Abstract
Objective:
To analyze the potential impact of aspirin therapy for long-term secondary prevention after stroke of undetermined etiology in resource-limited settings without access to neuroimaging to distinguish ischemic stroke from intracerebral hemorrhage (ICH).
Methods:
We conducted a decision analysis using a Markov state transition model. Sensitivity analyses were performed across the worldwide reported range of the proportion of strokes due to ICH and the 95% confidence intervals (CIs) of aspirin-associated relative risks in patients with ICH.
Results:
For patients with stroke of undetermined etiology, long-term aspirin was the preferred treatment strategy across the worldwide reported range of the proportion of strokes due to ICH. At 34% of strokes due to ICH (the highest proportion reported in a large epidemiologic study), the benefit of aspirin remained beyond the upper bounds of the 95% CIs of aspirin-associated post-ICH relative risks most concerning to clinicians (ICH recurrence risk and mortality risk if ICH recurs on aspirin). Based on the estimated 11,590,204 strokes in low- and middle-income countries in 2010, our model predicts that aspirin therapy for secondary stroke prevention in all patients with stroke in these countries could lead to an estimated yearly decrease of 84,492 recurrent strokes and 4,056 stroke-related mortalities.
Conclusions:
The concern that the risks of aspirin in patients with stroke of unknown etiology could outweigh the benefits is not supported by our model, which predicts that aspirin for secondary prevention in patients with stroke of undetermined etiology in resource-limited settings could lead to decreased stroke-related mortality and stroke recurrence.
In 2010, 63% of ischemic strokes and 80% of hemorrhagic strokes occurred in low- and middle-income countries (LMIC), leading to 57% of worldwide deaths and 64% of worldwide disability-adjusted life-years (DALYs) due to ischemic stroke (IS) and 84% of worldwide deaths and 86% of worldwide DALYs due to intracerebral hemorrhage (ICH).1,2 Age-specific mortality rates, mortality-to-incidence ratios, and DALYs for both IS and ICH are all higher in LMIC than in high-income countries.2
This disproportionate stroke burden in LMIC is due to limitations in resources for all aspects of stroke care, including prevention, treatment, and rehabilitation.3 In conjunction with risk factor modification, aspirin is an inexpensive and effective medication for secondary stroke prevention4–6; however, only 3.8% of patients with prior stroke in low-income countries take antiplatelet agents, compared to 53.1% in high-income countries.7 Lower rates of aspirin use may contribute to the burden of potentially preventable stroke-related disability and mortality in high-incidence low-resource settings.
One of us (A.L.B.) has worked in such settings, where colleagues have described that one reason aspirin is used less is that without access to CT to distinguish IS from ICH, clinicians must balance presumed risks of aspirin administration in patients with potential ICH against potential benefits of secondary prevention in patients with possible IS. Unfortunately, clinical predictors to distinguish IS from ICH are not sufficiently reliable to guide clinical decision-making.8 In order to assist clinicians practicing in resource-limited settings, we conducted a decision analysis to determine the impact of administering aspirin as long-term secondary preventive therapy to all patients after stroke when CT is not available to distinguish IS from ICH.
METHODS
Model structure.
