Background: AtALMT9 is an intracellular anion channel regulating stomata aperture.
Results: ATP interacts with the pore of AtALMT9 competing with vacuolar anions and modifying the voltage dependence of this ion channel.
Conclusion: Cytosolic nucleotides and vacuolar anions directly modulate the transport activity of AtALMT9.
Significance: Regulation of intracellular transporters is crucial for their physiological functions.
Keywords: ATP, Chloride Transport, Electrophysiology, Membrane Biophysics, Membrane Transport, Chloride Channels, Intracellular, Malate, Open Channel Blocker
Abstract
The aluminum-activated malate transporters (ALMTs) form a membrane protein family exhibiting different physiological roles in plants, varying from conferring tolerance to environmental Al3+ to the regulation of stomatal movement. The regulation of the anion channels of the ALMT family is largely unknown. Identifying intracellular modulators of the activity of anion channels is fundamental to understanding their physiological functions. In this study we investigated the role of cytosolic nucleotides in regulating the activity of the vacuolar anion channel AtALMT9. We found that cytosolic nucleotides modulate the transport activity of AtALMT9. This modulation was based on a direct block of the pore of the channel at negative membrane potentials (open channel block) by the nucleotide and not by a phosphorylation mechanism. The block by nucleotides of AtALMT9-mediated currents was voltage dependent. The blocking efficiency of intracellular nucleotides increased with the number of phosphate groups and ATP was the most effective cellular blocker. Interestingly, the ATP block induced a marked modification of the current-voltage characteristic of AtALMT9. In addition, increased concentrations of vacuolar anions were able to shift the ATP block threshold to a more negative membrane potential. The block of AtALMT9-mediated anion currents by ATP at negative membrane potentials acts as a gate of the channel and vacuolar anion tune this gating mechanism. Our results suggest that anion transport across the vacuolar membrane in plant cells is controlled by cytosolic nucleotides and the energetic status of the cell.
Introduction
Aluminum-activated malate transporters (ALMTs)3 form a membrane protein family exclusive to plants. These proteins have been found to play different important physiological roles. In roots ALMTs are involved in conferring tolerance to environmental Al3+ by extruding organic acids in the soil (1–3). In guard cells ALMTs mediate anion fluxes through the plasma and vacuolar membranes and are involved in the regulation of stomatal movements (3–6). The first ALMT channel described, TaALMT1, was identified as a gene associated to aluminum resistance (2). TaALMT1 and its homologue in Arabidopsis thaliana, AtALMT1, catalyze the exudation of malate across the plasma membrane of root cells, resulting in complexation of Al3+ (2, 3). Another identified plasma membrane targeted ALMT in A. thaliana is AtALMT12 (5). AtALMT12 is a component of the R-type/QUAC channel of guard cells that mediate the efflux of anions to induce stomata closure (5). AtALMT9 was the first vacuolar ALMT identified and characterized in A. thaliana (4). It was shown that AtALMT9 is able to mediate malate and fumarate currents into the vacuole of Arabidopsis mesophyll cells. Recently, AtALMT9 was shown to play a crucial role in guard cells where it functions as a malate-activated chloride channel involved in stomata opening (6). Moreover, AtALMT9 served as a model to investigate the structural organization of AtALMTs. It was reported that AtALMT9 assembles as a multimer/tetramer and a region involved in forming the permeation pathway was identified (7).
The aim of the present study was to address the question, why different members of the ALMT family exhibit such striking differences in the current-voltage relationships (i.e. I-V curve). The current-voltage relationship is a fundamental biophysical characteristic of ion transporters providing information on their physiological properties. AtALMT12, which constitutes part of the R-type/QUAC currents in the plasma membrane of guard cells, exhibits a characteristic “bell-shaped” I-V curve (5, 8). The bell shape of the I-V curve results from an abrupt change in channel conductance from positive to negative at very negative membrane potentials. This indicates that the channels start to close and the resulting ion current decrease after a critical membrane potential (5, 8). In contrast, the other ALMTs exhibit a monotonic voltage-dependent I-V curve. This fact indicates that the channels stay open at negative membrane potentials (2–4, 6, 9). The difference between the I-V characteristics of the ALMTs opens the question of the origin of such a difference. The R-type/quick-activating anion channel of Arabidopsis hypocotyl and guard cells was shown to be regulated by cytosolic nucleotides (10–13). In hypocotyl protoplasts it was proposed that cytosolic nucleotides are blockers acting as a “voltage-dependent gate” of the R-type/QUAC/AtALMT12 channel (13), a gating mode similar to what has been observed in mammalian KIR (potassium inward rectifier) with Mg2+ and polyamines (14, 15). Surprisingly, after the discovery that ALMTs are part of the R-type/QUAC current the effect of intracellular nucleotides on ALMTs has never been investigated.
In the present study we addressed the question of the origin of the different behaviors of I-V curves of the ALMTs. We used the vacuolar anion channel AtALMT9 as a model for ALMT channels with a monotonic I-V curve and found that the cytosolic nucleotides block and regulate AtALMT9. The block of AtALMT9 by cytosolic nucleotides is voltage-dependent and in the presence of cytosolic nucleotides the I-V curves becomes bell-shaped similarly to the one of the R-type/QUAC channel. Moreover, the block by cytosolic nucleotides is modulated by the concentration and the nature of the permeable anion present at the cytosolic and the vacuolar side of the channel. The anions at the vacuolar side affect the block by cytosolic nucleotides indicating that the cytosolic nucleotides and the vacuolar anions interact within the permeation pathway of AtALMT9. Our data provide new insight in the regulation of anion channels in the vacuolar membrane.
EXPERIMENTAL PROCEDURES
Overexpression of AtALMT9-GFP in Nicotiana benthamiana
Agrobacterium tumefaciens (GV3101) was transformed with plasmids containing the sequences of the AtALMT9WT channel and its point-mutated derivative AtALMT9K193E by electroporation. The Agrobacterium-mediated infiltration of 4-week-old N. benthamiana leaves was performed as described previously with slight modifications (16). After transient transformation tobacco plants were grown in the greenhouse (16 h light/8 h dark, 25 °C/23 °C, 100 to 200 μmol photons m−2 s−1, 60% relative humidity) for another 2–3 days and then used to isolate protoplasts for patch clamp experiments.
Electrophysiology
Mesophyll protoplasts from AtALMT9-GFP overexpressing tobacco leaves were isolated by enzymatic digestion. The enzyme solution contained 0.3% (w/v) cellulase R-10, 0.03% (w/v) pectolyase Y-23, 1 mm CaCl2, 500 mm sorbitol, and 10 mm MES, pH 5.3, 550 mOsm. Protoplasts were washed twice and resuspended in the same solution without enzymes. Vacuoles were released from mesophyll protoplasts by the addition of 5 mm EDTA and a slight osmotic shock (500 mOsm, see medium below). Transformed vacuoles exhibiting an AtALMT9-GFP signal were selected using an epifluorescence microscope. Membrane currents from tonoplast patches were recorded in the excised cytosolic-side out configuration with the patch clamp technique as described elsewhere (6, 7).
