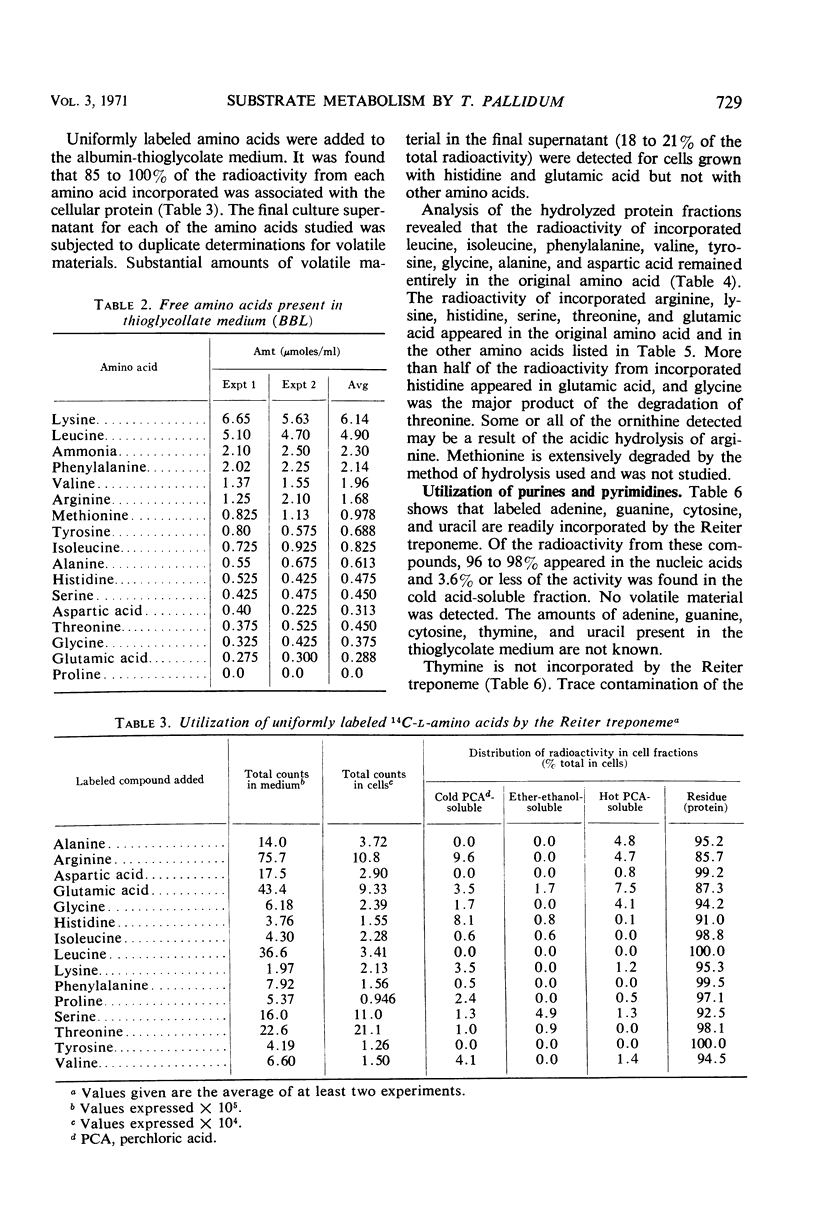
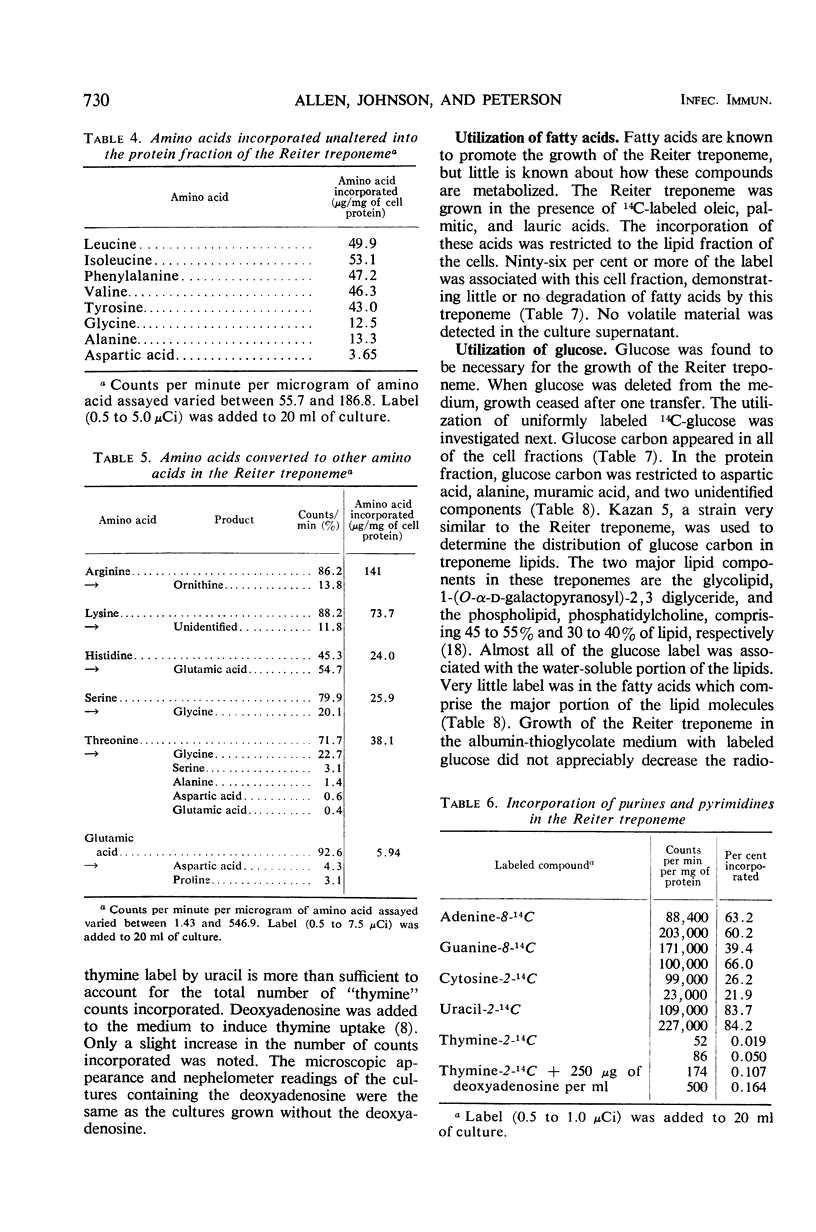
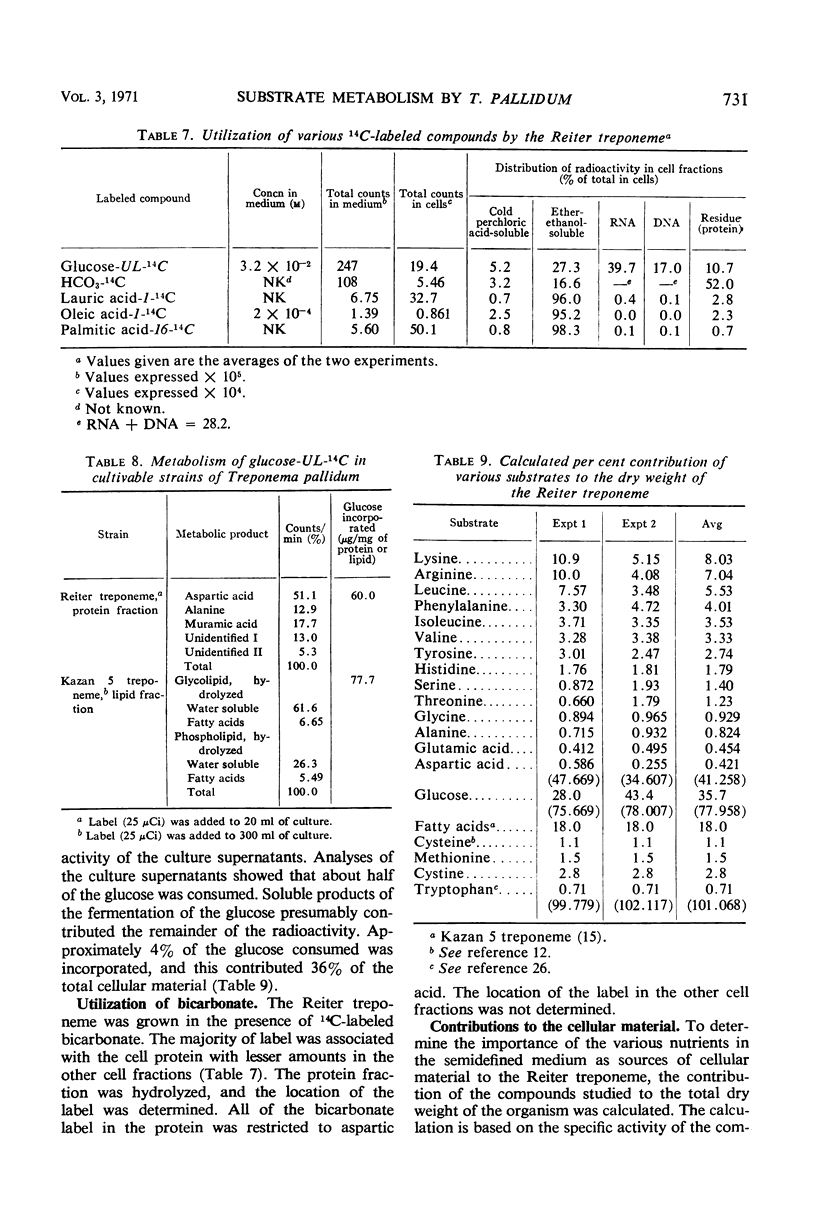
Abstract
The utilization of glucose, amino acids, fatty acids, bicarbonate, purines, and pyrimidines by the Reiter treponeme was studied by using carbon 14-labeled substrates. The distribution of carbon from the substrates into various cell components was determined. Radioactivity from labeled bicarbonate in the cellular protein was restricted to aspartic acid. The Reiter treponeme is capable of synthesizing glycine, serine, alanine, aspartic acid, glutamic acid, proline, and possibly ornithine. Phenylalanine, arginine, lysine, leucine, isoleucine, valine, threonine, and histidine do not appear to be synthesized by this treponeme. The Reiter treponeme cannot synthesize fatty acids, and thymine is not incorporated. Glucose is a major carbon and energy source. Arginine, histidine, serine, threonine, and glutamic acid are degraded by the Reiter treponeme and may serve as energy sources. It was calculated that exogenously supplied amino acids contribute 41 to 54% of the cellular material; fatty acids, 18%; and glucose, 28 to 43%.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBAN S. Studies on the metabolism of the Treponemata. I. Amino acid metabolism. J Bacteriol. 1954 Oct;68(4):493–497. doi: 10.1128/jb.68.4.493-497.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARBAN S. Studies on the metabolism of the treponemata. II. Transamination in the Reiter treponeme. J Bacteriol. 1956 Mar;71(3):274–277. doi: 10.1128/jb.71.3.274-277.