Background: Plasmodium falciparum invades red blood cells.
Results: We show that the merozoite surface proteins MSPDBL1 and MSPDBL2 are part of the large MSP1 complex.
Conclusion: The MSP1 complex acts as a platform for display of MSPDBL1 and MSPDBL2 on the merozoite surface.
Significance: This provides important information on the structure of the merozoite surface.
Keywords: Cell Invasion, Malaria, Parasite, Plasmodium, Protein Complex, Complex, falciparum, Infection, Merozoite
Abstract
Plasmodium falciparum is the causative agent of the most severe form of malaria in humans. The merozoite, an extracellular stage of the parasite lifecycle, invades erythrocytes in which they develop. The most abundant protein on the surface of merozoites is merozoite surface protein 1 (MSP1), which consists of four processed fragments. Studies indicate that MSP1 interacts with other peripheral merozoite surface proteins to form a large complex. Successful invasion of merozoites into host erythrocytes is dependent on this protein complex; however, the identity of all components and its function remain largely unknown. We have shown that the peripheral merozoite surface proteins MSPDBL1 and MSPDBL2 are part of the large MSP1 complex. Using surface plasmon resonance, we determined the binding affinities of MSPDBL1 and MSPDBL2 to MSP1 to be in the range of 2–4 × 10−7 m. Both proteins bound to three of the four proteolytically cleaved fragments of MSP1 (p42, p38, and p83). In addition, MSPDBL1 and MSPDBL2, but not MSP1, bound directly to human erythrocytes. This demonstrates that the MSP1 complex acts as a platform for display of MSPDBL1 and MSPDBL2 on the merozoite surface for binding to receptors on the erythrocyte and invasion.
Introduction
Invasion of merozoites during the asexual blood stage of Plasmodium falciparum is essential for parasite survival. This multistep process involves initial contact, reorientation, active invasion, and resealing and is very tightly controlled, involving specific ligand-receptor interactions between the erythrocyte and parasite for successful invasion. The surface localization of merozoite surface proteins (MSPs)3 could implicate them in the initial recognition and binding of host erythrocytes (1). As the parasite enters the host erythrocyte, the surface coat is shed into the bloodstream by protease cleavage of some of these proteins (1–4). The entire invasion process is highly efficient, requiring less than 60 s to be completed (5). Consequently, the host immune response has limited opportunity to respond to the free merozoites. However, repeated exposure to merozoites during infection with malaria can induce protective responses toward surface proteins, indicating that MSPs are exposed and targeted by the host immune system.
MSP1 is a glycosylphosphatidylinositol-anchored protein that has been widely studied as a vaccine candidate (for a review, see Ref.6; also see Ref. 7). It is also the most abundant protein on the merozoite surface. A C-terminally located glycosylphosphatidylinositol anchors this protein of 190–200 kDa on the merozoite surface (7). Prior to merozoite release, the precursor MSP1 is processed into four proteolytic products, p83, p30, p38, and p42 (8). These products remain associated and form a non-covalent complex on the merozoite surface. The p42 fragment is then processed further by a second protease, Subtilisin 2, to release the complex from the C-terminal MSP1-19 stub, which is internalized into newly invaded erythrocytes (3, 9, 10). The role of the MSP1 merozoite surface complex in erythrocyte invasion remains unclear, but recombinant fragments and parasite-derived forms of MSP1 have been implicated in the binding to erythrocyte receptors (11–15).
Processed forms of two proteins that peripherally associate with the merozoite surface have been shown to interact with the MSP1 complex (11–13). MSP636 binds to p38, and MSP722 binds to p83, p30, and p38 polypeptide fragments of MSP1 (14). MSP7 is derived from a poorly understood multigene family found on chromosome 13 of P. falciparum (15). Although the functional role of MSP7 in the MSP1 complex is not known, deletion of msp7 impairs parasite invasion of erythrocytes. MSP6 belongs to the MSP3 family of proteins that are all encoded by a cluster of genes on chromosome 10 of the P. falciparum genome and are defined by the presence of a conserved N-terminal five-amino acid motif (NLRN(A/G)) (16, 17). Like MSP6, none of the MSP3 family of proteins has a glycosylphosphatidylinositol anchor or transmembrane domain, and they are presumed to interact with the merozoite surface extrinsically through an interacting partner(s). Members of this family have high sequence similarity in their C-terminal regions that results in a cross-reactive immune response to these proteins (17). Members of the MSP3 family have low sequence similarity in their N-terminal regions, suggesting potential functional differences. They have been considered as potential vaccine candidates as antibodies against MSP6 efficiently inhibit parasite growth in vitro (14), whereas antibodies against MSP3 appear to interfere with parasite growth via a mechanism of antibody-dependent cellular inhibition (18–21).
The proteins MSPDBL1 (PF3D7_1035700) and MSPDBL2 (PF3D7_1036300) contain sequence motifs that define them as MSP3 family members (17, 22). In addition to the glutamate-rich C-terminal secreted polymorphic antigen associated with merozoite (SPAM) domain, they also contain a cysteine-rich region termed Duffy binding-like (DBL) domain. The DBL domain is defined by homology to other erythrocyte-binding proteins that include the Duffy-binding protein of Plasmodium vivax and erythrocyte binding antigen-175 of P. falciparum (23, 24). In both P. vivax Duffy-binding protein and P. falciparum erythrocyte binding antigen-175, the single and tandemly arranged DBL domains, respectively, are involved in interacting with host receptors to mediate invasion.
MSPDBL1 and MSPDBL2 both localize to the surface of the merozoite. As they do not possess transmembrane domains or glycosylphosphatidylinositol anchors, they are presumed to associate extrinsically through other merozoite surface proteins (22, 25). MSPDBL1 and MSPDBL2 are able to bind erythrocytes through their DBL domains, but as yet the receptors have not been identified (22, 26). This function suggests that they may be involved in the initial interaction of the merozoite with the host erythrocyte, and the ability of antibodies to MSPDBL1 to partially inhibit parasite growth is consistent with this (25). These proteins appear to be functionally important to parasite survival as they have been shown to be under balancing selection with one of the strongest selection signatures for P. falciparum proteins observed for MSPDBL2 (27, 28). Importantly, naturally acquired antibodies against the highly polymorphic DBL domain of MSPDBL2 have been shown to be significantly associated with protection from malaria (29). MSPDBL2 has also been implicated in potential resistance to halofantrine of P. falciparum parasites (28–31).
In this study, we used parasite-derived material to establish the interaction of MSPDBL1 and -2 with the MSP1 complex and validate these interactions with recombinant proteins to show that MSPDBL1 and -2 directly interact with MSP1 at nanomolar affinities. We have also begun to build the known MSP1 complex and established that MSP6 does not interact with either of the MSPDBL proteins in the assembled complex. Thus we propose that the role of the MSP1 complex is to act as a platform to display MSPDBL1 and MSPDBL2 for binding to their receptors on the host erythrocyte.
EXPERIMENTAL PROCEDURES
Ethics Statement
Antibodies were raised in mice and rabbits under the guidelines of the National Health and Medical Research Committee and the Public Health Service Policy on Humane Care and Use of Laboratory Animals. The specific details of our protocol were approved by the Royal Melbourne Hospital Animal Welfare Committee under approval number AEC2011.009. The use of human blood was approved by The Walter and Eliza Hall Institute of Medical Research Human Ethics Committee under approval number HREC86/17.
Parasites
Five P. falciparum 3D7 lines were used throughout this study: 3D7/DBL1HA, 3D7/DBL2HA, 3D7ΔMSPDBL1, 3D7ΔMSPDBL2, and 3D7. As described previously, the transfected parasite lines 3D7/DBL1HA and 3D7/DBL2HA have the human dihydrofolate reductase gene that confers resistance to the drug WR99210 (22). The hemagglutinin (HA)-tagged parasite lines were maintained in the presence of 5 nm WR99210. For the generation of 3D7ΔMSPDBL1 or 3D7ΔMSPDBL2, constructs were assembled in pCC1 and transfected as described (32). To harvest invasion supernatant, synchronized schizont stage parasites were allowed to rupture, and culture medium was harvested postinvasion by centrifugation at 9000 rpm for 10 min. To harvest late stage schizonts, cultures were centrifuged at 3000 rpm for 5 min and treated with saponin. Parasite proteins were extracted from saponin-lysed material in the presence of 0.1% Triton X-100 on ice for 10 min before centrifugation at 9000 rpm for 10 min with the addition of Complete protease inhibitors (Roche Applied Science) to minimize nonspecific proteolysis. Both supernatant and pellet were harvested for use in assays described.
