Background: The novel phosphotyrosine-binding domain (HYB) of Hakai forms an atypical, zinc-coordinated homodimer.
Results: C-terminal truncation of the HYB domain causes dramatic structural changes and it becomes a monomer, but in the presence of substrate, it becomes a dimer to recognize the substrate.
Conclusion: HYB dimerization is a unique prerequisite for substrate-binding activity of Hakai.
Significance: The HYB domain may orchestrate the function of Hakai in cancer and cell-cell contacts.
Keywords: E3 Ubiquitin Ligase, Nuclear Magnetic Resonance (NMR), Phosphotyrosine, Structural Biology, Zinc Finger
Abstract
Hakai, an E3 ubiquitin ligase, disrupts cell-cell contacts in epithelial cells and is up-regulated in human colon and gastric adenocarcinomas. Hakai acts through its phosphotyrosine-binding (HYB) domain, which bears a dimeric fold that recognizes the phosphotyrosine motifs of E-cadherin, cortactin, DOK1, and other Src substrates. Unlike the monomeric nature of the SH2 and phosphotyrosine-binding domains, the architecture of the HYB domain consists of an atypical, zinc-coordinated tight homodimer. Here, we report a C-terminal truncation mutant of the HYB domain (HYBΔC), comprising amino acids 106–194, which exists as a monomer in solution. The NMR structure revealed that this deletion mutant undergoes a dramatic structural change caused by a rearrangement of the atypical zinc-coordinated unit in the C terminus of the HYB domain to a C2H2-like zinc finger in HYBΔC. Moreover, using isothermal titration calorimetry, we show that dimerization of HYBΔC can be induced using a phosphotyrosine substrate peptide. This ligand-induced dimerization of HYBΔC is further validated using analytical ultracentrifugation, size-exclusion chromatography, NMR relaxation studies, dynamic light scattering, and circular dichroism experiments. Overall, these observations suggest that the dimeric architecture of the HYB domain is essential for the phosphotyrosine-binding property of Hakai.
Introduction
Phosphotyrosine (Tyr(P))3-binding domains are key determinants of specificity and selectivity in many signal transduction pathways and act by integrating Tyr(P) signals from upstream kinases to downstream effectors that regulate the complex physiology of eukaryotic cells (1–3). The SH2 domain was the first Tyr(P)-binding domain to be discovered (4, 5) and has since been extensively characterized (1, 6–10). The second domain to be identified with a capacity to bind tyrosine residues was the phosphotyrosine-binding domain (11). Subsequently, idiosyncratic Tyr(P)-binding domains have been observed in the C2 domain of protein kinase Cδ (12) and pyruvate kinase M2 (13), and we recently reported the existence of a third Tyr(P)-binding domain in Hakai, coined the HYB domain for Hakai phosphotyrosine (Tyr(P)) binding (14).
Hakai is an E3-ubiquitin ligase first noted for its role in regulating E-cadherin expression and disrupting cell-cell contacts in epithelial tissues (15, 16). Subsequent work has identified the elevated expression of Hakai in human colon and gastric adenocarcinomas (17, 18). The HYB domain interacts with other Src substrates, such as Cortactin, a structural protein involved in coordinating actin rearrangement during cell movement (19), and DOK1, a scaffolding protein that assists in the assembly of signaling complexes (20). Both of these proteins offer important functional contributions in the progression of cancer (21, 22), and both are regulated by the activity of Hakai (14). The novel features of the HYB domain and its interaction with various key molecules may indicate a physiologically important role for Hakai in cancer.
The structure of the HYB domain was determined by x-ray crystallography (14). It comprises amino acids 106–206, and forms an atypical, zinc-coordinated homodimer in an antiparallel, intertwined configuration, utilizing residues from the Tyr(P)-binding region of two Hakai monomers. The dimeric nature of the domain configures the formation of a Tyr(P)-binding pocket that recognizes specific phosphorylated tyrosine residues and flanking acidic amino acids of its substrates (14). The C-terminal region, which harbors the atypical zinc-coordination motif and key residues involved in the Tyr(P) interaction, plays an important role in the dimerization observed in the HYB domain. In the present study, we investigated the consequences of deleting residues from the C terminus of Hakai, whereas maintaining the essential residues involved in the Tyr(P) interaction and the atypical zinc-coordination of the E3 ligase. Through this structural truncation, we identified a C-terminal deletion mutant of Hakai (aa 106–194), herein referred to as HYBΔC, which exists as a monomer in solution and flips to a dimeric arrangement in the presence of a Tyr(P) substrate, with the incoming substrate inducing the conformational changes required to initiate dimerization of HYBΔC monomers. This monomeric to dimeric switch of HYBΔC in the presence of the phosphorylated substrate was further validated by biophysical studies. Taken together, our results suggest that the dimerization of Hakai Tyr(P)-binding domain is a unique prerequisite for substrate binding.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Sequences corresponding to aa 106–194 of Hakai were cloned into pGEX6P-1 vector (GE Healthcare) using BamHI and SalI restriction sites, and expressed as a GST fusion protein in Escherichia coli BL21 (DE3) cells. Cells were cultured in LB medium at 37 °C until the A600 nm reached 0.6–0.7. Cells were then incubated with 0.15 mm isopropyl 1-thio-β-d-galactopyranoside and 50 μm zinc sulfate, and then grown for an additional 20 h at 15 °C. Cells were collected by centrifugation (8000 × g, 10 min, 4 °C), and the pellets were resuspended in lysis buffer (50 mm BisTris, pH 6.5, 300 mm NaCl, 10 μm zinc chloride, 5% glycerol, 0.5% Triton X-100, 2 mm DTT, 1 mm phenylmethylsulfonyl fluoride) and homogenized using a French Press Cell Disrupter (Thermo Scientific, Wilmington, DE). Cell lysates were then centrifuged at 18,000 × g for 30 min at 4 °C (JA-25.50 fixed angle rotor centrifuge, Beckman Coulter, Fullerton, CA) and the supernatant was passed through glutathione-Sepharose resin (GE Healthcare) for 2–4 h at 4 °C. The resin was subsequently washed with a buffer containing 50 mm BisTris, pH 6.5, 300 mm NaCl, 5% glycerol, and 2 mm DTT, and the bound supernatant was then subjected to an overnight on-column cleavage at 4 °C with GST-PreScission Protease (GE Healthcare) to remove the GST tag. A major portion of GST and GST-PreScission Protease remained bound to the glutathione-Sepharose resin and the flow-through containing the partially purified, untagged proteins were further purified using a Superdex 75 size exclusion column (GE Healthcare) equilibrated with a buffer containing 50 mm sodium phosphate buffer, pH 6.5, and 5 mm DTT.
Circular Dichroism Spectrometry
Far UV spectra (260–190 nm) of HYB (aa 106–206) and HYBΔC (aa 106–194) in the absence and presence phosphorylated peptide of E-cadherin (residues 747–759) were measured using a Jasco J-810 spectropolarimeter in phosphate buffer, pH 6.5, at room temperature with a 0.1-cm path length and stoppered cuvettes. The protein concentration was maintained at 20 μm in all cases. Six scans were recorded, averaged, and the baseline subtracted. The scale on the CD spectra was normalized to mean residue ellipticity (MRE), which is measured in degrees cm2 dmol−1 residue−1 using the following equation (23),
where θ is raw ellipticity measured in machine units (millidegrees), MRW is mean residue weight (MRW = protein mean weight (in daltons)/(number of residues) for the protein, P is path length in cm, and CONC is protein concentration in mg/ml. The MRE is plotted against the corresponding wavelength.
Dynamic Light Scattering
Dynamic light scattering studies were carried out on a DynaPro Light Scattering instrument (Wyatt Technology Europe GmbH, Dernbach, Germany) with protein concentrations at A280 nm of 1.0, in buffer containing 50 mm sodium phosphate, pH 6.5, and 5 mm DTT.
NMR Spectroscopy
All NMR experiments were carried out at 25 °C on a Bruker Avance 800 MHz spectrometer equipped with a TXI cryogenic probe using 1 mm 13C,15N-labeled HYBΔC (aa 106–194) sample prepared in the buffer containing 50 mm sodium phosphate, pH 6.5, and 5 mm DTT. 1H, 13C, and 15N resonance assignments were achieved by measuring the three-dimensional HNCACB, three-dimensional CBCA(CO)NH (24), and three-dimensional CCH-TOCSY (25) spectra. Inter-proton distance restraints for structural calculation were obtained from three-dimensional 13C-edited NOESY-HSQC, three-dimensional 15N-edited NOESY-HSQC, and two-dimensional NOESY spectra using a 100-ms mixing time. A weakly aligned 15N-labeled sample (0.8 mm) was prepared using the same buffer by the addition of 6 mg/ml of filamentous phage Pf1 (from ASLA Biotech Ltd, Latvia) for residue dipolar coupling (RDC) measurements. 1DNH RDCs were measured using the In-Phase and Anti-Phase method (26). The RDC values were obtained by subtracting the reference value in isotropic solution. NMR spectra (two- and three-dimensional) were processed using the NMRPipe program (27), and data analysis was performed with the help of the Sparky program (28).
