Background: The lectin Yos9 engages protein determinants of ER-associated degradation (ERAD) substrates in vivo.
Results: Binding of Yos9 to model unfolded substrates in vitro triggers its aggregation.
Conclusion: Substrate-induced aggregation of Yos9 may play a role in the regulation of ERAD.
Significance: This work demonstrates specific binding of Yos9 to protein substrates and reveals a novel consequence of this interaction.
Keywords: Aggregation, Carbohydrate-binding Protein, Endoplasmic Reticulum (ER), Endoplasmic Reticulum-associated Protein Degradation (ERAD), Substrate Specificity, CPY*, Yos9
Abstract
A substantial fraction of nascent proteins delivered into the endoplasmic reticulum (ER) never reach their native conformations. Eukaryotes use a series of complementary pathways to efficiently recognize and dispose of these terminally misfolded proteins. In this process, collectively termed ER-associated degradation (ERAD), misfolded proteins are retrotranslocated to the cytosol, polyubiquitinated, and degraded by the proteasome. Although there has been great progress in identifying ERAD components, how these factors accurately identify substrates remains poorly understood. The targeting of misfolded glycoproteins in the ER lumen for ERAD requires the lectin Yos9, which recognizes the glycan species found on terminally misfolded proteins. In a role that remains poorly characterized, Yos9 also binds the protein component of ERAD substrates. Here, we identified a 45-kDa domain of Yos9, consisting of residues 22–421, that is proteolytically stable, highly structured, and able to fully support ERAD in vivo. In vitro binding studies show that Yos9(22–421) exhibits sequence-specific recognition of linear peptides from the ERAD substrate, carboxypeptidase Y G255R (CPY*), and binds a model unfolded peptide ΔEspP and protein Δ131Δ in solution. Binding of Yos9 to these substrates results in their cooperative aggregation. Although the physiological consequences of this substrate-induced aggregation remain to be seen, it has the potential to play a role in the regulation of ERAD.
Introduction
Nascent proteins destined for the secretory pathway enter the specialized environment of the endoplasmic reticulum (ER)4 as linear polypeptides that must fold into their native conformations. The complex topologies and numerous domains of many secreted proteins in conjunction with their high flux into the ER make folding of these proteins particularly challenging. Even with chaperone assistance, some of these proteins are not able to reach their native states and are considered terminally misfolded. To prevent transit of these nonfunctional proteins through the secretory system as well as formation of detrimental aggregates, eukaryotes efficiently eliminate them via a series of conserved pathways termed ER-associated degradation (ERAD). These pathways use multiple factors to recognize misfolded proteins and retrotranslocate them into the cytoplasm where they are ubiquitinated by the E3 ubiquitin ligase Hrd1 and degraded by the proteasome.
The ERAD machinery faces a challenging problem in specificity. Insufficient degradation causes accumulation of misfolded proteins, which is associated with disease, including neurodegenerative diseases such as Alzheimer disease (1), whereas excessive degradation causes the loss of potentially functional proteins as in cystic fibrosis (2). To ensure efficient disposal of misfolded glycoproteins found in the ER lumen, cells employ a two-part commitment signal. These ERAD substrates must display glycans with a terminal α1,6-linked mannose (3–5) as well as unfolded protein determinants that remain poorly defined (6). These requirements have been primarily characterized for the model substrate CPY*, which is the G255R variant of budding yeast carboxypeptidase Y (CPY) that is misfolded and degraded via ERAD (7). CPY* has four glycans, of which the one closest to the C terminus is necessary and sufficient to signal destruction of the full-length protein (4, 5). To affect ERAD of glycoproteins, the mannosidase Htm1 modifies substrate glycans (8) to reveal a terminal α1,6-linked mannose that is subsequently recognized by the ER-resident lectin, Yos9 (3, 9–11). Yos9 binds this signal glycan via its well conserved mannose receptor homology (MRH) domain, the selectivity of which was elucidated by the crystal structure of the MRH domain from the Yos9 human homolog, OS-9, in complex with α3,α6-mannopentaose (12). Although the proper glycan is necessary for ERAD of glycoproteins, it is not sufficient; these ERAD substrates must also possess some misfolded character.
Members of a “luminal surveillance complex” recognize the protein determinant required for ERAD, recruit substrates to Hrd1-associated machinery, and activate downstream events that commit substrates for degradation (13, 14). These events are orchestrated by Kar2, an ER-localized Hsp70, Hrd3, a membrane-bound component of the Hrd1 complex, and Yos9. As a chaperone, Kar2 plays a crucial role in maintaining the solubility of ERAD substrates prior to retrotranslocation (15). Hrd3 binds CPY* (14, 16) and has been proposed to act as a substrate recruitment factor for the Hrd1 ligase given its role in stabilizing (17, 18) and activating (14) the Hrd1 ligase. Yos9 is also able to directly interact with CPY*, and this binding is independent of its activity as a lectin. Previous work has shown that both wild type and a lectin mutant (R200A) of Yos9 immunoprecipitate CPY* with or without its glycans (9, 13, 19). Following these findings, Yos9 has been proposed to have additional roles in which it retains misfolded proteins in the ER (19, 20) and facilitates degradation of nonglycosylated proteins (21).
