Background: Roles of the multifunctional kinase PKCα in bone are unknown.
Results: Female Prkca−/− mice form bone in their medullary cavities associated with higher osteoblastic differentiation. Bone and spleen changes in Prkca−/− mice resemble features of Gaucher disease.
Conclusion: PKCα regulates osteoblast differentiation and bone architecture.
Significance: PKCα-targeting therapies may benefit low bone mass conditions, including Gaucher disease and osteoporosis.
Keywords: Bone, Estrogen, Gaucher Disease, Osteoblast, Protein Kinase C (PKC), Wnt Signaling, Mechanical Loading
Abstract
Bones' strength is achieved and maintained through adaptation to load bearing. The role of the protein kinase PKCα in this process has not been previously reported. However, we observed a phenotype in the long bones of Prkca−/− female but not male mice, in which bone tissue progressively invades the medullary cavity in the mid-diaphysis. This bone deposition progresses with age and is prevented by disuse but unaffected by ovariectomy. Castration of male Prkca−/− but not WT mice results in the formation of small amounts of intramedullary bone. Osteoblast differentiation markers and Wnt target gene expression were up-regulated in osteoblast-like cells derived from cortical bone of female Prkca−/− mice compared with WT. Additionally, although osteoblastic cells derived from WT proliferate following exposure to estradiol or mechanical strain, those from Prkca−/− mice do not. Female Prkca−/− mice develop splenomegaly and reduced marrow GBA1 expression reminiscent of Gaucher disease, in which PKC involvement has been suggested previously. From these data, we infer that in female mice, PKCα normally serves to prevent endosteal bone formation stimulated by load bearing. This phenotype appears to be suppressed by testicular hormones in male Prkca−/− mice. Within osteoblastic cells, PKCα enhances proliferation and suppresses differentiation, and this regulation involves the Wnt pathway. These findings implicate PKCα as a target gene for therapeutic approaches in low bone mass conditions.
Introduction
Age-related failure of bones' intrinsic ability to match their mass and architecture to their functional load bearing results in fragility and increased incidence of fractures characteristic of osteoporosis (1). Part of this process involves thinning of the load-bearing cortices of long bones due to expansion of the medullary cavity in both women and men (2, 3). This weakens the bone structure and predisposes to fracture (4). Deterioration of bone structure is a consequence of chronic failure of the cells that form bone (osteoblasts) to adequately compensate for the activity of those that resorb it (osteoclasts). This may in part be due to aging-related deficiencies in osteoblast proliferation and differentiation (5, 6). The identification of molecular pathways that enhance osteoblast function has led to novel agents entering clinical trials for the treatment of osteoporosis, including, most recently, neutralizing antibodies against the Wnt antagonist sclerostin, which potently inhibits bone formation (7).
Wnt signaling following sclerostin down-regulation naturally occurs in bones subjected to mechanical loading, correlating with subsequent bone formation (8–10). Osteoblast lineage cells' responses to their mechanical loading-engendered strain environment constitute the primary functional determinant of bone mass and architecture (11). The cellular outcomes of mechanical strain-initiated signaling include site-specific activation of bone formation through osteoblast differentiation and proliferation (9, 12, 13). Molecular mechanisms facilitating these responses include ligand-independent functions of the estrogen receptors (ERs)4 (14–16) and the Wnt/β-catenin signaling pathway (10, 17–19). Both Wnt and ER signaling are established drug targets for the treatment of osteoporosis. Both of these potently osteogenic pathways are also regulated by the ubiquitous kinase protein kinase Cα (PKCα) (20–23), leading us to hypothesize that PKCα may critically regulate osteoblast function.
PKCα has been implicated in major disease processes (24) in part through its role as a critical regulator of proliferation in various cell types (21, 22, 25), yet its roles in osteoblasts are largely unknown. PKCα regulates ERα activity in osteoblast-like cells (23, 26) and inhibits Wnt/β-catenin signaling in cancer cell types (27, 28). Exposure to mechanical strain rapidly activates PKCα in osteoblast-like cells (29), and PKC signaling has been implicated in the regulation of various mechanically responsive genes, including the osteoblast differentiation marker osteocalcin (30–32). A role for PKCα in regulating osteoblastic cell differentiation has been suggested previously (33). Furthermore, pharmacological activation of PKC signaling rescues defective proliferation of marrow-derived osteoblastic cells in a mouse model in Type I Gaucher disease (34). This disease involves debilitating osteoporosis and architectural deterioration together with hematological abnormalities associated with mutation of the GBA1 gene (34, 35). Generalized inhibition of PKC signaling has been proposed to contribute to the etiology of this disease (34, 36), but the effects of global impairment of PKC isoforms on Gaucher-related phenotypes have not been investigated.
In this study, we characterize the bone phenotype of previously generated Prkca−/− mice in which the Prkca gene was disrupted by homologous recombination to generate a mouse constitutively lacking expression of PKCα (37). This mouse has been used to demonstrate roles for the gene in a number of tissues. Cardiac contractility has been shown to be increased in Prkca−/− mice, and gene deletion protects against heart failure induced by pressure overload and dilated cardiomyopathy (37). PKCα has also been shown to mediate hypertonicity-stimulated urea transport in the collecting ducts of the kidney (38) and has been shown to be critical for secretion of granule contents from platelets (39). Here we show that targeted deletion of Prkca leads to a marked increase in endosteal bone formation, progressively invading the medullary cavity at diaphyseal sites of load-bearing bones in female mice. In the experiments reported here, we sought to establish the mechanisms involved by examining the effects on bone architecture in vivo of age, gender, loading, and ovariectomy in Prkca−/− compared with WT mice and in vitro on osteoblast proliferation and differentiation. We also documented similarities in the Prkca−/− mice with Gaucher disease in humans.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatment
17β-estradiol (E2) was from Sigma and dissolved in ethanol. Wnt3a was from Tocris (Bristol, UK) and dissolved in PBS according to the manufacturer's instructions. Calphostin C and phorbol 12-myristate 13-acetate (PMA) were from Tocris and dissolved in ethanol and dimethyl sulfoxide, respectively. Cells were maintained in phenol red-free DMEM containing 10% heat-inactivated FCS (PAA, Somerset, UK), 2 mM l-glutamine, 100 IU/ml penicillin, and 100 IU/ml streptomycin (Invitrogen) (complete medium) in a 37 ºC incubator at 5% CO2, 95% humidity as described previously (19).
Cortical long bone-derived mouse osteoblastic cell (CLBOb) extractions from adult female mice were as described previously (6, 15, 19) and were always used at passage 1. For differentiation studies, CLBObs were seeded at 25,000 cells/cm2 and treated with or without 50 μm ascorbic acid and 10 mm β-glycerol phosphate. Alkaline phosphatase assays were using p-nitrophenyl phosphate Sigma FastTM according to the manufacturer's instructions and normalized relative to total protein content using the crystal violet method (6, 40, 41). Mineralized nodules fixed in ice-cold methanol on ice for 5 min were air-dried, washed in phosphate-buffered saline, stained in 2% alizarin red solution, pH 4.2, for 5 min, and cleared under running water, also as reported previously (6, 40, 41).
For strain experiments, cells were cultured on custom-made plastic strips, and strain was applied as described previously (6, 30, 42) through a brief period of 600 cycles of four-point bending of the strips with a peak strain of 3,400 μϵ on a Zwick/Roëll materials testing machine (Zwick Testing Machines Ltd., Leominster, UK) with strain rates on and off of ∼24,000 μϵ/s, dwell times on and off of 0.7 s, and a frequency of 0.6 Hz.
To determine the effect of treatment with the PKC activator PMA, cells were cultured for 7 days and then treated twice with 0.1 μm PMA at 12-h intervals and harvested 12 h after the second treatment.
Proliferation Studies and Ki-67 Staining
Proliferation studies and Ki-67 staining, including in situ cell cycle analysis, were as described previously by our group (6, 19). For proliferation studies, CLBObs were seeded at an initial density of 10,000 cells/cm2, whereas Saos-2 cells were seeded at 5,000 cells/cm2 and allowed to settle overnight, flooded with complete medium for 24 h, and then serum-deprived in 2% charcoal/dextran-stripped serum overnight before strain or treatment. To determine cell number, random images of DAPI-stained nuclei were taken at ×4 magnification on a Leica DMRB microscope with an Olympus DP72 digital camera, binarized, and automatically analyzed using ImageJ (National Institutes of Health, version 1.46d). Ki-67-positive cells were counted using ImageJ on five randomly chosen images per slide at an original magnification of ×20. Cycle stage analysis was performed on 190 ± 22 positive nuclei/slide imaged at ×40. Key results were independently verified by two observers (G. L. G. and L. B. M.). Representative images of the pattern of Ki-67 distribution in Saos-2 cells and CLBObs in different stages of the cell cycle have been published previously by our group (6, 19).
Quantitative Reverse Transcriptase PCR
In order to isolate marrow and bone fractions, bones were rapidly dissected of all surrounding tissues, and the epiphyses were removed. The diaphyses containing marrow were placed upright in custom-made plastic straws inside 2-ml tubes, which were then centrifuged at 10,000 r.p.m. for 10 s at 4 °C. Bones were snap-frozen for RNA extraction with RNEasy Plus Universal kits (Qiagen, Sussex, UK), whereas marrow was lysed directly in RNEasy Plus lysis buffer (Qiagen) and stored at −80 °C.
qRT-PCR was performed as described previously (18, 43). Mouse β2-microglobulin (β2 mg), osteocalcin, and Wnt-induced secreted protein 2 (Wisp2) were as follows: β2 mg, ATGGCTCGCTCGGTGACCCT (forward) and TTCTCCGGTGGGTGGCGTGA (reverse); osteocalcin, CTGACCTCACAGATCCCAAGC (forward) and TGGTCTGATAGCTCGTCACAAG (reverse); Wisp2, GGTTTCACCTGCCTGCCGCT (forward) and TCACACACCCACTCGGGGCA (reverse).
All other primer sequences were from the Harvard PrimerBank (43) (Table 1). Gene panels representing osteoblast differentiation and Wnt targets were predetermined, and all quantified genes are presented here.
TABLE 1.