The cytosolic solution contained (i) 100 mm malic acid, adjusted to pH 7.5 with BisTris propane, (ii) 30, 50, and 100 mm malic acid, supplemented with nucleotides as indicated, adjusted to pH 7.5 with BisTris propane, and (iii) 100 mm Cl−, supplemented with nucleotides as indicated, adjusted to pH 7.5 with BisTris propane. The osmolarity was adjusted to 500 mOsm using sorbitol. The pipette solution contained (i) 112 mm malic acid, 5 mm HCl and was adjusted with BisTris propane to pH 6; (ii) 10 mm malic acid, 90 mm MES, 5 mm HCl and was adjusted with BisTris propane; (iii) 1 mm malic acid, 99 mm MES, 5 mm HCl, and was adjusted with BisTris propane; (iv) 100 mm HCl and was adjusted with BisTris propane to pH 6. The osmolarity was adjusted to 550 mOsm using sorbitol. The ionic condition (i) of cytosolic solution and (ii) the pipette solution is defined as control solution (ctrl). All chemicals were purchased from Sigma. Liquid junction potentials were measured according to Neher (17) and corrected when higher than ±2 mV.
The dose-response for the ATPfree inhibition of AtALMT9WT currents were fitted and analyzed with the Langmuir isotherm in the following form,
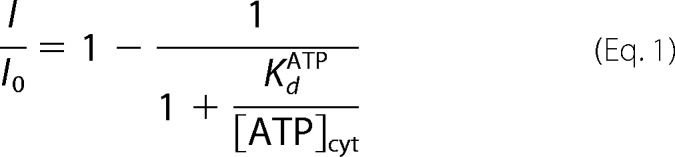 |
where I is the AtALMT9 current amplitude in the presence of ATPfree, I0 the AtALMT9 current under control solution, [ATP]cyt the cytosolic ATP concentration, and KdATP the dissociation constant of ATPfree.
To estimate the fraction of the electrical field that ATP traverses to reach its binding site in Fig. 1B, the voltage-dependent dissociation constant relationship was fitted with the equation described by Woodhull (18),
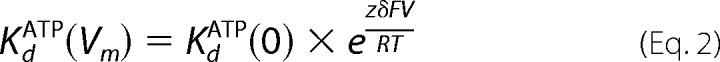 |
in which KdATP(Vm) is the voltage-dependent dissociation constant of ATP, KdATP(0) the dissociation constant of ATP at 0 mV, Vm is the transmembrane potential, z the valence of the blocker (−4 for ATP at pH 7.5), δ the fraction of the electrical membrane field traversed by the blocker, and F, R, and T the Faraday constant, gas constant, and absolute temperature, respectively.
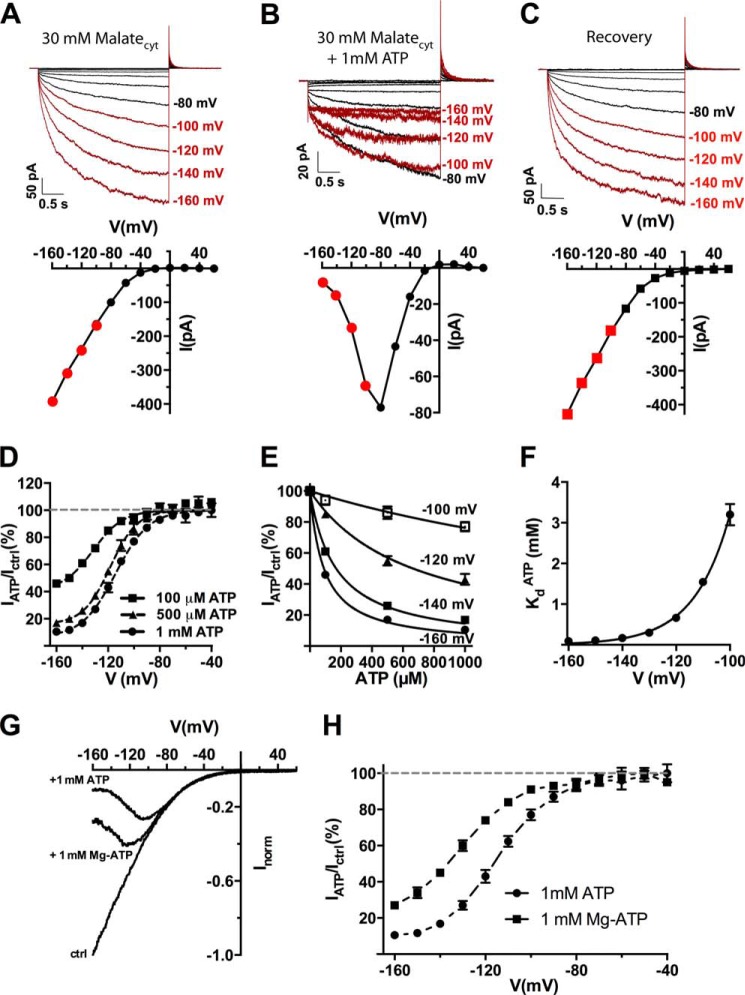
FIGURE 1.
Cytosolic ATP reversibly blocks AtALMT9-mediated currents in a voltage-dependent fashion. A–C, representative traces and corresponding I-V curves of excised cytosolic-side out patches from N. benthamiana vacuoles overexpressing AtALMT9 in 30 mm malatecyt, 100 mm malatevac (A), 30 mm malatecyt + 1 mm ATPfree, 100 mm malatevac (B), and recovery (C). Currents were evoked in response to 3-s voltage pulses ranging from +60 to −160 mV in −20 mV steps, followed by a tail pulse at +60 mV. The holding potential was set at +60 mV. Red highlights the currents at the membrane potentials where the ATP inhibition was effective. D, ratio between currents measured in the absence (Ictrl, 30 mm malatecyt, 100 mm malatevac) and presence of the indicated concentrations of ATPcyt (IATP, 30 mm malatecyt + X mm ATPfree, 100 mm malatevac) at different applied membrane potentials. Data were fitted with Equation 3 (see “Experimental Procedures”; dashed line) (n = 4–7). E, dose-response curves for ATPfree at different membrane potentials. Data were fitted with Equation 1 (see “Experimental Procedures”; solid lines) to derive the dissociation constant (KdATP). F, voltage dependence of the dissociation constant for ATP (KdATP) in 30 mm malatecyt, 100 mm malatevac. Solid line, data were fitted with Equation 2. G and H, inhibitory effect of Mg-ATP. G, normalized current-voltage curves were obtained with a voltage ramp (from +60 to −160 mV in 1.5 s) with 30 mm malatecyt, 100 mm malatevac and in 30 mm malatecyt, 100 mm malatevac in the presence of 1 mm ATPfree (+1 mm ATP) or Mg-ATP (1 mm Mg-ATP). H, residual currents (IATP/Ictrl; mean ± S.E., n = 3–7) plotted against the applied voltage in the presence of 1 mm ATPfree or Mg-ATP. Data were fitted with Equation 3 (dashed line). Error bars denote S.E.
