-independent manner. Removing HCO3− ions from cytoplasm also removes the substrate (CO2) for CAi; therefore a simple buffer substitution experiment would not be an appropriate test to distinguish the effects of H+ and HCO3− ions. However, it is generally accepted that H+ ions are more reactive than HCO3− ions. Furthermore, earlier observations of the acid response of store-operated calcium entry (64) and mTORC1 (65) were made in the absence of CO2/HCO3−, arguing for H+ ions as the key regulators.
Growing evidence points to a negative correlation between the expression of CAi isoforms, such as CAII, and cancer disease severity (11, 13–15). Importantly for disease progression, CAII down-regulation would help to protect cytoplasmic pH from pCO2 fluctuations and bestow cancer cell pH with a greater degree of autonomy from extracellular influences, such as those arising from intermittent blood flow (73). Conversely, rapid changes in blood flow would be registered by cells with high CAi activity. For example, mTOR-regulated metabolism and proliferation would be more responsive to fluctuating blood flow in cancer cells with higher CAi activity, particularly in regions close to aberrant blood vessels, which experience the sharpest pCO2 fluctuations. Incidentally, these tumor regions are also important for metastasis and nutrient sensing. In solid tumors made of cancer cells with high CAi activity surrounded by a fibroblast stroma of low CAi activity, pCO2 fluctuations would evoke distinct [H+] responses in the two cell types as illustrated by [Ca2+]i responses (Fig. 4).
In conclusion, this study demonstrates a novel role for CAi activity in cancer as a transducer of pCO2 fluctuations (which arise from intermittent blood flow) into a potent cytoplasmic signal (H+ ions). Cancer cells may alter the degree of temporal coupling between pCO2 and pHi by tuning cytoplasmic CAi activity, e.g. through CAII expression (Fig. 6). This work highlights the importance of dynamic aspects of pH signaling as a modality distinct from responses to stable pH changes.
This work was supported in part by the Association for International Cancer Research (to P. S. and A. H.) and Cancer Research UK via the Oxford Cancer Research Centre (to P. S. and A. H.).
- CA
- carbonic anhydrase
- CAi
- intracellular CA
- CAe
- extracellular CA
- mTOR
- mechanistic target of rapamycin
- pHi
- intracellular pH
- pCO2
- CO2 partial pressure
- pO2
- O2 partial pressure
- AM
- acetoxymethyl
- ATZ
- acetazolamide
- CAs
- soluble cytoplasmic CA
- NHE
- Na+/H+ exchange
- DMA
- 5-(N,N-dimethyl)amiloride
- [H+]i
- intracellular [H+]
- mTORC1
- mTOR complex 1
- S6K
- S6 kinase.
REFERENCES
- 1. Hulikova A., Vaughan-Jones R. D., Swietach P. (2011) Dual role of CO2/HCO3− buffer in the regulation of intracellular pH of three-dimensional tumor growths. J. Biol. Chem. 286, 13815–13826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boron W. F. (2004) Regulation of intracellular pH. Adv. Physiol. Educ. 28, 160–179 [DOI] [PubMed] [Google Scholar]
- 3. Hulikova A., Harris A. L., Vaughan-Jones R. D., Swietach P. (2013) Regulation of intracellular pH in cancer cell lines under normoxia and hypoxia. J. Cell. Physiol. 228, 743–752 [DOI] [PubMed] [Google Scholar]
- 4. Hulikova A., Swietach P. (2014) Rapid CO2 permeation across biological membranes: implications for CO2 venting from tissue. FASEB J. 28, 2762–2774 [DOI] [PubMed] [Google Scholar]
- 5. Geers C., Gros G. (2000) Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol. Rev. 80, 681–715 [DOI] [PubMed] [Google Scholar]
- 6. Supuran C. T. (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 7, 168–181 [DOI] [PubMed] [Google Scholar]
- 7. Maren T. H. (1967) Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol. Rev. 47, 595–781 [DOI] [PubMed] [Google Scholar]