Given that Yos9 can bind both the signal glycan as well as misfolded protein determinants, we hypothesized that Yos9 may contribute to the integration of both signals required for ERAD. To explore this possibility, we characterized the binding of misfolded proteins by Yos9 in vitro. Using both the full-length protein as well as a proteolytically derived variant of Yos9 that is functional in vivo, we show that Yos9 exhibits sequence-specific recognition of linear peptides from CPY* and that binding either a model unfolded protein or peptide in vitro results in the aggregation of Yos9 with its binding partner. This substrate-induced aggregation constitutes a novel property of Yos9 that has the potential to be biologically important for its function in ERAD.
EXPERIMENTAL PROCEDURES
Limited Proteolysis of Yos9
Limited proteolysis was carried out by treating 100 μg of purified Yos9 with 0.005 units of elastase (5 μl of 1 unit/ml, where 1 unit is defined as 9.6 units/mg) in a 250-μl reaction (10 mm Hepes, 150 mm NaCl, 1 mm CaCl2, 10% glycerol, pH 7.4) for 2.5 h at 23 °C and mixing continuously at 500 rpm. The reaction was quenched by the addition of guanidine chloride crystals until saturation and run on a gel, which was stained with Coomassie Blue R-250. Bands were cut out, and mass spectrometry was performed by the David King laboratory (University of California at Berkeley). The mass of the predominant cleavage product was confirmed to be 45,401–45,403 Da. For comparison, Yos9(22–421) is calculated to be 45,407 Da.
Yos9 Purification and Refolding
His-tagged Yos9 or Yos9(22–421) was purified as described previously (3), with the exception that the gel filtration was done in 10 mm Hepes, 150 mm NaCl, pH 7.4.
Circular Dichroism Spectroscopy
Measurements were conducted on a Jasco J-715 spectrometer in a 2-mm cuvette at 20 °C. Samples contained 0.05 mg/ml Yos9(22–421) in 0.1 m sodium phosphate, pH 7.4. The spectrum was recorded over the range of 195–250 nm at a scanning speed of 5 nm/min and a bandwidth of 1.0 nm.
Size Exclusion Chromatography (SEC) Multiangle Light Scattering (MALS)
Molecular weight determination for purified Yos9 and Yos9(22–421) was done by SEC (Shodex KW-803 column) with an Ettan LC (GE Healthcare) and in-line DAWN HELEOS MALS and OPTILab rEX differential refractive index detectors (Wyatt Technology Corp.). Data were analyzed by the ASTRA V software package. SEC was performed in 10 mm Hepes, 150 mm NaCl, pH 7.4.
Cycloheximide Degradation Assays
CPY* degradation was measured in a Saccharomyces cerevisiae S288C strain with a genomic copy of either Yos9 (wild type) or Yos9(22–421). The Yos9(22–421) strain (yJW1759) was constructed via a URA3 intermediate and retains both the endogenous promoter and terminator. Cycloheximide chase degradation assays were performed as described previously (3, 13). The HA epitope (on CPY*) was detected using a 12CA5 monoclonal antibody (Roche Applied Science) at 1:1000. Hexokinase was used as a loading control and was detected by an anti-hexokinase antibody (US Biologicals) at 1:1200.
Peptide Array
The peptide array (W1007 B) was made by the MIT Biopolymers Laboratory for the Peter Walter laboratory. The CPY* tiling arrays was composed of 18-mer peptides that tiled through the CPY* sequence 3 amino acids at a time. To calculate the affinity of Yos9 for different amino acids, a larger tiled peptide array was used that included sequences from CPY*, mouse insulin, myelin protein zero, protein 8ab from SARS-CoV virus, and peptides known to bind BiP (22, 23).
The arrays were processed according to a protocol described previously (24) with some modifications. The array was incubated in methanol for 10 min and then washed three times in buffer (10 mm Hepes, 150 mm NaCl, 0.05% Tween 20, pH 7.4) for 10 min. The array was then incubated for 1 h at room temperature with 1.1 μm Yos9(22–421). The array was then washed again three times for 10 min in the buffer above. Using a semi-dry transfer apparatus, bound Yos9(22–421) was transferred to a nitrocellulose membrane and detected with an anti-His6 tag antibody (Abcam 3H2201). The array was stripped before re-use (25).
Fluorescence Anisotropy
Synthetic peptides (Elim Biosciences) were purified to >90% purity, and the identities were confirmed by mass spectrometry. Peptides were labeled with the fluorophore fluorescein amidite (FAM). The following peptides were used in this study: ΔEspP-FAM (MKK HKR ILA LCF LGL LQS SYS FA K(5-FAM)-NH2); ΔEspP (MKK HKR ILA LCF LGL LQS SYS AAK KKK).
For binding of Yos9 to ΔEspP-FAM, changes in fluorescence anisotropy were measured on a Spectramax-M5 plate reader using a protocol described previously (24). Increasing concentrations of Yos9 were added to 50 nm FAM-labeled peptides in 20-μl reactions, and after a 30-min incubation, readings were made with λex = 485 nm and λem = 525 nm. Anisotropy values are reported as an average of three separate titrations.