Structure Determination
The solution structure for HYBΔC (aa 106–194) was calculated using the Xplor-NIH 2.24 software package (29). A total of 1087 NOE distance restraints, 21 hydrogen bonds, and 110 dihedral angle restraints were predicted by the TALOS program (30). Initial structures were calculated using torsion angle molecular dynamics protocol. Structure refinement was performed using a simulated annealing protocol, and 55 RDCs restraints were used in the cooling stage. The position of three zinc ions were determined by the distance restraints derived from the x-ray structure of HYB (PDB code 3VK6). The three zinc ions are bound to the HYBΔC through nine cysteines and three histidines exhibiting the topology Cys109-X2-Cys112-Zn1-Cys130-X2-Cys133, Cys125-X-His127-Zn2-Cys145-X2-Cys148, and Cys166-X5-Cys172-Zn3-His185-X4-His190, respectively. These zinc ions were included in the structural calculations with an approximately tetrahedral geometry. The distance constraints include four fixed distances for each zinc ion, where the zinc-sulfur (cysteine Sγ) bond distance was set to 2.3 Å and zinc-nitrogen (histidine Nϵ2) bond lengths was set to 2.0 Å, respectively. A final ensemble of the 20 lowest energy structures from 100 calculated structures was selected for the figure preparation using Chimera program.
15N Relaxation Measurements
The 15N single-labeled free HYBΔC sample (0.8 mm) and complex sample (∼1 mm peptide added) were used to measure the 15N relaxation time, T1 and T2, respectively. The 15N relaxation times, T1 and T1ρ, were measured at 25 °C using inversely detected two-dimensional NMR methods (31, 32) on a Bruker 800 Avance machine equipped with a TXI cryogenic probe. For the free HYBΔC sample, relaxation times, T1, were determined by collecting eight points with delays of 10, 250 (×3), 400, 500, 650, 750, and 850 ms using a recycle delay of 3 s and 8 scans. Relaxation times, T1ρ, were measured by collecting eight points with delays of 1, 30 (×2), 45, 60, 75, 90, and 110 ms using a spin-lock power of 1.6 kHz, a 2.0-s recycle delay, and 8 scans. For the complex sample, relaxation times, T1, were determined by collecting eight points with delays of 10, 250 (×2), 400, 500, 650, 750, and 1000 ms using a recycle delay of 3 s and 16 scans. Relaxation times, T1ρ, were measured by collecting eight points with delays of 1, 15, 30 (×2), 45, 60, 75, and 87 ms using a spin-lock power of 1.6 kHz, a 2.0-s recycle delay, and 16 scans. All data were recorded using 220 and 1280 complex points in t1 and t2 dimensions, respectively, and with spectral widths of 1946 Hz (15N) and 11160 Hz (1H). Relaxation times were fitted as single exponential decays to peak height data. The spin-spin relaxation time, T2, was calculated from T1ρ and T1 according to the following equation (32),
where θ = arctan(Δω/ω1), and Δω and ω1 are the resonance offset and spin-lock field strength, respectively. The experiment error was analyzed by repeating the T1 measurement (250 ms) three times. The relative standard deviation for HSQC peak intensities was about 0.99%. The relaxation constants were also given with the fitting errors. The differences in relaxation parameters observed were significantly higher than the standard deviation; thus, it was outside the error range.
Isothermal Titration Calorimetry
The phosphorylated peptide of E-cadherin (residues 747–759) was titrated at a molar concentration of 575 μm against 75 μm HYBΔC in a VP-ITC microcalorimeter (Microcal, Northhampton, UK) at 293 K. The titrations were carried out using 30 10-μl injections of the peptide into the sample cell containing HYBΔC and the data were analyzed with a one-site binding model using the Origin software package version 7.0 supplied by Microcal. All measurements were repeated three times.
Analytical Ultracentrifugation (AUC)
The oligomeric states of the HYBΔC in the absence and presence of the Tyr(P) peptide were investigated by monitoring their sedimentation properties in AUC sedimentation velocity experiments. Samples (400 μl) in 50 mm sodium phosphate, pH 6.5, and 5 mm DTT with an absorbance of 1.0 at 280 nm were used. Sedimentation velocity profiles were collected by monitoring the absorbance at 280 nm. The samples were centrifuged at 40,000 × g at 25 °C in a Beckman ProteomeLab XL-I centrifuge fitted with a four-hole AN-60 Ti rotor and double-sector aluminum centerpieces and equipped with absorbance optics. The scans were analyzed using the Sedfit program (33).
Chemical Shift Perturbation Analysis
Chemical shift perturbations of backbone amides by the binding of phospho-E-cadherin-(747–759) peptide to HYBΔC (peptide to protein ratio is 1.2:1) were calculated. The chemical shift perturbations were defined as Δδ = [(ΔδHN2 + ΔδN2/25)/2]0.5 for amide NH, where ΔδHN and ΔδN are the chemical shift differences of amide 1H, amide 15N, between the samples in the presence of phospho-E-cadherin-(747–759) peptide and in the absence of phospho-E-cadherin-(747–759) peptide. The NH HSQC assignments for HYBΔC complex were confirmed by three-dimensional 15N-edited NOESY-HSQC experiment.
RESULTS
HYBΔC Is a Monomer in Solution
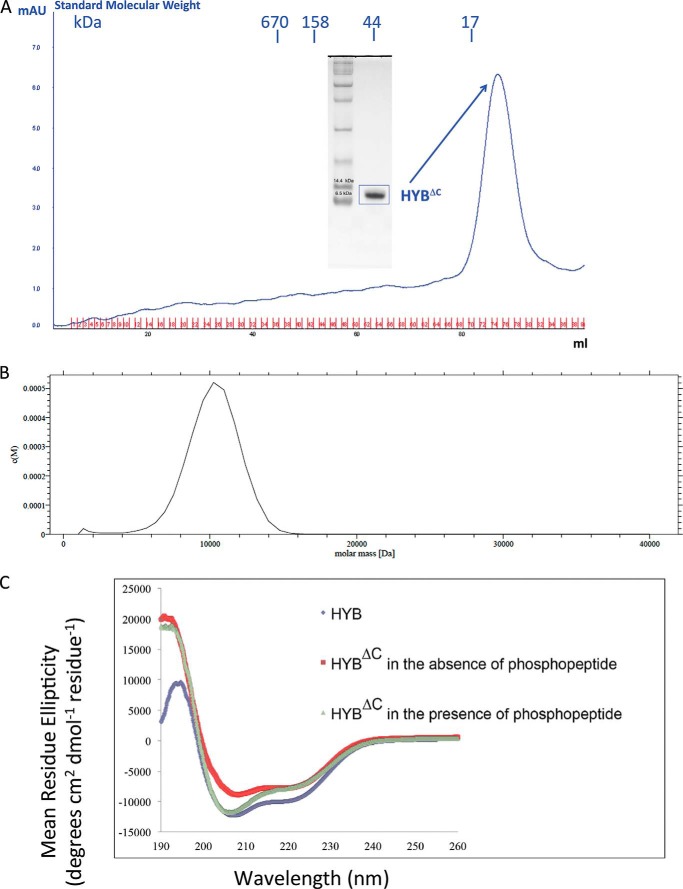
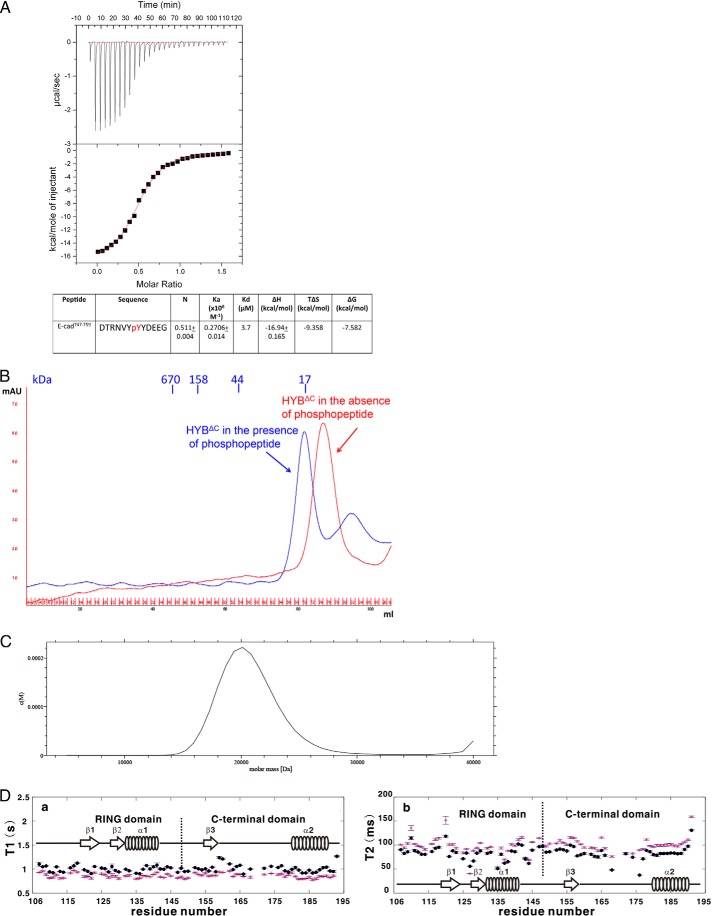
We previously demonstrated that the HYB domain consists of an atypical zinc-coordinated, intertwined homodimer formed by an anti-parallel arrangement of two Hakai (aa 106–206) monomers (14). The dimerization mainly occurs through the C-terminal region of Hakai (aa 106–206), which harbors key Tyr(P)-interacting residues as well as the atypical zinc-coordinated motif formed by two histidine residues (His185 and His190) and one cysteine residue (Cys172) from one monomer and a second cysteine residue (Cys166) from the adjacent monomer. In this study, we expressed and purified a C-terminal truncation mutant comprising residues 106–194, herein referred to as HYBΔC, which retains all of the residues involved in the atypical zinc-coordinated motif and the Tyr(P) interaction. The gel-filtration elution profile showed that HYBΔC had an apparent molecular weight equivalent to a monomeric subunit (Fig. 1A). Furthermore, the monomeric nature of HYBΔC was verified using AUC analysis, which showed a peak corresponding to an apparent molecular mass of monomeric HYBΔC (∼10,200 Da) (Fig. 1B). The dynamic light scattering experiments also revealed an apparent molecular weight of monomeric HYBΔC.