The fraction of currents not blocked by ATP in Figs. 1B, 2B, 3B, and 4B were fitted by a Boltzmann function with an offset,
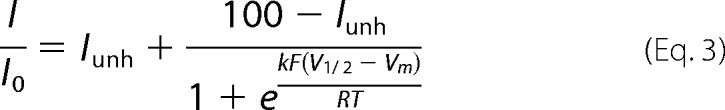 |
where I is the current amplitude in the presence of ATP, I0 is the current amplitude in a solution without ATP, Iunh is the minimum fraction of unblocked current by ATP, V½ is the potential at which current is half-blocked, Vm is the membrane potential, k is a slope factor, F, R, and T the Faraday constant, gas constant, and absolute temperature, respectively. Experiments were performed at room temperature (22–25 °C).
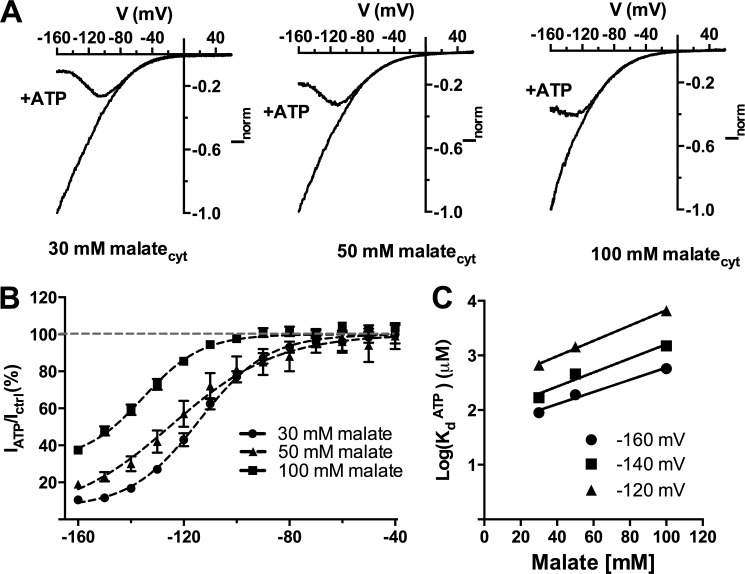
FIGURE 2.
Cytosolic ATP compete with cytosolic malate to inhibit AtALMT9-mediated currents. A, normalized current-voltage curves obtained with a voltage ramp protocol (from +60 to −160 mV in 1. 5 s) in cytosolic-side buffers containing various concentrations of malate (30, 50, and 100 mm malatecyt) with constant vacuolar side conditions (100 mm malatevac) in the presence (+ATP) or absence of 1 mm ATPfree. B, fraction of unblocked current in the presence of 1 mm ATPfree (IATP/Ictrl; mean ± S.E., n = 4–7) plotted against the applied voltage in different cytosolic malate concentrations. Data were fitted with Equation 3 (“Experimental Procedures”; Table 1; solid line). C, logarithmic plot of the dissociation constant for ATPfree (KdATP) at different applied membrane potentials (−120, −140, −160 mV) shown as a function of different cytosolic malate concentrations (30, 50, and 100 mm malatecyt). Data were fitted with a straight line with no theoretical significance to extrapolate the value of the dissociation constant at malate concentrations in the physiological range. Error bars denote S.E.
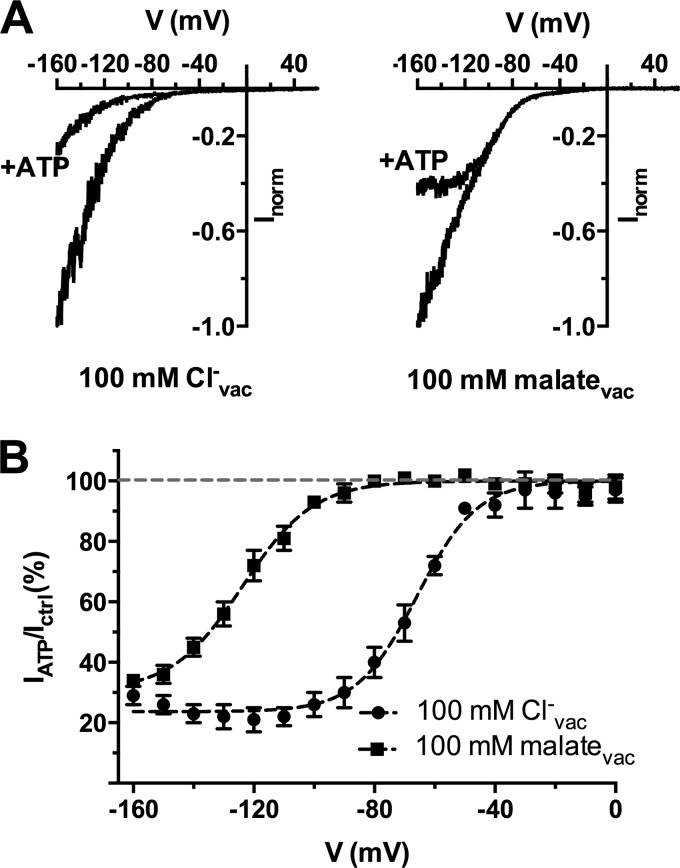
FIGURE 3.
Vacuolar anions affect the ATP-dependent block of AtALMT9-mediated chloride currents. A, normalized AtLMT9-mediated currents elicited by a voltage ramp from +60 to −160 mV in 1. 5 s (holding potential +60 mV) with a chloride-based cytosolic buffer in the presence (+ATP) or absence of 1 mm ATPfree. The vacuolar buffers contained 100 mm Cl− (left panel; 100 mm Clcyt− + 1 mm malatecyt, 100 mm Clvac−) or malate (right panel;100 mm Clcyt− + 1 mm malatecyt, 100 mm malatevac). B, fraction of unblocked current (IATP/Ictrl; mean ± S.E., n = 4) plotted against the applied membrane potential in the presence of 100 mm Cl− or 100 mm malate in the vacuolar buffer. Data were fitted with Equation 3 (“Experimental Procedures”; Table 1; dashed lines) yielding V½ = −66 ± 1 mV (100 mm Clvac−) and V½ = −124 ± 1 mV (100 mm malatevac). Error bars denote S.E.