For binding of Yos9 to Δ131Δ labeled with the fluorophore 5-((((2-iodoacetyl) amino)ethyl) amino)naphthalene-1-sulfonic acid (IAEDANS), changes in fluorescence anisotropy were measured on a Jobin Yvon fluorometer with excitation and emission monochromator slits both set to 7 nm, an integration time of 1 s, and with λex = 340 nm and λem = 480 nm. Increasing concentrations of Yos9 were added to 1.25 μm Δ131Δ. Values are reported as an average of four readings. IAEDANS-labeled Δ131Δ was donated by the Agard laboratory and made as described previously (26).
For both ΔEspP-FAM and IAEDANS-labeled Δ131Δ data sets, anisotropy (r) values were calculated from Equation 1,
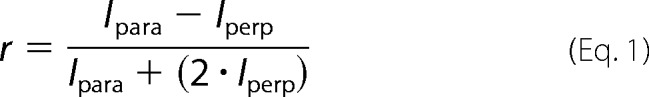 |
where Ipara and Iperp are the measured intensities when the excitation and emission polarizers are parallel or perpendicular, respectively. Binding constants were calculated by fitting the data to Equation 2,
 |
where [Yos9] is the concentration of Yos9; n is the Hill coefficient (assumed to be 1 for the case of Yos9 at low concentrations), and Kd is the dissociation constant. The variables rfree and rbound were fixed to the anisotropy of the peptide alone and the maximum anisotropy observed in the presence of Yos9, respectively.
Purification of 15N-Labeled Δ131Δ and Nuclear Magnetic Resonance (NMR) Spectroscopy
The expression and purification of Δ131Δ from Escherichia coli were performed as described previously (27) with the following modifications for isotopic labeling. Δ131Δ was expressed beginning with a 20-ml overnight starter culture in LB that was spun down and resuspended in 1 liter of M9 minimal growth media supplemented with 1 g/liter [15N]ammonium chloride and 0.5 g/liter ISOGRO growth supplement (Sigma).
HSQC data of Δ131Δ in buffer (25 mm MES, 150 mm NaCl, pH 6) were acquired on the University of California at Berkeley 900-MHz spectrometer with a cryoprobe using a fast HSQC pulse sequence from Mark Kelly (University of California at San Francisco). An initial HSQC of 143 μm Δ131Δ was acquired followed by HSQC after serial additions of Yos9(22–421). Data were processed with NMRPipe (28), and peak height analysis was performed with the ccpn analysis software. Δ131Δ assignments were transferred from a previously published study (29).
Dynamic Light Scattering (DLS)
DLS measurements were done on a DynaPro MS/X light scattering instrument (Wyatt Technology). The laser is a 55-milliwatt laser and 826.6 nm; detection angle is 90°. Samples were prepared in 10 mm Hepes, 100 mm NaCl, 1 mm CaCl2, 10 mm MgCl2, pH 7.4, and solutions were filtered immediately prior to mixing. The laser power was 100%. Data were analyzed with Dynamics Version 6 software. Histograms represent the average of three independent data sets (except for Yos9(22–421) plus bovine serum albumin (BSA) for which there was only a single measurement at the specified concentration) each with at least 10 measurements.
Aggregate Centrifugation Assay
Protein solutions were centrifuged for 15 min at 20,000 × g after which the supernatant was separated from the pellet. SDS-loading buffer was added to samples, and they were run on a 4–12% SDS-polyacrylamide gel in MES buffer followed by staining with Coomassie Blue R-250.
RESULTS
Truncated Variant of Yos9 Is Folded and Functional in Vivo
To assist in the biophysical characterization of Yos9, we sought to define a compact, stable domain that retained functionality in vivo. To this end, we carried out limited proteolysis of purified, recombinant Yos9. Using elastase, we identified a well defined 45-kDa fragment that by mass spectrometry corresponded to residues 22–421 (Fig. 1, A and B) and was similar to a truncation variant previously shown to be structured by circular dichroism (30). This fragment, Yos9(22–421), does not include the signal sequence or C-terminal residues that are part of a region predicted to be disordered by FoldIndex (31). Importantly, when integrated into the genome in place of the full-length protein, Yos9(22–421) facilitates CPY* degradation in a manner indistinguishable from full-length Yos9 (Fig. 1C). Thus, Yos9(22–421) represents a compact, folded domain that is sufficient to support Yos9-mediated degradation of a glycoprotein in vivo.
FIGURE 1.
Truncated variant of Yos9 is folded and functional. A, schematic of the Yos9 domain architecture. Dark gray, signal sequence (SS); black, mannose receptor homology (MRH) domain; light gray, truncated variant Yos9(22–421). B, limited proteolysis of Yos9 with elastase. C, degradation of CPY* after inhibition of translation with cycloheximide. Gray, wild type yeast; black, genomic Yos9(22–421). D, circular dichroism spectra of Yos9(22–421). E, SEC-MALS of Yos9(22–421) (top) and Yos9 (bottom). Black, molecular weight; gray, normalized refractive index.