FIGURE 1.
Gel filtration, analytical ultracentrifugation, and circular dichroism of the HYBΔC (aa 106–194). A, gel filtration profile of HYBΔC. HYBΔC was loaded onto a calibrated Superdex-75 gel filtration chromatography column. The elution profile suggests that HYBΔC exists as a monomer. The SDS-PAGE analysis of HYBΔC depicts the purity and the molecular mass of the peak fraction (∼10 kDa). B, AUC analysis of HYBΔC. The monomeric nature of HYBΔC was studied using sedimentation velocity analysis. The molecular mass profile of HYBΔC indicates that the protein exists as a monomer in solution, with an apparent molecular mass of 10 kDa. C, circular dichroism analysis shows a significant difference in the secondary structure composition between HYBΔC and the untruncated HYB domain. Furthermore, in the presence of the phosphotyrosine peptide ligand (E-cadherin-(747–759)), the CD spectrum of HYBΔC shows marked resemblance with that of the HYB domain, suggesting a conformational change in HYBΔC in the presence of the phosphorylated ligand.
Circular Dichroism Analysis
Circular dichroism (CD) analysis revealed significant differences in the secondary structure composition between the monomeric HYBΔC and the dimeric HYB domain (Fig. 1C). In the presence of the phosphorylated peptide of E-cadherin (corresponding to residues 747–759), the CD spectra demonstrated conformational changes to the HYBΔC. These significant differences in the conformation of HYBΔC in the CD spectral analysis motivated us to determine the NMR structure of HYBΔC to understand the structural basis of this ligand-associated conformational change.
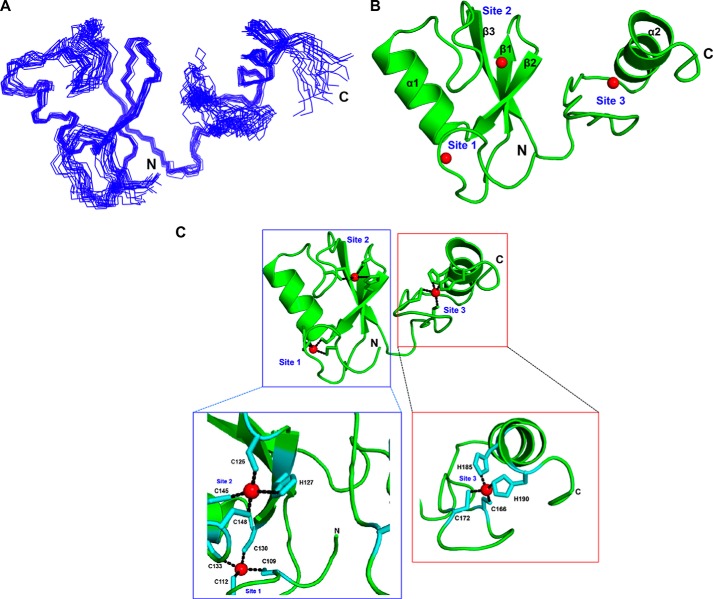
NMR Structure of HYBΔC
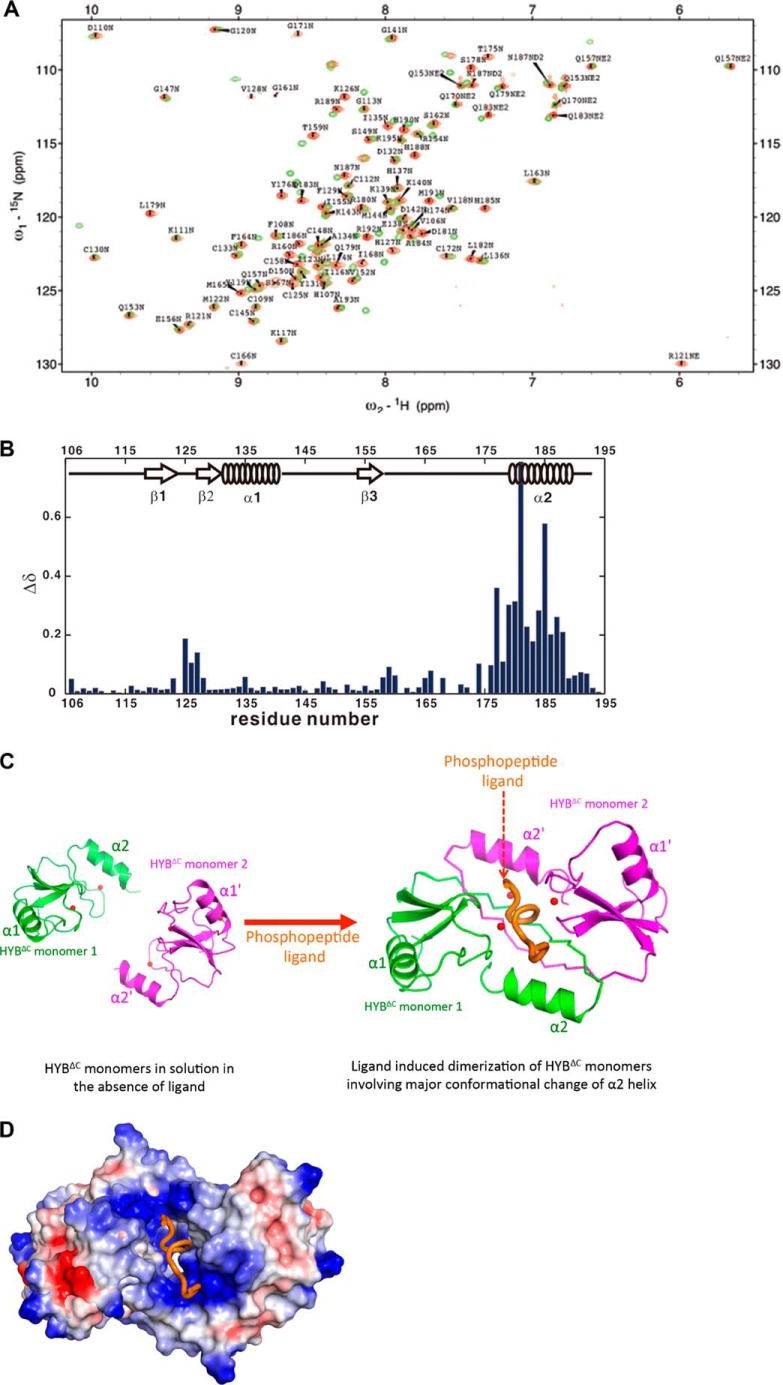
To gain structural insights into the tertiary fold of HYBΔC, we solved the solution NMR structure. The NMR structure of HYBΔC was refined to a final r.m.s. deviation of 0.63 ± 0.21 Å (backbone) (Table 1) for the 20 best structures (Fig. 2, A and B). The NMR structure of HYBΔC revealed a monomeric fold, comprising an N-terminal RING domain and a C-terminal zinc-coordinated domain bearing a C2H2-type zinc finger (ZnF) configuration (Fig. 2, B and C). As expected from knowledge of the HYB structure, the solution structure of HYBΔC contained three zinc-coordination sites. The RING domain (residues 106–148) was shown to adopt a typical RING architecture containing two β-strands (β1 and β2) and one α-helix (α1), which engage two zinc ions. The C-terminal domain (residues 148–194) lay in close association with the N-terminal RING domain by means of the third β-strand, β3, which formed an anti-parallel β-sheet arrangement with β1 and β2 of the RING domain. The C-terminal domain contained the third zinc-coordination site of HYBΔC, formed by the residues Cys166, Cys172, His185, and His190, resembling a typical C2H2 ZnF motif (Fig. 2C); the two cysteines (Cys166 and Cys172) formed a hairpin loop and the two histidines (His185 and His190) were found juxtaposed to the α-helix (α2).