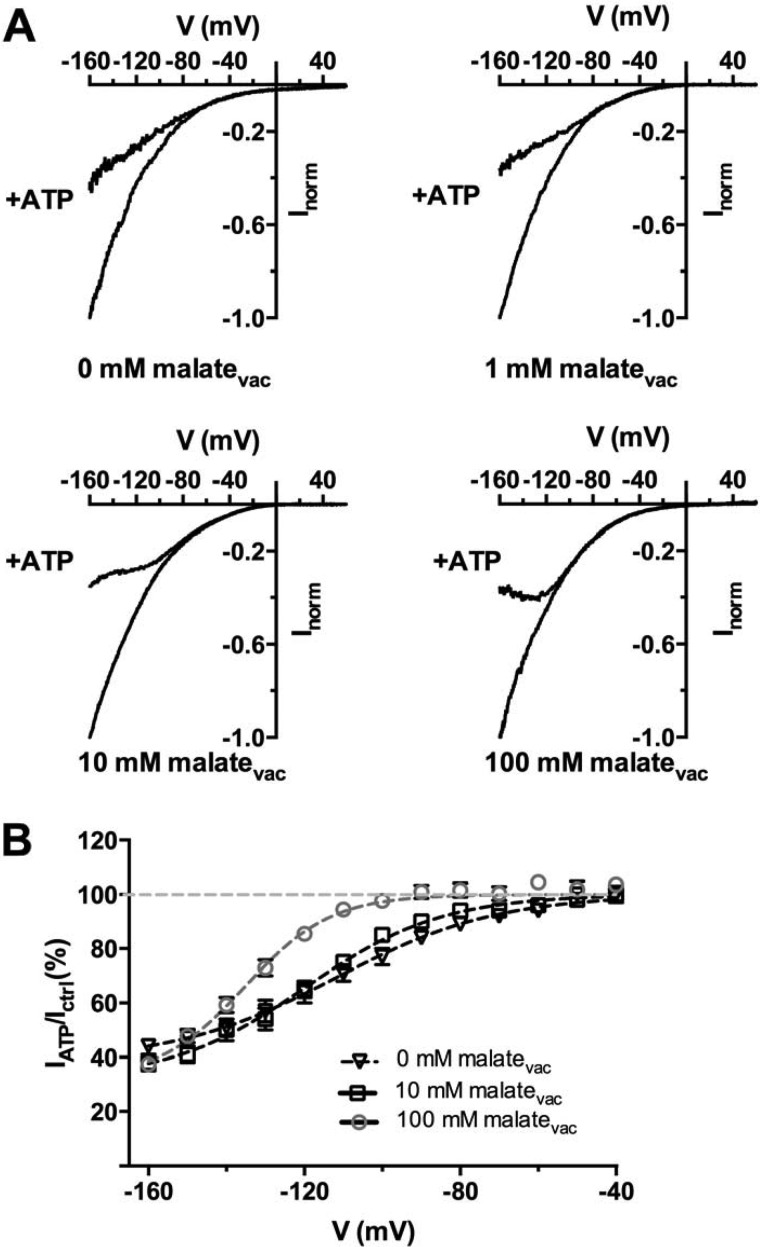
FIGURE 4.
Vacuolar malate relieves cytosolic ATP inhibition. A, currents elicited by a voltage ramp from +60 to −160 mV in 1.5 s (holding potential +60 mV) in the presence of a cytosolic-side buffer containing 100 mm malate and vacuolar-side buffers containing 0 mm malate (100 mm malatecyt/100 mm MESvac), 1 mm malate (100 mm malatecyt, 1 mm malate + 99 mm MESvac), 10 mm malate (100 mm malatecyt, 10 mm malate + 90 mm MESvac), 100 mm malate (100 mm malatecyt, 100 mm malatevac), in the absence or presence of 1 mm ATPfree (+ATP) in cytosolic-side buffer. B, ratios (IATP/ICtrl, mean ± S.E., n = 5–7) between currents recorded in the presence (IATP) and absence (Ictrl) of 1 mm ATPfree with the progressive increase of vacuolar malate concentration as indicated (cytosolic buffer was kept constant with 100 mm malate). The solid lines represent data fit with Equation 3 (“Experimental Procedures”; Table 1). Error bars denote S.E.
Nucleotides
ATP was tested either as a Mg2+ chelate or as a Tris salt (free ATP as indicated). AMP and ADP were tested as sodium salt. AMPPNP was Li3+ salt. GTP was Tris salt. All nucleotides were purchased from Sigma.
RESULTS
Cytosolic Nucleotides Inhibit AtALMT9-mediated Currents
To test the effect of cytosolic nucleotides on AtALMT9-mediated currents we performed patch clamp experiments in excised cytosolic-side out configuration using vacuoles extracted from N. bentamiana protoplasts that transiently over-expressed AtALMT9-GFP. Under control conditions (30 mm malatecyt/100 mm malatevac), voltage pulses starting from a holding potential of +60 mV ranging to −160 in −20 mV steps induced the activation of time-dependent inward malate currents (Fig. 1A) (4, 6, 7). When 1 mm ATPfree was applied at the cytosolic side of the vacuolar membrane (30 mm malatecyt + 1 mm ATPcyt/100 mm malatevac) AtALMT9-mediated currents were reversibly inhibited (Fig. 1B), and similar results were obtained when 1 mm Mg-ATP was applied (Fig. 1G). The presence of 1 mm ATPfree in the cytosolic buffer changed the I-V characteristic of AtALMT9 from monotonic in control conditions to non-monotonic (i.e. bell-shaped) (Fig. 1B, lower panel). Notably, the inhibitory effect of ATPfree was significantly higher compared with Mg-ATP (Fig. 1H). This indicates that the inhibition is mainly or even exclusively due to ATPfree and not MgATP, the form used in energization processes. The inhibition by ATP is strongly voltage dependent, being more pronounced at more negative membrane potentials (Fig. 1B). At −160 mV, the maximum membrane potential we could apply, the currents in the presence of 1 mm ATPfree were reduced to 10.5 ± 1.2% of the control currents (30 mm malatecyt + 1 mm ATPcyt/100 mm malatevac; Fig. 1D). The voltage dependence of the inhibition could be adequately described by a Boltzmann function (Equation 3 under “Experimental Procedures”) with an “offset” indicating that at a given ATPfree concentration the inhibition never reached 100% at any applied membrane potential (Fig. 1D). We found that the dissociation constant for ATP (KdATP) is a function of the membrane potential with KdATP = 90 ± 5 μm at −160 mV (Fig. 1, E and F). Using the Woodhull formalism (18) to analyze the voltage dependence of KdATP we could estimate that ATP transverse ≈50% of the applied membrane potential (Fig. 1F).