In vitro, Yos9(22–421) also behaved similarly to the full-length protein. Following renaturation, recombinant Yos9(22–421) appeared folded by circular dichroism in agreement with previous studies (3, 30) indicating secondary structure similar to that of the full-length protein (Fig. 1D). In addition, Yos9(22–421) binds fluorescently labeled mannotriose with an affinity similar to that observed for Yos9 (supplemental Fig. S1). Currently, there is no consensus in the literature regarding the oligomeric state of Yos9 (3, 30). Previous work hypothesized that Yos9 exists as a trimer (3), but this was based on its volume of elution from a size exclusion column, which may have overestimated the molecular weight due to the presence of disordered regions. In another study, the crystal structure of a portion of Yos9 (Yos9(226–424)) showed that it could form a dimer (30). The authors (30) confirmed this interaction via solution measurements in vitro, but they could not detect the dimer or find evidence of its function in vivo. We determined the molecular weight of both full-length and Yos9(22–421) in solution using size exclusion chromatography multiangle light scattering (SEC-MALS), and we found that these weights were consistent with both species existing as monomers (Fig. 1E). Although the molecular weights were constant over a range of concentrations (8–116 μm), it is possible that a low affinity dimer could dissociate within the SEC column producing the observed results.
Yos9(22–421) Binds Specific Regions of CPY* in Vitro
To determine whether Yos9 specifically recognizes protein components of an ERAD substrate, we first identified binding sites for Yos9 along the sequence of CPY*. Using a peptide array created by tiling along the entire sequence of CPY*, we queried the binding of Yos9(22–421) to specific 18-amino acid segments of CPY* presented as linear epitopes (as they might be encountered in the context of an unfolded or misfolded protein) (Fig. 2A). We quantified the observed binding pattern by averaging Yos9 binding intensities for each amino acid along the sequence of CPY* and found that Yos9(22–421) exhibited a binding signature highlighting four regions of enhanced affinity (Fig. 2B). This signature also appears to be specific as Ire1, the luminal sensor for the unfolded protein response (UPR), shows a different binding signature with the same CPY* peptide array (24). Although none of the CPY* peptides were glycosylated, Yos9(22–421) bound to areas near the positions of the second, third, and fourth glycans as well as near the region that Ire1 binds most strongly (24). An analysis of the amino acid composition of the peptides bound suggested that Yos9(22–421) preferentially binds peptides rich in leucine, arginine, and lysine (supplemental Fig. S2). The predilection for basic residues may be informative but should be interpreted with caution because the isoelectric point of Yos9 is 4.79.
FIGURE 2.

Yos9(22–421) binds specific regions of CPY*. A, binding of Yos9(22–421) to a peptide array tiling along the sequence of CPY* and detected with an anti-His antibody. B, quantification of Yos9(22–421) binding to each amino acid of CPY* by averaging the intensities of peptide spots containing a given amino acid. ss, signal sequence.
Yos9 Binds a Model Misfolded Peptide and Protein in Solution
Although we wanted to verify the binding of peptides from the CPY* array in solution, many of the peptides attached to the array were unlikely to be soluble. Therefore, we turned to two well studied models of unfolded proteins, the peptide ΔEspP and the protein Δ131Δ, which are both soluble despite the fact that one is a peptide with hydrophobic character and the other exists in a predominantly unfolded state.
ΔEspP is a 24-amino acid peptide derived from a signal sequence that is “moderately hydrophobic but unusually basic” (32). The solubility imparted by the basic residues has made it useful for studying binding by both the signal recognition particle (32) and the UPR sensor Ire1 (24). We measured the affinity of Yos9 for ΔEspP modified with the fluorescein derivative FAM using fluorescence anisotropy. Yos9 bound ΔEspP-FAM with an affinity of Kd = 0.94 ± 0.26 μm when fit to a noncooperative binding curve (Fig. 3A). The truncated version Yos9(22–421) also bound ΔEspP-FAM (Kd = 2.27 ± 0.58 μm). The affinity of Yos9 for ΔEspP-FAM was greater than that of BSA for the peptide (Kd = 3.53 ± 1.25 μm), and the latter interaction was weakened substantially by the addition of detergent indicating that it was likely due to nonspecific interactions.
FIGURE 3.

Yos9 binds ΔEspP and Δ131Δ in vitro. A, fluorescence anisotropy of 50 nm ΔEspP-FAM binding to Yos9. Kd is 0.94 ± 0.26 μm. B, fluorescence anisotropy of Yos9 titrated into 1.25 μm IAEDANS-labeled Δ131Δ. Kd is 38.8 ± 6.7 μm.
To determine whether ΔEspP bound Yos9 at a single saturable site, we attempted to compete fluorescently labeled ΔEspP-FAM off of Yos9 with unlabeled ΔEspP. When preincubating either 1 or 10 μm Yos9 with 50 nm ΔEspP-FAM and titrating ΔEspP up to 250 μm, we did not observe the decrease in anisotropy expected if ΔEspP displaced ΔEspP-FAM on Yos9 (addressed further below). Therefore, we turned to another model misfolded substrate, Δ131Δ, to determine whether Yos9 contains a unique site for substrate binding and whether it binds a specific portion of the substrate.