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- Breznak J. A., Canale-Parola E. Spirochaeta aurantia, a pigmented, facultatively anaerobic spirochete. J Bacteriol. 1969 Jan;97(1):386–395. doi: 10.1128/jb.97.1.386-395.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle H., Steinman H. G. The Nutritional Requirements of Treponemata: I. Arginine, Acetic Acid, Sulfur-containing Compounds, and Serum Albumin as Essential Growth-promoting Factors for the Reiter Treponeme. J Bacteriol. 1948 Aug;56(2):163–176. [PMC free article] [PubMed] [Google Scholar]
- FULTON J. D., SMITH P. J. Carbohydrate metabolism in Spirochaeta recurrentis. 1. The metabolism of spirochaetes in vivo and in vitro. Biochem J. 1960 Sep;76:491–499. doi: 10.1042/bj0760491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULTON J. D., SPOONER D. F. The metabolism of leptospira icterohaemorrhagiae in vitro. Exp Parasitol. 1956 Mar;5(2):154–177. doi: 10.1016/0014-4894(56)90012-1. [DOI] [PubMed] [Google Scholar]
- HANCOCK R., McMANUS F. Carbon dioxide fixation in the synthesis of aspartic acid by a strain of Staphylococcus aureus. Biochim Biophys Acta. 1960 Jul 29;42:152–154. doi: 10.1016/0006-3002(60)90762-9. [DOI] [PubMed] [Google Scholar]
- Hespell R. B., Canale-Parola E. Carbohydrate metabolism in Spirochaeta stenostrepta. J Bacteriol. 1970 Jul;103(1):216–226. doi: 10.1128/jb.103.1.216-226.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. C., GARY N. D. NUTRITION OF LEPTOSPIRA POMONA. II. FATTY ACID REQUIREMENTS. J Bacteriol. 