List of PrimerBank IDs for PCR primers used in this study
| Gene | PrimerBank ID |
|---|---|
| Osteoblast markers | |
| Runx2 (also a Wnt target) | 225690525b1 |
| Osterix | 18485518a1 |
| Collagen 1 A1 (Col1A1) | 118131144b1 |
| Osteoprotegerin (also a Wnt target) | 113930715b1 |
| Receptor activator of NF-κB ligand (RANKL) | 114842414b1 |
| Osteocalcin | 13811695a1 |
| Wnt targets | |
| cMyc | 293629266b3 |
| Cyclin D1 (CCND1) | 119672895b1 |
| Axin2 | 158966712b1 |
| Cyr61 | 239937453b1 |
| Adipocyte markers | |
| Peroxisome proliferator-activated receptor γ (PPARγ) | 187960104b1 |
| cAMP element-binding protein α (C/EBPα) | 131886531b2 |
| Osteoclast markers | |
| Receptor activator of NF-κB (RANK) | 110350008b1 |
| Tartrate-resistant acid phosphatase (TRAP) | 156151432b1 |
Western Blotting
Cells were cultured as for proliferation studies and lysed in radioimmune precipitation buffer containing protease inhibitors (Sigma). Lysates were sonicated prior to quantification of protein content by a bicinchonic acid assay (Sigma). Lysate protein content was standardized to 500 μg/ml and solubilized in reducing Laemmli sample buffer. Proteins were resolved by SDS-PAGE and then transferred to PVDF membranes. Membranes were blocked with 10% BSA and subjected to immunoblotting with anti-PKCα (New England Biolabs Ltd., Hitchin, UK); anti-PKCβ, -δ, -ϵ, and -θ (BD Biosciences); and anti-PKCγ (Insight Biotechnology Ltd., Wembley, UK) and α-tubulin (Sigma) as a loading control.
Histochemistry
Sclerostin immunodetection on decalcified bone sections was as described previously (9). Hematoxylin and eosin (H&E) staining was done following standard protocols.
Hematological Analysis
Blood was taken from 4-month-old mice via cardiac puncture into 50 mm EDTA (1:10, v/v). Samples were immediately analyzed on a Horiba Pentra ES60 hematology analyzer (Horiba UK Ltd., Northampton, UK).
Determination of Bone Structure and the Effects of Sciatic Neurectomy, Ovariectomy, and Castration
Prkca−/− mice were as described previously (39). All procedures complied with the UK Animals (Scientific Procedures) Act 1986 and were reviewed and approved by the University of Bristol ethics committee. Breeding pairs of Prkca+/− mice were crossed to generate Prkca−/− and Prkca+/+ for experimentation as littermate matched pairs. Following sacrifice, legs were stored in 70% ethanol, and whole femur or tibia was imaged by microcomputed tomography (μCT) using the SkyScan 1172 system (SkyScan, Kontich, Belgium) with a voxel size of 4.8 μm (110 μm3). The scanning, reconstruction, and method of analysis have been reported previously (6, 44) and were performed according to American Society for Bone and Mineral Research guidelines (45).
Sciatic neurectomy, ovariectomy, and castration were performed to investigate the effects of these interventions on intramedullary bone. Sciatic neurectomy was as described previously by our group (44). Female mice were subjected to unilateral sciatic neurectomy of the right tibia at 15 weeks of age and sacrificed 3 weeks later at 18 weeks of age. Ovariectomy was also performed as described previously by our group (46). Female mice were ovariectomized at 8 weeks of age and sacrificed 10 weeks later at 18 weeks of age. Similarly, male mice were castrated at 8 weeks of age and sacrificed 10 weeks later at 18 weeks of age. The left limbs of ovariectomized or castrated mice were compared with the left control limbs of non-operated mice of the same age.
Statistical Analysis
Data are presented as means ± S.E. Comparisons between two groups, including comparisons within gender, were by Student's t test following Levene's test for homogeneity of variance. Comparisons between more than two groups were by analysis of variance with post hoc Bonferroni or Games-Howel tests. Genotype by age interactions and genotype by intervention interactions were determined by mixed model analysis with Bonferroni adjustment performed in SPSS (version 17).
RESULTS
PKCα Deletion Causes Age-dependent and Sex- and Site-specific Changes in Bone Structure
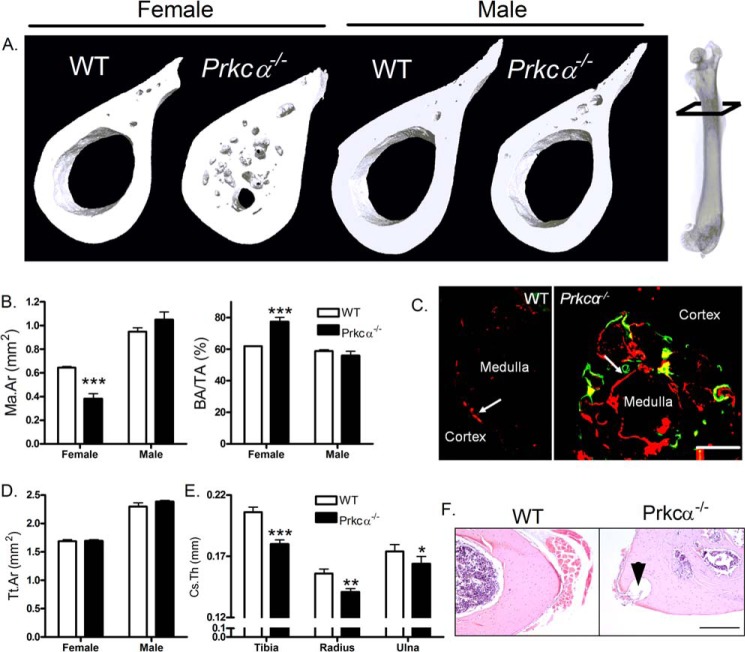
Bone structure was investigated by μCT, which revealed that the medullary cavity of female, but not male, Prkca−/− mice was characterized by the invasion of disorganized bone in the diaphyses of the femur (Fig. 1A), humerus, and tibia. This invasion was sufficient to significantly reduce the area of the medullary cavity and increase bone area fraction (Fig. 1B) due to endosteal bone formation (Fig. 1C). Total tissue area enclosed within the periosteum was not significantly different between either male or female WT and Prkca−/− mice (Fig. 1D), suggesting that PKCα specifically influences the endosteal surface. Male Prkca−/− mice had reduced cortical thickness in various skeletal sites (Fig. 1E), but this parameter could not be accurately assessed in females with extensive intramedullary bone or cortical pores. Both males and females developed large cavities throughout the diaphyseal cortices (Fig. 1F). Although reminiscent of the cavities that carry blood vessels in normal bone, these are much larger than any seen in WT mice. No differences in body weight or bone lengths were observed between WT and Prkca−/− mice (not shown).
FIGURE 1.
Prkca deletion causes intramedullary bone formation in female mice. A, representative three-dimensional μCT reconstructions showing 30% of the bone's length from the proximal end, indicated on the radiograph, in 22-week-old WT and Prkca−/− female and male mice. B, μCT quantification of medullary area (Ma.Ar) and bone area per tissue area (BA/TA) in female and male WT and Prkca−/− 22-week-old mice (n = 5). C, dynamic histomorphometry with calcein (green) and alizarin (red) fluorochromes illustrating medullary double-labeling in the tibia midshaft of 18-week-old female Prkca−/− but not WT mice (scale bar, 0.5 mm). D, total tissue area (Tt.ar) was quantified by μCT 30% of the femur's length from its proximal end in 22-week-old male and female WT and Prkca−/− mice. E, male Prkca−/− mice had lower cortical thickness (Cs.Th) in the midshaft of the tibia, radius, and ulna than WT males as determined by μCT analysis. F, cortical holes, indicated by the arrowhead, were observed in all long bones tested in Prkca−/− male and female mice. H&E staining illustrates a hole in the humerus of a female Prkca−/− (scale bar, 1 mm). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus WT of the same gender. Error bars, S.E.
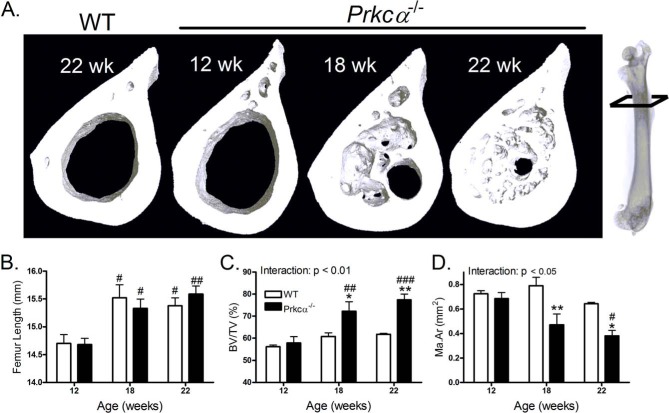
The intramedullary bone phenotype was not evident in 12-week-old mice but became evident by 18 weeks (Fig. 2A). In both genotypes, 12-week-old female mice had shorter femurs than 18-week-old mice and were thus not skeletally mature (Fig. 2B). 12-Week-old Prkca−/− mice had significantly lower cortical thickness before intramedullary bone was observed (WT = 0.18 ± 0.006 and Prkca−/− = 0.16 ± 0.005, p < 0.05). However, the presence of intramedullary bone increased the bone area fraction at 18 weeks and even more so at 22 weeks of age in Prkca−/− mice (Fig. 2C). This was because of a progressive reduction in medullary area (Fig. 2D). Consequently, significant genotype by age interactions were detected by mixed model analysis for both marrow area and bone area fraction (Fig. 2, C and D).
FIGURE 2.
Intramedullary bone invasion progresses with age in adult female Prkca−/− mice. A, representative three-dimensional reconstructions of the femoral 30% site in a 22-week WT mouse and 12-, 18-, or 22-week-old female Prkca−/− mice indicating medullary in-filling with age. B, quantification of femoral length in female mice of the indicated ages. Shown are quantifications of bone area per tissue area (BA/TA) (C) and medullary area (Ma.Ar) (D) in female 12-week-old (n = 6), 18-week-old (n = 6), and 22-week-old (n = 5) mice. Statistical significance of the genotype by age interactions is indicated. #, p < 0.05; ##, p < 0.01; ###, p < 0.001 versus 12-week-old mice of the same genotype. Bars, mean ± S.E. (error bars) *, p < 0.05; **, p < 0.01 versus WT mice of the same age.