TABLE 1.
NMR data and structure determination details for HYBΔC
| NMR data | |
|---|---|
| All NOE distance restraintsa | 1087 |
| Intra-residue | 224 |
| Sequential (|i–j| = 1) | 338 |
| Medium range (1 <|i–j|<5) | 193 |
| Long range (|i–j|≥5) | 344 |
| Hydrogen bond restraints | 21 |
| Dihedral angle restraints (ϕ, ψ)b | 110 |
| Residual dipolar coupling restraints | 55 |
| Energy statistics X-PLOR energy (kcal mol−1) | |
| Enoe | 76.1 ± 4.8 |
| Ecdih | 1.26 ± 0.26 |
| Erdc | 20.2 ± 3.7 |
| Deviations from idealized covalent geometryc | |
| R.m.s. deviations of bond lengths (Å) | 0.0031 ± 0.0031 |
| R.m.s. deviations of bond angles (°) | 0.430 ± 0.015 |
| R.m.s. deviations of improper angles (°) | 0.358 ± 0.011 |
| Deviations from experimental restraints | |
| R.m.s. deviations of distance restraints (Å) | 0.0369 ± 0.0012 |
| R.m.s. deviations of dihedral angle restraints (°) | 0.265 ± 0.074 |
| Ramachandran plot analysis (%)d | |
| Residues in allowed region | 96.9% |
| Residues in generally allowed regions | 2.3% |
| Residues in disallowed regions | 0.8% |
| Average R.m.s. deviations from mean structure (Å)e | |
| Heavy atoms | 1.29 ± 0.21 |
| Backbone atoms (N, CA, C′, O) | 0.63 ± 0.21 |
a The distance restraints were obtained by classifying the NOE cross peaks into three categories: strong (1.8–2.9 Å), medium (1.8–3.5 Å), and weak (1.8–5.0 Å).
b Dihedral angles of backbone φ and ψ were predicted by TALOS (30) using the chemical shifts of Cα, Cβ, Hα, N, and HN.
c Twenty lowest-energy conformers with no NOE violations greater than 0.3 Å and no torsion angle violations greater than 3° were selected from 100 conformers to represent the NMR ensembles.
d Calculated with PROCHECK-NMR (66).
e Calculated with MOLMOL (67) over secondary structure region α1 (131–140), α2 (179–190), β1 (119–123), β2 (127–130) and β3 (154–158).
FIGURE 2.
NMR structure of HYBΔC (aa 106–194). A, superposition of 20 energy-minimized conformers representing the NMR structure of HYBΔC. B, ribbon diagram of the lowest-energy conformer representing the three-dimensional NMR structure of HYBΔC. Each monomer contains three zinc-coordination sites: Sites 1, 2, and 3. The zinc ions are shown as red spheres. C, metal coordinations in the NMR structure of HYBΔC. The coordination of zinc ions in the RING domain and the C-terminal domain of HYBΔC are shown.
The overall structure of HYBΔC was compared with other proteins present in the Protein Data Bank using the DALI server (34). The DALI search showed several structural homologs containing a similar RING domain, but none bore any resemblance beyond amino acid residue 166 of the C-terminal domain of HYBΔC, the C2H2 ZnF site. This suggested that combination of the N-terminal RING domain and the C-terminal domain containing the C2H2 ZnF adopts a novel fold in HYBΔC. To determine whether any protein in the PDB database contained a fold resembling the C2H2 ZnF of HYBΔC, a second DALI search was performed for the localized fold comprising amino acid residues 159–194 of HYBΔC. The results showed only four proteins that contain a fold similar to the C2H2 ZnF of HYBΔC: zinc finger protein 406 (PDB code 2elx), SPIF2 (PDB code 1sp2), RAS-related protein RAB-5A (PDB code 3mjh), and SWI5 (PDB code 1zfd), bearing a sequence similarity of 30, 27, 28, and 19%, and Z-scores of 2.4, 2.3, 2.1, and 2.1, respectively. These proteins are transcription factors, with the exception of RAB-5A, which is involved in Rab5 binding using the distinctive N-terminal C2H2 zinc finger (35).
Comparison of the Structure of HYBΔC with HYB Domain
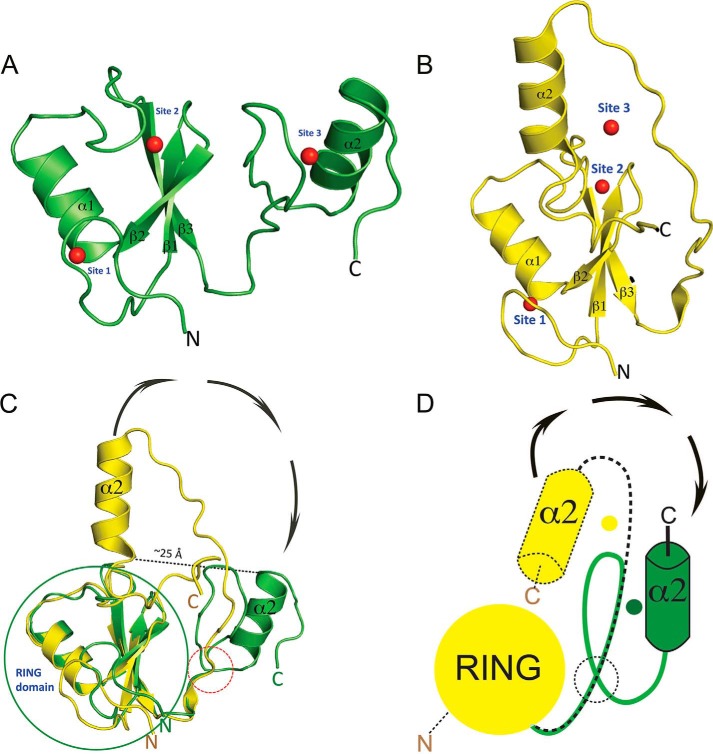
To analyze the structural differences between monomeric HYBΔC and HYB domain, the monomeric HYBΔC was superimposed onto that of one of the monomeric counterparts of Hakai (aa 106–206). The structural segment corresponding to residues 106–166 of HYBΔC and one monomer of the HYB domain were found to be similar, with a r.m.s. deviation of 0.9 Å (Fig. 3). This implies that the RING domain (residues 106–148) and the fold of the linker sequence (residues 149–166) overlap in both HYBΔC and the monomer of the HYB domain. Structural differences arose, however, from residue 167 onward, which corresponds to the C-terminal zinc-coordination site and the terminal α-helix (α2). The C-terminal helix α2 of HYBΔC was flipped by 180° and is separated from the α2 of HYB monomeric counterpart by a distance of 25 Å (Fig. 3C). Fig. 4, A–F, show comparisons between the monomeric HYBΔC and the dimeric fold of the HYB domain. From these comparisons, it became apparent that the differences in the folding arose from changes in the C2H2 ZnF in HYBΔC, with all of the zinc-coordination residues situated on the same monomeric chain of HYBΔC; this is in contrast to the sharing of zinc ions between the two monomers in the atypical zinc-coordination motif of the HYB domain. Thus, the NMR structure provides the basis for the existence of HYBΔC as a monomer in solution and bearing a different fold, likely because its lacks the atypical zinc-binding motif, which is critical for the dimerization of the full-length HYB domain. The overall topology of HYBΔC and the HYB domain is compared in Fig. 4, E and F.