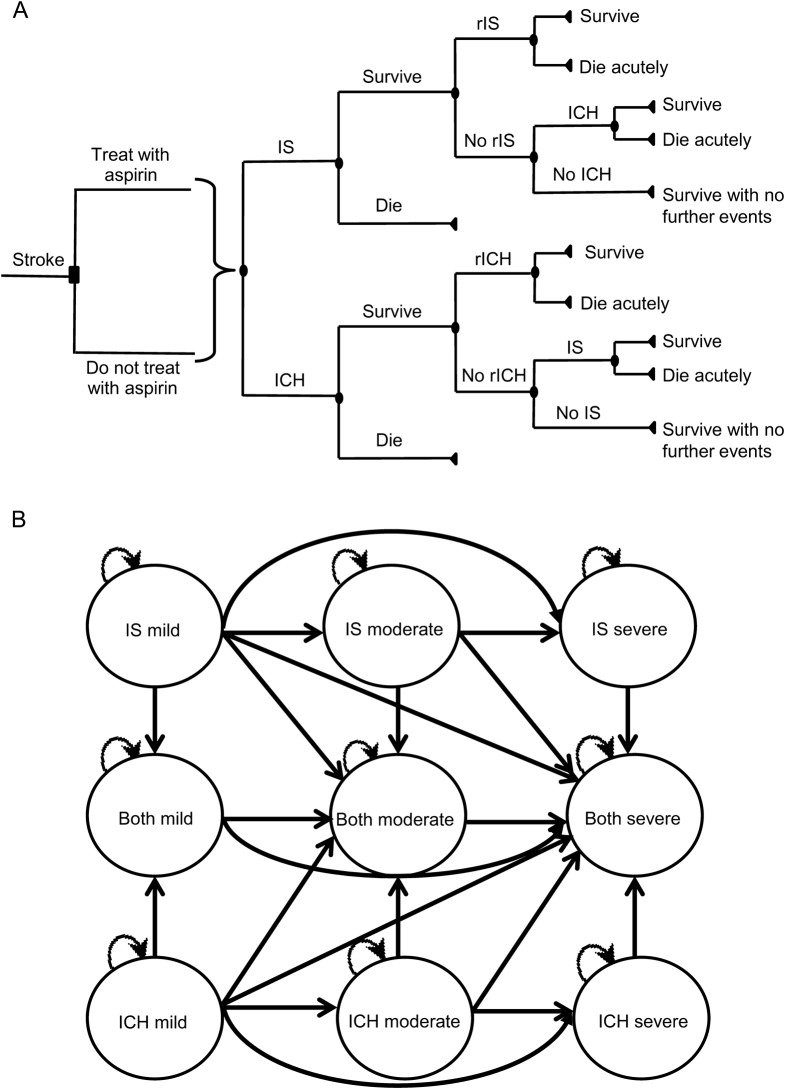
We constructed a Markov state transition model to evaluate the potential outcomes of 2 strategies for long-term secondary prevention after stroke of undetermined etiology: administering aspirin to all patients vs not administering aspirin to any patients. We conducted our decision analysis using TreeAge software (Williamstown, MA). In each yearly cycle, patients could have an IS or ICH, survive without further stroke, or die without further stroke (figure 1A). All patients entered the first cycle after a single stroke of unknown etiology with resultant mild, moderate, or severe disability. After an IS or ICH during a yearly cycle, patients could stay at the same level of disability, progress to a worse state of disability, or die acutely from the event, but they could never return to a lesser state of disability (figure 1B). For example, if a patient with moderate disability from an initial stroke had another stroke and survived, the disability state of the patient could either remain at moderate or worsen to severe, depending on the severity of the second stroke. If patients with prior IS had ICH or patients with prior ICH had IS, patients entered a “both” state in which they stayed until death. As with patients in IS or ICH states, in each yearly cycle, patients in “both” states could die without further events, survive without further events, or incur IS or ICH (after which they could stay at the same level of disability, progress to a worse state of disability, or die acutely from the event, but they could never return to a lesser state of disability). Patients in “both” states carried the yearly risk of IS of patients with prior IS and the yearly risk of ICH of patients with prior ICH. There were therefore 10 possible health states: IS (with mild, moderate, or severe disability), ICH (with mild, moderate, or severe disability), both IS and ICH (with mild, moderate, or severe disability), and dead (figure 1B).
Figure 1. Model structure.
(A) Schematic of decision tree. For clarity, levels of disability are not shown. (B) State diagram showing possible transitions between states. For clarity, the possible transition to death from each of the 9 states is not shown. ICH = intracerebral hemorrhage; IS = ischemic stroke; rICH = recurrent ICH; rIS = recurrent ischemic stroke.
Background assumptions.