Expression of MSPDBL1 and MSPDBL2 Constructs in Escherichia coli
Four constructs each for MSPDBL1 and MSPDBL2 were designed for expression in E. coli corresponding to the full-length protein (FL), the DBL domain (DBL), the SPAM domain and leucine-like zipper domain (SL), and only the SPAM domain (SO) (see Fig. 2A). For MSPDBL1 FL and MSPDBL2 FL, codon-optimized synthetic genes (GeneArt) were generated from residues Ser105–Lys697 and Asp127–Asn762, respectively, for expression in E. coli. The synthetic genes were digested with BamHI and XhoI and ligated into the pPROEXHTb vector containing an N-terminal His6 tag (Invitrogen). MSPDBL1 and MSPDBL2 DBL constructs were generated as described previously (22). For all other constructs, the following primers were designed for PCR amplification from synthetic genes: MSPDBL1 SL-F (5′-ggatccGCACTGCCTGGCACCAATATTATT-3′), MSPDBL1 SL-R (5′-tcgagttaTTTCTGAAACAGGTCGGTCATATCTTC-3′), MSPDBL1 SO-F (5′-ggatccGCACTGCCTGGCACCAATATTATT-3′), MSPDBL1 SO-R (5′-tcgagttaATCCTCATCCACTTTGCTGATGCT-3′), MSPDBL2 SL-F (5′-gatccCTGGAACAGCATAGCAAAGAAGATGTT-3′), MSPDBL2 SL-R (5′-tcgagttaATTTTTAAACAGGTTGGTAATATCTTTGCTC-3′), MSPDBL2 SO-F (5′-gatccCTGGAACAGCATAGCAAAGAAGATGTT-3′), and MSPDBL2 SO-R (5′-tcgagttaTTTTTCGTCTTTTTCGCTGTTGTTAAAGC-3′). The lowercase letters represent BamHI and XhoI restriction sites. PCR-amplified products were ligated into a pPROEXHTb vector (Invitrogen) with an N-terminal His6 tag for purification purposes.
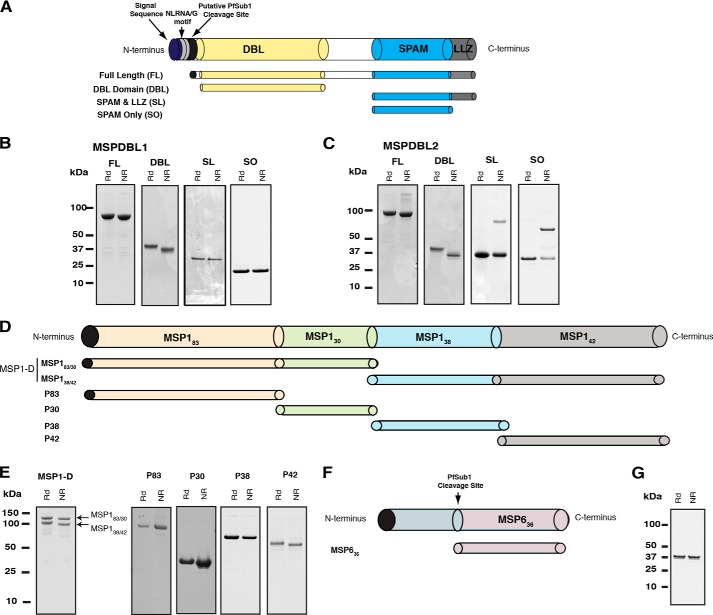
FIGURE 2.
Recombinant MSPDBL1, MSPDBL2, MSP1, and MSP6 proteins expressed in E. coli. A, schematic representation of MSPDBL1 and -2 and the four recombinant proteins encompassing different domains: FL, DBL, SL, and SO. B, Coomassie Brilliant Blue-stained gels of final protein products of MSPDBL1 domains: FL, 71.7 kDa; DBL, 38.9 kDa; SL, 22.7 kDa; and SO, 16.6 kDa. C, Coomassie Brilliant Blue-stained gels of final protein products of MSPDBL2 domains: FL, 76.4 kDa; DBL, 38.9 kDa; SL, 24.6 kDa; and SO, 18.7 kDa with an N-terminal His6 tag under non-reducing (NR) and reducing (Rd) conditions with molecular mass standards in kDa denoted (left). D, schematic representation of MSP1 and the six recombinant proteins expressed in E. coli: MSP1-D (heterodimer of MSP183/30 and MSP138/42), p83, p30, p38, and p42. E, Coomassie Brilliant Blue-stained gels of final protein products of MSP1-D (102 + 89.7 kDa) and the N-terminally His6 tagged subunits p83 (83.5 kDa), p30 (25.0 kDa), p38 (49.1 kDa), and p42 (46.7 kDa) with an N-terminal His6 tag under non-reducing (NR) and reducing (Rd) conditions with molecular mass standards in kDa denoted. F, schematic representation of MSP6 with a putative PfSub1 cleavage site where MSP636 was designed to include the C-terminal region of this site. G, final purified MSP636 (28.1 kDa) on SDS-PAGE under reducing (Rd) and non-reducing conditions (NR).
Expression and Purification of MSP1-D, p42, p38, p30, p83, and MSP636
Constructs were expressed and purified as described previously to obtain pure proteins (14).
Expression of MSPDBL1 and MSPDBL2
Constructs were transformed into BL21(DE3) E. coli strains for expression as described previously (22). Briefly, bacteria were grown to an A600 of ∼0.6 before the addition of 1 mm isopropyl β-d-1-thiogalactopyranoside. Cultures were grown for a further 3 h before harvesting by centrifugation. The cells were lysed by sonication and processed either as insoluble or soluble proteins.
Purification of Insoluble Proteins
MSPDBL1 and MSPDBL2 FL, DBL, and SL were expressed as insoluble proteins. The insoluble fraction was resolubilized in 6 m guanidine HCl, pH 8.0 before purification on a nickel-nitrilotriacetic acid (Ni-NTA) column under reducing conditions. The proteins of interest were eluted in 8 m urea, pH 8.0 in the presence of 1 m imidazole. The pooled proteins were then refolded in 20 mm Bis-Tris, pH 7.0, 100 mm NaCl, 1 m urea in the presence of 1 mm reducing glutathione and 1 mm oxidizing glutathione to promote disulfide bond formation and left to refold for 30 h at 25 °C.
Purification of MSPDBL1 SO and MSPDBL2 SO
Proteins were produced in E. coli as soluble proteins with a His6 tag. E. coli extracts were loaded on an Ni-NTA column equilibrated with wash buffer (50 mm Tris, pH 8.0, 100 mm NaCl, 20 mm imidazole) before eluting with 50 mm Tris, pH 8.0, 100 mm NaCl, 400 mm imidazole.
Purification of MSPDBL1 and MSPDBL2 Recombinant Proteins
The refolded material from FL, DBL, SL, and SO Ni-NTA elutions were dialyzed into 20 mm Hepes, pH 7.0, 20 mm NaCl before loading onto a Sepharose Q column. Proteins were eluted in a linear gradient (1–100%) with elution buffer containing 1 m NaCl. Fractions containing proteins of interest were pooled and concentrated for further purification using a Sephadex 200 16/60 column (GE Healthcare) into 20 mm Hepes, pH 7.0, 150 mm NaCl. Purity was examined by SDS-PAGE (Invitrogen). Reduced samples were achieved through the addition of β-mercaptoethanol. Gels were Coomassie-stained and subsequently destained in methanol/acetic acid.
Recombinant MSP1 and MSPDBLFL Co-immunoprecipitation
Recombinant proteins at 7 μg each were allowed to incubate overnight at 4 °C. The resulting mixture was then added to 10 μg of antibody mixture (mAb5.2, anti-MSP1; mAb7D11, anti-MSPDBL2 FL; and mAb2A7, anti-MSPDBL1 FL) in a final volume of 200 μl. Following a 1-h incubation, 50 μl of Sepharose G-agarose was added for a further 1 h. Postincubation the mixture was centrifuged and washed twice with phosphate-buffered saline (PBS)-Tween 20 before eluting with sample buffer supplemented with β-mercaptoethanol. The proteins were analyzed by SDS-PAGE with both Coomassie staining and Western blotting to positively identify proteins of interest.
Immunoblotting and Antibodies
Proteins were separated by 4–12% Bis-Tris SDS-PAGE (Invitrogen). Standard Western blotting procedures were performed using nitrocellulose (0.45 μm), and the immunoblots were processed with enhanced chemiluminescence (ECL) substrates (Pierce). For all Western blots, recombinant proteins were detected with antibodies at 1:1000 dilution. Antibodies used to detect MSP1-D were anti-MSP1 monoclonal 5.2, anti-MSP1 monoclonal m195, anti-MSP1 polyclonal R645, and pooled hyperimmune sera derived from humans. MSPDBL1 was detected using anti-MSPDBL1 monoclonal 2A7 and anti-MSPDBL1 polyclonal R1277 raised against full-length recombinant MSPDBL1. MSPDBL2 was detected using anti-MSPDBL2 monoclonal 7D11 and anti-MSPDBL2 polyclonal R1296 raised against full-length recombinant MSPDBL2. Detection of MSP6 was through the use of pooled hyperimmune sera.
Surface Plasmon Resonance
Binding of recombinant proteins was monitored by surface plasmon resonance (SPR) using the Biacore 2000 system (GE Healthcare). CM5 (Xantec) chip surfaces were prepared by immobilization of MSP1-D at 15 μg/ml in acetate buffer, pH 5.0; MSPDBL1 FL at 15 μg/ml in sodium acetate buffer, pH 4.0; and MSPDBL2 FL at 15 μg/ml in acetate buffer, pH 3.8 using standard amine coupling methods aiming for 10,000 resonance units. Final immobilization of 5719.0, 7761.6, and 7342.9 resonance units was achieved for MSP1-D, MSPDBL1 FL, and MSPDBL2 FL, respectively. Experiments were performed at 25 °C at a flow rate of 20 μl/min in 10 mm Hepes, pH 7.4, 100 mm NaCl, 0.005% (v/w) Tween 20, 3.4 mm EDTA with a contact time of 200 s and a dissociation time of 200 s. The chip surface was regenerated with a single 20-s injection of 50% ethylene glycol at 30 μl/min. The buffer difference was subtracted from blank cell lanes, which were coupled in the absence of protein. Data were processed using BIAevaluation software, and steady-state affinity was obtained using a one-site binding fit with Prism. Concentrations were chosen so that the contribution of mass transport limitation was negligible.