- 8. Sly W. S., Hu P. Y. (1995) Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu. Rev. Biochem. 64, 375–401 [DOI] [PubMed] [Google Scholar]
- 9. Ivanov S., Liao S. Y., Ivanova A., Danilkovitch-Miagkova A., Tarasova N., Weirich G., Merrill M. J., Proescholdt M. A., Oldfield E. H., Lee J., Zavada J., Waheed A., Sly W., Lerman M. I., Stanbridge E. J. (2001) Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am. J. Pathol. 158, 905–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wykoff C. C., Beasley N., Watson P. H., Campo L., Chia S. K., English R., Pastorek J., Sly W. S., Ratcliffe P., Harris A. L. (2001) Expression of the hypoxia-inducible and tumor-associated carbonic anhydrases in ductal carcinoma in situ of the breast. Am. J. Pathol. 158, 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou R., Huang W., Yao Y., Wang Y., Li Z., Shao B., Zhong J., Tang M., Liang S., Zhao X., Tong A., Yang J. (2013) CA II, a potential biomarker by proteomic analysis, exerts significant inhibitory effect on the growth of colorectal cancer cells. Int. J. Oncol. 43, 611–621 [DOI] [PubMed] [Google Scholar]
- 12. Parkkila S., Lasota J., Fletcher J. A., Ou W. B., Kivelä A. J., Nuorva K., Parkkila A. K., Ollikainen J., Sly W. S., Waheed A., Pastorekova S., Pastorek J., Isola J., Miettinen M. (2010) Carbonic anhydrase II. A novel biomarker for gastrointestinal stromal tumors. Mod. Pathol. 23, 743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiang W. L., Chu S. C., Yang S. S., Li M. C., Lai J. C., Yang S. F., Chiou H. L., Hsieh Y. S. (2002) The aberrant expression of cytosolic carbonic anhydrase and its clinical significance in human non-small cell lung cancer. Cancer Lett. 188, 199–205 [DOI] [PubMed] [Google Scholar]
- 14. Wang N., Chen Y., Han Y., Zhao Y., Liu Y., Guo K., Jiang Y. (2012) Proteomic analysis shows down-regulations of cytoplasmic carbonic anhydrases, CAI and CAII, are early events of colorectal carcinogenesis but are not correlated with lymph node metastasis. Tumori 98, 783–791 [DOI] [PubMed] [Google Scholar]
- 15. Sheng W., Dong M., Zhou J., Li X., Dong Q. (2013) Down regulation of CAII is associated with tumor differentiation and poor prognosis in patients with pancreatic cancer. J. Surg. Oncol. 107, 536–543 [DOI] [PubMed] [Google Scholar]
- 16. Nordfors K., Haapasalo J., Korja M., Niemelä A., Laine J., Parkkila A. K., Pastorekova S., Pastorek J., Waheed A., Sly W. S., Parkkila S., Haapasalo H. (2010) The tumour-associated carbonic anhydrases CA II, CA IX and CA XII in a group of medulloblastomas and supratentorial primitive neuroectodermal tumours: an association of CA IX with poor prognosis. BMC Cancer 10, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pastorekova S., Zatovicova M., Pastorek J. (2008) Cancer-associated carbonic anhydrases and their inhibition. Curr. Pharm. Des. 14, 685–698 [DOI] [PubMed] [Google Scholar]
- 18. Swietach P., Patiar S., Supuran C. T., Harris A. L., Vaughan-Jones R. D. (2009) The role of carbonic anhydrase 9 in regulating extracellular and intracellular pH in three-dimensional tumor cell growths. J. Biol. Chem. 284, 20299–20310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Swietach P., Wigfield S., Cobden P., Supuran C. T., Harris A. L., Vaughan-Jones R. D. (2008) Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growths. J. Biol. Chem. 283, 20473–20483 [DOI] [PubMed] [Google Scholar]
- 20. Chiche J., Ilc K., Laferrière J., Trottier E., Dayan F., Mazure N. M., Brahimi-Horn M. C., Pouysségur J. (2009) Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 69, 358–368 [DOI] [PubMed] [Google Scholar]