As in our previous decision analysis,9 we made the following background assumptions to reflect the scenario facing clinicians in low-income countries: neuroimaging is not available to distinguish between IS and ICH; thrombolysis with IV tissue plasminogen activator (tPA) is not available (or cannot be administered without CT to determine whether radiologic contraindications to thrombolysis are present)10,11; aspirin is the only antiplatelet agent available (given the expense of clopidogrel and dipyridamole); and anticoagulation is not feasible (given lack of access to monitoring of prothrombin time and partial thromboplastin time in resource-limited settings). We assumed that patients presenting with stroke had either IS or ICH but not subarachnoid hemorrhage (SAH) because the incidence of SAH is far less than that of IS or ICH (accounting for 7% of strokes in low-income countries),12 SAH generally presents with a clinical syndrome distinct from IS or ICH, and SAH would be expected to be almost uniformly fatal in resource-limited settings without timely access to advanced neurocritical care. Our model also assumed that patients being considered for long-term secondary prevention with aspirin had survived for at least 1 month following their initial stroke, because most data on the effects of aspirin on long-term outcomes after ICH describe patients who survived beyond the first month after ICH.13,14
Data.
Data used in the decision analysis are summarized in table 1.
Table 1.
Data used in the model
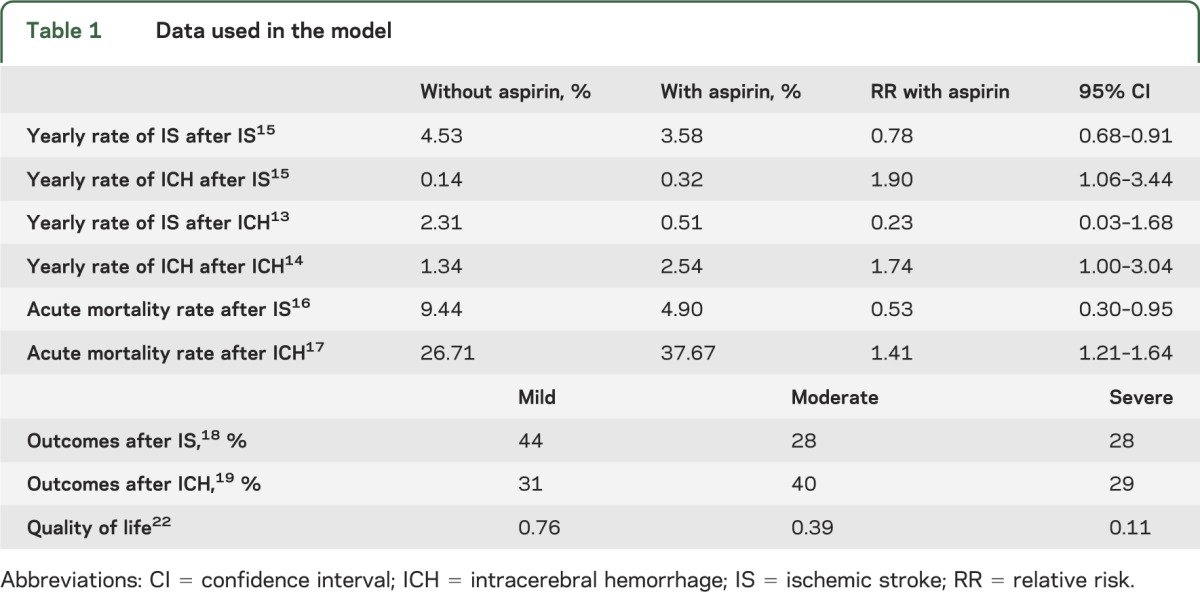
Risks of secondary events after initial IS and ICH and aspirin's effects on these risks.
The yearly rates of recurrent IS and post-IS ICH after initial IS and aspirin's effects on the risks of these outcomes were obtained from the largest meta-analysis of aspirin secondary stroke prevention trials.15 The yearly rates of recurrent ICH and post-ICH IS after initial ICH and aspirin's effects on the risk of these outcomes come from the largest studies providing data on the effects of aspirin on the risks of IS after ICH13 and recurrent ICH14 in patients who survived beyond the first month after ICH. Aspirin has also been demonstrated to decrease mortality in patients who are taking it at the time of IS16 and to increase mortality in patients taking it at the time of ICH,17 and both of these factors were incorporated into our model (table 1).
Outcomes after IS and ICH.