Competition Enzyme-linked Immunosorbent Assays (ELISAs)
Plates were coated overnight at 4 °C with 100 μl of MSP6, MSPDBL1, or MSPDBL2 to obtain a final concentration of 2 μg/ml before combinations of MSP1/MSP6, MSP1/MSPDBL1, and MSP1/MSPDBL2 were added onto the coat for a further 4 h. Wells were washed three times in wash buffer before 100 μl of anti-MSP1 monoclonal 5.2 was allowed to incubate at 1:1000 dilution. After 2 h, wells were washed three times with wash buffer and incubated with 100 μl of goat anti-mouse HRP (Millipore) at 1:4000 dilution. After 1 h, wells were washed three times with wash buffer and two times with PBS. 100 μl of peroxidase substrate was added and allowed to incubate for 5 min before the reaction was stopped with 100 μl of 1 m phosphoric acid. The absorbance at 450 nm was collected and tabulated for analysis.
Erythrocyte Binding Assays
Fresh whole blood samples were obtained from the Australian Red Cross. Recombinant proteins were incubated with human erythrocytes (50-μl packed volume) in PBS for 2 h at room temperature in 300 μl. The mixture was passed through 400 μl of dibutyl phthalate (Sigma) and centrifuged. The supernatant was discarded, and the pellet was washed twice with RPMI 1640 medium/Hepes before eluting in PBS with 1 m NaCl. The resulting supernatant was subjected to SDS-PAGE followed by immunoblotting. For antibody inhibition binding assays, a titration of either polyclonal or preimmune antibodies between 0 and 4 mg/ml was added to 0.2 μg of MSPDBL1 FL or MSPDBL2 FL and allowed to incubate for 1 h before being subjected to a standard binding assay as described above.
Growth Inhibition Assay
Late schizont stage parasites at 0.2% parasitemia were added to fresh erythrocytes at 1% hematocrit in 96-well round bottom microtiter plates. Affinity-purified anti-MSPDBL1 antibodies between 0 and 1 mg/ml were added to the culture at a final dilution of 1:10 prior to reinvasion. After incubation for two cycles (96 h) of parasite growth, the parasitemia for each individual well was determined by flow cytometry where parasitemia of trophozoite stage parasites stained with ethidium bromide was expressed as a percentage of growth compared with a non-inhibitory antibody control (33).
RESULTS
MSPDBL1 and MSPDBL2 Form a Complex with MSP1
To determine whether MSPDBL1 and MSPDBL2 were present on the merozoite surface as a complex with other proteins, immunoprecipitation experiments were performed with culture supernatants from P. falciparum 3D7 parasites in which the genes encoding MSPDBL1 and MSPDBL2 were tagged with HA epitopes (22). Anti-HA antibodies immunoprecipitated MSPDBL1 from 3D7/DBL1HA (Fig. 1A) and MSPDBL2 from 3D7/DBL2HA culture supernatants (Fig. 1B). Under non-reducing conditions on SDS-PAGE, full-length MSP1 (∼190 kDa) and a smaller fragment (∼180 kDa) were found to co-precipitate with both MSPDBL1 and MSPDBL2. In reciprocal immunoprecipitation experiments with 3D7/DBL1HA culture supernatant using anti-MSP1 antibodies, both MSPDBL1 and MSP1 were detected (Fig. 1A). Similarly, both MSPDBL2 and MSP1 were detected in the 3D7/DBL2HA supernatants when immunoprecipitated with the anti-MSP1 antibodies (Fig. 1B). This confirmed that MSPDBL1 and MSPDBL2 co-precipitate with MSP1, consistent with them being part of a large complex on the merozoite surface.
FIGURE 1.
MSPDBL1 and -2 are components of the MSP1 complex. Invasion supernatant harvested postinvasion from 3D7/DBL1HA, 3D7/DBL2HA, and 3D7 parasite lines was used for immunoprecipitation experiments with either anti-HA monoclonal or anti-MSP1 polyclonal antibodies, and bound proteins were detected by immunoblotting with rat anti-HA monoclonal antibodies (Roche Applied Science) under reducing conditions or mouse anti-MSP130 monoclonal m195 under non-reducing conditions (A and B). Anti-HA antibodies pulled down both MSPDBL1 and MSP1 (A) from 3D7/DBL1HA parasites and MSPDBL2 and MSP1 from 3D7/DBL2HA parasites (B). In the control lane, anti-HA antibodies did not pull down either MSPDBL or MSP1 from 3D7 parasites. In reciprocal experiments, anti-MSP1 antibodies pulled down both MSPDBL1 and MSP1 from 3D7/DBL1HA parasites and MSPDBL2 and MSP2 from 3D7/DBL2HA parasites, indicating the interaction of MSP1 with MSPDBL1 and MSPDBL2. In the control assay, anti-MSP1 antibodies were able to pull down native MSP1 material from 3D7 invasion supernatant.
MSPDBL1, MSPDBL2, MSP1, and MSP6 Recombinant Proteins
To further analyze the interaction of MSPDBL1 and MSPDBL2 with MSP1, constructs were designed, expressed, and purified from E. coli that consisted of different domains from each protein (Fig. 2, A, D, and F). These included the full-length MSPDBL1 and -2 proteins beginning from a putative PfSub1 cleavage site (FL) (4), DBL, SL, and SO. All constructs were assessed as having greater than 90% purity by SDS-PAGE (Fig. 2, B and C). These proteins ran slightly higher than their apparent size on SDS-PAGE probably due to the glutamate-rich regions as observed previously for other acidic proteins (13, 34, 35). Proteins that contained the DBL domain (DBL and FL) showed a differential shift in molecular weight when electrophoresed in the presence of reducing and non-reducing sample buffers, indicating the presence of disulfide bonds. In addition, due to a single cysteine residue in the SL and SO constructs of MSPDBL2, samples run under non-reducing conditions showed a higher molecular weight species corresponding to a dimeric form of the protein that could be disrupted in the presence of a reducing agent.
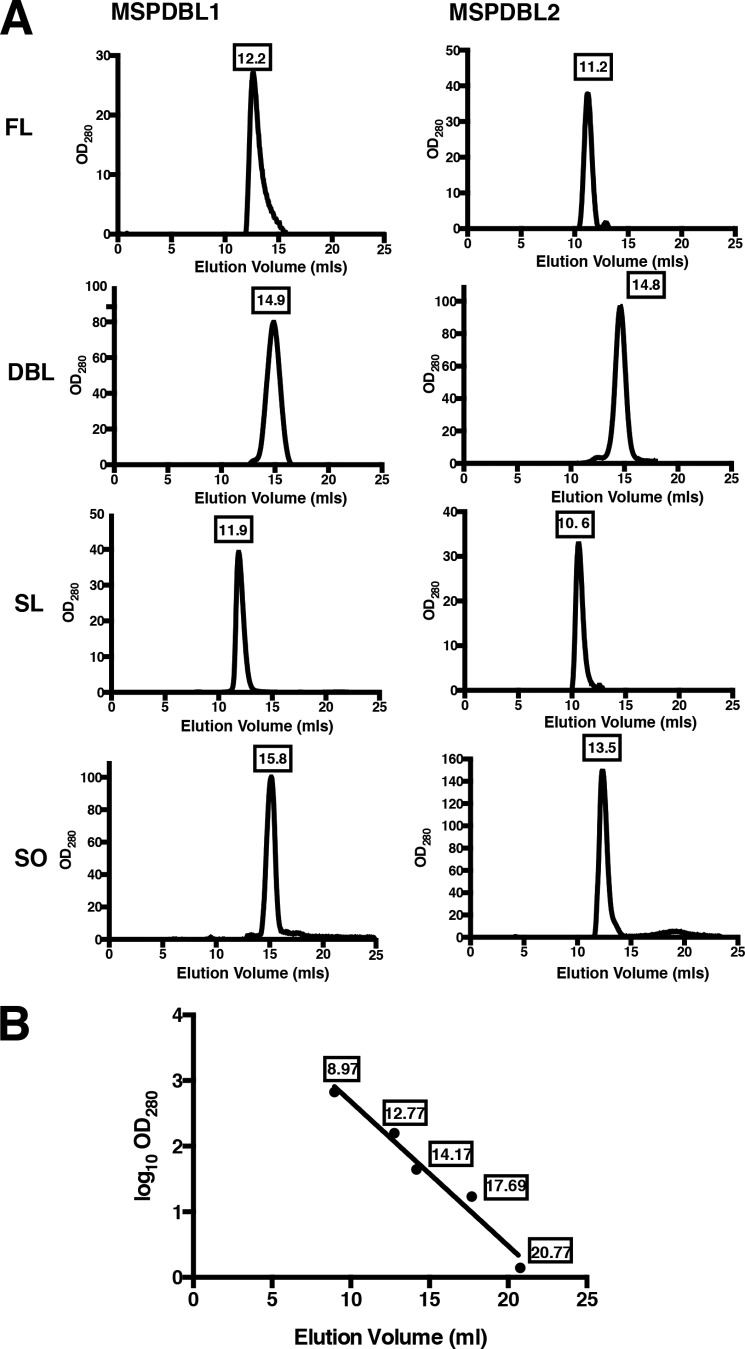
During size exclusion chromatography purification of the various constructs, it was noted that FL and SL constructs that had the leucine-like zipper domain present exhibited an increased mobility on the column, eluting at a volume corresponding to an oligomeric form (Fig. 3 and Table 1) as compared with globular standards (Fig. 3). In the absence of the leucine-like zipper domain in MSPDBL1 SO (16.6 kDa), the protein eluted at ∼26 kDa with the difference in size probably due to the elongated nature of the molecule (data not shown). MSPDBL2 SO eluted at a size of ∼81 kDa, roughly 4 times the molecular mass. The extra cysteine residue present in the SPAM domain could be responsible for this, allowing the SPAM domain of MSPDBL2 to form a higher oligomeric state in the absence of the leucine-like zipper domain. MSPDBL1 and MSPDBL2 DBL domains both eluted at volumes corresponding to their molecular weights. This result is consistent with the previous observation that the C-terminal leucine-like zipper domain of the MSP3 family is responsible for oligomerization (36).