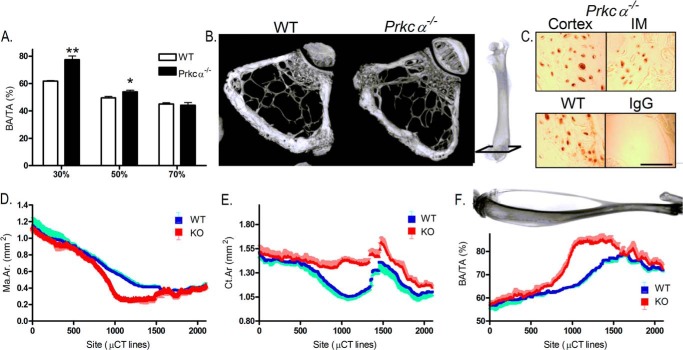
Intramedullary bone was consistently localized to ∼30% of the femur's length from the proximal end and did not extend into the cancellous regions at the bones' ends (Fig. 3, A and B). Expression of the mature osteocyte product sclerostin was detected by immunohistochemistry, indicating the presence of mature osteocytes within the intramedullary bone (Fig. 3C). A similar distribution was observed in the tibia, where we fully characterized the location of this intramedullary bone by adapting the method of μCT analysis such that 2,000 cross-sectional measurements were made along the length of the tibia between 20 and 80% of the bone's length. This demonstrated a significant deviation in bone area due to bony invasion of the tibial medullary cavity only in the mid-diaphysis (Fig. 3, D–F). The distribution of intramedullary bone in the tibia approximately corresponds to the region of bone predicted by ourselves and others to experience the greatest bending (strain) during axial load bearing (10, 47).
FIGURE 3.
Intramedullary bone expansion in the bones of female Prkca−/− mice occurs at specific sites. A, quantification of bone area per tissue area (BA/TA) in three different sites along the length of the femur of 22-week-old female WT and Prkca−/− mice (n = 5). B, representative three-dimensional reconstructions of μCT images of female WT and Prkca−/− trabecular bone in the distal femur. Images represent 100 μCT lines taken 100 lines above the growth plate, approximately indicated on the radiograph. C, immunolocalization of sclerostin in the humeral diaphysis from a female Prkca−/− mouse, indicating sclerostin expression in both the IM and cortical bone. WT cortical bone is shown as a positive control, and a non-immune IgG is shown as a negative control. Scale bar, 250 μm. D–F, approximately 2,000 measurements were made along the length of the tibia of female WT or Prkca−/− mice (n = 3 in each case), with each measurement representing a single μCT line (4.8 μm). Shown are medullary area (Ma.Ar) (D), cortical area (Ct.Ar) (E), and bone area per tissue area (BA/TA) (F). Bars and dots, mean ± S.E. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus WT at the same site.
Prkca Deletion Parallels Aspects of Gaucher Disease
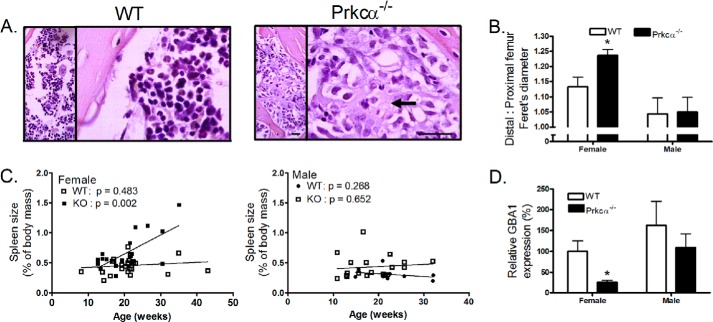
Sites of intramedullary bone and surrounding marrow were further characterized by histology. Within the marrow, amorphous eosinophilic cells reminiscent of Gaucher cells (48) were observed in regions with intramedullary bone (Fig. 4A). Type I Gaucher disease is a condition associated with debilitating skeletal pathologies (49) and has previously been suggested to involve suppression of PKC signaling (34, 36). Other similarities with Gaucher disease observed in Prkca−/− mice, including reduction in cortical thickness described above and impaired platelet aggregation previously reported (39), led us to investigate further parallels. Platelet number has previously been reported not to be significantly different between WT and Prkca−/− mice (50), and further hematological analysis revealed no significant differences in circulating total white blood cell numbers between WT (n = 4) and Prkca−/− (n = 8) mice (Prkca−/− 96 ± 5% of WT, p = 0.28).
FIGURE 4.
Deletion of Prkca−/− mimics features of type 1 Gaucher disease in female mice. A, representative images of female WT and Prkca−/− bone stained with hematoxylin and eosin showing Gaucher-like cells in the Prkca−/− marrow in the same region as the IM bone infiltration. Scale bar, 50 μm. B, Feret's diameter was calculated 25% (proximal) and 75% (distal) of the femur's length from its proximal end. The ratio of distal to proximal in 22-week-old mice of each genotype is shown. C, spleen weights of WT and Prkca−/− mice of each sex, sacrificed at different ages for different uses, expressed as a proportion of body weight. p values shown are for the slope indicating progression with age only in the female Prkca−/−. D, marrow from 22-week-old WT and Prkca−/− mice was harvested, and Gba1 expression was analyzed by qRT-PCR. Bars, mean ± S.E. (error bars) (n = 5). *, p < 0.05 versus WT.
Human Gaucher patients develop a distinct “Erlenmeyer flask” deformity in which the proximal femur narrows relative to the distal femur (49). The ratio of Feret's diameter (maximum diameter) between the distal and proximal femur was greater in female Prkca−/− than in WT mice, indicating a similar narrowing of the proximal relative to the distal femur (Fig. 4B). Splenomegaly is another common feature of Gaucher disease (49). Progressive, age-related splenomegaly was observed in female but not male Prkca−/− mice (Fig. 4C). To determine whether Prkca deletion alters expression of the gluocerebrosidase 1 (Gba1) gene causally mutated in Gaucher disease (49), bone marrow was collected from 22-week-old male and female Prkca−/− mice, and Gba1 expression was quantified by qRT-PCR. Gba1 expression was lower in marrow from female but not male Prkca−/− mice relative to WT (Fig. 4D).
PKCα Influences the Balance between Osteoblastic Proliferation and Differentiation in Vitro
Marrow-derived osteoblastic cells from a mouse model of Gaucher disease have previously been reported to have impaired proliferation that could be rescued by PKC activation (34). In order to investigate whether differences in osteoblast proliferation and differentiation might contribute to the bony invasion of the medullary cavity in female Prkca−/− mice, we investigated the effect of Prkca deletion on primary cultures of osteoblast-like cells derived from the load-bearing cortices of mouse long bones (CLBObs). CLBObs have been extensively characterized by our group and are able to respond to osteogenic stimuli, including Wnts, estradiol, and physiological mechanical strain (15, 18, 19, 31, 47, 51, 52). Robust PKCα expression was detected in CLBObs from WT mice, whereas no expression was detected in cells from Prkca−/− mice by Western blotting (data not shown). None of the other PKC isoforms tested (PKCβ, PKCγ, PKCσ, and PKCϵ) were expressed differently between cells of the two genotypes (data not shown). PKCθ was not detected in either genotype (data not shown).
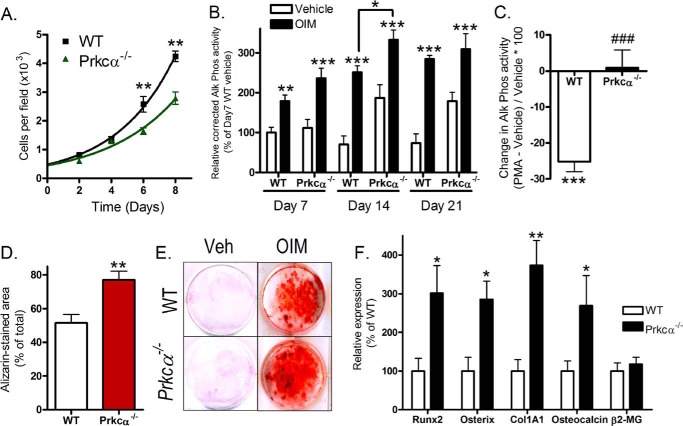
CLBObs from adult female Prkca−/− mice were less proliferative than those in similar cultures derived from WT mice (Fig. 5A). Ki-67 in situ cell cycle analysis revealed that this was due to a greater proportion of cells from Prkca−/− mice being in a Ki-67-negative, quiescent state with no significant differences observed between proliferating cells in different stages of the cell cycle (not shown). Consistent with a shift in the proliferation-differentiation balance away from proliferation, CLBObs from female Prkca−/− mice showed spontaneously increased activity of the early osteoblastic differentiation marker alkaline phosphatase by 14 days of culture (Fig. 5B). Osteogenesis induction medium increased alkaline phosphatase activity further in both WT and Prkca−/− cultures (Fig. 5B), whereas activation of PKC signaling by phorbol ester PMA (0.1 μm) reduced alkaline phosphatase activity in WT but not Prkca−/− cultures (Fig. 5C). By day 21 of treatment with osteogenesis induction medium, cells from female Prkca−/− mice had mineralized a greater proportion of their cell culture surface than cells from WT mice (Fig. 5, D and E). Expression of markers of osteoblastic differentiation was also higher in CLBOb cultures from Prkca−/− than in those from WT mice (Fig. 5F).
FIGURE 5.
Osteoblast-like cells from female Prkca−/− mice have an enhanced differentiation state in vitro. CLBObs were derived from female WT and Prkca−/− mice. A, growth curves were determined by counting the cell number at the indicated time points. B, CLBObs derived from WT and Prkca−/− mice were cultured for the indicated period of time with or without treatment with osteogenesis induction medium (OIM). Alkaline phosphatase activity was determined and normalized to total protein content (n = 12). Unless indicated, comparisons are relative to vehicle-treated cultures of the same genotype at the same time point. C, cultures from WT and Prkca−/− mice were treated with the PKC activator PMA. Alkaline phosphatase activity was determined normalized to total protein content, and the percentage change in activity relative to vehicle-treated controls is shown. D, quantification of the proportion of culture area stained with alizarin red after 21 days of treatment with osteogenesis induction medium (n = 12). E, representative cultures from WT and Prkca−/− mice fixed following 21 days of treatment with vehicle (veh) or OIM and stained with alizarin red. F, qRT-PCR quantification of osteoblastic differentiation markers in CLBObs after 14 days of culture (n = 12). β2-MG housekeeping gene expression is shown per μg of RNA. Bars, mean ± S.E. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus WT controls. ###, p < 0.001 versus the percentage change in WT cultures.