The distribution of outcomes after IS (mild, moderate, or severe disability) comes from the placebo group in a meta-analysis of IV tPA trials,18 and the distribution of outcomes after ICH comes from a recent trial of blood pressure control in acute ICH.19 Modified Rankin Scale (mRS) score of 0–1 was considered mild disability, mRS 2–3 moderate disability, and mRS 4–5 severe disability. Quality of life adjustment factors (Q) for mild, moderate, and severe disability due to stroke were the same as those used in other stroke-related decision analyses,20,21 taken from a time tradeoff analysis of the effect of stroke on quality of life.22 We assumed a fixed level of disability for each health state until death. As has been noted in prior stroke-related decision analyses, this may underestimate early disability and overestimate later disability but nevertheless yields a plausible estimation of disability over the lifespan.20 Yearly rates of death without further stroke event are taken from standard life tables23 with the addition of 0.08 excess annual mortality due to stroke, as in prior stroke-related decision analyses.20
Epidemiology of IS and ICH.
In a study of stroke risk factors in 3,000 patients in 22 countries, the percentage of first strokes caused by ICH ranged from 9% in high-income counties to as high as 34% in parts of Africa.24 The latter figure correlates closely with the proportion of strokes due to ICH in low-income countries estimated in the 2010 Global Burden of Disease study (35%).2 Although the percentage of strokes due to ICH in sub-Saharan Africa has been reported to be as high as 60% in smaller studies,25–27 ICH may be overrepresented in these small series due to the severity of illness required for presentation to referral centers with the capacity to perform brain imaging and conduct such studies.3
Analyses.
Our base case was a hypothetical 69-year-old patient with stroke of undetermined etiology in an LMIC, since 69 was the average age of incident stroke in LMIC in 2010.1 We used 34% as the proportion of strokes due to ICH in the base case analysis, because this is the highest proportional incidence of ICH reported in a large epidemiologic study.24 We conducted sensitivity analyses across the worldwide range of reported proportions of stroke caused by ICH (9%–60%) and on all aspirin-associated relative risks (RRs) across their 95% confidence intervals (CIs) to assess the threshold values below which aspirin was the preferred long-term treatment strategy. In order to assess the impact of simultaneous variation of 2 risk parameters, we conducted 2-way sensitivity analyses varying both aspirin-associated post-ICH RRs and proportion of first strokes caused by ICH. Outcomes were expressed in quality-adjusted life-years (QALYs).
RESULTS
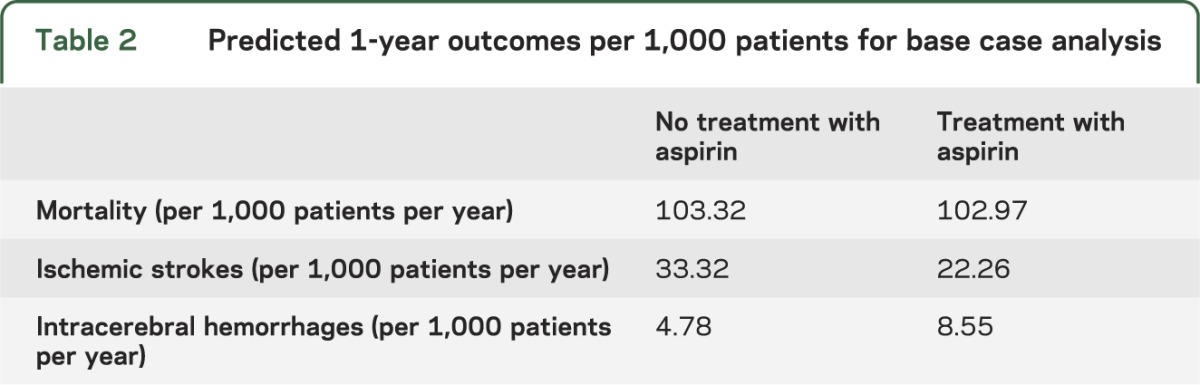
Aspirin treatment for long-term secondary prevention after stroke of undetermined etiology was the preferred strategy across the entire reported range of the proportion of strokes due to ICH worldwide (9%–60%) (figure 2A). For the base case (a hypothetical 69-year-old with prior stroke of unknown etiology in an LMIC where ICH causes 34% of strokes), aspirin yielded 3.38 QALYs, whereas no treatment resulted in 3.32 QALYs. In addition, in the base case analysis, aspirin is predicted to prevent approximately 11 IS per 1,000 patients per year at a cost of approximately 4 more ICH per 1,000 patients per year (resulting in a net decrease in 7 strokes per 1,000 patients per year). Aspirin is also predicted to decrease yearly mortality from 103.32 deaths per 1,000 patients per year to 102.97 per 1,000 (table 2). Applying these figures to the estimated 11,590,204 strokes in LMIC in 2010,1 treating patients with aspirin for secondary prevention after stroke of unknown etiology would be predicted to lead to an estimated yearly decrease of 84,492 recurrent strokes and 4,056 stroke-related mortalities compared to no aspirin therapy.