FIGURE 3.
Size exclusion chromatography of the MSPDBL1 and MSPDBL2 constructs. The chromatograms for MSPDBL1 and MSPDBL2 FL, DBL, SL, and SO are plotted. The linear regression was derived from molecular standards: thyroglobulin (670 kDa), bovine γ-globulin (158 kDa), chicken ovalbumin (44 kDa), equine myoglobulin (17 kDa), and vitamin B12 (1.4 kDa) with elution volumes of 8.97, 12.77, 14.17, 17.69, and 20.77 ml, respectively. Experimentally derived results were fitted to y = −0.11x + 4.881 where r2 = 0.9701 and are reported in Table 1.
TABLE 1.
Molecular mass of the MSPDBL1 and -2 constructs compared with that obtained experimentally
| Sample | Molecular mass | Experimental molecular mass |
|---|---|---|
| kDa | kDa | |
| MSPDBL1 | ||
| FL | 71.7 | 157 |
| DBL | 38.9 | 40 |
| SL | 22.7 | 186 |
| SO | 16.6 | 26 |
| MSPDBL2 | ||
| FL | 76.4 | 265 |
| DBL | 38.9 | 41 |
| SL | 24.6 | 352 |
| SO | 18.7 | 81 |
To further confirm that the proteins were indeed refolded, both the FL constructs of MSPDBL1 and MSPDBL2 were subjected to far-UV CD spectroscopy (Fig. 4). Deconvolution of data using the CONTINLL (CD Pro Software) algorithm showed that MSPDBL1 FL (Fig. 4A) contained ∼22% α-helix, 25% β-sheet, 21% turn, and 32% disorder, whereas MSPDBL2 FL (Fig. 4B) had ∼24% α-helix, 19% β-sheet, 24% turn, and 33% disorder (Table 2).
FIGURE 4.
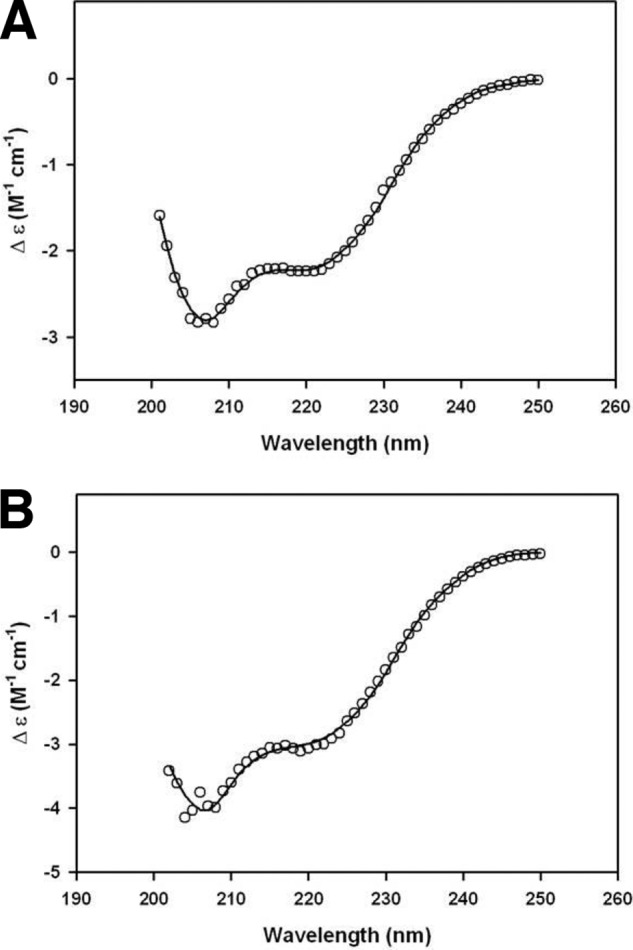
Circular dichroism spectra of MSPDBL1 and MSPDBL2 FL. The change in extinction coefficient is plotted as a function of wavelength for MSPDBL1 FL (A) and MSPDBL2 FL (B) at an initial concentration of 0.5 mg/ml. The data (open circles) were collected within a 4-s averaging time and at 20 °C, and the solid lines represent the nonlinear least square regression analysis best fits using the CONTINLL algorithm from the CDPro software package using the SP29 protein database. All results resulted in a root mean square deviation ≤0.081, and the secondary structure proportions are reported in Table 2.
TABLE 2.
Deconvolution of circular dichroism data
| Sample | α-Helix | β-Sheet | Turn | Disorder |
|---|---|---|---|---|
| % | % | % | % | |
| MSPDBL1 FL | 22 | 25 | 21 | 32 |
| MSPDBL2 FL | 24 | 19 | 24 | 33 |
Previously, it has been shown that the MSP1-D can be reconstituted from its processed products p83, p30, p38, and p42 (Fig. 2D) (37). To probe the interaction of MSPDBL1 and -2 with the MSP1 complex, these processed products were expressed, and complexes were formed as described previously (37). This included the MSP183/30 and MSP138/42 polypeptides as a complex (MSP1-D) as well as p83, p30, p38, and p42 individually (Fig. 2E). Additionally, the MSP6 protein, which forms a complex with MSP1, was expressed as a fragment starting from the PfSub1 cleavage site (Fig. 2F) and purified as described previously (Fig. 2G) (14).
The MSP1 and MSPDBL Interaction
To further confirm the direct interaction among MSPDBL1, -2, and MSP1, recombinant MSP1-D was incubated with FL MSPDBL1 or MSPDBL2. The mixture was immunoprecipitated with monoclonal antibodies specific to MSPDBL1, MSPDBL2, or MSP1. The immunoprecipitated proteins were visualized on Coomassie-stained gels to show that both anti-MSP1 and anti-MSPDBL1 monoclonal antibodies pulled down MSP1-D and MSPDBL1 together (Fig. 5A). Similar results were obtained for MSP1-D and MSPDBL2 (Fig. 5A), indicating that recombinant MSP1-D complex interacted with both MSPDBL1 and MSPDBL2. Protein identities were confirmed by immunoblotting where reciprocal immunoprecipitation experiments showed that anti-MSP1 monoclonal antibodies immunoprecipitated both MSPDBL1 and MSPDBL2, whereas the anti-MSPDBL1 and anti-MSPDBL2 monoclonal antibodies immunoprecipitated MSP1-D (Fig. 5B). Equivalent immunoprecipitations with recombinant protein apical membrane antigen 1 (AMA1) were performed as negative controls. The interaction between MSPDBL1 and -2 with MSP1 was also detected using an ELISA where binding can be titrated with an increasing amount of MSP1-D incubated with MSPDBL1 and MSPDBL2 (Fig. 5C). The binding affinity of the MSP1-MSPDBL1 and MSP1-MSPDBL2 interactions was assayed using SPR. FL MSPDBL1 and MSPDBL2 proteins bound to MSP1-D with a steady-state affinity of 170 and 450 nm, respectively, as determined using a one-site binding model, consistent with the immunoprecipitation and ELISA experiments (Fig. 5, D and E).
FIGURE 5.
Probing the formation of a complex between MSP1-D and MSPDBL1 or MSPDBL2. A, co-immunoprecipitation of MSP1-D with MSPDBL1 (top) or MSP1-D with MSPDBL2 (bottom) with either anti-MSP1 monoclonal (5.2) or anti-MSPDBL monoclonal antibodies (MSPDBL1, 2A7; MSPDBL2, 7D11) isolated a complex containing both recombinant MSP1 and MSPDBL. Samples were analyzed by SDS-PAGE and stained with Coomassie Blue with molecular weights indicated on the left. B, the same samples were subjected to immunoblot analysis with either anti-MSP1 or MSPDBL monoclonal antibodies as described above, confirming that MSP1 interacts with MSPDBL1 and MSPDBL2. In the bottom panel, MSP1 and MSPDBL were allowed to interact with His6-tagged AMA1 prior to immunoprecipitation with either anti-AMA1 monoclonal 3F9 or anti-MSP1 monoclonal 5.2 and MSPDBL monoclonal antibodies (MSPDBL1, 2A7; MSPDBL2, 7D11). Immunoblots show that anti-MSP1 monoclonal and anti-MSPDBL antibodies immunoprecipitate MSP1-D and MSPDBL but not AMA1. C, MSPDBL1 and MSPDBL2 bind to MSP1. ELISA plates were coated with MSPDBL1, MSPDBL2, or AMA1 at 2 μg/ml. A 2-fold dilution of MSP1 was added at 0–2 μg/ml. Binding was detected using anti-MSP1 monoclonal 5.2 and quantified at A450. Binding of AMA1 is a negative control. The binding was fitted to a one-site-specific binding curve where y = A450max(x)/(Kd + x). D and E, SPR sensorgrams of MSPDBL1 FL (D) and MSPDBL2 FL (E) binding to MSP1-D between concentrations of 0.03125 and 2 μm. The maximum responses were plotted to a one-site total binding model to determine the steady-state affinity (Kd). R.U., resonance units; IP, immunoprecipitation. Error bars represent standard deviation (S.D.).