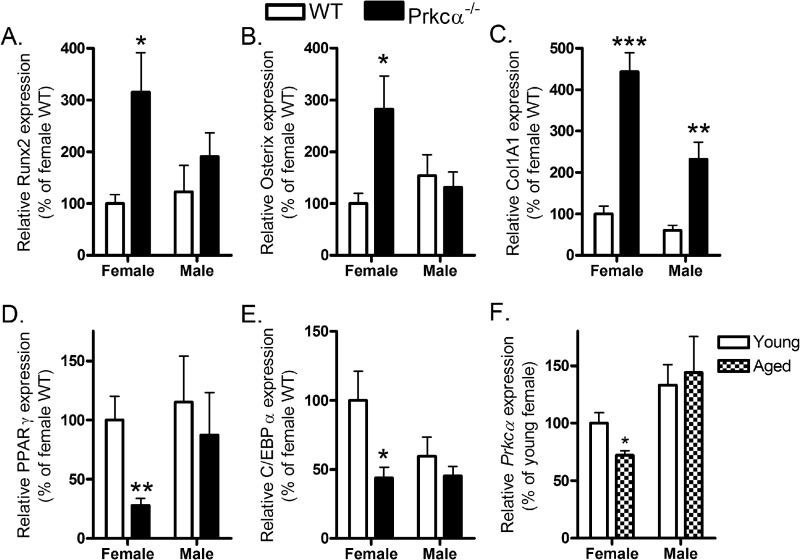
To validate these findings in vivo, osteoblastic differentiation markers were quantified in both marrow and cortical bone extracted from 22-week-old female and male Prkca−/− mice. Runx2 and osterix expression was significantly elevated in marrow from female, but not male, Prkca−/− mice (Fig. 6, A and B), whereas collagen 1 A1 (Col1A1) was elevated in Prkca−/− mice of both genders (Fig. 6C). Surprisingly, none of these differences were observed in the bone tissue predominantly representing terminally differentiated osteocytes; nor were osteoclast-related markers differently expressed (not shown). Consistent with enhanced osteoblast lineage commitment at an early stage of differentiation, markers of adipogenic differentiation were significantly lower in marrow from female but not male Prkca−/− mice (Fig. 6, D and E). We also detected an effect of age on Prkca expression; levels were lower in marrow of 19-month-old (aged) female, not male, WT mice compared with levels in 18-week-old (adult) mice (Fig. 6F).
FIGURE 6.
Prkca deletion alters marrow expression of differentiation markers in a sex-specific manner. Marrow was harvested from pooled tibiae and femurs of male and female WT and Prkca−/− mice and processed by qRT-PCR analysis of the osteoblastic differentiation markers Runx2 (A), osterix (B), and Col1A1 (C) and the adipogenic markers PPARγ (D) and C/EBPα (E) (n = 5). F, Prkca expression was quantified in marrow from young adult (17-week-old) or aged (19-month-old) male and female mice (n = 8). Bars, mean ± S.E. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus the respective WT controls.
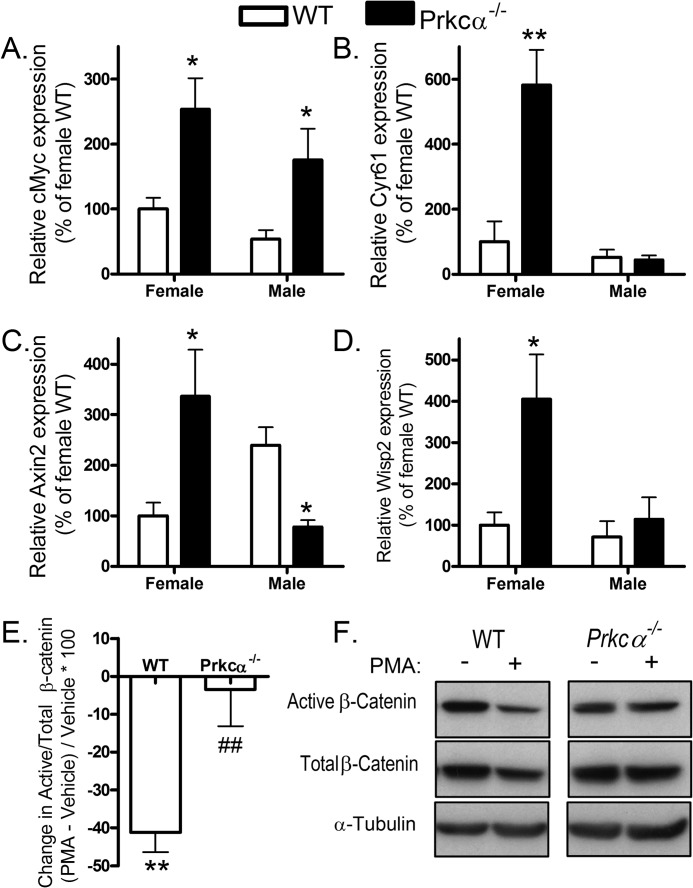
Because the Wnt pathway is a critical regulator of osteoblast differentiation, we investigated whether Wnt signaling was altered in Prkca−/− mice by quantifying the expression of selected Wnt target genes in marrow and bone from Prkca−/− and WT mice. The proliferating cell marker cMyc was overexpressed in marrow from both female and male Prkca−/− mice (Fig. 7A). Cyr61, which is involved in the promotion of osteoblast differentiation by Wnts (53), was dramatically elevated in female but not male Prkca−/− marrow (Fig. 7B). Female Prkca−/− marrow had elevated, whereas males had reduced, expression of Axin2 (Fig. 7C), and female, but not male, Prkca−/− marrow had elevated expression of Wisp2 relative to WT (Fig. 7D). Thus, in the marrow of female mice, deletion of PKCα up-regulated the expression of all Wnt target genes tested. Furthermore, PKC activation with PMA reduced the proportion of β-catenin in the active (dephosphorylated) form in CLBObs from WT but not Prkca−/− mice (Fig. 7, E and F), demonstrating that PKCα suppresses active β-catenin in a manner that cannot be redundantly achieved by activation of other PKC isoforms in these cells.
FIGURE 7.
Prkca suppresses Wnt/β-catenin signaling. A–D, marrow was harvested from pooled tibiae and femurs of male and female WT and Prkca−/− mice and processed by qRT-PCR analysis of the proliferation marker cMyc (A) and other direct Wnt target genes Cyr61 (B), Axin2 (C), and Wisp2 (D) (n = 5). E and F, CLBObs from WT and Prkca−/− female mice were treated with a 0.1 μm concentration of the PKC activator PMA twice at 12-h intervals and lysed 12 h following the second treatment. Active (dephosphorylated) and total β-catenin levels were determined by Western blotting relative to α-tubulin. E, the percentage change in active versus total β-catenin was calculated (n = 6). F, representative Western blots. Bars, mean ± S.E. (error bars). *, p < 0.05; **, p < 0.01 for the effect of PMA treatment; ##, p < 0.01 versus the change in WT controls.
PKCα Promotes Osteoblastic Proliferation in Vitro following Strain and Estradiol, not Wnt3a
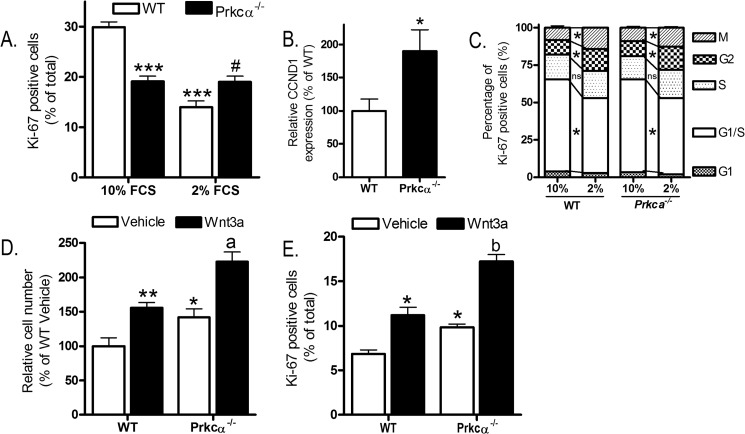
To investigate the role of PKCα in proliferation of osteoblastic cells following mitogenic stimuli, CLBObs were first serum-depleted in 2% charcoal/dextran-stripped (c/d) fetal calf serum. As expected (54), this reduced proliferation of WT CLBObs but surprisingly had no effect on the proportion of cells stained positive for Ki-67 in cultures from female Prkca−/− mice (Fig. 8A). Expression of the proliferating cell marker cyclin D1 was confirmed to be greater in serum-depleted CLBObs from Prkca−/− than in those from WT mice (Fig. 8B). Serum depletion altered the distribution of proliferating cells in the cell cycle similarly in both genotypes, such that the only difference between the genotypes was the initial step whereby quiescent cells become Ki-67-positive (Fig. 8C). Treatment with Wnt3a (10 ng/ml), known to increase osteoblastic cell proliferation (19), similarly increased cell number and the proportion of cells stained for Ki-67 in both genotypes (Fig. 8, D and E).
FIGURE 8.
Prkca deletion alters recruitment of osteoblast-like cells to the cell cycle but not Wnt3a-induced proliferation. A, CLBObs female from WT and Prkca−/− mice were cultured under permissive (10% FCS) or serum-depleted (2% c/d FCS, used for subsequent proliferation studies) for 24 h. The proportion of cells stained positive for Ki-67 was determined. B, CCND1 expression was quantified in qRT-PCR in serum-depleted (2% c/d FCS) subconfluent cultures of CLBObs from WT and Prkca−/− female mice (n = 6). C, the proportion of Ki-67-positive cells with a pattern of staining consistent with the indicated cell cycle stages was determined in CLBObs from female WT and Prkca−/− mice cultured under permissive (10% FCS) or serum-depleted (2% c/d FCS, used for subsequent proliferation studies) for 24 h (n = 8). Cells were treated with Wnt3a and fixed 48 h later for cell number analysis (C) or 24 h later for Ki-67 analysis (D). Bars, mean ± S.E. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus WT controls. #, p < 0.05 versus WT with 2% FCS. a, p < 0.05; b, p < 0.01 versus Wnt3a-treated cells from Prkca−/− mice.