- 21. McIntyre A., Patiar S., Wigfield S., Li J. L., Ledaki I., Turley H., Leek R., Snell C., Gatter K., Sly W. S., Vaughan-Jones R. D., Swietach P., Harris A. L. (2012) Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin Cancer Res. 18, 3100–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loewenstein W. R., Kanno Y. (1966) Intercellular communication and the control of tissue growth: lack of communication between cancer cells. Nature 209, 1248–1249 [DOI] [PubMed] [Google Scholar]
- 23. Sterling D., Reithmeier R. A., Casey J. R. (2001) A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J. Biol. Chem. 276, 47886–47894 [DOI] [PubMed] [Google Scholar]
- 24. Li X., Liu Y., Alvarez B. V., Casey J. R., Fliegel L. (2006) A novel carbonic anhydrase II binding site regulates NHE1 activity. Biochemistry 45, 2414–2424 [DOI] [PubMed] [Google Scholar]
- 25. Becker H. M., Hirnet D., Fecher-Trost C., Sültemeyer D., Deitmer J. W. (2005) Transport activity of MCT1 expressed in Xenopus oocytes is increased by interaction with carbonic anhydrase. J. Biol. Chem. 280, 39882–39889 [DOI] [PubMed] [Google Scholar]
- 26. Villafuerte F. C., Swietach P., Youm J. B., Ford K., Cardenas R., Supuran C. T., Cobden P. M., Rohling M., Vaughan-Jones R. D. (2014) Facilitation by intracellular carbonic anhydrase of Na+-HCO3− co-transport but not Na+/H+ exchange activity in the mammalian ventricular myocyte. J. Physiol. 592, 991–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boron W. F. (2010) Evaluating the role of carbonic anhydrases in the transport of HCO3−-related species. Biochim. Biophys. Acta 1804, 410–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Swietach P., Vaughan-Jones R. D., Harris A. L. (2007) Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 26, 299–310 [DOI] [PubMed] [Google Scholar]
- 29. Baudelet C., Cron G. O., Ansiaux R., Crokart N., DeWever J., Feron O., Gallez B. (2006) The role of vessel maturation and vessel functionality in spontaneous fluctuations of T2*-weighted GRE signal within tumors. NMR Biomed. 19, 69–76 [DOI] [PubMed] [Google Scholar]
- 30. Braun R. D., Lanzen J. L., Dewhirst M. W. (1999) Fourier analysis of fluctuations of oxygen tension and blood flow in R3230Ac tumors and muscle in rats. Am. J. Physiol. Heart Circ. Physiol. 277, H551–H568 [DOI] [PubMed] [Google Scholar]
- 31. Chaplin D. J., Hill S. A. (1995) Temporal heterogeneity in microregional erythrocyte flux in experimental solid tumours. Br. J. Cancer 71, 1210–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kimura H., Braun R. D., Ong E. T., Hsu R., Secomb T. W., Papahadjopoulos D., Hong K., Dewhirst M. W. (1996) Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Res. 56, 5522–5528 [PubMed] [Google Scholar]
- 33. Mesquita R. C., Han S. W., Miller J., Schenkel S. S., Pole A., Esipova T. V., Vinogradov S. A., Putt M. E., Yodh A. G., Busch T. M. (2012) Tumor blood flow differs between mouse strains: consequences for vasoresponse to photodynamic therapy. PLoS One 7, e37322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dewhirst M. W. (1998) Concepts of oxygen transport at the microcirculatory level. Semin. Radiat. Oncol. 8, 143–150 [DOI] [PubMed] [Google Scholar]
- 35. Brurberg K. G., Graff B. A., Olsen D. R., Rofstad E. K. (2004) Tumor-line specific pO2 fluctuations in human melanoma xenografts. Int. J. Radiat. Oncol. Biol. Phys. 58, 403–409 [DOI] [PubMed] [Google Scholar]
- 36. Brurberg K. G., Graff B. A., Rofstad E. K. (2003) Temporal heterogeneity in oxygen tension in human melanoma xenografts. Br. J. Cancer 89, 350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cárdenas-Navia L. I., Mace D., Richardson R. A., Wilson D. F., Shan S., Dewhirst M. W. (2008) The pervasive presence of fluctuating oxygenation in tumors. Cancer Res. 68, 5812–5819 [DOI] [PubMed] [Google Scholar]