Dissecting the Binding Regions of MSPDBL1 and -2 to MSP1
To delineate the region(s) of MSPDBL required for binding to MSP1 using SPR, fragments corresponding to DBL, SL, and SO were assayed for binding to full-length MSP1. Binding affinities of MSPDBL1 DBL and SL to MSP1-D were 390 nm and 1.3 μm (Fig. 6, A and B), respectively. For MSPDBL2 DBL and SL to MSP1-D, binding affinities were 350 nm and 1.0 μm (Fig. 6, D and E), respectively. The SO construct did not bind to MSP1 (Fig. 6, C and F). These results show that both the DBL domain and SL regions were able to bind individually to MSP1 and that the DBL domain bound with a stronger affinity to MSP1 than did the SL region. In addition, without the LLZ, the SPAM domain was unable to interact with MSP1.
FIGURE 6.
Dissecting the interactions of MSP1-D with MSPDBL FL, DBL, SL, and SO. SPR sensorgrams (left panels) show binding of MSP1-D to MSPDBL1 DBL (A), SL (B), and SO (C) and MSPDBL2 DBL (D), SL (E), and SO (F) between concentrations of 0.03125 and 10 μm. The resulting maximum responses were plotted to a one-site total binding model to determine the steady-state affinity (Kd), where error bars represent S.D. (right panels). The binding affinity of MSP1-D to MSPDBL1 DBL and SL was determined to be 390 nm and 1.3 μm, respectively. No binding was observed for both MSPDBL1 SO and MSPDBL2 SO to MSP1-D. MSPDBL2 DBL and SL constructs bound to MSP1 at 350 nm and 1.0 μm, respectively. R.U., resonance units.
MSPDBL1 and MSPDBL2 Interact with p42, p38, and p83 but Not p30 of MSP1
MSP1 is processed into four different fragments prior to invasion. To understand how MSPDBL1 and MSPDBL2 bound to this complex, we tested their ability to interact with the processed fragments of MSP1 (p42, p38, p30, and p83). Using SPR, we detected binding of three of four MSP1 fragments, namely p42, p38, and p83, to MSPDBL1 FL at binding affinities of 1.1 μm, 860 nm, and 1.4 μm, respectively (Fig. 7, A–D). Similarly, FL MSPDBL2 bound to MSP1 fragments at 840 nm for p42, 2.5 μm for p38, and 1.9 μm for p83 (Fig. 7, E–H). It is evident from the SPR assays that MSPDBL1 and MSPDBL2 bound to fragments of MSP1 with weaker affinity as compared with the entire MSP1 protein assembled for which the interaction appeared to be 2–8 times stronger. This suggests that each MSP1 fragment had an additive effect in anchoring MSPDBL1 and MSPDBL2 to the complex. It has been shown previously that MSP6 binds to p38 and MSP7 binds to p38, p30, and p83, demonstrating specificity in the binding regions of these peripheral proteins to MSP1 fragments (14). We describe for the first time that the binding of MSPDBL1 and MSPDBL2 to the MSP1 complex involves the p42, p38, and p83 processed products of MSP1.
FIGURE 7.
Dissecting the interactions of MSPDBL1/2 with MSP1-D fragments. Shown is the analysis of the interaction of MSPDBL1 with p42 (A), p38 (B), p30 (C), and p83 (D) and MSPDBL2 with p42 (E), p38 (F), p30 (G), and p38 (H). SPR assays determined that both MSPDBL1 and MSPDBL2 bound to three of the four MSP1 fragments, p42, p38, and p83. Sensorgrams (left panels) are shown at 2-fold increases in concentration between 0.03125 and 4 μm. The resulting maximum responses were plotted to a one-site total binding model where the steady-state affinity (Kd) was determined (right panels). MSPDBL1 bound to p42 at 1.1 μm, p38 at 860 nm, and p83 at 1.4 μm, whereas MSPDBL2 bound to p42 at 840 nm, p38 at 2.5 μm, and p83 at 1.9 μm. R.U., resonance units. Error bars represent S.D.
Probing the Interactions among MSP6, MSPDBL, and MSP1
MSPDBL1 and MSPDBL2 both bound to p38 of MSP1, the fragment with which MSP6 interacts (Fig. 7, B and F) (14, 37). To determine whether the MSP1-interacting proteins MSPDBL1 and MSPDBL2 also bound with MSP6 in the complex, MSP6 was flowed over immobilized MSP1-D, MSPDBL1 FL, and MSPDBL2 FL. We observed that MSP6 bound to MSP1 with an affinity of 79 nm (Fig. 8A); however, MSP6 showed no detectable binding to either MSPDBL1 or MSPDBL2 (Fig. 8, B and C).
FIGURE 8.
MSP1, MSPDBL, and MSP6 form a three-component complex. The interaction of MSP6 with either MSP1-D, MSPDBL1, or MSPDBL2 was monitored with SPR. The SPR sensorgrams show the binding responses of MSP6 to immobilized MSP1-D (A), MSPDBL1 (B), and MSPDBL2 (C) between 0.03125 and 4 μm. Where binding was observed in A, the maximum response at each concentration was plotted to a one-site total binding model, and the steady-state affinity (Kd) of the MSP1-MSP6 interaction was determined to be 79 nm. D, immunoprecipitation assays using MSP1-MSPDBL1-MSP6 or MSP1-MSPDBL2-MSP6 showed that all three components were pulled down using anti-MSP1 and anti-MSPDBL antibodies but not with anti-AMA1 antibodies. E, anti-MSP1 5.2 monoclonal antibodies only detect MSP1. ELISA plates were coated with a 2 μg/ml concentration of either MSP1, MSP6, MSPDBL1, or MSPDBL2, and anti-MSP1 5.2 monoclonal antibodies were used to detect binding. Measurements were taken at A450. F, competition ELISA. Plates were coated with 2 μg/ml MSP6 to assay the effect of site-specific saturation of MSP1 in the presence of either MSP6, MSPDBL1, or MSPDBL2 at 10 μg/ml and MSP1-D at 2 μg/ml. Upon incubation of MSP1-MSP6, MSP1-MSPDBL1, or MSP1-MSPDBL2 to the MSP6-coated plate, binding was detected with anti-MSP1 mAb5.2 and secondary anti-mouse antibodies conjugated to HRP. Absorbance values at A450 were measured. G, ELISA plates were coated with 2 μg/ml FL MSPDBL1 or MSPDBL2. A 2-fold titration of MSP1-D across the concentration range of 0–6.4 μg/ml was allowed to incubate with 10 μg/ml MSP6, and binding was detected as described above. All ELISA experiments (F and G) were repeated, and similar results were obtained. The error bars represent the S.E. of four independent assays. R.U., resonance units; IP, immunoprecipitation.
To assay whether MSPDBL1 and MSPDBL2 formed a complex with MSP6 and MSP1, recombinant FL MSPDBL1 or MSPDBL2 was allowed to interact with MSP6 and MSP1-D before immunoprecipitating with anti-MSPDBL1, anti-MSPDBL2, anti-MSP1, or anti-AMA1 monoclonal antibodies. Anti-MSP1 monoclonal antibodies brought down either MSPDBL1 and MSP6 or MSPDBL2 and MSP6 with MSP1-D (Fig. 8D). All three proteins (MSPDBL1, MSP6, and MSP1-D) were pulled down with anti-MSPDBL1 antibodies. A similar observation was made with immunoprecipitations using anti-MSPDBL2 antibodies. Because MSPDBL1 and MSPDBL2 did not appear to interact with MSP6, the presence of MSP6 in the eluate suggests that MSP6 associated only with MSP1 and that MSPDBL1 and MSPDBL2 were also part of the three-component complex.
Competition ELISAs were performed to determine whether MSPDBL1, MSPDBL2, and MSP6 bound to the same regions on MSP1. To eliminate the possibility of cross-reactivity of anti-MSP1 antibodies to MSP6, MSPDBL1, and MSPDBL2, the plate was coated with each of the four proteins, and anti-MSP1 antibodies were used to show that the monoclonal anti-MSP1 antibodies did not cross-react with MSP6, MSPDBL1, and MSPDBL2 (Fig. 8E). In the competition assay, excess amounts of MSP6 were allowed to incubate with MSP1-D before the complex was subjected to a coated plate of MSP6, MSPDBL1, or MSPDBL2 (Fig. 8F). We observed that the binding sites of MSP6 on MSP1 were blocked when the binding of MSP6-MSP1 complex to an MSP6-coated plate was drastically reduced. In comparison, MSPDBL1-MSP1 or MSPDBL2-MSP1 complexes were still able to bind to the MSP6-coated plate. This indicates that the binding site that MSP6 occupies on MSP1 is not the same as the MSPDBL1 or MSPDBL2 binding site. Similarly, titrating the amount of MSP1-D in the presence of MSP6 in excess showed no blocking effect for the interaction of MSP1 with MSPDBL1 or MSPDBL2, suggesting that the binding pockets for MSPDBL1/2 and MSP6 are in different positions (Fig. 8G).