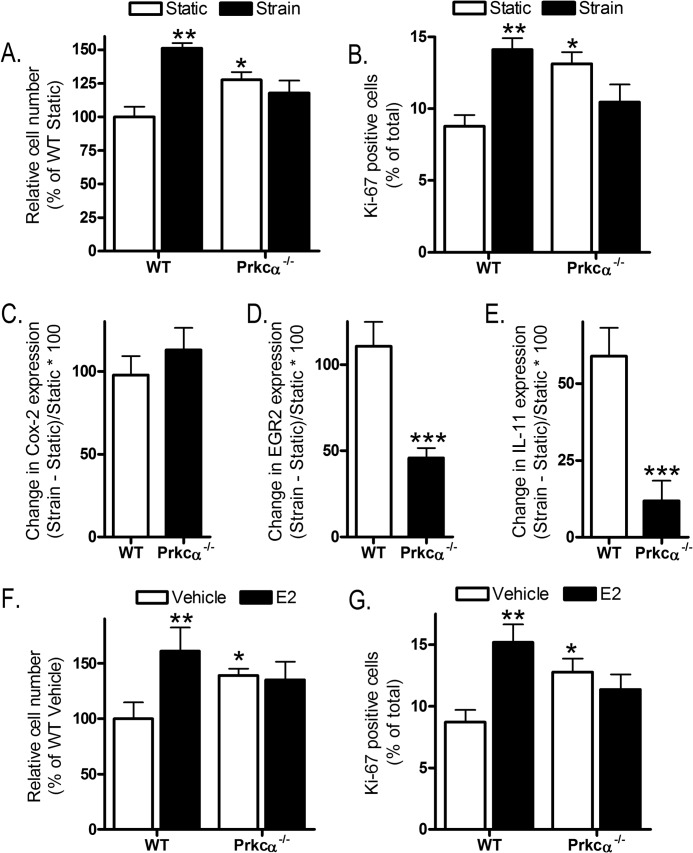
Given that canonical Wnt signaling is activated in osteoblastic cells subjected to mechanical strain (18) and contributes to the mechanisms by which strain induces osteoblastic cell proliferation (19, 53, 55), we exposed cells to mechanical strain by four-point bending of their substrate. This increased proliferation of WT-derived CLBObs, as expected (19, 53, 55), but did not increase proliferation of cells similarly derived from Prkca−/− mice (Fig. 9, A and B). The involvement of PKCα in strain-responsive signaling was further investigated by comparing the expression of the known strain target genes Cox-2, Egr2, and IL-11 (15, 31). In both genotypes, Cox-2 was up-regulated to a similar degree 1 h after strain (Fig. 9C). In contrast, although Egr2 was significantly up-regulated in both genotypes, this was to a significantly lower extent in cells from Prkca−/− mice (Fig. 9D). IL-11 was up-regulated in CLBObs from WT but not Prkca−/− mice (Fig. 9E).
FIGURE 9.
Prkca deletion prevents increased proliferation following mechanical strain or estradiol and alters strain-related gene regulation. CLBObs from female WT and Prkca−/− mice were subjected to strain and fixed 48 h later for cell number analysis (A) or 24 h later for Ki-67 analysis (B). C and E, cells were subjected to strain or kept as static controls and harvested 1 h later to quantify Cox-2 (C), EGR2 (D), and IL-11 (E). The percentage changes in expression in strained versus static control slides are shown (n = 12–15). F and G, cells were treated with 0.1 μm E2 and fixed 48 h later to count cell number or 24 h later for Ki-67 analysis. Bars for proliferation data represent the mean ± S.E. (error bars) (n = 8). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus WT controls.
Intriguingly, this pattern of gene regulation following strain is similar to that observed in CLBObs lacking the activation function 1 domain of ERα (15), the receptor that facilitates osteoblast proliferation following both strain and estradiol treatment (19, 56). Estradiol treatment (0.1 μm) was unable to increase cell number or Ki-67 positivity in CLBObs from Prkca−/− mice as it did in cultures from WT mice (Fig. 9, F and G). Treatment with Wnt3a, strain, or estradiol did not alter the cell cycle distribution of proliferating cells in either genotype (not shown).
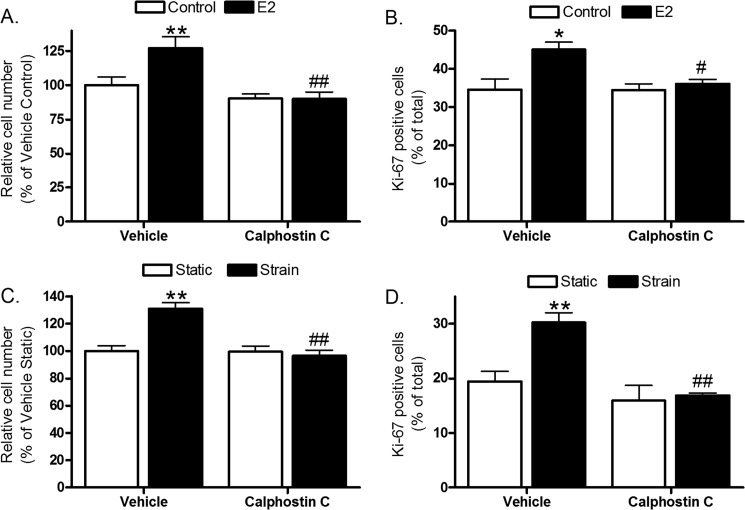
PKC involvement in estradiol- and strain-induced osteoblast proliferation was substantiated in cells of the human female osteoblastic Saos-2 line in which PKC signaling was blocked by pretreatment with 0.1 μm photoactivated calphostin C before strain or estradiol treatment (Fig. 10, A–D). Thus, osteoblast proliferation in response to these anabolic stimuli is impaired when PKC signaling is inhibited.
FIGURE 10.
PKC inhibition prevents human osteoblastic cell proliferation following strain and estradiol. Saos-2 cells were treated with 1 μm E2 (A and B) or subjected to strain with or without 30-min pretreatment with 0.1 μm photoactivated calphostin C (C and D) and fixed 36 h later for cell number analysis (A and C) or 24 h later for Ki-67 analysis (B and D). Bars, means ± S.E. (error bars) (n = 8). *, p < 0.05; **, p < 0.01 versus vehicle controls. #, p < 0.05; ##, p < 0.01 versus the second bar in each graph.
Disuse Prevents Intramedullary Bone Invasion in Female Prkca−/− Mice
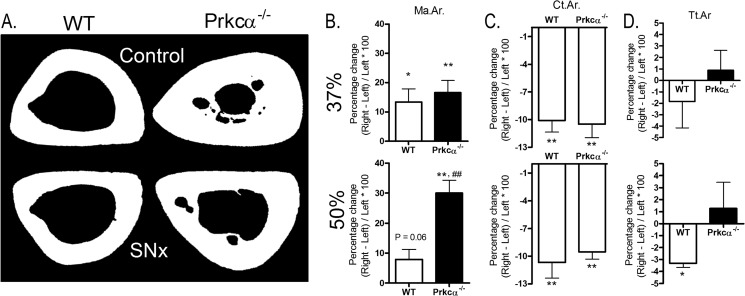
The inability of osteoblast-like cells from Prkca−/− mice to proliferate following strain or estradiol treatment, together with the gender and site specificity of the IM bone phenotype in Prkca−/− mice, led us to hypothesize that IM bone formation may be related to load bearing and/or circulating estrogens. To investigate the influence of load bearing, we substantially reduced it from the right tibiae of Prkca−/− and WT mice by unilateral sciatic neurectomy and compared subsequent bone mass with that in the contralateral left tibia, which acted as an internal control. The effect of Prkca deletion on the response to disuse was assessed at 37% of the bone's length from the proximal end, as reported previously (44). At this site, the bone structure was similar between WT and Prkca−/− mice, and the absence of PKCα in Prkca−/− mice did not influence bone loss (Fig. 11, A–D), which was not significantly different from that in WT at this site.
FIGURE 11.
Disuse influences intramedullary bone in the tibia of female Prkca−/− mice. Female Prkca−/− mice and WT controls (n = 6) were subjected to unilateral right sciatic neurectomy (SNx), causing disuse of the right tibia, at 15 weeks of age and sacrificed 3 weeks later. Left limbs served as internal controls. The effect of sciatic neurectomy was determined at 37 and 50% of the bone's length from the proximal end. A, representative cross-sectional images are shown at the 50% site. The percentage change in medullary area (Ma.Ar) (B), cortical area (Ct.Ar) (C), and total tissue area (Tt.Ar.) (D) was determined by comparison with the left control limbs by μCT. Bars, mean percentage change ± S.E. (error bars). *, p < 0.05; **, p < 0.01, indicating the effect of neurectomy. ##, p < 0.01; ###, p < 0.001 versus the percentage change in WT mice.
However, at the 50% site where IM bone is present in the Prkca−/− mice, disuse resulted in significantly less IM bone (Fig. 11A), such that the medullary area of neurectomized limbs in Prkca−/− mice was not significantly different from that of the control limbs of WT mice (p = 0.16). The overall reduction in bone area was similar between the two genotypes due to significantly smaller total tissue area in the disused limb of WT but not Prkca−/− mice (Fig. 11, B and C). This suggests that the invasion of the medullary cavity by intramedullary bone in female Prkca−/− mice is promoted by load bearing.
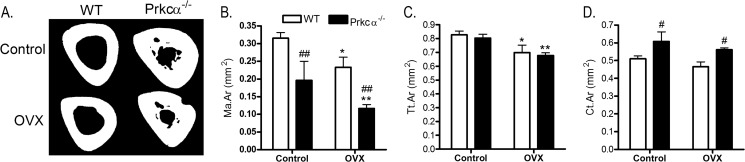
The effect of ovarian hormones on IM bone formation was investigated by subjecting young female mice to ovariectomy (OVX). OVX was performed at 8 weeks of age, before IM bone forms, and the presence of IM bone was analyzed 10 weeks later. OVX resulted in smaller marrow area and smaller total tissue area in both WT and Prkca−/− mice (Fig. 12, A–D). These effects of OVX were similar in both genotypes (genotype by OVX interaction, p > 0.8, as determined by mixed models). Bone area in OVX Prkca−/− mice was greater than in non-ovariectomized wild-type mice (p < 0.05), demonstrating that loss of ovarian hormones did not prevent IM bone development (Fig. 12A).
FIGURE 12.
Ovariectomy does not prevent intramedullary bone in the tibia of female Prkca−/− mice. Female Prkca−/− mice (n = 7) and WT controls (n = 5) were subjected to ovariectomy (OVX) at 8 weeks of age and sacrificed 10 weeks later. Their left limbs were compared with the left limbs of non-ovariectomized controls. A, representative cross-sectional images are shown. Medullary area (Ma.Ar; B), total tissue area (Tt.Ar; C), and cortical area (Ct.Ar; D) were determined by μCT 50% of the tibia's length from the proximal end. Bars, mean percentage change ± S.E. (error bars). *, p < 0.05; **, p < 0.01, indicating the effect of OVX. #, p < 0.05; ##, p < 0.01 versus similarly treated WT mice.