The block of AtALMT9-mediated currents by ATP was influenced by the cytosolic concentration of the conductive anion (i.e. malate). Cytosolic ATP inhibited AtALMT9 currents more at lower cytosolic malate concentrations (Fig. 2, A and B). Conversely, the KdATP was also dependent on the concentration of cytosolic malate. Reducing cytosolic malate concentrations led to a decreased KdATP value (Fig. 2C). The dependence of the inhibition by cytosolic ATP on the concentration of permeable anion suggested a competition mechanism between cytosolic ATP and malate. To extrapolate whether this modulation occurs in the physiological range, a KdATP of ∼50 μm at −160 mV could be determined for malate concentrations in the physiological range (400∼800 μm) (19–21). These results indicate that the modulation observed by free ATP occurs in the physiological range, since free ATP concentrations deduced from cytosolic ATP and Mg2+ concentrations are in the range of 30 to 100 μm (22).
The Anions in the Vacuolar Lumen Affect the Inhibition of AtALMT9 by Cytosolic Nucleotides
Recently, we have shown that AtALMT9 is also permeable to chloride and that it is involved in chloride accumulation in the vacuole (6). Thus, we tested the effect of free ATP on AtALMT9-mediated chloride currents. Similarly to what was observed when malate was the main permeable anion, 1 mm ATPfree strongly inhibited AtALMT9-mediated chloride currents to 29 ± 3% of the ATPfree conditions at −160 mV (100 mm Clcyt− + 1 mm malatecyt/100 mm Clvac−; Fig. 3). Intriguingly, the I-V curve in chloride conditions inhibited by ATP remained monotonic and did not display a bell-shaped behavior in the investigated voltage range at all tested concentrations of cytosolic ATP (Fig. 3A, left). In these conditions the ATP inhibition started to be effective at membrane potentials more negative than −50 mV (Fig. 3B). However, when chloride was the main permeable anion in the cytosolic solution and 100 mm malate was in the vacuolar buffer (100 mm Clcyt− + 1 mm malatecyt, 100 mm malatevac), the inhibitory effect induced by 1 mm ATPfree (Fig. 3A, right) resembled that observed in the malate-based cytosolic solution (Figs. 1 and 2). Indeed, under these conditions the inhibitory effect of cytosolic ATP starts abruptly at lower membrane potentials (−100 mV). The analysis of the voltage dependence of the ATP blockade shows that the presence of malate instead of Cl− in the vacuole shifts the effect of cytosolic ATP toward more negative potentials (Fig. 3B). Indeed, the half-activation voltage (V½) was −66 ± 1 and −124 ± 1 mV with 100 mm Cl− or 100 mm malate in the vacuole, respectively (Fig. 3B, Table 1). The maximal inhibition obtained at hyperpolarized voltages appeared to depend only slightly on the nature of the vacuolar anion, being 24 ± 1 and 31 ± 2% in the presence of Cl− or malate in the vacuole, respectively (Fig. 3B, Table 1).
TABLE 1.
Parameters of the Boltzmann fits (Equation 3) in different experimental conditions
IATP/I0 is the ratio between the current measured in the presence of ATP and in cytosolic solutions without ATP. Iunh is the residual current in presence of ATP, V½ is the potential at which half of the current was blocked. Data are presented as mean ± S.D.
| Ionic conditions |
IATP/I0 at −160 mV | Parameters |
Location | |||
|---|---|---|---|---|---|---|
| Cytosolic | Vacuolar | ATPcyt | V½ | Iunh | ||
| mm | % | mV | % | |||
| 30 mm Malate | 100 mm Malate | 0.1 | 46 ± 1 | −131 ± 4 | 40 ± 7 | Fig. 1B |
| 30 mm Malate | 100 mm Malate | 0.5 | 17 ± 1 | −118 ± 1 | 16 ± 3 | Fig. 1B |
| 30 mm Malate | 100 mm Malate | 1 | 10 ± 1 | −114 ± 0.8 | 6 ± 2 | Fig. 1B |
| 50 mm Malate | 100 mm Malate | 1 | 19 ± 2 | −119 ± 4 | 11 ± 5 | Fig. 2B |
| 100 mm Malate | 100 mm Malate | 1 | 37 ± 2 | −135 ± 2 | 32 ± 5 | Fig. 2B |
| 100 mm Cl− | 100 mm Cl− | 1 | 29 ± 3 | −66 ± 1 | 24 ± 1 | Fig. 3B |
| 100 mm Cl− | 100 mm Malate | 1 | 34 ± 2 | −124 ± 1 | 31 ± 2 | Fig. 3B |
| 100 mm Malate | 0 mm Malate | 1 | 44 ± 2 | −112 ± 4 | 38 ± 4 | Fig. 4B |
| 100 mm Malate | 1 mm Malate | 1 | 42 ± 1 | −113 ± 2 | 39 ± 3 | Fig. 4B |
| 100 mm Malate | 10 mm Malate | 1 | 38 ± 3 | −119 ± 3 | 31 ± 4 | Fig. 4B |
| 100 mm Malate | 100 mm Cl− | 1 | 41 ± 3 | −135 ± 13 | 7 ± 16 | Fig. 4B |
To evaluate whether the presence of malate in the vacuole was affecting the ATP inhibition we progressively raised the vacuolar concentration of malate (0 mm malatevac + 100 mm MESvac, 1 mm malatevac + 99 mm MESvac, 10 mm malatevac + 90 mm MESvac, 100 mm malatevac) while keeping constant the cytosolic solution (100 mm malatecyt). The ATP block was shifted toward more negative membrane potentials by vacuolar malate (Fig. 4B). Moreover, in the absence of vacuolar malate the inhibition by 1 mm free ATP did not induce a bell-shaped I-V curve (Fig. 4A) similar to what observed in Cl− conditions (100 mm Clcyt−, 100 mm Clvac−). The V½ was shifted toward more negative values by vacuolar malate (Fig. 4B, Table 1). Thus, the permeable anion in the vacuolar buffer affected the inhibitory behavior of cytosolic ATP on AtALMT9. This further suggests that cytosolic ATP and malate compete for a binding site that is likely to be located in the conduction pathway of AtALMT9 at the cytosolic side.