Δ131Δ is a 131-residue fragment of staphylococcal nuclease (149 residues in full length) that is globally unfolded but compact (27, 29, 33). Its residual structure has been extensively characterized by NMR spectroscopy, and due to its well dispersed HSQC spectrum, one can readily map regions of Δ131Δ that interact with binding partners. This was recently illustrated with the chaperone Hsp90, which recognizes Δ131Δ via a single region (26). We first determined whether Yos9 bound Δ131Δ using fluorescence anisotropy. Full-length Yos9 bound Δ131Δ modified with the fluorophore IAEDANS with an apparent affinity of Kd = 38.8 ± 6.7 μm (Fig. 3B).
We next attempted to identify where Yos9 bound along the sequence of Δ131Δ. Using NMR, we acquired an HSQC titration series of Δ131Δ with increasing concentrations of Yos9(22–421) (Fig. 4A). As we added Yos9(22–421), peaks on the Δ131Δ spectrum uniformly lost intensity. Using 41 unambiguously assigned peaks, we calculated peak heights associated with Δ131Δ residues as increasing amounts of Yos9(22–421) were added (Fig. 4B). The simultaneous loss of peak intensity along the entire sequence of Δ131Δ is consistent with either multiple nonspecific contacts between Yos9(22–421) and Δ131Δ or increasing aggregation as they approached equimolar concentrations.
FIGURE 4.
Yos9 forms aggregates in the presence of binding substrate. A, HSQC spectra of 15N-Δ131Δ with the addition of increasing amounts of Yos9(22–421). Δ131Δ:Yos9(22–421) molar ratios are as follows: gray, 1:0; navy, 1:0.26; purple, 1:0.53; red, 1:0.78. B, intensity of Δ131Δ peak height for each of the 41 assigned peaks after addition of Yos9(22–421). Peak height was calculated as a fraction of that for Δ131Δ alone. C and D, DLS-derived values for the average hydrodynamic radius of the given species. E, samples of Yos9(22–421) and Δ131Δ alone or mixed were centrifuged and split into pellet (P) and supernatant (S) fractions.
Yos9 Forms Aggregates in the Presence of Binding Substrate
To directly assess whether Yos9 formed aggregates with Δ131Δ, we used DLS, which can be used to derive the hydrodynamic radius of a species in solution. DLS measurements showed that Yos9, Yos9(22–421), and Δ131Δ did not aggregate when alone in solution (Fig. 4C), but when mixed at or above concentrations of 1 μm they reproducibly formed large aggregates (Fig. 4D). The aggregation is highly cooperative, as we did not observe oligomers of intermediate sizes with a range of Yos9 and Δ131Δ concentrations (supplemental Fig. S3). Yos9 also aggregated in the presence of ΔEspP, which may explain the inability of ΔEspP to compete ΔEspP-FAM off of Yos9 in the anisotropy experiments. Of note, in some batches of ΔEspP, it formed oligomers on its own, and DLS experiments with ΔEspP should be interpreted with caution. Importantly, Yos9 did not form aggregates in the presence of BSA (Fig. 4D).
Although it appeared that Yos9 selectively aggregated in the presence of binding substrates, we were not able to identify conditions that attenuated this process; changes in solution conditions such as salt, pH, magnesium (which stabilizes Δ131Δ), and calcium (which stabilizes some ER-resident proteins) did not impact aggregation. We also used available structural data for a portion of Yos9 to probe the effect of targeted mutations on aggregation. The crystal structure of Yos9(226–424) shows it arranged as a dimer in the asymmetric unit in which each monomer contains a hydrophobic groove that could potentially accommodate an extended polypeptide (30). We reasoned that either interactions with itself through the dimer interface or with substrate through the putative binding groove could drive aggregation. We recombinantly expressed two Yos9 variants with mutations in conserved residues in either the dimer interface (F383A/L393D) or the hydrophobic groove (Y318A/L329A/I377A), and we found that both aggregated in a manner indistinguishable from wild type Yos9 (supplemental Fig. S4).
In an independent measure of aggregation propensity, we assessed the degree with which Yos9 and Δ131Δ form aggregates that can be cleared by centrifugation. Alone in solution, Yos9(22–421) and Δ131Δ stay in the supernatant when centrifuged, but upon mixing, some portion of each comes down in the pellet (Fig. 4E). This was observed at concentrations as low as 1 μm. Of note, although the aggregates were composed of both proteins in all cases, Δ131Δ was more abundant than Yos9 in the pelleted fraction.
DISCUSSION
Yos9, an essential lectin in glycoprotein ERAD, binds glycans specifically found on terminally misfolded proteins in the ER. Although this role has been characterized in a number of studies (3, 9–11), Yos9 also engages the protein component of misfolded proteins, whose unfolded character is necessary to affect ERAD (9, 13). Here, we explore how Yos9 recognizes this protein determinant and the potential consequences of this interaction. To facilitate biophysical characterization of Yos9, we defined a truncated variant that is compact, ordered, and fully functional in the degradation of CPY*. Removal of the disordered C terminus via limited proteolysis gave a remarkably well defined fragment, Yos9(21–422), that contains both portions of Yos9 for which there are known structures as follows: Yos9(226–424) (30) and the MRH domain of the human homolog OS-9 (12), which corresponds approximately to Yos9 residues 117–239. The truncated variant characterized here may facilitate further structural studies that address all functions of Yos9 without the potential issues incurred by the conformational flexibility of the C terminus.