FIGURE 3.
Comparison of monomeric HYBΔC with monomeric Hakai of the dimeric HYB domain. A and B, schematic representations of HYBΔC (aa 106–194; green) and the Hakai (aa 106–206; yellow) monomeric counterpart (PDB code 3vk6) of the HYB domain, respectively, in the same orientation. C, superposition of HYBΔC on Hakai reveals an overlap with a r.m.s. deviation of 0.9 Å for a stretch of residues from 106 to 166, which contains the RING domain and part of the C-terminal domain in both structures. Differences in the structure arise after residue Cys166 (highlighted by a red dotted circle, lower right of the figure). Beyond this, the folds of HYBΔC and the HYB monomeric counterpart are completely different, with the helix α2 flipping by 180° and separating by ∼25 Å. The relative rotations that result in the conformational change are indicated by arrows orientated in a clockwise direction. D, schematic representation of the conformational changes in C of the structures of HYBΔC and Hakai (aa 106–206) of the HYB domain with the relative rotation resulting in the observed structural changes. The yellow dot represents the original position of the C-terminal zinc ion at Site 3 of the monomeric Hakai counterpart in the dimeric HYB domain, and the green dot represents the new position of the Site 3 zinc ion in HYBΔC.
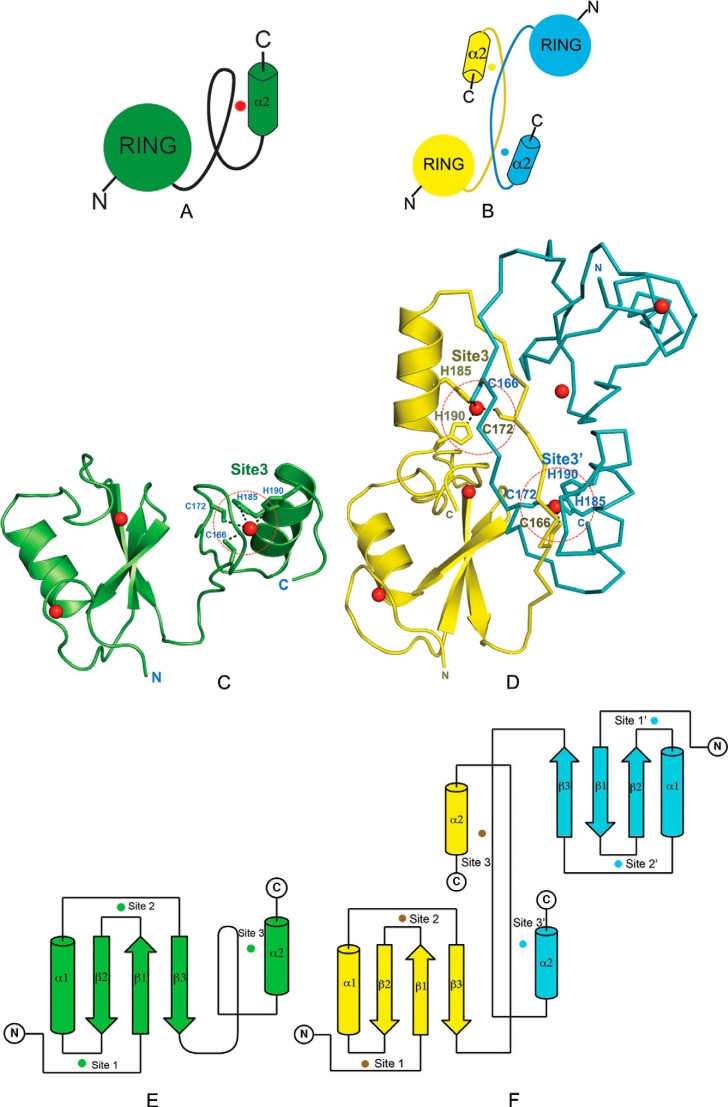
FIGURE 4.
C2H2 ZnF in the C-terminal domain of HYBΔC is critical for its monomeric fold. Analysis of the structural differences between HYBΔC and HYB (A) and the dimeric fold of HYB domain containing paired Hakai (aa 106–206) (PDB code 3vk6) monomers (B). The red dot in A represents the zinc ion in the C-terminal domain of HYBΔC, whereas the cyan and yellow dots in B represent the C-terminal zinc ion shared between the respective monomers of the dimeric HYB-fold. C, the monomeric fold of HYBΔC (green) contains a C2H2 ZnF in the C-terminal domain (highlighted by red dotted circle). D, the HYB domain (PDB code 3vk6) contains a dimeric fold of paired Hakai (aa 106–206) monomers (yellow monomer in ribbon representation; cyan in schematic representation for clarity). In monomeric HYBΔC, all four zinc-coordinating residues are situated in the same monomeric chain. Comparatively, the dimeric fold of the HYB domain is formed by the sharing of two zinc ions (red spheres) between the Hakai monomers, such that three of the four zinc-binding residues come from one monomer (His185, His190, and Cys172) and the fourth from the adjacent monomer (Cys166). The zinc-coordinating side chains are shown as sticks. The structural rearrangement arises because of a systematic reshuffling among the zinc-coordinating residues in the monomeric and dimeric conformers. Topology of HYBΔC (E) is compared with that of the HYB domain (PDB code 3vk6) (F). The zinc ions are shown as spheres (color coded according to the monomer colors) and the three zinc-coordination sites are indicated for each monomer (Sites 1, 2, and 3). In HYBΔC, Site 3 is formed by four zinc-coordination residues, all situated from the same monomer. For the HYB domain, Site 3 and Site 3′ share residues from both monomers.
HYBΔC Binds Phospho-E-cadherin Ligand as a Dimer
We next questioned whether monomeric HYBΔC could still engage with a phosphorylated substrate ligand. Hence, we conducted isothermal titration calorimetry (ITC) experiments to investigate the binding property of HYBΔC with the peptide ligand corresponding to our previously established Tyr(P) motif of E-cadherin, comprising residues 747–759 (14). The ITC experiment revealed that HYBΔC binds with the Tyr(P) ligand of E-cadherin with a binding stoichiometry of 2:1 (HYBΔC:Tyr(P) ligand) and an affinity value of Kd = 3.7 μm (Fig. 5A). This indicates that the phospho-E-cadherin-(747–759) ligand interacts with a dimeric form of HYBΔC.
FIGURE 5.
ITC, gel filtration profiles, AUC, and NMR relaxation studies of HYBΔC with phospho-E-cadherin-(747–759). A, the tyrosine-phosphorylated E-cadherin-(747–759) was titrated against HYBΔC using ITC. The top panel shows the heat release profile after baseline correction and the lower panel indicates the binding isotherm for the interaction. The dissociation constant (Kd) and binding stoichiometry (N) are shown in the table. B, comparison of gel filtration profiles of HYBΔC in the presence (blue) and absence (red) of phospho-E-cadherin-(747–759) using a Superdex 75 gel filtration column. The elution profile suggests that HYBΔC exists as a dimer in the presence of ligand but as a monomer in the absence of the ligand. C, AUC analysis of HYBΔC in the presence of phospho-E-cadherin-(747–759) ligand. The ligand-induced dimerization of HYBΔC was studied using sedimentation velocity analysis. The results show that the protein exists as a dimer in the presence of the ligand with an apparent molecular mass of 20,000 Da. D, 15N relaxation T1 (a) and T2 (b) values as well as the error values for each residues in free HYBΔC sample (labeled as a red star) and phospho-E-cadherin747–759 bound complex (labeled as ●). The results show the T1 and T2 values change in the presence and absence of the substrate peptide. The secondary structures and domain boundary of HYBΔC also are illustrated in the middle of the figure. The missing residues and weak intensity residues (except Val128) are located in the region between β3 and α2.
To further understand the dimeric nature of this interaction, HYBΔC was separated on a calibrated gel-filtration column in the presence and absence of the ligand. A comparison of these two elution profiles showed that HYBΔC elutes as a dimer in the presence of the ligand and as a monomer in its apo form (Fig. 5B). To further verify this presumed ligand-induced dimerization of HYBΔC, sedimentation velocity AUC experiments were conducted with the samples containing HYBΔC in the presence of the phospho-E-cadherin-(747–759) peptide. The analysis of the AUC data using Sedfit (33) showed that the apparent molecular mass of HYBΔC in the presence of ligand is 20,000 Da (Fig. 5C), which is equivalent to twice the monomeric molecular weight of HYBΔC. Interestingly, the 15N relaxation data clearly showed a change in the T1 and T2 values in the presence and absence of the substrate peptide (Fig. 5D). The average T1 and T2 values for the free HYBΔC were 0.88 s and 120 ms, respectively. However, upon binding to the peptide, the average T1 and T2 values for the complex were 1.02 s and 100 ms, respectively. These results indicate that a high molecular weight complex is formed upon binding. Dynamic light scattering results further confirmed these findings, with an apparent molecular weight corresponding to a dimer in the presence of the substrate peptide. The combined results suggest that HYBΔC switches from a monomeric conformation to a dimeric form in the presence of a Tyr(P)-containing ligand.