MSPDBL1 and MSPDBL2 Recombinant Proteins Bind Erythrocytes
MSPDBL1 and MSPDBL2 have been shown to bind erythrocytes via unknown receptors (22, 26). The ability of the recombinant protein domains to bind to the host cell was tested in erythrocyte binding assays. As expected, constructs that contained a DBL domain (FL and DBL) bound to erythrocytes (Fig. 9A). Trypsin, chymotrypsin, and neuraminidase treatment of erythrocytes had no effect on the binding of the FL constructs of MSPDBL1 and -2 to erythrocytes, consistent with previous results (Fig. 9B) (22). This is in comparison with PfRh4.9, a recombinant fragment of PfRH4 that is known to bind to complement receptor 1 (38). These data show that MSPDBL1 and -2 FL recombinant proteins were correctly folded and functional. Surprisingly, the SL construct from both proteins also bound to erythrocytes, and this was through the LLZ domain as the SO polypeptide did not bind (Fig. 9A). It is not clear whether this apparent binding is functionally relevant as this region may not normally be exposed on the merozoite surface. The homologous region in another MSP3 family member, MSP6, has been shown to be involved in multimerization and binding to MSP1 (14). In comparison with MSPDBL1 and -2 proteins, MSP6, which lacks a DBL domain, did not show any binding to erythrocytes. Similarly, the MSP1-D complex did not bind to erythrocytes (Fig. 9A) (38). Taken together these data suggest that MSPDBL1 and MSPDBL2 form a complex with MSP1 on the merozoite surface to mediate binding to the host erythrocyte.
FIGURE 9.
MSPDBL1 and MSPDBL2, but not MSP1 or MSP6, bind to human erythrocytes. A, 3 μg of recombinant FL, DBL, SL, and SO proteins of MSPDBL1 and MSPDBL2 together with MSP1, MSP6, and Rh4.9 (control) were subjected to either binding in the presence of red blood cells (+) or not subjected to binding as a molecular weight reference (−). Bound proteins were eluted in the presence of 1 m NaCl and separated by SDS-PAGE before analysis by immunoblotting with anti-MSPDBL1 polyclonal R1277, anti-MSPDBL2 polyclonal R1296, hyperimmune sera for MSP6 and MSP1, or anti-Rh4.9 monoclonal 10C9 as indicated. B, MSPDBL1 and MSPDBL2 erythrocyte binding activity is not affected by enzyme treatments. 3 μg each of MSPDBL1 FL, MSPDBL2 FL, and Rh4.9 were incubated with red blood cells untreated (U) or treated with neuraminidase (N), trypsin (T), or chymotrypsin (C), and binding was detected in immunoblots. Rh4.9 was used as a control for successful enzyme treatments. C, full-length MSPDBL1 and MSPDBL2 recombinant proteins incubated with anti-MSPDBL1, anti-MSPDBL2, or preimmune sera at final concentrations between 0 and 4 mg/ml were added to red blood cells (RBCs). The bound proteins were eluted and analyzed on immunoblots with anti-MSPDBL-specific antibodies as described above. D, MSPDBL1 and MSPDBL2 proteins are not produced in 3D7ΔMSPDBL1 and 3D7ΔMSPDBL2, respectively. Late stage schizonts were harvested and extracted using Tris/NaCl/EDTA/Triton X-100, and protein expression was tested in each of 3D7, 3D7ΔMSPDBL1, and 3D7ΔMSPDBL2 using anti-MSPDBL1 polyclonal R1277, anti-MSPDBL2 polyclonal R1296, anti-SERA5 polyclonal, and anti-AMA1 polyclonal sera. E, parasite growth was reduced in 3D7 wild type and 3D7/DBL2KO lines in the presence of anti-MSPDBL1 antigen-specific antibodies. Final concentrations of 0–1 mg/ml anti-MSPDBL1 affinity-purified antibodies were added to the culture. The observed inhibition was concentration-dependent. In the presence of anti-MSPDBL1 antibodies at 1 mg/ml, growth decreased by 33.66 ± 4.82% (***, p = 0.0001) in 3D7 and 41.95 ± 3.454% (***, p = 0.0067) in 3D7ΔMSPDBL2 using Fisher's exact test. Growth was measured as a percentage of non-inhibitory growth in PBS, and error bars represent the S.E. of three separate experiments in duplicate.
Polyclonal antibodies derived from rabbit immunizations against the FL constructs of MSPDBL1 and -2 were assayed for their ability to inhibit binding of MSPDBL1 and -2 to erythrocytes. Our erythrocyte binding assays indicate that polyclonal anti-MSPDBL1 and anti-MSPDBL2 antibodies were able to block binding of MSPDBL1 and -2 to erythrocytes in a dose-dependent manner where almost full blocking of binding was achieved at 4.0 mg/ml (Fig. 9C). The equivalent preimmune serum was unable to prevent binding of MSPDBL1 or MSPDBL2 to erythrocytes, showing that the blocking effect was mediated through antibodies against MSPDBL1 and MSPDBL2.
MSPDBL1 and MSPDBL2 have very similar characteristics, and it is possible that both may play similar roles in the MSP1 complex. To delineate the function of these two proteins, we generated single gene knock-outs of both mspdbl1 and mspdbl2 parasites in the 3D7 parental line. The 3D7ΔMSPDBL1 and 3D7ΔMSPDBL2 parasite lines were confirmed to have the corresponding genes disrupted (data not shown) and lacked expression of the proteins (Fig. 9D). Our attempts to knock out both genes together were not successful, suggesting that they share an essential function.
To test the effects of targeting both MSPDBL1 and MSPDBL2 together, we used affinity-purified antibodies specific for MSPDBL1 on 3D7ΔMSPDBL2 parasites that lacked expression of MSPDBL2 (Fig. 9E). Affinity-purified antibodies to MSPDBL1 were able to inhibit 3D7 parental parasite growth by up to ∼30% at 1 mg/ml. When the same assay was performed on 3D7ΔMSPDBL2 parasites, we saw a decrease in growth by up to 40% at 1 mg/ml (Fig. 9E). This shows that, although antibodies to MSPDBL1 did not appear to be significantly more potent in the absence of MSPDBL2 function, inhibitory antibodies present can disrupt parasite growth.
DISCUSSION
There are several lines of evidence suggesting that the MSP1 complex plays an essential role in normal growth and survival in the blood stage of the parasite. Antibodies targeting epitopes distributed throughout the MSP1 molecule, including some monoclonal antibodies directed against MSP1-19, inhibit invasion by blocking the secondary processing of the protein and thus the growth of parasite in vitro (20, 39, 40). Furthermore, it has not been possible to disrupt the gene encoding this protein (41). Current evidence suggests that MSP1 is involved in merozoite invasion of the erythrocyte, although there is little understanding of its exact role. In this study, we have shown that the erythrocyte-binding proteins MSPDBL1 and MSPDBL2 associate with MSP1 at the merozoite surface. This complex provides a platform for MSPDBL1 and MSPDBL2 to bind with their receptor(s) on the erythrocyte surface.
The MSP1 complex is shed into the culture supernatant upon successful invasion of the parasite (9), and known components include MSP6 and MSP7 (12, 14). We have shown using native parasite material in immunoprecipitation experiments that MSPDBL1 and -2 are also part of the MSP1 complex. Recombinant forms of MSPDBL1, MSPDBL2, and MSP1 allowed us to confirm these interactions and show that they bound to the p42, p38, and p83 fragments of the proteolytically cleaved MSP1 protein (Fig. 10). The binding of MSPDBL1 and MSPDBL2 to MSP1 can occur through both the SPAM domain and DBL domain. It was also observed that that the full-length MSPDBL1 and MSPDBL2 bound individual domains of MSP1 with weaker affinity compared with the assembled complex. This suggests that MSPDBL1 and -2 have multiple binding sites on MSP1 and that these contribute to the overall affinity of binding and stability of the complex.
FIGURE 10.

MSPDBL1 and MSPDBL2 form a complex with MSP1 to mediate interaction with erythrocytes during invasion. A schematic representation of MSPDBL in complex with MSP1 based on our data and published reports shows that MSPDBL interacts with p42, p38, and p83 of MSP1, whereas MSP6 interacts exclusively with MSP1. This MSP1-MSPDBL complex interacts with an unknown receptor on the surface of erythrocytes to mediate invasion. We have represented MSPDBL1 and -2 proteins in the complex as a single unit; however, it is likely that they are present as oligomers as suggested by our data (Fig. 3 and Table 1).
Both MSPDBL1 and -2 interacted with p42, p38, and p83 of MSP1, suggesting multiple binding interfaces. In contrast, MSP6 bound only p38 of MSP1 (14), and we have shown that it does not bind MSPDBL1 and -2 (Fig. 8). Our results indicate that MSPDBL1 and MSPDBL2 interact with MSP1 at different sites on the p38 fragment compared with that of MSP6 (14). Based on our observations, we propose that MSPDBL1 and MSPDBL2 would be embedded within MSP1 so that the entire complex is stabilized for its interaction with host erythrocytes (Fig. 10). It is not clear whether MSPDBL1 and MSPDBL2 have the same overall function; however, current evidence suggests that this is likely. They both have the same domain structure and appear to bind to the MSP1 complex in the same way through p42, p38, and p83. They both bind to a specific receptor on the erythrocyte (22, 26), and it has not been possible to disrupt both the mspdbl1 and mspdbl2 genes in the same parasite line, suggesting that they provide a redundant function for each other (41). It is currently not clear whether MSPDBL1 and -2 are present within the same MSP1 complex or whether there are multiple forms of the MSP1 complex that consist of different proteins, including MSPDBL1 or -2, and this may be important with respect to the function(s) of these complexes.