1963 May;85:976–982. doi: 10.1128/jb.85.5.976-982.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. C., ROGERS P. METABOLISM OF LEPTOSPIRAE. I. UTILIZATION OF AMINO ACIDS AND PURINE, AND PYRIMIDINE BASES. Arch Biochem Biophys. 1964 Sep;107:459–470. doi: 10.1016/0003-9861(64)90302-9. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Livermore B. P., Jenkin H. M., Eggebraten L. Lipids of Treponema pallidum Kazan 5. Infect Immun. 1970 Nov;2(5):606–609. doi: 10.1128/iai.2.5.606-609.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Livermore B. P., Walby J. K., Jenkin H. M. Lipids of parasitic and saprophytic leptospires. Infect Immun. 1970 Sep;2(3):286–291. doi: 10.1128/iai.2.3.286-291.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Rogers P. Metabolism of leptospires. II. The action of 8-azaguanine. Can J Microbiol. 1967 Dec;13(12):1621–1629. doi: 10.1139/m67-212. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Livermore B. P., Johnson R. C. Isolation and characterization of a glycolipid from Treponema pallidum, Kazan 5. Biochim Biophys Acta. 1970 Jul 14;210(2):315–318. doi: 10.1016/0005-2760(70)90176-1. [DOI] [PubMed] [Google Scholar]
- OYAMA V. I., STEINMAN H. G., EAGLE H. The nutritional requirements of treponemata. V. A detoxified lipide as the essential growth factor supplied by crystallized serum albumin. J Bacteriol. 1953 May;65(5):609–616. doi: 10.1128/jb.65.5.609-616.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINMAN H. G., EAGLE H. Nutritional requirements of Treponemata. II. Pantothenic acid, glutamine, and phenylalanine as additional growth-promoting factors for the Reiter treponeme. J Bacteriol. 