Proposed Model of Tyr(P) Recognition by HYBΔC
To understand the mode of Tyr(P) recognition by HYBΔC, we undertook chemical shift perturbation analyses of HYBΔC in the presence of phospho-E-cadherin-(747–759). The results showed a significant shift for α2 helix in the presence of phospho-E-cadherin-(747–759) (Fig. 6, A and B). This chemical shift perturbation upon ligand binding with HYBΔC bears marked similarity to the one observed in our previous study wherein the 15N dimeric HYB domain was titrated with the phospho-E-cadherin-(747–759) peptide (14). Given that the presence of a Tyr(P) ligand changes the oligomerization, we analyzed the proposed dimeric configuration formed by the HYBΔC monomers in the presence of Tyr(P) ligand. We compared the HYBΔC monomer structure with the corresponding monomer of the untruncated HYB domain and observed a major conformational change in the α2 helix of the HYBΔC (Figs. 3 and 4). Our observations from the solution experiments, structural analysis, chemical shift perturbation data, and previous mutational data (14) suggest that the Tyr(P) ligand induces the dimerization to create a binding pocket in HYBΔC for substrate recognition (Fig. 6C). The electrostatic surface potential representation of HYBΔC in the phospho-E-cadherin-(747–759) peptide complex model suggests that the Tyr(P) along with the surrounding acidic residues on the phospho-E-cadherin-(747–759) peptide potentially interact with the positively charged HYBΔC binding pocket (Fig. 6D).
FIGURE 6.
Proposed model of Tyr(P) recognition by HYBΔC. A, an overlay of the HN-HSQC spectra of HYBΔC in the absence (green) or presence (red) of phospho-E-cadherin-(747–759) peptide. B, chemical shift perturbations of backbone amides by the binding of phospho-E-cadherin-(747–759) peptide to HYBΔC. The chemical shift perturbations were defined as Δδ = [(ΔδHN2 + ΔδN2/25)/2]0.5 for amide NH, where ΔδHN and ΔδN are the chemical shift differences of amide 1H, amide 15N, between the samples in the presence and absence of phospho-E-cadherin-(747–759) peptide. The NH HSQC assignments for HYBΔC complex were confirmed by three-dimensional 15N-edited NOESY-HSQC experiment. The empty regions represent no information available for residues in those regions because the NH HSQC peaks of those residues were invisible or unassigned. The secondary structures of HYBΔC also are illustrated in the middle of the figure. C, a proposed model of Tyr(P) recognition by the ligand-induced dimeric configuration of the HYBΔC monomers. The respective monomers are shown in green and magenta, with the peptide ligand in orange. The zinc ions are shown as red spheres. The HYBΔC·Tyr(P) peptide complex model was generated based on the chemical shift data as well as our previous mutational and binding studies (14), which showed the interaction of His127, Tyr176, His185, and Arg189 of HYB with the E-cadherin. Although the interacting side chains from the Tyr(P) peptide are not yet clearly established, our previous mutational studies showed that Asp750, Val752, Tyr(P)754, Asp756, and Glu757 are the interacting sites from the phospho-E-cadherin-(747–759) peptide (14). D, an electrostatic surface potential representation of the model presented in C suggests that the Tyr(P) along with its surrounding acidic residues of the phospho-E-cadherin-(747–759) interacts with the positively charged HYBΔC binding pocket. The Tyr(P) peptide ligand is shown in orange.
DISCUSSION
Phosphotyrosine-binding domains are critical, modular components that bind to phosphorylated tyrosine residues in acceptor proteins to create multiprotein complexes and regulate several intracellular signaling pathways (1, 8, 36). Dysregulation of these pathways is often associated with oncogenic transformation (37), rendering Tyr(P)-binding domains as attractive targets for directed therapies (38–40). So far, all of the major Tyr(P)-binding domains, including SH2 and phosphotyrosine-binding domains, tend to be monomeric (7, 41), barring the few exceptions where they function as homodimers. These exceptions include the SH2 domains of Grb10, Grb14, Grb7, APS, and SH2-B (7, 41–43). In addition, in STAT proteins, tyrosine phosphorylation mediated dimerization has been reported to occur via SH2 domains (44–46). The recently identified Tyr(P)-binding fold in Hakai, HYB, bears a novel dimeric fold consisting of two atypical ZnFs shared between the paired Hakai (aa 106–206) monomers that exist in an intertwined configuration across the flexible C-terminal region (14). Previously we showed that the atypical, intermolecular zinc coordination is necessary for the dimerization of Hakai and hence its ability to interact with the substrate (14). Mutations of these residues involved in intermolecular zinc coordination results in dimer to monomer transition; and also abrogate the ability of full-length Hakai to bind its targets like E-cadherin and Cortactin, as revealed in the mammalian cell based studies (14). In this study, we showed that deletion of the flexible C-terminal region of HYB also results a monomeric fold in solution. A similar observation was recently reported in the C-terminal domain of SARS-CoV main protease (Mpro-C), where the truncation of the disordered C-terminal helix results in its transition from a dimer to a monomer (47). In yet another study, a dimer/tetramer equilibrium observed in Escherichia coli DNA mismatch repair protein MutS is converted into a monomer/dimer equilibrium upon deletion of the C-terminal 53 amino acids (48).
To understand the structural basis for this monomeric conformation of HYBΔC, we determined its solution NMR structure. The NMR structure revealed that the characteristic atypical ZnF, which plays a key role in the dimeric fold of the HYB domain, is replaced by a C2H2-type ZnF in HYBΔC, formed by two cysteines and two histidines, all situated on the same monomer. This represents a unique monomeric to dimeric switching mechanism.
One of the best studied physiological functions of the HYB domain is its interaction with and regulation of tyrosine-phosphorylated E-cadherin at cell-cell junctions (14, 15, 49, 50). As such, we selected a tyrosine-phosphorylated E-cadherin-(747–759) peptide as a model substrate to further assess this monomeric conformation of HYBΔC and to determine whether it is still functional. The ITC interaction studies showed that the ligand binds with the protein only in its dimeric form. This result is consistent with our previous studies that a dimeric HYB fold is necessary to create the Tyr(P) binding pocket; this was also validated with full-length Hakai in cell-based experiments (14). A series of solution studies further validated this switch to a dimeric conformation of HYBΔC in the presence of the Tyr(P) E-cadherin-(747–759) ligand. Furthermore, all of these experiments were carried out at different concentrations of HYBΔC and suggest that the ligand-induced dimerization of HYBΔC is independent of protein concentration. Previous studies have demonstrated ligand-mediated dimerization as a novel mechanism for protein-carbohydrate recognition (51, 52). The present study extends upon this paradigm to Tyr(P) signaling and regulation in Hakai.
Protein dimerization is an important regulatory mechanism in signal transduction (53–55). Numerous RING finger ubiquitin ligase family members require dimerization for their function; for example, cIAP, XIAP, RNF4, SIAH, and TRAF2 act as homodimers (56–60), whereas heterodimers form between MDM2 and MDMX, BRCA1 and BARD1, and RING1b and BMI1, respectively (61). Previous studies have shown that dimerization is often necessary for the interaction of the E2 ubiquitin conjugate with the RING domain (56). However, the dimerization of Hakai monomers reported in this study likely represents a unique mechanism, as the incoming substrate, rather than association with the RING domain, mediates the dimerization. This might represent a critical mechanism to facilitate the conformational switch Hakai requires for substrate binding and thus for the ubiquitination of the substrates such as E-cadherin.
In conclusion, we have identified a novel monomeric fold of HYBΔC, which impairs its Tyr(P) binding property. However, in the presence of the Tyr(P) ligand, the monomeric HYBΔC becomes a dimer to create the Tyr(P) binding pocket to engage the substrate. The findings from this study suggest that the dimeric architecture of the HYB domain is necessary to engage the Tyr(P) ligand, which is in sharp contrast to all the other known Tyr(P)-binding domains that predominantly function as monomers. Selectively targeting the dimeric interface of therapeutically important enzymes has emerged as an attractive method of allosteric inhibition (62–65). The importance of the dimeric architecture of HYB in Tyr(P) substrate binding demonstrated by the present study makes it an ideal target for the design of selective allosteric inhibitors that abrogate HYB dimerization and potentially act as novel therapeutic interventions against cancer.