Our studies show that Yos9 exhibits sequence specificity when recognizing polypeptides. Binding of Yos9(21–422) to an array of CPY*-derived peptides presented as linear epitopes revealed four regions of CPY* preferentially bound by Yos9. The existence of multiple binding sites is consistent with the previous finding that local perturbations near any of the four glycans on CPY* allow the glycan proximal to the defect to signal degradation via ERAD (6). These perturbations may function to simply unfold CPY* sufficiently to expose linear polypeptides that are bound by Yos9 while it surveys the identity of the adjacent glycan. Because protein-glycan interactions are generally weak and involve multiple contacts to increase the avidity of binding (34), Yos9 may use unfolded protein determinants as handles with which to facilitate the interrogation of glycan structures.
Yos9(22–421) preferentially binds sequences rich in hydrophobic and basic amino acids. Although unfolded proteins are often identified via exposed hydrophobic regions that are normally buried within the core of folded proteins, the role of basic residues may not be as readily apparent. Yet, the ER Hsp70 chaperone BiP (Kar2 in yeast) is known to bind regions rich in both basic and hydrophobic residues (22, 23, 35–37). Although work with a number of CPY/CPY* fusions has shown that Yos9 and BiP have different binding specificities (19), Kar2 has been shown to recognize regions of CPY* involved in ERAD (6). Thus, Yos9 and BiP/Kar2 may broadly share some of the same binding principles, which is consistent with studies showing that Yos9 retains misfolded proteins in the ER in addition to its role as a lectin (19, 20).
Solution studies complementary to those with the peptide array revealed an unexpected characteristic of Yos9. Binding of Yos9 to a peptide, ΔEspP, or a longer unfolded protein, Δ131Δ, resulted in the cooperative formation of large scale aggregates.
Although aggregation complicates the interpretation of the binding data, it is an interesting phenomenon with potential functional significance. Multiple studies indicate that the oligomerization of the Hrd1 complex may play a role in the regulation of ERAD. Endogenous Hrd1 forms high molecular weight complexes whose formation is regulated by the transmembrane protein Usa1 (38, 39). This oligomerization is required for ERAD of soluble glycoproteins in yeast (40) and appears to be conserved in the mammalian system in which the protein Herp binds HRD1 oligomers (41). Although it remains unclear exactly how Hrd1 oligomerization regulates ERAD activity, it is possible that Yos9 may modulate this process when bound to substrate. The interaction of Yos9 with Hrd3 (13, 16, 38), which interacts with and is essential for the stability of Hrd1 (17, 18), may facilitate transfer of information from Yos9 to the Hrd1 complex.
Oligomerization also plays a central role in the regulation of another key pathway involved in responding to misfolded proteins, the UPR. Ire1, the primary sensor in the yeast UPR, is a transmembrane protein whose oligomerization in response to unfolded proteins helps activate downstream signaling pathways central to the UPR (42–45). Recently, it was shown that direct binding of peptides in vitro induces Ire1 oligomerization through a specific interface that may become exposed as a result of a substrate-induced conformational change (24). Yos9 could employ a similar mechanism to facilitate downstream signaling to the Hrd1 complex.
In summary, Yos9 exhibits striking aggregation exclusively in the presence of its binding substrates. The physiological consequences of this process and how it may be modulated by other ERAD components such as Hrd1 and Hrd3 remain to be seen, but the structural characterization of a compact, stable Yos9 variant, such as Yos9(22–421), is a potential starting place to elucidate its physiological roles.
Supplementary Material
Acknowledgments
We thank D. King for performing the mass spectrometry on the Yos9 fragment; M. Kelly for NMR assistance; T. Street, D. Agard, B. Gardner, and P. Walter for reagents and advice; E. Q. Toyama for helpful discussions; and Y. C. Liu, E. Costa, and J. Dunn for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant U01 GM098254 (to J. S. W.).

This article contains supplemental Figs. S1–S4.
- ER
- endoplasmic reticulum
- ERAD
- ER-associated degradation
- CPY
- carboxypeptidase Y
- CPY*
- carboxypeptidase Y G255R
- MRH
- mannose 6-phosphate receptor homology
- SEC
- size exclusion chromatography
- MALS
- multiangle light scattering
- FAM
- fluorescein amidite
- IAEDANS
- 5-((((2-iodoacetyl)amino)ethyl)amino)naphthalene-1-sulfonic acid
- DLS
- dynamic light scattering
- UPR
- unfolded protein response
- HSQC
- heteronuclear single quantum coherence.