Figure 2. One- and 2-way sensitivity analyses.
In all 1-way sensitivity analyses (A–D), the x-axis displays the parameter for which sensitivity analysis is being performed and the y-axis represents quality-adjusted life-years (QALYs). In all 2-way sensitivity analyses (E and F), the x-axis represents the proportion of initial strokes due to intracerebral hemorrhage (ICH) and the y-axis represents a relative risk (RR) associated with aspirin in patients with prior ICH. In 1-way sensitivity analyses, the threshold at which the preferred strategy changes is marked by a vertical dashed line; aspirin treatment is preferred to no treatment to the left of this threshold value. In 2-way sensitivity analyses, the region favoring aspirin treatment is shaded blue and the region favoring no treatment is shaded red. In A and B, the base case proportion of strokes due to ICH (34%) is denoted by an arrow. In C and D, the base case value for the RR is denoted by a closed circle and its 95% confidence interval (CI) by a horizontal line. In E and F, the intersection of the base case value for proportion of initial strokes due to ICH and the RR is indicated by an asterisk, and dashed lines denote the 95% CIs of the RRs. (A) One-way sensitivity analysis of the proportion of initial strokes due to ICH. (B) One-way sensitivity analysis of age at time of first stroke. (C) One-way sensitivity analysis of the RR of recurrent ICH associated with aspirin after initial ICH. (D) One-way sensitivity analysis of the RR of acute mortality associated with aspirin if a patient has an ICH while on aspirin. (E) Two-way sensitivity analysis of the RR of recurrent ICH associated with aspirin and proportion of initial strokes due to ICH. (F) Two-way sensitivity analysis of the RR of acute mortality associated with aspirin if a patient has an ICH while on aspirin and proportion of initial strokes due to ICH.
Table 2.
Predicted 1-year outcomes per 1,000 patients for base case analysis
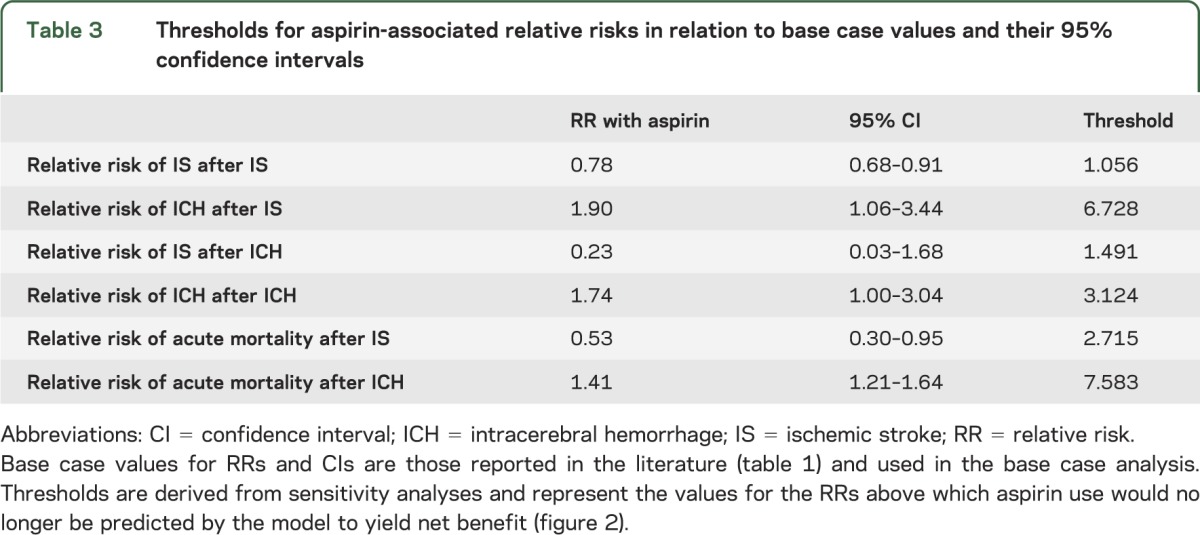
The predicted benefit of aspirin was preserved across the lifespan from age 20 to age 99, with greater benefit at younger ages (figure 2B). In sensitivity analyses for all aspirin-related RRs across their 95% CIs, the benefit of aspirin was maintained up to thresholds beyond the upper bound of the 95% CIs for all aspirin-associated RRs with the exception of one: the RR of subsequent IS after initial ICH (table 3). However, the threshold value of 1.49 for this RR indicates that aspirin would actually have to increase the risk of IS after initial ICH by nearly 50% for no treatment to be favored, which seems clinically implausible.