The Inhibition of AtALMT9 by Cytosolic Nucleotides does Not Require ATP Hydrolysis
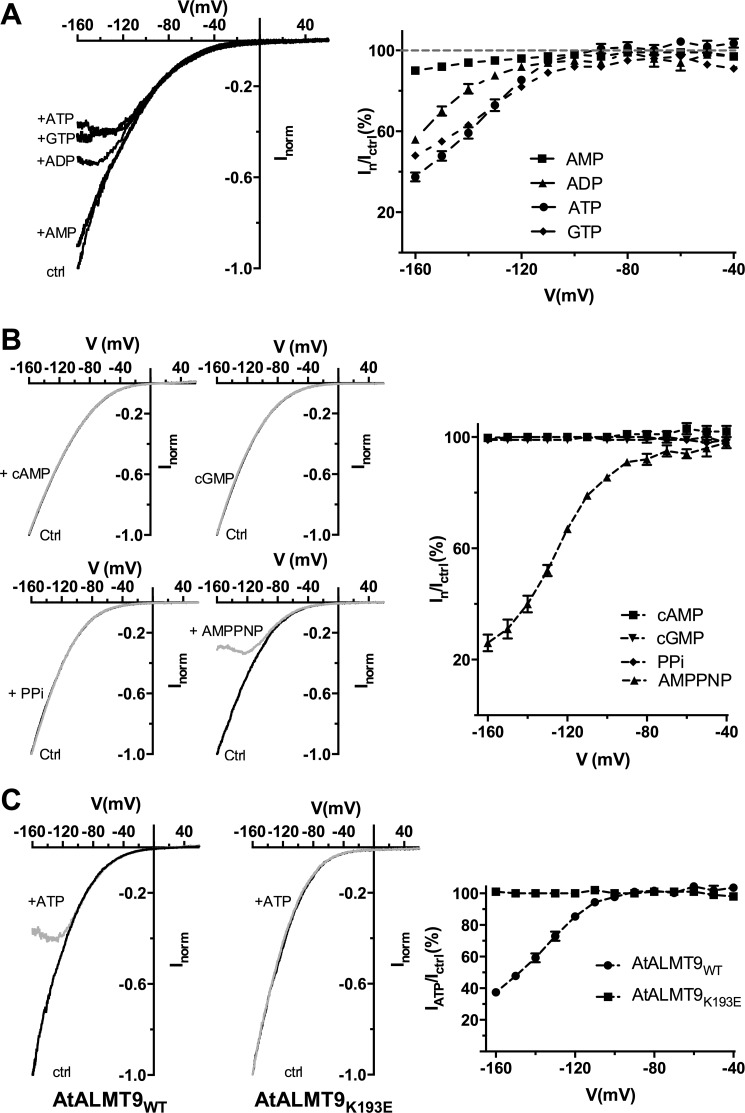
Our data suggest that cytosolic ATP interact with AtALMT9 directly blocking the pore. However, to definitively exclude the possibility that the effect of ATP was based on a phosphorylation/dephosphorylation mechanism, we used a non-hydrolysable analog of ATP, AMPPNP. In the presence of 1 mm AMPPNP in the cytosolic solution (100 mm malatecyt + 1 mm ATP or AMPPNPcyt, 100 mm malatevac), the amplitudes of AtALMT9-mediated currents at −160 mV were reduced to 26.0 ± 3.0% (Fig. 5B). AMPPNP showed a similar and even stronger inhibitory effect compared with ATP (Table 1, Fig. 5B). This demonstrates that no phosphorylation of AtALMT9 occurs under the experimental setup. We further analyzed the relative importance of the polyphosphate and nucleoside moieties in the block of AtALMT9-mediated currents. We found that the progressive reduction of the number of phosphate groups, and thus of negative charges, strongly impacted the inhibitory effect of the nucleotides with ADP being less efficient than ATP (IADP/Ictrl = 56 ± 2% at −160 mV) and AMP even less (IAMP/Ictrl = 90 ± 1% at −160 mV) (Fig. 5A). Differently, the nucleoside part seems to have a marginal role because 1 mm free GTP showed an effect similar to ATP (IGTP/Ictrl = 48 ± 2% at −160 mV; Fig. 5A). These data suggest that the number of charges carried by the nucleotide is fundamental for the block of AtALMT9-mediated currents. Cyclic nucleotides such as cAMP and cGMP and the inorganic pyrophosphate (PPi) induced no significant reduction of AtALMT9-mediated currents (Fig. 5B). Previously, we found that lysine 193 is part of the permeation pathway of AtALMT9 and its mutation (K193E) affects both anion permeation rectification and efficiency of citrate to block the currents (7). Thus, in an attempt to find a site of interaction of ATP with the channel, we tested whether the mutation of Lys-193 impacted the ATP blockade. We found that the currents mediated by the AtALMT9K193E variants were completely insensitive to 1 mm ATPfree in the cytosolic side solution (100 mm malate + 1 mm ATPcyt, 100 mm malatevac; Fig. 5B). These data suggest that cytosolic nucleotides interact with Lys-193 and that the blocking mechanism was similar to the one observed previously for citrate. Therefore, ATP is likely to block AtALMT9-mediated currents by obstructing the AtALMT9 pour.
FIGURE 5.
Effect of the non-hydrolysable nucleotide AMPPNP and different cytosolic nucleotides on AtALMT9-mediated malate currents. A, left panel: representative currents elicited by a voltage ramp (from +60 to −160 mV in 1.5 s, holding potential +60 mV) in 100 mm malatecyt, 100 mm malatevac (ctrl), and in 100 mm malatecyt, 100 mm malatevac + 1 mm of different nucleotides in the cytosolic-side buffer: ATP, ADP, AMP, GTP. Right panel: mean current ratios between currents in the presence of 1 mm of the indicated nucleotide (In) and control conditions (ICtrl) at different membrane potentials (n = 4–7). B, left panel: I-V curves elicited as in A in 100 mm malatecyt and 100 mm malatevac (Ctrl) and 100 mm malatecyt and 100 mm malatevac in the presence of 1 mm cAMP, cGMP, PPi, and AMPPNP (gray traces). Right panel: mean current ratios between currents in the presence of 1 mm of the indicated nucleotide (In) and control conditions (Ictrl) at different membrane potentials of cAMP, cGMP, PPi, and AMPPNP and currents measured (n = 3–4). C, left panel: representative currents obtained as in A in excised cytosolic-side out patches from vacuoles overexpressing AtALMT9WT and AtALMT9K193E (n = 6) in the presence (+ATP) or absence of 1 mm ATPfree (100 mm malatecyt, 100 mm malatevac). Right panel: mean current ratios of AtALMT9WT and AtALMT9K193E currents in the presence (IATP) or absence (Ictrl) of 1 mm ATPfree at different membrane potentials. Dashed lines indicate the tendency but have no theoretical meaning. Error bars denote S.E.