This work was supported by MOE (Tier 2) Grant R154000625112.
The NMR data and structure of HYBΔC have been deposited in the Biological Magnetic Resonance Bank under accession number BMRB 25008.
The atomic coordinates and structure factors (code 2mq1) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- Tyr(P)
- phosphotyrosine
- aa
- amino acid(s)
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- HYB
- Hakai Tyr(P)-binding domain
- RDC
- residue dipolar coupling
- AUC
- analytical ultracentrifugation
- HSQC
- heteronuclear single quantum coherence
- r.m.s.
- root mean square
- ITC
- isothermal titration calorimetry
- PDB
- Protein Data Bank
- SH2
- Src homology 2.
REFERENCES
- 1. Yaffe M. B. (2002) Phosphotyrosine-binding domains in signal transduction. Nat. Rev. Mol. Cell Biol. 3, 177–186 [DOI] [PubMed] [Google Scholar]
- 2. Deribe Y. L., Pawson T., Dikic I. (2010) Post-translational modifications in signal integration. Nat. Struct. Mol. Biol. 17, 666–672 [DOI] [PubMed] [Google Scholar]
- 3. Hunter T. (2009) Tyrosine phosphorylation: thirty years and counting. Curr. Opin. Cell Biol. 21, 140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeClue J. E., Sadowski I., Martin G. S., Pawson T. (1987) A conserved domain regulates interactions of the v-fps protein-tyrosine kinase with the host cell. Proc. Natl. Acad. Sci. U.S.A. 84, 9064–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sadowski I., Stone J. C., Pawson T. (1986) A noncatalytic domain conserved among cytoplasmic protein-tyrosine kinases modifies the kinase function and transforming activity of Fujinami sarcoma virus P130gag-fps. Mol. Cell. Biol. 6, 4396–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Forman-Kay J. D., Pawson T. (1999) Diversity in protein recognition by PTB domains. Curr. Opin. Struct. Biol. 9, 690–695 [DOI] [PubMed] [Google Scholar]
- 7. Liu B. A., Jablonowski K., Raina M., Arcé M., Pawson T., Nash P. D. (2006) The human and mouse complement of SH2 domain proteins-establishing the boundaries of phosphotyrosine signaling. Mol. Cell 22, 851–868 [DOI] [PubMed] [Google Scholar]
- 8. Pawson T., Nash P. (2003) Assembly of cell regulatory systems through protein interaction domains. Science 300, 445–452 [DOI] [PubMed] [Google Scholar]
- 9. Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. (1993) SH2 domains recognize specific phosphopeptide sequences. Cell 72, 767–778 [DOI] [PubMed] [Google Scholar]
- 10. Filippakopoulos P., Müller S., Knapp S. (2009) SH2 domains: modulators of nonreceptor tyrosine kinase activity. Curr. Opin. Struct. Biol. 19, 643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kavanaugh W. M., Williams L. T. (1994) An alternative to SH2 domains for binding tyrosine-phosphorylated proteins. Science 266, 1862–1865 [DOI] [PubMed] [Google Scholar]
- 12. Benes C. H., Wu N., Elia A. E., Dharia T., Cantley L. C., Soltoff S. P. (2005) The C2 domain of PKCδ is a phosphotyrosine binding domain. Cell 121, 271–280 [DOI] [PubMed] [Google Scholar]
- 13. Christofk H. R., Vander Heiden M. G., Wu N., Asara J. M., Cantley L. C. (2008) Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 452, 181–186 [DOI] [PubMed] [Google Scholar]
- 14. Mukherjee M., Chow S. Y., Yusoff P., Seetharaman J., Ng C., Sinniah S., Koh X. W., Asgar N. F., Li D., Yim D., Jackson R. A., Yew J., Qian J., Iyu A., Lim Y. P., Zhou X., Sze S. K., Guy G. R., Sivaraman J. (2012) Structure of a novel phosphotyrosine-binding domain in Hakai that targets E-cadherin. EMBO J. 31, 1308–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujita Y., Krause G., Scheffner M., Zechner D., Leddy H. E., Behrens J., Sommer T., Birchmeier W. (2002) Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 4, 222–231 [DOI] [PubMed] [Google Scholar]
- 16. Pece S., Gutkind J. S. (2002) E-cadherin and Hakai: signalling, remodeling or destruction? Nat. Cell Biol. 4, E72–E74 [DOI] [PubMed] [Google Scholar]
- 17. Figueroa A., Kotani H., Toda Y., Mazan-Mamczarz K., Mueller E. C., Otto A., Disch L., Norman M., Ramdasi R. M., Keshtgar M., Gorospe M., Fujita Y. (2009) Novel roles of hakai in cell proliferation and oncogenesis. Mol. Biol. Cell 20, 3533–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abella V., Valladares M., Rodriguez T., Haz M., Blanco M., Tarrío N., Iglesias P., Aparicio L. A., Figueroa A. (2012) miR-203 regulates cell proliferation through its influence on Hakai expression. PloS One 7, e52568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu H., Reynolds A. B., Kanner S. B., Vines R. R., Parsons J. T. (1991) Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol. Cell. Biol. 11, 5113–5124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mashima R., Hishida Y., Tezuka T., Yamanashi Y. (2009) The roles of Dok family adapters in immunoreceptor signaling. Immunol. Rev. 232, 273–285 [DOI] [PubMed] [Google Scholar]
- 21. Noh S. J., Baek H. A., Park H. S., Jang K. Y., Moon W. S., Kang M. J., Lee D. G., Kim M. H., Lee J. H., Chung M. J. (2013) Expression of SIRT1 and cortactin is associated with progression of non-small cell lung cancer. Pathol. Res. Pract. 209, 365–370 [DOI] [PubMed] [Google Scholar]
- 22. Siouda M., Yue J., Shukla R., Guillermier S., Herceg Z., Creveaux M., Accardi R., Tommasino M., Sylla B. S. (2012) Transcriptional regulation of the human tumor suppressor DOK1 by E2F1. Mol. Cell. Biol. 32, 4877–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whitmore L., Wallace B. A. (2008) Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers 89, 392–400 [DOI] [PubMed] [Google Scholar]
- 24. Bax A., Grzesiek S. (1993) Methodological advances in protein NMR. Acc. Chem. Res. 26, 131–138 [Google Scholar]
- 25. Fesik S. W., Eaton H. L., Olejniczak E. T., Zuiderweg E. R. P., McIntosh L. P., Dahlquist F. W. (1990) 2D and 3D NMR spectroscopy employing carbon-13/carbon-13 magnetization transfer by isotropic mixing: spin system identification in large proteins. J. Am. Chem. Soc. 112, 886–888 [Google Scholar]
- 26. Ottiger M., Delaglio F., Bax A. (1998) Measurement of J and dipolar couplings from simplified two-dimensional NMR spectra. J. Magn. Reson. 131, 373–378 [DOI] [PubMed] [Google Scholar]
- 27. Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 28. Goddard T. D., Kneller D. G. (2004) SPARKY 3. University of California, San Francisco, CA [Google Scholar]
- 29. Schwieters C. D., Kuszewski J. J., Tjandra N., Clore G. M. (2003) The Xplor-NIH NMR molecular structure determination package. J. Magn. Reson. 160, 65–73 [DOI] [PubMed] [Google Scholar]
- 30. Cornilescu G., Delaglio F., Bax A. (1999) Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J. Biomol. NMR 13, 289–302 [DOI] [PubMed] [Google Scholar]
- 31. Farrow N. A., Muhandiram R., Singer A. U., Pascal S. M., Kay C. M., Gish G., Shoelson S. E., Pawson T., Forman-Kay J. D., Kay L. E. (1994) Backbone dynamics of a free and phosphopeptide-complexed Src homology 2 domain studied by 15N NMR relaxation. Biochemistry 33, 5984–6003 [DOI] [PubMed] [Google Scholar]
- 32. Akke M., Palmer A. G. (1996) Monitoring macromolecular motions on microsecond to millisecond time scales by R1ρ-R1 constant relaxation time NMR spectroscopy. J. Am. Chem. Soc. 118, 911–912 [Google Scholar]