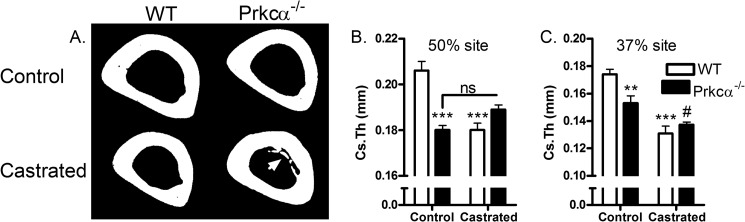
We therefore next investigated whether the lack of intramedullary bone in the long bones of male Prkca−/− mice is due to androgens in males rather than high levels of ovarian steroids in females. 10 weeks following castration, small amounts of intramedullary bone were observed in the tibia of Prkca−/− but none in WT mice (Fig. 13A). Remarkably, this IM bone in male mice occurred in the tibial midshaft (50% of the bone's length) at the same site as it did in female Prkca−/− mice. Castration significantly reduced tibial midshaft cortical thickness in the WT but not Prkca−/− mice, potentially due to the presence of intramedullary bone at this site (Fig. 13B). At the proximal 37% site of the tibia without intramedullary bone, castration reduced cortical thickness in both WT and Prkca−/− mice (Fig. 13C). The presence of IM bone was not sufficient to significantly alter marrow area or cortical bone area relative to castrated WT mice (not shown).
FIGURE 13.
The cortical response to castration is site-specifically altered in Prkca−/− mice due to intramedullary bone formation in the tibial midshaft. A, representative two-dimensional μCT images of control and castrated male WT and Prkca−/− mice. The arrow indicates the presence of intramedullary bone only observed in Prkca−/− mice. Cortical thickness (Cs.Th) was quantified in the tibial midshaft (50%) (B) and the proximal 37% site (C). Bars, mean ± S.E. (error bars); controls, n = 6; WT castrated, n = 4; Prkca−/− castrated, n = 6. ns, not significant. **, p < 0.01, *** p < 0.001 versus WT control. #, p < 0.05 versus control Prkca−/− mice.
DISCUSSION
Our observation of a bone phenotype in female mice with Prkca deletion led us to investigate the potential involvement of PKCα in key osteoregulatory signaling pathways in whole bones in vivo and in osteoblasts in vitro. Prkca deletion had no effect on the medullary cavity in young mice, but this situation changed with maturity between 12 and 22 weeks of age when female, but not male, Prkca−/− mice formed diaphyseal intramedullary bone in various long bones, leaving their periosteal dimensions unaffected. This phenotype is thus remarkable for its age and gender specificity as well as its consistent presence in restricted bone sites.
Histological investigation of these sites led us to identify Gaucher-like cells in the bone marrow in affected regions of the medullary cavity. Various other recognized features of Gaucher disease have been documented, predominantly in female Prkca−/− mice, including marrow infiltration, loss of GBA1 expression, splenomegaly, reduced cortical thickness, bone vascular changes, and impaired platelet aggregation (35, 39, 57, 58). Inhibition of PKC signaling due to sphingolipid accumulation is believed to contribute to the etiology of Gaucher disease (36), and our findings are consistent with this hypothesis, demonstrating that selective global deletion of the Prkca isoform is sufficient to mimic aspects of the disease. However, loss of PKCα cannot explain all features of Gaucher disease, including the increase in bone resorption in patients (35) compared with the predominant phenotype of deregulated endosteal formation in these mice. Thus, although we do not consider Prkca−/− mice a model of Gaucher disease, they may provide insights into its pathogenesis, particularly with relation to bone involvement previously suggested to involve PKC (34).
Osteoblastic cells from a mouse model of Gaucher disease had previously been reported to have deficits in their differentiation and proliferation (34). In the present study, osteoblast differentiation markers were higher in the marrow of Prkca−/− mice relative to their WT counterparts, and increased osteoblastic differentiation was also observed in vitro using osteoblastic cells derived from the long bones of Prkca−/− mice relative to WT. Consistent with a switch in the balance from osteoblast proliferation toward differentiation, osteoblastic cells from Prkca−/− mice were less proliferative than WT-derived cells under permissive culture conditions. The initial step of recruitment to the cell cycle was the only difference observed between the two genotypes, illustrating the role of PKCα as a signaling node promoting proliferation in response to numerous mitogenic stimuli. However, osteoblastic cells from Prkca−/− mice retain the ability to increase their proliferation, as demonstrated by their response to Wnt3a, which was similar to that observed in cells derived from WT mice.
Exposure to a short period of dynamic mechanical strain change did not increase proliferation of cells from Prkca−/− mice as it did in WT-derived cells, and the strain-related up-regulation of EGR2 and IL-11 was also deficient in osteoblastic cells lacking Prkca. Given that it is the responses of such resident bone cells to the strains experienced during habitual loading that determine bone architecture (11), perturbation of the signaling axes involved may account for the reduction in cortical thickness and Erlenmeyer flask-like architecture observed in Prkca−/− mice, which parallel changes in Gaucher patient femora (35).
However, not all responses to strain are dependent on PKCα because Cox-2 up-regulation by strain was similar in both genotypes. This pattern of Cox-2, Egr2, and IL-11 regulation following strain observed in cells from Prkca−/− mice is similar to the regulation of these genes in osteoblastic cells lacking the activation function 1 domain of ERα (15). Given that the ERα activation function 1 domain mediates its interactions with other proteins, including PKC (59), these findings suggest that PKCα and ERα contribute to the same signaling pathways initiated in osteoblastic cells by strain. ERα is also required for osteoblastic cells to increase their proliferation following strain and following estradiol treatment (19, 56), and deletion of Prkca also prevented osteoblastic proliferation following strain or estradiol treatment in the present study. PKCα and ERα physically interact in osteoblastic cells in a complex involving c-Src (23), suggesting a potential mechanism for cooperation between these proteins within the same signaling cascades. Furthermore, PKC signaling reduces ER signaling in overconfluent osteoblastic cells, which attain a more differentiated state (26, 60), potentially acting as a cellular context-specific “break” on ER signaling that is lost in Prkca−/− mice. Although none of the myriad ERα transgenic mice generated thus far have been reported to form intramedullary bone, it is intriguing that ERα deletion only impairs the osteogenic response to loading in female, not male, mice (15, 61). This sex-specific facilitation of bone's adaptation to loading by ERα is ligand-independent and non-genomic (15), suggesting that it involves interactions with signaling molecules potentially including PKCα.
Because it is well established that sex hormones and mechanical loading both involve signaling through the ERs (15, 53, 61), we investigated the effect of load bearing on bone structure in Prkca−/− mice through sciatic neurectomy-induced disuse and the potential role of systemic ovarian hormones through castration or ovariectomy prior to the development of intramedullary bone. The change in cortical bone area triggered by disuse was not influenced by loss of PKCα, suggesting that the increase in resorption due to disuse is not significantly impaired by loss of PKCα. However, disuse prevented any significant invasion of the medullary cavity in female Prkca−/− mice. Based on our current studies, we cannot exclude the possibility that, in addition to inducing disuse, sciatic neurectomy prevented medullary invasion by reducing sympathetic stimulation. Although some authors have reported that the sympathetic nervous system is involved in bone loss caused by hind limb suspension (62, 63), sympathetic blockade does not alter bone gain following loading or bone loss due to neurectomy-induced disuse (64). Disuse, be it through sciatic neurectomy or hind limb suspension, reduces Wnt signaling (9, 65). Thus, an alternative hypothesis is that disuse prevents medullary bone formation by locally suppressing the increase in Wnt signaling observed in the bones of female Prkca−/− mice relative to WT.
In Prkca−/− males, castration resulted in intramedullary bone formation at the same skeletal sites where it was observed in female Prkca−/− mice. However, the amount of intramedullary bone formed in castrated male Prkca−/− mice was considerably less than in female Prkca−/− mice of the same age. This is not surprising because bone had only 10 weeks to form (from castration to sacrifice). Androgen signaling has previously been reported to suppress bone's response to loading (66), which could explain this result and supports our conclusion that PKCα signaling may influence bone's response to load bearing. This situation contrasts with that following ovariectomy, which did not alter intramedullary invasion. Thus, site-specific intramedullary bone formation in female Prkca−/− mice occurs independently of ovarian hormones but requires physiological load bearing.
Endosteal responses to disuse normally change with age, such that medullary expansion with disuse occurs in mature but not growing animals (67). This may also be relevant to the age dependence of bone formation observed in the marrow of skeletally mature female Prkca−/− mice. Disuse-induced bone loss involves suppression of Wnt signaling (65), and perturbing components of the Wnt pathway has previously been shown to have gender-specific effects on bone mass (68) and on the responses to disuse (17). The bases for these gender-specific effects are largely unknown, in part because the interactions between Wnt and androgen signaling in bone have not been studied as extensively as those between Wnt and estrogen signaling. The findings of this study suggest that androgen signaling may suppress bone phenotypes observed in female mice.
Our findings demonstrate for the first time that PKCα is a regulator of the important Wnt signaling pathway in osteoblasts. In vivo changes, particularly in gene expression, must be interpreted with caution, given that the model used in these studies is a germ line deletion of PKCα, although in vitro studies support there being cell-autonomous roles for PKCα in osteoblasts. PKCα suppresses Wnt signaling, as evidenced by the reduced proportion of β-catenin in the active form and reduced activity of the Wnt target gene alkaline phosphatase following PKC activation when PKCα is present. Because Wnt signaling is critical for osteoblast lineage commitment (69, 70), this suggests a mechanism whereby PKCα suppresses osteoblast differentiation. It is also possible that loss of PKCα may have, directly or indirectly, altered Wnt protein levels. However, given that previous publications have demonstrated that PKCα interacts with canonical Wnt signaling at the level of β-catenin in other cell types (27, 28), this possibility was not pursued in the current study.
In conclusion, deletion of Prkca in mice in vivo leads to age-related bony invasion of the medullary cavity at specific sites of the long bones in young adult female but not male mice. This invasion occurs despite ovariectomy, does not occur in the absence of functional load bearing, and is observed in orchidectomized PKCα male mice. The effects of PKCα, at least in female mice, include suppression of osteoblastic differentiation and suppression of Wnt target gene expression, revealing a novel role for PKCα in the regulation of Wnt signaling in osteoblastic cells. In the absence of PKCα, neither strain nor estradiol are capable of recruiting quiescent osteoblasts to the cell cycle, although their capacity to proliferate in response to Wnt3a is not diminished. From these data, we infer that in female, but not male, mice, PKCα acts as a suppressor of loading-related bone formation on the endosteum, with no discernible effect on the periosteum. Prkca deletion in female mice phenocopies some aspects of Gaucher disease in humans. As a molecular regulator of osteoblastic activity, PKCα may be a suitable target for therapeutic approaches to various bone disorders.