DISCUSSION
ALMTs form a family of anion channels playing diverse important functions in plants. Among the different ALMTs, AtALMT9 is the first member of the family shown to be localized in the vacuolar membrane (4). In vivo AtALMT9 acts as a malate-activated chloride channel, playing an important role in stomatal opening (6). A further study provided evidence that AtALMT9 forms tetramers and the fifth transmembrane α-helix (TMα5) possibly forms the permeation pathway (7). Because the I-V characteristic is a fundamental property of ion channels, in the present work we aimed to find a reason for the different I-V characteristic displayed by different members of the ALMT family. AtALMT1 and AtALMT9 display a monotonic and moderately voltage-dependent inward rectifying I-V characteristics (3, 4). Differently, AtALMT12/QUAC1 displays an I-V characteristic with a strong voltage dependence especially at membrane potentials below −100 mV (5). Earlier studies on Arabidopsis hypocotyl protoplasts showed that cytosolic ATP regulates the R-type currents (12, 13). Thus, a model of the R-type channel that is gated by the cytosolic nucleotides was proposed. The vacuolar localization of AtALMT9 appears to be very convenient because it allows the reversible application of nucleotides on the cytosolic side of the membrane, enabling to directly probe the effect of nucleotides on I-V characteristic of AtALMT9.
The two main results we present in this work are that cytosolic nucleotides reversibly inhibit AtALMT9-mediated currents and that in the presence of cytosolic nucleotides the I-V characteristic of AtALMT9 changes from monotonic to bell shaped (Fig. 1). In other terms the presence of ATP in the cytosolic solution is sufficient to make AtALMT9 display the same bell-shaped I-V characteristic as AtALMT12/QUAC1. This intriguing result suggests that the typical bell-shaped I-V characteristic is not a unique property of this clade of the ALMT family and that it does not result from an intrinsic voltage sensor in the ion channel protein. The understanding of the voltage dependence of AtALMT9 and AtALMT12 is relevant because of their roles in regulating stomatal movement (5, 6). Our data indicate that the effect of ATP on AtALMT9 currents does not rely on a phosphorylation mechanism but directly by blocking the permeation pathway of the channel. Indeed, the non-hydrolysable ATP analog AMPPNP is able to block AtALMT9-mediated currents. AMPPNP presents a stronger inhibitory effect compared with ATP. This might be due to the presence of a nitrogen atom, forming an imido bond between the β and γ phosphorus, instead of an oxygen atom forming an anhydride bond. This difference in the phosphoric moiety between the two nucleotides might influence the interaction between the blocker and the channel, consequently affecting the strength of the inhibition. Moreover, several lines of evidence point at an “open-channel block” mechanism. (i) The effect is voltage dependent and more pronounced at negative voltages at which the channels' open probability is higher; (ii) the dissociation constant (KdATP) is voltage-dependent, indicating that ATP enters the transmembrane electrical field; (iii) the current is more noisy at membrane potentials at which ATP is effective (Fig. 1A); (iv) ATP and the permeating anion compete because the KdATP becomes lower when malate concentrations decrease (Fig. 2C). It is interesting to note that this is in line with the nucleotide-dependent gating mechanism of the R-type/QUAC channel in the plasma membrane proposed by Colcombet et al. (13). Moreover, our results provide a strong molecular basis to interpret these findings by the identification of Lys-193 as a possible interaction site of ATP with AtALMT9 (Fig. 5B). Notably, in a previous study we found Lys-193 to be part of a putative pore forming region (7), further supporting the idea that ATP interacts with the pore region of AtALMT9.
Because the inhibition of AtALMT9 by ATP in malate-based solutions transform the I-V curve of this channel from monotonic to bell shaped, similar to the one of R-type channels, our data support the idea that AtALMT12/QUAC1 is gated by cytosolic nucleotides and that the typical I-V characteristic of this channel comes from this voltage-dependent block. It has to be noted that in all the situations in which AtALMT12/QUAC1 has been measured ATP was present in significant amounts in the cytosolic solution (5, 8). Moreover, the only attempt to remove ATP from the cytosolic solution revealed a change of the bell-shaped I-V characteristic to monotonic one (13). As a corollary our data also provide a simple and straightforward interpretation of a phenomenon that R-type currents are activated by extracellular malate (23–25). In this work we found that vacuolar malate (which correspond in our configuration to extracellular malate) is able to release the ATP block (Figs. 3 and 4), consequently inducing activation of the AtALMT9 currents. Thus, the malate activation of AtALMT12/QUAC1 would result from a release of ATP block by the presence of extracellular malate.
It is interesting to address the question whether the ATP block of AtALMT9 currents can be relevant in a physiological context. Even if presently our data do not directly answer this question we can try to insert them in a physiological framework. The inhibition by ATP starts to be significant at tonoplast membrane potentials between −60 and −80 mV, which is probably within the physiological range (Fig. 2B) (26). Recently, we have shown that AtALMT9 is important in guard cells function for stomatal opening (6). Indeed, during stomatal opening AtALMT9 drives the influx of anions into the vacuole. The influx of anion via AtALMT9 is driven by hyperpolarized vacuolar membrane potentials that are maintained through H+-ATPases (27–29). During stomatal opening the high hydrolytic activity of the proton pump consumes ATP, decreasing its concentration in the cytosol. Subsequently, ATP is converted into AMP (30, 31), which is nearly inactive in blocking AtALMT9-meditated anion currents. Thus, a drop of cytosolic ATP levels could induce a release of the AtALMT9 blockage and consequently facilitate anion accumulation in the vacuole. In addition, the accumulation of malate in the vacuolar lumen, which is known to occur in parallel to chloride accumulation, is also able to remove the ATP blockade (Figs. 3 and 4). This blockade release would consequently facilitate anion uptake into the vacuole. Therefore, both cytosolic and vacuolar malate can regulate AtALMT9 activity with a direct activation (6) and a release of ATP block, respectively. It is interesting to note that AtALMT9 is the second vacuolar anion transporter after AtCLCa (32) whose activity is inhibited by cytosolic ATP. This further suggests that the regulation of vacuolar anion transporters and channels activity by cytosolic nucleotides has a physiological relevance. Moreover, the modulation of vacuolar anion transporters by cytosolic nucleotides suggests a direct connection between accumulation of anions in the vacuole and the energetic status of the cell.
Considering the data published so far, it is very likely that plasma membrane-localized ALMTs exhibit a similar modulation. In this case the modulation of the channel activity by ATP may play an even more important role, because during hyperpolarization events the inhibition of the channel is nearly complete.
This work was supported by grants from the Chinese Scholarship Council (to J. Z.) and the Swiss National Foundation (to E. M. and A. D. A).
- ALMT
- aluminum-activated malate transporter
- BisTris propane
- 1,3-bis[tris(hydroxymethyl)methylamino]propane
- AMPPNP
- 5′-adenylyl-β,γ-imidodiphosphate
- AUAC
- quick-activating anion channel.