- 38. Dewhirst M. W., Kimura H., Rehmus S. W., Braun R. D., Papahadjopoulos D., Hong K., Secomb T. W. (1996) Microvascular studies on the origins of perfusion-limited hypoxia. Br. J. Cancer Suppl. 27, S247–S251 [PMC free article] [PubMed] [Google Scholar]
- 39. Dewhirst M. W., Braun R. D., Lanzen J. L. (1998) Temporal changes in pO2 of R3230AC tumors in Fischer-344 rats. Int. J. Radiat. Oncol. Biol. Phys. 42, 723–726 [DOI] [PubMed] [Google Scholar]
- 40. Magat J., Jordan B. F., Cron G. O., Gallez B. (2010) Noninvasive mapping of spontaneous fluctuations in tumor oxygenation using 19F MRI. Med. Phys. 37, 5434–5441 [DOI] [PubMed] [Google Scholar]
- 41. Lanzen J., Braun R. D., Klitzman B., Brizel D., Secomb T. W., Dewhirst M. W. (2006) Direct demonstration of instabilities in oxygen concentrations within the extravascular compartment of an experimental tumor. Cancer Res. 66, 2219–2223 [DOI] [PubMed] [Google Scholar]
- 42. Cárdenas-Navia L. I., Yu D., Braun R. D., Brizel D. M., Secomb T. W., Dewhirst M. W. (2004) Tumor-dependent kinetics of partial pressure of oxygen fluctuations during air and oxygen breathing. Cancer Res. 64, 6010–6017 [DOI] [PubMed] [Google Scholar]
- 43. Bhujwalla Z. M., Artemov D., Ballesteros P., Cerdan S., Gillies R. J., Solaiyappan M. (2002) Combined vascular and extracellular pH imaging of solid tumors. NMR Biomed. 15, 114–119 [DOI] [PubMed] [Google Scholar]
- 44. Garcia-Martin M. L., Martinez G. V., Raghunand N., Sherry A. D., Zhang S., Gillies R. J. (2006) High resolution pHe imaging of rat glioma using pH-dependent relaxivity. Magn. Reson. Med. 55, 309–315 [DOI] [PubMed] [Google Scholar]
- 45. Sevick E. M., Jain R. K. (1988) Blood flow and venous pH of tissue-isolated Walker 256 carcinoma during hyperglycemia. Cancer Res. 48, 1201–1207 [PubMed] [Google Scholar]
- 46. Cardone R. A., Casavola V., Reshkin S. J. (2005) The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 5, 786–795 [DOI] [PubMed] [Google Scholar]
- 47. Parks S. K., Chiche J., Pouysségur J. (2013) Disrupting proton dynamics and energy metabolism for cancer therapy. Nat. Rev. Cancer 13, 611–623 [DOI] [PubMed] [Google Scholar]
- 48. Schwab A., Fabian A., Hanley P. J., Stock C. (2012) Role of ion channels and transporters in cell migration. Physiol. Rev. 92, 1865–1913 [DOI] [PubMed] [Google Scholar]
- 49. Pastorek J., Pastoreková S., Callebaut I., Mornon J. P., Zelník V., Opavský R., Zat'ovicová M., Liao S., Portetelle D., Stanbridge E. J., Zavada J., Burny A., Kettmann R. (1994) Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene 9, 2877–2888 [PubMed] [Google Scholar]
- 50. Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. (1979) Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18, 2210–2218 [DOI] [PubMed] [Google Scholar]
- 51. Eisner D. A., Kenning N. A., O'Neill S. C., Pocock G., Richards C. D., Valdeolmillos M. (1989) A novel method for absolute calibration of intracellular pH indicators. Pflugers Arch. 413, 553–558 [DOI] [PubMed] [Google Scholar]
- 52. Schroeder M. A., Ali M. A., Hulikova A., Supuran C. T., Clarke K., Vaughan-Jones R. D., Tyler D. J., Swietach P. (2013) Extramitochondrial domain rich in carbonic anhydrase activity improves myocardial energetics. Proc. Natl. Acad. Sci. U.S.A. 110, E958–E967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Elvidge G. P., Glenny L., Appelhoff R. J., Ratcliffe P. J., Ragoussis J., Gleadle J. M. (2006) Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1α, HIF-2α, and other pathways. J. Biol. Chem. 281, 15215–15226 [DOI] [PubMed] [Google Scholar]
- 54. Wykoff C. C., Beasley N. J., Watson P. H., Turner K. J., Pastorek J., Sibtain A., Wilson G. D., Turley H., Talks K. L., Maxwell P. H., Pugh C. W., Ratcliffe P. J., Harris A. L. (2000) Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 60, 7075–7083 [PubMed] [Google Scholar]