Recombinant and native forms of MSP142 have been found to bind to a fragment of band 3 or heparin-like molecules, which are found on the erythrocyte surface (42, 43). However, we did not observe any detectable binding of MSP1 or fragments of this protein to erythrocytes. Instead, we observed strong binding of both MSPDBL1 and MSPDBL2 directly to erythrocytes through unknown receptors. Although we cannot completely rule out binding of MSP1 directly to erythrocytes, it does occur indirectly through MSPDBL1 and MSPDBL2 as part of the complex. Therefore a function of MSP1 is to act as a platform on the merozoite for binding of these proteins to receptors during the invasion of erythrocytes.
The MSP1 complex on the surface of the merozoite is exposed to the host immune system. Indeed, the importance of this complex has been elucidated in numerous studies where antibodies targeting individual members of this complex, including samples recovered from semi-immune individuals, have been shown to inhibit parasite growth in vitro (20, 44–47). MSPDBL1 and MSPDBL2 have a SPAM domain similar to MSP6, and the highly conserved C-terminal region of these proteins may be an option for eliciting cross-reactive immune responses, which may target multiple members of the MSP3 family (48, 49).
The LLZ motif in the conserved C-terminal region of MSPDBL1 and -2 has been identified as an important region for successful oligomerization for members of the MSP3 family (14, 36). We observed that truncation of the C-terminal LLZ from MSPDBL1 and MSPDBL2 prevented interaction between them and MSP1, a result consistent with previous data for the MSP636 and MSP1 interaction where only a tetrameric MSP636 is able to bind to MSP1 (14). This indicates that oligomerization is important for successful incorporation of MSPDBL1 and -2 into the MSP1 complex, although this remains to be assessed in future studies. Further evaluation showed that deletion of the LLZ motif from the SPAM domain of MSPDBL1 and MSPDBL2 was sufficient to prevent binding of this fragment to host erythrocytes. Although the exact mechanism remains to be ascertained, this supports the importance of an oligomeric form of the SPAM domain for function.
The DBL domains of MSPDBL1 and MSPDBL2 are extremely polymorphic, indicating that they are under high levels of selective pressure presumably from the host immune system (27–29). This is indicative of the important roles of the DBL domains within MSPDBL1 and -2 and is consistent with the inability to disrupt the function of both within the same parasite. Indeed, evidence is accumulating that these proteins are important targets of immunity, and increased exposure to these antigens, in particular MSPDBL2, has been associated with reduced risk of malaria (50). We have shown in this study that antibodies targeting both MSPDBL1 and MSPDBL2 had the ability to directly inhibit binding to host erythrocytes. In addition, the assessment of the growth inhibitory potential of anti-MSPDBL1-specific antibodies showed a reduction of overall parasite growth using modest antibody concentrations. Taken together, these results suggest that these two molecules are potential targets within the MSP1 complex that can be exploited as a way to disrupt host-parasite interaction. However, the high level of polymorphism of the DBL domains is a significant hurdle that would need to be overcome for these to be considered as vaccine candidates.
In summary, we have shown that MSPDBL1 and -2 are components of the MSP1 complex on the merozoite surface and likely play a critical role in the initial interaction of the parasite with the host cell. The MSP1 complex provides a platform for the presentation of MSPDBL1 and -2 for binding to their receptors on the erythrocyte. Further work is required to fully understand the structure of the MSP1 complex and whether it exists in different forms containing varying subunits on the merozoite surface. We are progressively building this complex and investigating how the MSPDBL proteins orient and interact with other elements of the complex such as MSP7. Although much work has to be conducted to further assess the suitability of MSPDBLs as vaccine candidates, we believe that further structural insights into the MSPDBL-MSP1 complex would be essential for understanding the MSP1 complex as an entity and its role in invasion.
Acknowledgments
We thank the Red Cross Blood Service (Melbourne, Australia) for supply of red blood cells and serum and the Walter and Eliza Hall Institute of Medical Research Monoclonal Laboratory for growing the monoclonal antibodies.
This work was supported in part by National Health and Medical Research Council of Australia Grant 637406, by Victorian State Government Operational Infrastructure Support, and by the Australian Government National Health and Medical Research Council Independent Research Institutes Infrastructure Support Scheme.
- MSP
- merozoite surface protein
- SPAM
- secreted polymorphic antigen associated with merozoite
- DBL
- Duffy binding-like
- FL
- full-length protein
- SL
- the SPAM domain and leucine-like zipper domain
- SO
- only the SPAM domain
- Ni-NTA
- nickel-nitrilotriacetic acid
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- AMA1
- apical membrane antigen 1
- LLZ
- leucine-like zipper.
REFERENCES
- 1. Boyle M. J., Wilson D. W., Beeson J. G. (2013) New approaches to studying Plasmodium falciparum merozoite invasion and insights into invasion biology. Int. J. Parasitol. 43, 1–10 [DOI] [PubMed] [Google Scholar]
- 2. Blackman M. J., Chappel J. A., Shai S., Holder A. A. (1993) A conserved parasite serine protease processes the Plasmodium falciparum merozoite surface protein-1. Mol. Biochem. Parasitol. 62, 103–114 [DOI] [PubMed] [Google Scholar]
- 3. Harris P. K., Yeoh S., Dluzewski A. R., O'Donnell R. A., Withers-Martinez C., Hackett F., Bannister L. H., Mitchell G. H., Blackman M. J. (2005) Molecular identification of a malaria merozoite surface sheddase. PLoS Pathog. 1, 241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silmon de Monerri N. C., Flynn H. R., Campos M. G., Hackett F., Koussis K., Withers-Martinez C., Skehel J. M., Blackman M. J. (2011) Global identification of multiple substrates for Plasmodium falciparum SUB1, an essential malarial processing protease. Infect. Immun. 79, 1086–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilson P. R., Crabb B. S. (2009) Morphology and kinetics of the three distinct phases of red blood cell invasion by Plasmodium falciparum merozoites. Int. J. Parasitol. 39, 91–96 [DOI] [PubMed] [Google Scholar]
- 6. Holder A. A., Blackman M. J. (1994) What is the function of MSP-1 on the malaria merozoite? Parasitol. Today 10, 182–184 [DOI] [PubMed] [Google Scholar]
- 7. Holder A. A. (2009) The carboxy-terminus of merozoite surface protein 1: structure, specific antibodies and immunity to malaria. Parasitology 136, 1445–1456 [DOI] [PubMed] [Google Scholar]
- 8. McBride J. S., Heidrich H. G. (1987) Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol. Biochem. Parasitol. 23, 71–84 [DOI] [PubMed] [Google Scholar]
- 9. Blackman M. J., Holder A. A. (1992) Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1. Mol. Biochem. Parasitol. 50, 307–315 [DOI] [PubMed] [Google Scholar]
- 10. Blackman M. J., Scott-Finnigan T. J., Shai S., Holder A. A. (1994) Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 180, 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stafford W. H., Günder B., Harris A., Heidrich H. G., Holder A. A., Blackman M. J. (1996) A 22 kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol. Biochem. Parasitol. 80, 159–169 [DOI] [PubMed] [Google Scholar]
- 12. Pachebat J. A., Ling I. T., Grainger M., Trucco C., Howell S., Fernandez-Reyes D., Gunaratne R., Holder A. A. (2001) The 22 kDa component of the protein complex on the surface of Plasmodium falciparum merozoites is derived from a larger precursor, merozoite surface protein 7. Mol. Biochem. Parasitol. 117, 83–89 [DOI] [PubMed] [Google Scholar]
- 13. Trucco C., Fernandez-Reyes D., Howell S., Stafford W. H., Scott-Finnigan T. J., Grainger M., Ogun S. A., Taylor W. R., Holder A. A. (2001) The merozoite surface protein 6 gene codes for a 36 kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol. Biochem. Parasitol. 112, 91–101 [DOI] [PubMed] [Google Scholar]
- 14. Kauth C. W., Woehlbier U., Kern M., Mekonnen Z., Lutz R., Mücke N., Langowski J., Bujard H. (2006) Interactions between merozoite surface proteins 1, 6, and 7 of the malaria parasite Plasmodium falciparum. J. Biol. Chem. 281, 31517–31527 [DOI] [PubMed] [Google Scholar]
- 15. Kadekoppala M., O'Donnell R. A., Grainger M., Crabb B. S., Holder A. A. (2008) Deletion of the Plasmodium falciparum merozoite surface protein 7 gene impairs parasite invasion of erythrocytes. Eukaryot. Cell 7, 2123–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gardner M. J., Hall N., Fung E., White O., Berriman M., Hyman R. W., Carlton J. M., Pain A., Nelson K. E., Bowman S., Paulsen I. T., James K., Eisen J. A., Rutherford K., Salzberg S. L., Craig A., Kyes S., Chan M. S., Nene V., Shallom S. J., Suh B., Peterson J., Angiuoli S., Pertea M., Allen J., Selengut J., Haft D., Mather M. W., Vaidya A. B., Martin D. M., Fairlamb A. H., Fraunholz M. J., Roos D. S., Ralph S. A., McFadden G. I., Cummings L. M., Subramanian G. M., Mungall C., Venter J. C., Carucci D. J., Hoffman S. L., Newbold C., Davis R. W., Fraser C. M., Barrell B. (2002) Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419, 498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh S., Soe S., Weisman S., Barnwell J. W., Pérignon J. L., Druilhe P. (2009) A conserved multi-gene family induces cross-reactive antibodies effective in defense against Plasmodium falciparum. PLoS One 4, e5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soe S., Theisen M., Roussilhon C., Aye K. S., Druilhe P. (2004) Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect. Immun. 72, 247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oeuvray C., Bouharoun-Tayoun H., Gras-Masse H., Bottius E., Kaidoh T., Aikawa M., Filgueira M. C., Tartar A., Druilhe P. (1994) Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84, 1594–1602 [PubMed] [Google Scholar]