This work was supported by the Wellcome Trust Grants 092045/Z/10/Z and 088560/Z/09/Z (to G. L. G. and L. B. M.) and by British Heart Foundation Grant RG/10/006/28299 (to A. P.).
- ER
- estrogen receptor
- CLBOb
- cortical long bone-derived mouse osteoblastic cell
- PMA
- phorbol 12-myristate 13-acetate
- qRT-PCR
- quantitative RT-PCR
- μCT
- microcomputed tomography
- IM
- intramedullary
- OVX
- ovariectomy
- c/d
- charcoal/dextran-stripped.
REFERENCES
- 1. Lanyon L., Skerry T. (2001) Postmenopausal osteoporosis as a failure of bone's adaptation to functional loading: a hypothesis. J. Bone Miner. Res. 16, 1937–1947 [DOI] [PubMed] [Google Scholar]
- 2. Rachner T. D., Khosla S., Hofbauer L. C. (2011) Osteoporosis: now and the future. Lancet 377, 1276–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vanderschueren D., Vandenput L., Boonen S., Lindberg M. K., Bouillon R., Ohlsson C. (2004) Androgens and bone. Endocr. Rev. 25, 389–425 [DOI] [PubMed] [Google Scholar]
- 4. Zebaze R. M., Ghasem-Zadeh A., Bohte A., Iuliano-Burns S., Mirams M., Price R. I., Mackie E. J., Seeman E. (2010) Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet 375, 1729–1736 [DOI] [PubMed] [Google Scholar]
- 5. Kassem M., Marie P. J. (2011) Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell 10, 191–197 [DOI] [PubMed] [Google Scholar]
- 6. Meakin L. B., Galea G. L., Sugiyama T., Lanyon L. E., Price J. S. (2014) Age-related impairment of bones' adaptive response to loading in mice is associated with gender-related deficiencies in osteoblasts but no change in osteocytes. J. Bone Miner. Res. 29, 1859–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Padhi D., Jang G., Stouch B., Fang L., Posvar E. (2011) Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J. Bone Miner. Res. 26, 19–26 [DOI] [PubMed] [Google Scholar]
- 8. Robling A. G., Niziolek P. J., Baldridge L. A., Condon K. W., Allen M. R., Alam I., Mantila S. M., Gluhak-Heinrich J., Bellido T. M., Harris S. E., Turner C. H. (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 283, 5866–5875 [DOI] [PubMed] [Google Scholar]
- 9. Moustafa A., Sugiyama T., Prasad J., Zaman G., Gross T. S., Lanyon L. E., Price J. S. (2012) Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos. Int. 23, 1225–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tu X., Rhee Y., Condon K. W., Bivi N., Allen M. R., Dwyer D., Stolina M., Turner C. H., Robling A. G., Plotkin L. I., Bellido T. (2012) Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 50, 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skerry T. M. (2006) One mechanostat or many? Modifications of the site-specific response of bone to mechanical loading by nature and nurture. J. Musculoskelet. Neuronal Interact. 6, 122–127 [PubMed] [Google Scholar]
- 12. Pead M. J., Skerry T. M., Lanyon L. E. (1988) Direct transformation from quiescence to bone formation in the adult periosteum following a single brief period of bone loading. J. Bone Miner. Res. 3, 647–656 [DOI] [PubMed] [Google Scholar]
- 13. Turner C. H., Owan I., Alvey T., Hulman J., Hock J. M. (1998) Recruitment and proliferative responses of osteoblasts after mechanical loading in vivo determined using sustained-release bromodeoxyuridine. Bone 22, 463–469 [DOI] [PubMed] [Google Scholar]
- 14. Lee K. C. L., Jessop H., Suswillo R., Zaman G., Lanyon L. E. (2004) The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of oestrogen receptor-α and -β. J. Endocrinol. 182, 193–201 [DOI] [PubMed] [Google Scholar]
- 15. Windahl S. H., Saxon L., Borjesson A. E., Lagerquist M. K., Frenkel B., Henning P., Lerner U. H., Galea G. L., Meakin L. B., Engdahl C., Sjögren K., Antal M. C., Krust A., Chambon P., Lanyon L. E., Price J. S., Ohlsson C. (2013) Estrogen receptor-α is required for the osteogenic response to mechanical loading in a ligand-independent manner involving its activation function 1 but not 2. J. Bone Miner. Res. 28, 291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aguirre J. I., Plotkin L. I., Gortazar A. R., Millan M. M., O'Brien C. A., Manolagas S. C., Bellido T. (2007) A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J. Biol. Chem. 282, 25501–25508 [DOI] [PubMed] [Google Scholar]
- 17. Saxon L. K., Jackson B. F., Sugiyama T., Lanyon L. E., Price J. S. (2011) Analysis of multiple bone responses to graded strains above functional levels, and to disuse, in mice in vivo show that the human Lrp5 G171V high bone mass mutation increases the osteogenic response to loading but that lack of Lrp5 activity reduces it. Bone 49, 184–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Armstrong V. J., Muzylak M., Sunters A., Zaman G., Saxon L. K., Price J. S., Lanyon L. E. (2007) Wnt/β-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor α. J. Biol. Chem. 282, 20715–20727 [DOI] [PubMed] [Google Scholar]
- 19. Galea G. L., Meakin L. B., Sugiyama T., Zebda N., Sunters A., Taipaleenmaki H., Stein G. S., van Wijnen A. J., Lanyon L. E., Price J. S. (2013) Estrogen receptor α mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor β. J. Biol. Chem. 288, 9035–9048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oster H., Leitges M. (2006) Protein kinase C α but not PKCζ suppresses intestinal tumor formation in ApcMin/+ mice. Cancer Res. 66, 6955–6963 [DOI] [PubMed] [Google Scholar]
- 21. Gwak J., Jung S. J., Kang D. I., Kim E. Y., Kim D. E., Chung Y. H., Shin J. G., Oh S. (2009) Stimulation of protein kinase Cα suppresses colon cancer cell proliferation by down-regulation of β-catenin. J. Cell Mol. Med. 13, 2171–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang J. Z., O'Flatharta C., Harvey B. J., Thomas W. (2008) Membrane ERα-dependent activation of PKCα in endometrial cancer cells by estradiol. Steroids 73, 1110–1122 [DOI] [PubMed] [Google Scholar]
- 23. Longo M., Brama M., Marino M., Bernardini S., Korach K. S., Wetsel W. C., Scandurra R., Faraggiana T., Spera G., Baron R., Teti A., Migliaccio S. (2004) Interaction of estrogen receptor alpha with protein kinase C α and c-Src in osteoblasts during differentiation. Bone 34, 100–111 [DOI] [PubMed] [Google Scholar]
- 24. Konopatskaya O., Poole A. W. (2010) Protein kinase Cα: disease regulator and therapeutic target. Trends Pharmacol. Sci. 31, 8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haughian J. M., Reno E. M., Thorne A. M., Bradford A. P. (2009) Protein kinase C α-dependent signaling mediates endometrial cancer cell growth and tumorigenesis. Int. J. Cancer 125, 2556–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Longo M., Peruzzi B., Fortunati D., De Luca V., Denger S., Caselli G., Migliaccio S., Teti A. (2006) Modulation of human estrogen receptor α F promoter by a protein kinase C/c-Src-dependent mechanism in osteoblast-like cells. J. Mol. Endocrinol. 37, 489–502 [DOI] [PubMed] [Google Scholar]
- 27. Gwak J., Cho M., Gong S. J., Won J., Kim D. E., Kim E. Y., Lee S. S., Kim M., Kim T. K., Shin J. G., Oh S. (2006) Protein-kinase-C-mediated β-catenin phosphorylation negatively regulates the Wnt/β-catenin pathway. J. Cell Sci. 119, 4702–4709 [DOI] [PubMed] [Google Scholar]
- 28. Lee J. M., Kim I. S., Kim H., Lee J. S., Kim K., Yim H. Y., Jeong J., Kim J. H., Kim J. Y., Lee H., Seo S. B., Kim H., Rosenfeld M. G., Kim K. I., Baek S. H. (2010) RORα attenuates Wnt/β-catenin signaling by PKCα-dependent phosphorylation in colon cancer. Mol. Cell 37, 183–195 [DOI] [PubMed] [Google Scholar]
- 29. Geng W. D., Boskovic G., Fultz M. E., Li C., Niles R. M., Ohno S., Wright G. L. (2001) Regulation of expression and activity of four PKC isozymes in confluent and mechanically stimulated UMR-108 osteoblastic cells. J. Cell. Physiol. 189, 216–228 [DOI] [PubMed] [Google Scholar]
- 30. Galea G. L., Sunters A., Meakin L. B., Zaman G., Sugiyama T., Lanyon L. E., Price J. S. (2011) Sost down-regulation by mechanical strain in human osteoblastic cells involves PGE2 signaling via EP4. FEBS Lett. 585, 2450–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zaman G., Sunters A., Galea G. L., Javaheri B., Saxon L. K., Moustafa A., Armstrong V. J., Price J. S., Lanyon L. E. (2012) Loading-related regulation of transcription factor EGR2/Krox-20 in bone cells is ERK1/2 protein-mediated and prostaglandin, Wnt signaling pathway-, and insulin-like growth factor-I axis-dependent. J. Biol. Chem. 287, 3946–3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kido S., Kuriwaka-Kido R., Umino-Miyatani Y., Endo I., Inoue D., Taniguchi H., Inoue Y., Imamura T., Matsumoto T. (2010) Mechanical stress activates Smad pathway through PKCδ to enhance interleukin-11 gene transcription in osteoblasts. PLoS One 5, e13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakura A., Higuchi C., Yoshida K., Yoshikawa H. (2011) PKCα suppresses osteoblastic differentiation. Bone 48, 476–484 [DOI] [PubMed] [Google Scholar]
- 34. Mistry P. K., Liu J., Yang M., Nottoli T., McGrath J., Jain D., Zhang K., Keutzer J., Chuang W. L., Chuang W. L., Mehal W. Z., Zhao H., Lin A., Mane S., Liu X., Peng Y. Z., Li J. H., Agrawal M., Zhu L. L., Blair H. C., Robinson L. J., Iqbal J., Sun L., Zaidi M. (2010) Glucocerebrosidase gene-deficient mouse recapitulates Gaucher disease displaying cellular and molecular dysregulation beyond the macrophage. Proc. Natl. Acad. Sci. U.S.A. 107, 19473–19478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wenstrup R. J., Roca-Espiau M., Weinreb N. J., Bembi B. (2002) Skeletal aspects of Gaucher disease: a review. Br. J. Radiol. 75, A2–A12 [DOI] [PubMed] [Google Scholar]