REFERENCES
- 1. Delhaize E., Ryan P. R., Hebb D. M., Yamamoto Y., Sasaki T., Matsumoto H. (2004) Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proc. Natl. Acad. Sci. U.S.A. 101, 15249–15254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sasaki T., Yamamoto Y., Ezaki B., Katsuhara M., Ahn S. J., Ryan P. R., Delhaize E., Matsumoto H. (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J. 37, 645–653 [DOI] [PubMed] [Google Scholar]
- 3. Hoekenga O. A., Maron L. G., Piñeros M. A., Cançado G. M., Shaff J., Kobayashi Y., Ryan P. R., Dong B., Delhaize E., Sasaki T., Matsumoto H., Yamamoto Y., Koyama H., Kochian L. V. (2006) AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 103, 9738–9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kovermann P., Meyer S., Hörtensteiner S., Picco C., Scholz-Starke J., Ravera S., Lee Y., Martinoia E. (2007) The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J. 52, 1169–1180 [DOI] [PubMed] [Google Scholar]
- 5. Meyer S., Mumm P., Imes D., Endler A., Weder B., Al-Rasheid K. A., Geiger D., Marten I., Martinoia E., Hedrich R. (2010) AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 63, 1054–1062 [DOI] [PubMed] [Google Scholar]
- 6. De Angeli A., Zhang J., Meyer S., Martinoia E. (2013) AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat. Commun. 4, 1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang J., Baetz U., Krügel U., Martinoia E., De Angeli A. (2013) Identification of a probable pore forming domain in the multimeric vacuolar anion channel AtALMT9. Plant Physiol. 163, 830–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sasaki T., Mori I. C., Furuichi T., Munemasa S., Toyooka K., Matsuoka K., Murata Y., Yamamoto Y. (2010) Closing plant stomata requires a homolog of an aluminum-activated malate transporter. Plant Cell Physiol. 51, 354–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meyer S., Scholz-Starke J., De Angeli A., Kovermann P., Burla B., Gambale F., Martinoia E. (2011) Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation. Plant J. 67, 247–257 [DOI] [PubMed] [Google Scholar]
- 10. Hedrich R., Busch H., Raschke K. (1990) Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J. 9, 3889–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schulz-Lessdorf B., Lohse G., Hedrich R. (1996) GCAC1 recognizes the pH gradient across the plasma membrane: a pH-sensitive and ATP-dependent anion channel links guard cell membrane potential to acid and energy metabolism. Plant J. 10, 993–1004 [Google Scholar]
- 12. Thomine S., Guern J., Barbier-Brygoo H. (1997) Voltage-dependent anion channel of Arabidopsis hypocotyls: nucleotide regulation and pharmacological properties. J. Membr. Biol. 159, 71–82 [DOI] [PubMed] [Google Scholar]
- 13. Colcombet J., Thomine S., Guern J., Frachisse J. M., Barbier-Brygoo H. (2001) Nucleotides provide a voltage-sensitive gate for the rapid anion channel of Arabidopsis hypocotyl cells. J. Biol. Chem. 276, 36139–36145 [DOI] [PubMed] [Google Scholar]
- 14. Lopatin A. N., Makhina E. N., Nichols C. G. (1994) Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372, 366–369 [DOI] [PubMed] [Google Scholar]
- 15. Lu Z. (2004) Mechanism of rectification in inward-rectifier K+ channels. Annu. Rev. Physiol. 66, 103–129 [DOI] [PubMed] [Google Scholar]
- 16. Yang K. Y., Liu Y., Zhang S. (2001) Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc. Natl. Acad. Sci. U.S.A. 98, 741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neher E. (1992) Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 207, 123–131 [DOI] [PubMed] [Google Scholar]
- 18. Woodhull A. M. (1973) Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 61, 687–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gerhardt R., Heldt H. W. (1984) Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiol. 75, 542–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Winter H., Robinson D. G., Heldt H. W. (1994) Subcellular volumes and metabolite concentrations in spinach leaves. Planta 191, 180–190 [Google Scholar]
- 21. Farré E. M., Tiessen A., Roessner U., Geigenberger P., Trethewey R. N., Willmitzer L. (2001) Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, organic acids, amino acids, and sugar alcohols in potato tubers using a nonaqueous fractionation method. Plant Physiol. 127, 685–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yazaki Y., Asukagawa N., Ishikawa Y., Ohta E., Sakata M. (1988) Estimation of cytoplasmic free Mg2+ levels and phosphorylation potentials in mung bean root tips by in vivo 31P NMR spectroscopy. Plant Cell Physiol. 29, 919–924 [Google Scholar]
- 23. Hedrich R., Marten I. (1993) Malate-induced feedback-regulation of plasma-membrane anion channels could provide a Co2 sensor to guard-cells. EMBO J. 12, 897–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raschke K. (2003) Alternation of the slow with the quick anion conductance in whole guard cells effected by external malate. Planta 217, 651–657 [DOI] [PubMed] [Google Scholar]
- 25. Mumm P., Imes D., Martinoia E., Al-Rasheid K. A., Geiger D., Marten I., Hedrich R. (2013) C-terminus mediated voltage gating of Arabidopsis guard cell anion channel QUAC1. Mol. Plant 6, 1550–1563 [DOI] [PubMed] [Google Scholar]
- 26. Martinoia E., Meyer S., De Angeli A., Nagy R. (2012) Vacuolar transporters in their physiological context. Annu. Rev. Plant Biol. 63, 183–213 [DOI] [PubMed] [Google Scholar]
- 27. Gaxiola R. A., Palmgren M. G., Schumacher K. (2007) Plant proton pumps. FEBS Lett. 581, 2204–2214 [DOI] [PubMed] [Google Scholar]
- 28. Duby G., Boutry M. (2009) The plant plasma membrane proton pump ATPase: a highly regulated P-type ATPase with multiple physiological roles. Pflugers Arch. 457, 645–655 [DOI] [PubMed] [Google Scholar]
- 29. Palmgren M. G., Nissen P. (2011) P-Type ATPases. Annu. Rev. Biophys. 40, 243–266 [DOI] [PubMed] [Google Scholar]
- 30. Gout E., Bligny R., Douce R. (1992) Regulation of intracellular pH values in higher plant cells: carbon-13 and phosphorus-31 nuclear magnetic resonance studies. J. Biol. Chem. 267, 13903–13909 [PubMed] [Google Scholar]
- 31. Xia J. H., Saglio P., Roberts J. (1995) Nucleotide levels do not critically determine survival of maize root-tips acclimated to a low-oxygen environment. Plant Physiol. 108, 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Angeli A., Moran O., Wege S., Filleur S., Ephritikhine G., Thomine S., Barbier-Brygoo H., Gambale F. (2009) ATP binding to the C terminus of the Arabidopsis thaliana nitrate/proton antiporter, AtCLCa, regulates nitrate transport into plant vacuoles. J. Biol. Chem. 284, 26526–26532 [DOI] [PMC free article] [PubMed] [Google Scholar]