- 20. Woehlbier U., Epp C., Kauth C. W., Lutz R., Long C. A., Coulibaly B., Kouyaté B., Arevalo-Herrera M., Herrera S., Bujard H. (2006) Analysis of antibodies directed against merozoite surface protein 1 of the human malaria parasite Plasmodium falciparum. Infect. Immun. 74, 1313–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roussilhon C., Oeuvray C., Müller-Graf C., Tall A., Rogier C., Trape J. F., Theisen M., Balde A., Pérignon J. L., Druilhe P. (2007) Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med 4, e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hodder A. N., Czabotar P. E., Uboldi A. D., Clarke O. B., Lin C. S., Healer J., Smith B. J., Cowman A. F. (2012) Insights into Duffy binding-like domains through the crystal structure and function of the merozoite surface protein MSPDBL2 from Plasmodium falciparum. J. Biol. Chem. 287, 32922–32939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Batchelor J. D., Zahm J. A., Tolia N. H. (2011) Dimerization of Plasmodium vivax DBP is induced upon receptor binding and drives recognition of DARC. Nat. Struct. Mol. Biol. 18, 908–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tolia N. H., Enemark E. J., Sim B. K., Joshua-Tor L. (2005) Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell 122, 183–193 [DOI] [PubMed] [Google Scholar]
- 25. Sakamoto H., Takeo S., Maier A. G., Sattabongkot J., Cowman A. F., Tsuboi T. (2012) Antibodies against a Plasmodium falciparum antigen PfMSPDBL1 inhibit merozoite invasion into human erythrocytes. Vaccine 30, 1972–1980 [DOI] [PubMed] [Google Scholar]
- 26. Wickramarachchi T., Cabrera A. L., Sinha D., Dhawan S., Chandran T., Devi Y. S., Kono M., Spielmann T., Gilberger T. W., Chauhan V. S., Mohmmed A. (2009) A novel Plasmodium falciparum erythrocyte binding protein associated with the merozoite surface, PfDBLMSP. Int. J. Parasitol. 39, 763–773 [DOI] [PubMed] [Google Scholar]
- 27. Ochola L. I., Tetteh K. K., Stewart L. B., Riitho V., Marsh K., Conway D. J. (2010) Allele frequency-based and polymorphism-versus-divergence indices of balancing selection in a new filtered set of polymorphic genes in Plasmodium falciparum. Mol. Biol. Evol. 27, 2344–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amambua-Ngwa A., Tetteh K. K., Manske M., Gomez-Escobar N., Stewart L. B., Deerhake M. E., Cheeseman I. H., Newbold C. I., Holder A. A., Knuepfer E., Janha O., Jallow M., Campino S., Macinnis B., Kwiatkowski D. P., Conway D. J. (2012) Population genomic scan for candidate signatures of balancing selection to guide antigen characterization in malaria parasites. PLoS Genet. 8, e1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tetteh K. K., Stewart L. B., Ochola L. I., Amambua-Ngwa A., Thomas A. W., Marsh K., Weedall G. D., Conway D. J. (2009) Prospective identification of malaria parasite genes under balancing selection. PLoS One 4, e5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Tyne D., Park D. J., Schaffner S. F., Neafsey D. E., Angelino E., Cortese J. F., Barnes K. G., Rosen D. M., Lukens A. K., Daniels R. F., Milner D. A., Jr., Johnson C. A., Shlyakhter I., Grossman S. R., Becker J. S., Yamins D., Karlsson E. K., Ndiaye D., Sarr O., Mboup S., Happi C., Furlotte N. A., Eskin E., Kang H. M., Hartl D. L., Birren B. W., Wiegand R. C., Lander E. S., Wirth D. F., Volkman S. K., Sabeti P. C. (2011) Identification and functional validation of the novel antimalarial resistance locus PF10_0355 in Plasmodium falciparum. PLoS Genet. 7, e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Tyne D., Uboldi A. D., Healer J., Cowman A. F., Wirth D. F. (2013) Modulation of PF10_0355 (MSPDBL2) alters Plasmodium falciparum response to antimalarial drugs. Antimicrob. Agents Chemother. 57, 2937–2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maier A. G., Rug M., O'Neill M. T., Brown M., Chakravorty S., Szestak T., Chesson J., Wu Y., Hughes K., Coppel R. L., Newbold C., Beeson J. G., Craig A., Crabb B. S., Cowman A. F. (2008) Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell 134, 48–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lopaticki S., Maier A. G., Thompson J., Wilson D. W., Tham W. H., Triglia T., Gout A., Speed T. P., Beeson J. G., Healer J., Cowman A. F. (2011) Reticulocyte and erythrocyte binding-like proteins function cooperatively in invasion of human erythrocytes by malaria parasites. Infect. Immun. 79, 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McColl D. J., Silva A., Foley M., Kun J. F., Favaloro J. M., Thompson J. K., Marshall V. M., Coppel R. L., Kemp D. J., Anders R. F. (1994) Molecular variation in a novel polymorphic antigen associated with Plasmodium falciparum merozoites. Mol. Biochem. Parasitol. 68, 53–67 [DOI] [PubMed] [Google Scholar]
- 35. Nicholls S. C., Hillman Y., Lockyer M. J., Odink K. G., Holder A. A. (1988) An S antigen gene from Plasmodium falciparum contains a novel repetitive sequence. Mol. Biochem. Parasitol. 28, 11–19 [DOI] [PubMed] [Google Scholar]
- 36. Burgess B. R., Schuck P., Garboczi D. N. (2005) Dissection of merozoite surface protein 3, a representative of a family of Plasmodium falciparum surface proteins, reveals an oligomeric and highly elongated molecule. J. Biol. Chem. 280, 37236–37245 [DOI] [PubMed] [Google Scholar]
- 37. Kauth C. W., Epp C., Bujard H., Lutz R. (2003) The merozoite surface protein 1 complex of human malaria parasite Plasmodium falciparum: interactions and arrangements of subunits. J. Biol. Chem. 278, 22257–22264 [DOI] [PubMed] [Google Scholar]
- 38. Tham W. H., Schmidt C. Q., Hauhart R. E., Guariento M., Tetteh-Quarcoo P. B., Lopaticki S., Atkinson J. P., Barlow P. N., Cowman A. F. (2011) Plasmodium falciparum uses a key functional site in complement receptor type-1 for invasion of human erythrocytes. Blood 118, 1923–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blackman M. J., Heidrich H.-G., Donachie S., McBride J. S., Holder A. A. (1990) A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172, 379–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Woehlbier U., Epp C., Hackett F., Blackman M. J., Bujard H. (2010) Antibodies against multiple merozoite surface antigens of the human malaria parasite Plasmodium falciparum inhibit parasite maturation and red blood cell invasion. Malar. J. 9, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cowman A. F., Crabb B. S. (2006) Invasion of red blood cells by malaria parasites. Cell 124, 755–766 [DOI] [PubMed] [Google Scholar]
- 42. Goel V. K., Li X., Chen H., Liu S. C., Chishti A. H., Oh S. S. (2003) Band 3 is a host receptor binding merozoite surface protein 1 during the Plasmodium falciparum invasion of erythrocytes. Proc. Natl. Acad. Sci. U.S.A. 100, 5164–5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boyle M. J., Wilson D. W., Richards J. S., Riglar D. T., Tetteh K. K., Conway D. J., Ralph S. A., Baum J., Beeson J. G. (2010) Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proc. Natl. Acad. Sci. U.S.A. 107, 14378–14383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Riley E. M., Allen S. J., Wheeler J. G., Blackman M. J., Bennett S., Takacs B., Schönfeld H. J., Holder A. A., Greenwood B. M. (1992) Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 14, 321–337 [DOI] [PubMed] [Google Scholar]
- 45. Egan A. F., Morris J., Barnish G., Allen S., Greenwood B. M., Kaslow D. C., Holder A. A., Riley E. M. (1996) Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J. Infect Dis. 173, 765–769 [DOI] [PubMed] [Google Scholar]
- 46. O'Donnell R. A., de Koning-Ward T. F., Burt R. A., Bockarie M., Reeder J. C., Cowman A. F., Crabb B. S. (2001) Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 193, 1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fan Y. T., Wang Y., Ju C., Zhang T., Xu B., Hu W., Chen J. H. (2013) Systematic analysis of natural antibody responses to P. falciparum merozoite antigens by protein arrays. J. Proteomics 78, 148–158 [DOI] [PubMed] [Google Scholar]
- 48. Singh S., Soe S., Mejia J. P., Roussilhon C., Theisen M., Corradin G., Druilhe P. (2004) Identification of a conserved region of Plasmodium falciparum MSP3 targeted by biologically active antibodies to improve vaccine design. J. Infect. Dis. 190, 1010–1018 [DOI] [PubMed] [Google Scholar]
- 49. Demanga C. G., Daher L. J., Prieur E., Blanc C., Pérignon J. L., Bouharoun-Tayoun H., Druilhe P. (2010) Toward the rational design of a malaria vaccine construct using the MSP3 family as an example: contribution of antigenicity studies in humans. Infect. Immun. 78, 486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tetteh K. K., Osier F. H., Salanti A., Kamuyu G., Drought L., Failly M., Martin C., Marsh K., Conway D. J. (2013) Analysis of antibodies to newly described Plasmodium falciparum merozoite antigens supports MSPDBL2 as a predicted target of naturally acquired immunity. Infect. Immun. 81, 3835–3842 [DOI] [PMC free article] [PubMed] [Google Scholar]