- 55. Leem C. H., Vaughan-Jones R. D. (1998) Out-of-equilibrium pH transients in the guinea-pig ventricular myocyte. J. Physiol. 509, 471–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Junge W., McLaughlin S. (1987) The role of fixed and mobile buffers in the kinetics of proton movement. Biochim. Biophys. Acta 890, 1–5 [DOI] [PubMed] [Google Scholar]
- 57. Swietach P., Zaniboni M., Stewart A. K., Rossini A., Spitzer K. W., Vaughan-Jones R. D. (2003) Modelling intracellular H+ ion diffusion. Prog. Biophys. Mol. Biol. 83, 69–100 [DOI] [PubMed] [Google Scholar]
- 58. Stewart A. K., Boyd C. A., Vaughan-Jones R. D. (1999) A novel role for carbonic anhydrase: cytoplasmic pH gradient dissipation in mouse small intestinal enterocytes. J. Physiol. 516, 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Spitzer K. W., Skolnick R. L., Peercy B. E., Keener J. P., Vaughan-Jones R. D. (2002) Facilitation of intracellular H+ ion mobility by CO2/HCO3− in rabbit ventricular myocytes is regulated by carbonic anhydrase. J. Physiol. 541, 159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Becker H. M., Klier M., Schüler C., McKenna R., Deitmer J. W. (2011) Intramolecular proton shuttle supports not only catalytic but also noncatalytic function of carbonic anhydrase II. Proc. Natl. Acad. Sci. U.S.A. 108, 3071–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Johnson D. E., Casey J. R. (2011) Cytosolic H+ microdomain developed around AE1 during AE1-mediated Cl−/HCO3− exchange. J. Physiol. 589, 1551–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. (1988) pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3−. Am. J. Physiol. Cell Physiol. 255, C844–C856 [DOI] [PubMed] [Google Scholar]
- 63. Vaupel P., Kallinowski F., Okunieff P. (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 49, 6449–6465 [PubMed] [Google Scholar]
- 64. Mancarella S., Wang Y., Deng X., Landesberg G., Scalia R., Panettieri R. A., Mallilankaraman K., Tang X. D., Madesh M., Gill D. L. (2011) Hypoxia-induced acidosis uncouples the STIM-Orai calcium signaling complex. J. Biol. Chem. 286, 44788–44798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Balgi A. D., Diering G. H., Donohue E., Lam K. K., Fonseca B. D., Zimmerman C., Numata M., Roberge M. (2011) Regulation of mTORC1 signaling by pH. PLoS One 6, e21549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Laplante M., Sabatini D. M. (2012) mTOR signaling in growth control and disease. Cell 149, 274–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Magnuson B., Ekim B., Fingar D. C. (2012) Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 441, 1–21 [DOI] [PubMed] [Google Scholar]
- 68. Gullino P. M., Grantham F. H., Smith S. H., Haggerty A. C. (1965) Modifications of the acid-base status of the internal milieu of tumors. J. Natl. Cancer Inst. 34, 857–869 [PubMed] [Google Scholar]
- 69. Feng Z., Zhang H., Levine A. J., Jin S. (2005) The coordinate regulation of the p53 and mTOR pathways in cells. Proc. Natl. Acad. Sci. U.S.A. 102, 8204–8209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Frame S., Cohen P. (2001) GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 359, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pouysségur J., Dayan F., Mazure N. M. (2006) Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441, 437–443 [DOI] [PubMed] [Google Scholar]
- 72. Berridge M. J. (1997) The AM and FM of calcium signalling. Nature 386, 759–760 [DOI] [PubMed] [Google Scholar]
- 73. Fang J. S., Gillies R. D., Gatenby R. A. (2008) Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression. Semin. Cancer Biol. 18, 330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]