REFERENCES
- 1. Chiti F., Dobson C. M. (2006) Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 [DOI] [PubMed] [Google Scholar]
- 2. Younger J. M., Chen L., Ren H.-Y., Rosser M. F., Turnbull E. L., Fan C.-Y., Patterson C., Cyr D. M. (2006) Sequential quality-control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell 126, 571–582 [DOI] [PubMed] [Google Scholar]
- 3. Quan E. M., Kamiya Y., Kamiya D., Denic V., Weibezahn J., Kato K., Weissman J. S. (2008) Defining the glycan destruction signal for endoplasmic reticulum-associated degradation. Mol. Cell 32, 870–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kostova Z., Wolf D. H. (2005) Importance of carbohydrate positioning in the recognition of mutated CPY for ER-associated degradation. J. Cell Sci. 118, 1485–1492 [DOI] [PubMed] [Google Scholar]
- 5. Spear E. D., Ng D. T. (2005) Single, context-specific glycans can target misfolded glycoproteins for ER-associated degradation. J. Cell Biol. 169, 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xie W., Kanehara K., Sayeed A., Ng D. T. (2009) Intrinsic conformational determinants signal protein misfolding to the Hrd1/Htm1 endoplasmic reticulum-associated degradation system. Mol. Biol. Cell 20, 3317–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finger A., Knop M., Wolf D. H. (1993) Analysis of two mutated vacuolar proteins reveals a degradation pathway in the endoplasmic reticulum or a related compartment of yeast. Eur. J. Biochem. 218, 565–574 [DOI] [PubMed] [Google Scholar]
- 8. Clerc S., Hirsch C., Oggier D. M., Deprez P., Jakob C., Sommer T., Aebi M. (2009) Htm1 protein generates the N-glycan signal for glycoprotein degradation in the endoplasmic reticulum. J. Cell Biol. 184, 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhamidipati A., Denic V., Quan E. M., Weissman J. S. (2005) Exploration of the topological requirements of ERAD identifies Yos9p as a lectin sensor of misfolded glycoproteins in the ER lumen. Mol. Cell 19, 741–751 [DOI] [PubMed] [Google Scholar]
- 10. Kim W., Spear E. D., Ng D. T. (2005) Yos9p detects and targets misfolded glycoproteins for ER-associated degradation. Mol. Cell 19, 753–764 [DOI] [PubMed] [Google Scholar]
- 11. Szathmary R., Bielmann R., Nita-Lazar M., Burda P., Jakob C. A. (2005) Yos9 protein is essential for degradation of misfolded glycoproteins and may function as lectin in ERAD. Mol. Cell 19, 765–775 [DOI] [PubMed] [Google Scholar]
- 12. Satoh T., Chen Y., Hu D., Hanashima S., Yamamoto K., Yamaguchi Y. (2010) Structural basis for oligosaccharide recognition of misfolded glycoproteins by OS-9 in ER-associated degradation. Mol. Cell 40, 905–916 [DOI] [PubMed] [Google Scholar]
- 13. Denic V., Quan E. M., Weissman J. S. (2006) A luminal surveillance complex that selects misfolded glycoproteins for ER-associated degradation. Cell 126, 349–359 [DOI] [PubMed] [Google Scholar]
- 14. Gauss R., Sommer T., Jarosch E. (2006) The Hrd1p ligase complex forms a linchpin between ER-lumenal substrate selection and Cdc48p recruitment. EMBO J. 25, 1827–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishikawa S. I., Fewell S. W., Kato Y., Brodsky J. L., Endo T. (2001) Molecular chaperones in the yeast endoplasmic reticulum maintain the solubility of proteins for retrotranslocation and degradation. J. Cell Biol. 153, 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gauss R., Jarosch E., Sommer T., Hirsch C. (2006) A complex of Yos9p and the HRD ligase integrates endoplasmic reticulum quality control into the degradation machinery. Nat. Cell Biol. 8, 849–854 [DOI] [PubMed] [Google Scholar]
- 17. Gardner R. G., Swarbrick G. M., Bays N. W., Cronin S. R., Wilhovsky S., Seelig L., Kim C., Hampton R. Y. (2000) Endoplasmic reticulum degradation requires lumen to cytosol signaling. Transmembrane control of Hrd1p by Hrd3p. J. Cell Biol. 151, 69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Plemper R. K., Bordallo J., Deak P. M., Taxis C., Hitt R., Wolf D. H. (1999) Genetic interactions of Hrd3p and Der3p/Hrd1p with Sec61p suggest a retro-translocation complex mediating protein transport for ER degradation. J. Cell Sci. 112, 4123–4134 [DOI] [PubMed] [Google Scholar]
- 19. Izawa T., Nagai H., Endo T., Nishikawa S. (2012) Yos9p and Hrd1p mediate ER retention of misfolded proteins for ER-associated degradation. Mol. Biol. Cell 23, 1283–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Benitez E. M., Stolz A., Wolf D. H. (2011) Yos9, a control protein for misfolded glycosylated and non-glycosylated proteins in ERAD. FEBS Lett. 585, 3015–3019 [DOI] [PubMed] [Google Scholar]
- 21. Jaenicke L. A., Brendebach H., Selbach M., Hirsch C. (2011) Yos9p assists in the degradation of certain nonglycosylated proteins from the endoplasmic reticulum. Mol. Biol. Cell 22, 2937–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flynn G. C., Chappell T. G., Rothman J. E. (1989) Peptide binding and release by proteins implicated as catalysts of protein assembly. Science 245, 385–390 [DOI] [PubMed] [Google Scholar]