- 36. Hannun Y. A., Bell R. M. (1987) Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science 235, 670–674 [DOI] [PubMed] [Google Scholar]
- 37. Braz J. C., Gregory K., Pathak A., Zhao W., Sahin B., Klevitsky R., Kimball T. F., Lorenz J. N., Nairn A. C., Liggett S. B., Bodi I., Wang S., Schwartz A., Lakatta E. G., DePaoli-Roach A. A., Robbins J., Hewett T. E., Bibb J. A., Westfall M. V., Kranias E. G., Molkentin J. D. (2004) PKC-α regulates cardiac contractility and propensity toward heart failure. Nat. Med. 10, 248–254 [DOI] [PubMed] [Google Scholar]
- 38. Wang Y., Klein J. D., Froehlich O., Sands J. M. (2013) Role of protein kinase C-α in hypertonicity-stimulated urea permeability in mouse inner medullary collecting ducts. Am. J. Physiol. Renal Physiol. 304, F233–F238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Konopatskaya O., Gilio K., Harper M. T., Zhao Y., Cosemans J. M., Karim Z. A., Whiteheart S. W., Molkentin J. D., Verkade P., Watson S. P., Heemskerk J. W., Poole A. W. (2009) PKCα regulates platelet granule secretion and thrombus formation in mice. J. Clin. Invest. 119, 399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang L., Zhao R., Shi X., Wei T., Halloran B. P., Clark D. J., Jacobs C. R., Kingery W. S. (2009) Substance P stimulates bone marrow stromal cell osteogenic activity, osteoclast differentiation, and resorption activity in vitro. Bone 45, 309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cao J. J., Singleton P. A., Majumdar S., Boudignon B., Burghardt A., Kurimoto P., Wronski T. J., Bourguignon L. Y., Halloran B. P. (2005) Hyaluronan increases RANKL expression in bone marrow stromal cells through CD44. J. Bone Miner. Res. 20, 30–40 [DOI] [PubMed] [Google Scholar]
- 42. Galea G. L., Price J. S., Lanyon L. E. (2013) Estrogen receptors' roles in the control of mechanically adaptive bone (re)modeling. Bonekey Rep. 2, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spandidos A., Wang X., Wang H., Seed B. (2010) PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 38, D792–D799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sugiyama T., Meakin L. B., Browne W. J., Galea G. L., Price J. S., Lanyon L. E. (2012) Bones' adaptive response to mechanical loading is essentially linear between the low strains associated with disuse and the high strains associated with the lamellar/woven bone transition. J. Bone Miner. Res. 27, 1784–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bouxsein M. L., Boyd S. K., Christiansen B. A., Guldberg R. E., Jepsen K. J., Müller R. (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 25, 1468–1486 [DOI] [PubMed] [Google Scholar]
- 46. Sugiyama T., Galea G. L., Lanyon L. E., Price J. S. (2010) Mechanical loading-related bone gain is enhanced by tamoxifen but unaffected by fulvestrant in female mice. Endocrinology 151, 5582–5590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rawlinson S. C., Mosley J. R., Suswillo R. F., Pitsillides A. A., Lanyon L. E. (1995) Calvarial and limb bone cells in organ and monolayer culture do not show the same early responses to dynamic mechanical strain. J. Bone Miner. Res. 10, 1225–1232 [DOI] [PubMed] [Google Scholar]
- 48. Sokolowska B., Skomra D., Czartoryska B., Tomczak W., Tylki-Szymańska A., Gromek T., Dmoszyńska A. (2011) Gaucher disease diagnosed after bone marrow trephine biopsy: a report of two cases. Folia Histochem. Cytobiol. 49, 352–356 [DOI] [PubMed] [Google Scholar]
- 49. Piran S., Roberts A., Patterson M. A., Amato D. (2009) The clinical course of untreated Gaucher disease in 22 patients over 10 years: hematological and skeletal manifestations. Blood Cells Mol. Dis. 43, 289–293 [DOI] [PubMed] [Google Scholar]
- 50. Williams C. M., Harper M. T., Poole A. W. (2014) PKCα negatively regulates in vitro proplatelet formation and in vivo platelet production in mice. Platelets 25, 62–68 [DOI] [PubMed] [Google Scholar]
- 51. Zaman G., Pitsillides A. A., Rawlinson S. C., Suswillo R. F., Mosley J. R., Cheng M. Z., Platts L. A., Hukkanen M., Polak J. M., Lanyon L. E. (1999) Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J. Bone Miner. Res. 14, 1123–1131 [DOI] [PubMed] [Google Scholar]
- 52. Sunters A., Armstrong V. J., Zaman G., Kypta R. M., Kawano Y., Lanyon L. E., Price J. S. (2010) Mechano-transduction in osteoblastic cells involves strain-regulated estrogen receptor α-mediated control of insulin-like growth factor (IGF) I receptor sensitivity to ambient IGF, leading to phosphatidylinositol 3-kinase/AKT-dependent Wnt/LRP5 receptor-independent activation of β-catenin signaling. J. Biol. Chem. 285, 8743–8758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee K., Jessop H., Suswillo R., Zaman G., Lanyon L. (2003) Endocrinology: bone adaptation requires oestrogen receptor-α. Nature 424, 389. [DOI] [PubMed] [Google Scholar]
- 54. Javaheri B., Sunters A., Zaman G., Suswillo R. F., Saxon L. K., Lanyon L. E., Price J. S. (2012) Lrp5 is not required for the proliferative response of osteoblasts to strain but regulates proliferation and apoptosis in a cell autonomous manner. PLoS One 7, e35726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Robinson J. A., Chatterjee-Kishore M., Yaworsky P. J., Cullen D. M., Zhao W., Li C., Kharode Y., Sauter L., Babij P., Brown E. L., Hill A. A., Akhter M. P., Johnson M. L., Recker R. R., Komm B. S., Bex F. J. (2006) Wnt/β-catenin signaling is a normal physiological response to mechanical loading in bone. J. Biol. Chem. 281, 31720–31728 [DOI] [PubMed] [Google Scholar]
- 56. Cheng M. Z., Rawlinson S. C., Pitsillides A. A., Zaman G., Mohan S., Baylink D. J., Lanyon L. E. (2002) Human osteoblasts' proliferative responses to strain and 17β-estradiol are mediated by the estrogen receptor and the receptor for insulin-like growth factor I. J. Bone Miner. Res. 17, 593–602 [DOI] [PubMed] [Google Scholar]
- 57. Barton N. W., Brady R. O., Dambrosia J. M., Di Bisceglie A. M., Doppelt S. H., Hill S. C., Mankin H. J., Murray G. J., Parker R. I., Argoff C. E. (1991) Replacement therapy for inherited enzyme deficiency: macrophage-targeted glucocerebrosidase for Gaucher's disease. N. Engl. J. Med. 324, 1464–1470 [DOI] [PubMed] [Google Scholar]
- 58. Gillis S., Hyam E., Abrahamov A., Elstein D., Zimran A. (1999) Platelet function abnormalities in Gaucher disease patients. Am. J. Hematol. 61, 103–106 [DOI] [PubMed] [Google Scholar]
- 59. Lahooti H., Thorsen T., Aakvaag A. (1998) Modulation of mouse estrogen receptor transcription activity by protein kinase C δ. J. Mol. Endocrinol. 20, 245–259 [DOI] [PubMed] [Google Scholar]
- 60. Migliaccio S., Wetsel W. C., Fox W. M., Washburn T. F., Korach K. S. (1993) Endogenous protein kinase-C activation in osteoblast-like cells modulates responsiveness to estrogen and estrogen receptor levels. Mol. Endocrinol. 7, 1133–1143 [DOI] [PubMed] [Google Scholar]
- 61. Saxon L. K., Galea G., Meakin L., Price J., Lanyon L. E. (2012) Estrogen receptors α and β have different gender-dependent effects on the adaptive responses to load bearing in cancellous and cortical bone. Endocrinology 153, 2254–2266 [DOI] [PubMed] [Google Scholar]
- 62. Swift J. M., Hogan H. A., Bloomfield S. A. (2013) β-1 adrenergic agonist mitigates unloading-induced bone loss by maintaining formation. Med. Sci. Sports Exerc. 45, 1665–1673 [DOI] [PubMed] [Google Scholar]
- 63. Kondo H., Nifuji A., Takeda S., Ezura Y., Rittling S. R., Denhardt D. T., Nakashima K., Karsenty G., Noda M. (2005) Unloading induces osteoblastic cell suppression and osteoclastic cell activation to lead to bone loss via sympathetic nervous system. J. Biol. Chem. 280, 30192–30200 [DOI] [PubMed] [Google Scholar]
- 64. Marenzana M., De Souza R. L., Chenu C. (2007) Blockade of β-adrenergic signaling does not influence the bone mechano-adaptive response in mice. Bone 41, 206–215 [DOI] [PubMed] [Google Scholar]
- 65. Lin C., Jiang X., Dai Z., Guo X., Weng T., Wang J., Li Y., Feng G., Gao X., He L. (2009) Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/β-catenin signaling. J. Bone Miner. Res. 24, 1651–1661 [DOI] [PubMed] [Google Scholar]
- 66. Callewaert F., Bakker A., Schrooten J., Van Meerbeek B., Verhoeven G., Boonen S., Vanderschueren D. (2010) Androgen receptor disruption increases the osteogenic response to mechanical loading in male mice. J. Bone Miner. Res. 25, 124–131 [DOI] [PubMed] [Google Scholar]
- 67. Uhthoff H. K., Jaworski Z. F. (1978) Bone loss in response to long-term immobilisation. J. Bone Joint Surg. Br. 60, 420–429 [DOI] [PubMed] [Google Scholar]
- 68. Noh T., Gabet Y., Cogan J., Shi Y., Tank A., Sasaki T., Criswell B., Dixon A., Lee C., Tam J., Kohler T., Segev E., Kockeritz L., Woodgett J., Müller R., Chai Y., Smith E., Bab I., Frenkel B. (2009) Lef1 haploinsufficient mice display a low turnover and low bone mass phenotype in a gender- and age-specific manner. PLoS One 4, e5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gaur T., Lengner C. J., Hovhannisyan H., Bhat R. A., Bodine P. V., Komm B. S., Javed A., van Wijnen A. J., Stein J. L., Stein G. S., Lian J. B. (2005) Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 280, 33132–33140 [DOI] [PubMed] [Google Scholar]
- 70. Joeng K. S., Schumacher C. A., Zylstra-Diegel C. R., Long F., Williams B. O. (2011) Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Dev. Biol. 359, 222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]