1950 Jul;60(1):57–68. doi: 10.1128/jb.60.1.57-68.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINMAN H. G., EAGLE H., OYAMA V. I. Nutritional requirements of Treponemata. IV. The total nitrogen requirements of the Reiter Treponeme. J Biol Chem. 1953 Feb;200(2):775–785. [PubMed] [Google Scholar]
- STEINMAN H. G., EAGLE H., OYAMA V. I. The nutritional requirements of Treponemata. III. A defined medium for cultivation of the Reiter treponeme. J Bacteriol. 1952 Aug;64(2):265–269. doi: 10.1128/jb.64.2.265-269.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S., Listgarten M., Hubersak C., Cotmore J., Clark A. Morphological and biochemical differentiation of three types of small oral spirochetes. J Bacteriol. 1969 Jun;98(3):878–882. doi: 10.1128/jb.98.3.878-882.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUBER H., CANNEFAX G. R., HANSON A. W., RUSSELL H. Enzymes of treponemes. A comparative study. Exp Med Surg. 1962;20:324–328. [PubMed] [Google Scholar]
- VELDKAMP H. Isolation and characteristics of Treponema zuelzerae nov. spec., and anaerobic, free-living spirochete. Antonie Van Leeuwenhoek. 1960;26:103–125. doi: 10.1007/BF02538999. [DOI] [PubMed] [Google Scholar]
- Victor R., Lachica F., Hartman P. A. Carbon dioxide fixation and the synthesis of aspartic acid by S. faecium var. Durans. Biochem Biophys Res Commun. 1968 Aug 21;32(4):691–695. doi: 10.1016/0006-291x(68)90294-5. [DOI] [PubMed] [Google Scholar]