- 23. Blond-Elguindi S., Cwirla S. E., Dower W. J., Lipshutz R. J., Sprang S. R., Sambrook J. F., Gething M. J. (1993) Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell 75, 717–728 [DOI] [PubMed] [Google Scholar]
- 24. Gardner B. M., Walter P. (2011) Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 333, 1891–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carter J. M., Loomis-Price L. (2004) B cell epitope mapping using synthetic peptides. Curr. Protoc. Immunol. 2004 May;Chapter 9:Unit 9.4; 10.1002/0471142735.im0904s60 [DOI] [PubMed] [Google Scholar]
- 26. Street T. O., Lavery L. A., Agard D. A. (2011) Substrate binding drives large-scale conformational changes in the Hsp90 molecular chaperone. Mol. Cell 42, 96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alexandrescu A. T., Abeygunawardana C., Shortle D. (1994) Structure and dynamics of a denatured 131-residue fragment of staphylococcal nuclease: a heteronuclear NMR study. Biochemistry 33, 1063–1072 [DOI] [PubMed] [Google Scholar]
- 28. Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 29. Alexandrescu A. T., Shortle D. (1994) Backbone dynamics of a highly disordered 131 residue fragment of staphylococcal nuclease. J. Mol. Biol. 242, 527–546 [DOI] [PubMed] [Google Scholar]
- 30. Hanna J., Schütz A., Zimmermann F., Behlke J., Sommer T., Heinemann U. (2012) Structural and biochemical basis of Yos9 protein dimerization and possible contribution to self-association of 3-hydroxy-3-methylglutaryl-coenzyme A reductase degradation ubiquitin-ligase complex. J. Biol. Chem. 287, 8633–8640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prilusky J., Felder C. E., Zeev-Ben-Mordehai T., Rydberg E. H., Man O., Beckmann J. S., Silman I., Sussman J. L. (2005) FoldIndex: a simple tool to predict whether a given protein sequence is intrinsically unfolded. Bioinformatics 21, 3435–3438 [DOI] [PubMed] [Google Scholar]
- 32. Peterson J. H., Woolhead C. A., Bernstein H. D. (2003) Basic amino acids in a distinct subset of signal peptides promote interaction with the signal recognition particle. J. Biol. Chem. 278, 46155–46162 [DOI] [PubMed] [Google Scholar]
- 33. Wang Y., Shortle D. (1995) The equilibrium folding pathway of staphylococcal nuclease: identification of the most stable chain-chain interactions by NMR and CD spectroscopy. Biochemistry 34, 15895–15905 [DOI] [PubMed] [Google Scholar]
- 34. Dam T. K., Brewer C. F. (2004) Multivalent protein-carbohydrate interactions: isothermal titration microcalorimetry studies. Methods Enzymol. 379, 107–128 [DOI] [PubMed] [Google Scholar]
- 35. Flynn G. C., Pohl J., Flocco M. T., Rothman J. E. (1991) Peptide-binding specificity of the molecular chaperone BiP. Nature 353, 726–730 [DOI] [PubMed] [Google Scholar]
- 36. de Crouy-Chanel A., Kohiyama M., Richarme G. (1996) Specificity of DnaK for arginine/lysine and effect of DnaJ on the amino acid specificity of DnaK. J. Biol. Chem. 271, 15486–15490 [DOI] [PubMed] [Google Scholar]
- 37. Rüdiger S., Germeroth L., Schneider-Mergener J., Bukau B. (1997) Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 16, 1501–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carvalho P., Goder V., Rapoport T. A. (2006) Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell 126, 361–373 [DOI] [PubMed] [Google Scholar]
- 39. Horn S. C., Hanna J., Hirsch C., Volkwein C., Schütz A., Heinemann U., Sommer T., Jarosch E. (2009) Usa1 functions as a scaffold of the HRD-ubiquitin ligase. Mol. Cell 36, 782–793 [DOI] [PubMed] [Google Scholar]
- 40. Carvalho P., Stanley A. M., Rapoport T. A. (2010) Retrotranslocation of a misfolded luminal ER protein by the ubiquitin-ligase Hrd1p. Cell 143, 579–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kny M., Standera S., Hartmann-Petersen R., Kloetzel P.-M., Seeger M. (2011) Herp regulates Hrd1-mediated ubiquitylation in a ubiquitin-like domain-dependent manner. J. Biol. Chem. 286, 5151–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shamu C. E., Walter P. (1996) Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 15, 3028–3039 [PMC free article] [PubMed] [Google Scholar]
- 43. Credle J. J., Finer-Moore J. S., Papa F. R., Stroud R. M., Walter P. (2005) On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 102, 18773–18784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Korennykh A. V., Egea P. F., Korostelev A. A., Finer-Moore J., Zhang C., Shokat K. M., Stroud R. M., Walter P. (2009) The unfolded protein response signals through high-order assembly of Ire1. Nature 457, 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li H., Korennykh A. V., Behrman S. L., Walter P. (2010) Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc. Natl. Acad. Sci. U.S.A. 107, 16113–16118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