- 33. Brown P. H., Schuck P. (2006) Macromolecular size-and-shape distributions by sedimentation velocity analytical ultracentrifugation. Biophys. J. 90, 4651–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holm L., Kääriäinen S., Rosenström P., Schenkel A. (2008) Searching protein structure databases with DaliLite v.3. Bioinformatics 24, 2780–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mishra A., Eathiraj S., Corvera S., Lambright D. G. (2010) Structural basis for Rab GTPase recognition and endosome tethering by the C2H2 zinc finger of early endosomal autoantigen 1 (EEA1). Proc. Natl. Acad. Sci. U.S.A. 107, 10866–10871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Evans J. V., Ammer A. G., Jett J. E., Bolcato C. A., Breaux J. C., Martin K. H., Culp M. V., Gannett P. M., Weed S. A. (2012) Src binds cortactin through an SH2 domain cystine-mediated linkage. J. Cell Sci. 125, 6185–6197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pawson T. (2002) Regulation and targets of receptor tyrosine kinases. Eur. J. Cancer 38, S3–S10 [DOI] [PubMed] [Google Scholar]
- 38. Burke T. R., Jr., Yao Z. J., Liu D. G., Voigt J., Gao Y. (2001) Phosphoryltyrosyl mimetics in the design of peptide-based signal transduction inhibitors. Biopolymers 60, 32–44 [DOI] [PubMed] [Google Scholar]
- 39. Machida K., Mayer B. J. (2005) The SH2 domain: versatile signaling module and pharmaceutical target. Biochim. Biophys. Acta 1747, 1–25 [DOI] [PubMed] [Google Scholar]
- 40. Sawyer T. K., Bohacek R. S., Dalgarno D. C., Eyermann C. J., Kawahata N., Metcalf C. A., 3rd, Shakespeare W. C., Sundaramoorthi R., Wang Y., Yang M. G. (2002) SRC homology-2 inhibitors: peptidomimetic and nonpeptide. Mini Rev. Med. Chem. 2, 475–488 [DOI] [PubMed] [Google Scholar]
- 41. Stein E. G., Ghirlando R., Hubbard S. R. (2003) Structural basis for dimerization of the Grb10 Src homology 2 domain. Implications for ligand specificity. J. Biol. Chem. 278, 13257–13264 [DOI] [PubMed] [Google Scholar]
- 42. Depetris R. S., Hu J., Gimpelevich I., Holt L. J., Daly R. J., Hubbard S. R. (2005) Structural basis for inhibition of the insulin receptor by the adaptor protein Grb14. Mol. Cell 20, 325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hu J., Hubbard S. R. (2005) Structural characterization of a novel Cbl phosphotyrosine recognition motif in the APS family of adapter proteins. J. Biol. Chem. 280, 18943–18949 [DOI] [PubMed] [Google Scholar]
- 44. Darnell J. E., Jr. (1997) STATs and gene regulation. Science 277, 1630–1635 [DOI] [PubMed] [Google Scholar]
- 45. Soler-Lopez M., Petosa C., Fukuzawa M., Ravelli R., Williams J. G., Müller C. W. (2004) Structure of an activated Dictyostelium STAT in its DNA-unbound form. Molecular cell 13, 791–804 [DOI] [PubMed] [Google Scholar]
- 46. Wenta N., Strauss H., Meyer S., Vinkemeier U. (2008) Tyrosine phosphorylation regulates the partitioning of STAT1 between different dimer conformations. Proc. Natl. Acad. Sci. U.S.A. 105, 9238–9243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kang X., Zhong N., Zou P., Zhang S., Jin C., Xia B. (2012) Foldon unfolding mediates the interconversion between M(pro)-C monomer and 3D domain-swapped dimer. Proc. Natl. Acad. Sci. U.S.A. 109, 14900–14905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Manelyte L., Urbanke C., Giron-Monzon L., Friedhoff P. (2006) Structural and functional analysis of the MutS C-terminal tetramerization domain. Nucleic Acids Res. 34, 5270–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ishiyama N., Ikura M. (2012) The three-dimensional structure of the cadherin-catenin complex. Subcell. Biochem. 60, 39–62 [DOI] [PubMed] [Google Scholar]
- 50. Ishiyama N., Lee S. H., Liu S., Li G. Y., Smith M. J., Reichardt L. F., Ikura M. (2010) Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell 141, 117–128 [DOI] [PubMed] [Google Scholar]
- 51. Flint J., Nurizzo D., Harding S. E., Longman E., Davies G. J., Gilbert H. J., Bolam D. N. (2004) Ligand-mediated dimerization of a carbohydrate-binding molecule reveals a novel mechanism for protein-carbohydrate recognition. J. Mol. Biol. 337, 417–426 [DOI] [PubMed] [Google Scholar]
- 52. Sánchez-Vallet A., Saleem-Batcha R., Kombrink A., Hansen G., Valkenburg D. J., Thomma B. P., Mesters J. R. (2013) Fungal effector Ecp6 outcompetes host immune receptor for chitin binding through intrachain LysM dimerization. eLife 2, e00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Austin D. J., Crabtree G. R., Schreiber S. L. (1994) Proximity versus allostery: the role of regulated protein dimerization in biology. Chem. Biol. 1, 131–136 [DOI] [PubMed] [Google Scholar]
- 54. Klemm J. D., Schreiber S. L., Crabtree G. R. (1998) Dimerization as a regulatory mechanism in signal transduction. Annu. Rev. Immunol. 16, 569–592 [DOI] [PubMed] [Google Scholar]
- 55. Marianayagam N. J., Sunde M., Matthews J. M. (2004) The power of two: protein dimerization in biology. Trends Biochem. Sci. 29, 618–625 [DOI] [PubMed] [Google Scholar]
- 56. Nakatani Y., Kleffmann T., Linke K., Condon S. M., Hinds M. G., Day C. L. (2013) Regulation of ubiquitin transfer by XIAP, a dimeric RING E3 ligase. Biochem. J. 450, 629–638 [DOI] [PubMed] [Google Scholar]
- 57. Liew C. W., Sun H., Hunter T., Day C. L. (2010) RING domain dimerization is essential for RNF4 function. Biochem. J. 431, 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mace P. D., Linke K., Feltham R., Schumacher F. R., Smith C. A., Vaux D. L., Silke J., Day C. L. (2008) Structures of the cIAP2 RING domain reveal conformational changes associated with ubiquitin-conjugating enzyme (E2) recruitment. J. Biol. Chem. 283, 31633–31640 [DOI] [PubMed] [Google Scholar]
- 59. Park Y. C., Burkitt V., Villa A. R., Tong L., Wu H. (1999) Structural basis for self-association and receptor recognition of human TRAF2. Nature 398, 533–538 [DOI] [PubMed] [Google Scholar]
- 60. Polekhina G., House C. M., Traficante N., Mackay J. P., Relaix F., Sassoon D. A., Parker M. W., Bowtell D. D. (2002) Siah ubiquitin ligase is structurally related to TRAF and modulates TNF-α signaling. Nat. Struct. Biol. 9, 68–75 [DOI] [PubMed] [Google Scholar]
- 61. Metzger M. B., Hristova V. A., Weissman A. M. (2012) HECT and RING finger families of E3 ubiquitin ligases at a glance. J. Cell Sci. 125, 531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Andréola M. L. (2009) Therapeutic potential of peptide motifs against HIV-1 reverse transcriptase and integrase. Curr. Pharm. Des. 15, 2508–2519 [DOI] [PubMed] [Google Scholar]
- 63. Huber K. L., Ghosh S., Hardy J. A. (2012) Inhibition of caspase-9 by stabilized peptides targeting the dimerization interface. Biopolymers 98, 451–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lebon F., Ledecq M. (2000) Approaches to the design of effective HIV-1 protease inhibitors. Curr. Med. Chem. 7, 455–477 [DOI] [PubMed] [Google Scholar]
- 65. McMillan K., Adler M., Auld D. S., Baldwin J. J., Blasko E., Browne L. J., Chelsky D., Davey D., Dolle R. E., Eagen K. A., Erickson S., Feldman R. I., Glaser C. B., Mallari C., Morrissey M. M., Ohlmeyer M. H., Pan G., Parkinson J. F., Phillips G. B., Polokoff M. A., Sigal N. H., Vergona R., Whitlow M., Young T. A., Devlin J. J. (2000) Allosteric inhibitors of inducible nitric oxide synthase dimerization discovered via combinatorial chemistry. Proc. Natl. Acad. Sci. U.S.A. 97, 1506–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Laskowski R. A., Rullmannn J. A., MacArthur M. W., Kaptein R., Thornton J. M. (1996) AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 8, 477–486 [DOI] [PubMed] [Google Scholar]
- 67. Koradi R., Billeter M., Wüthrich K. (1996) MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graph. 14, 51–55 [DOI] [PubMed] [Google Scholar]