Table 3.
Thresholds for aspirin-associated relative risks in relation to base case values and their 95% confidence intervals
Sensitivity analyses for the 2 aspirin-associated RRs after ICH of most concern to clinicians caring for patients with strokes of unknown type are shown in figure 2C (RR of recurrent ICH after ICH) and figure 2D (RR of acute mortality if a patient were to be on aspirin at the time of subsequent ICH). Notably, the RR of recurrent ICH due to aspirin after initial ICH would have to be 1.8-fold higher than the reported RR to favor not treating with aspirin, and the RR of acute mortality if a patient were to be on aspirin at the time of subsequent ICH would have to be 5.4-fold higher than the reported RR to favor no aspirin treatment. These results suggest that aspirin treatment for long-term secondary stroke prevention after stroke of unknown etiology is favored across a broad range of clinically plausible parameter estimates.
Two-way sensitivity analyses demonstrate that aspirin treatment is preferred across the entire worldwide range of the proportion of strokes caused by ICH up to post-ICH aspirin-associated RRs beyond the base case values drawn from the literature (figure 2, E and F).
DISCUSSION
As of 2004, there were only 65 CT scanners in all of sub-Saharan Africa (excluding South Africa),28 representative of access to CT in many low-income regions. Guidelines for stroke care in high-income countries are contingent upon the availability of CT and therefore are not applicable to resource-limited settings without access to neuroimaging. Although the augmentation of clinical and technological resources for diagnostic and treatment capacity in neurology in resource-limited settings is critical, in the interim, clinicians on the front lines need context-sensitive clinical guidelines. Where guidelines and evidence are unavailable or inapplicable,29,30 decision analysis models can provide useful estimates of relative risks and benefits of treatment strategies.20,31
Clinicians in resource-limited settings face a challenging dilemma in the care of patients with stroke: do the potential benefits of secondary IS prevention with aspirin outweigh the potential risks of aspirin in patients with possible ICH when stroke etiology cannot be determined? Risk aversion regarding administration of potentially beneficial treatments with risk of high-valence adverse effects is a common phenomenon. One commonly cited example is that of anticoagulation in elderly patients with atrial fibrillation at elevated fall risk, given concern for intracranial hemorrhage.21,32 However, decision analysis in that setting suggests that the risk of recurrent IS far outweighs fall-related intracranial hemorrhage risk, favoring anticoagulation for stroke prevention in spite of fall risk.21 Similarly, the assumption that the potential long-term risks of aspirin could outweigh the potential long-term benefits in patients with stroke of unknown etiology is not supported by our model, which predicts that long-term aspirin therapy could lead to overall benefits in QALYs, yearly mortality, and yearly risk of further stroke after stroke of undetermined etiology. This predicted benefit is robust across the worldwide range of the proportion of strokes due to ICH up to values higher than those reported in the literature for potentially concerning RRs associated with aspirin administration after ICH.
Our study has several limitations related to the data utilized in the model. First, these data were collected predominantly in high-income countries and therefore may not necessarily reflect outcomes in low-income regions where cerebrovascular neuroepidemiology and effects of antiplatelets on outcomes are less well understood and where stroke therapies such as statins, anticoagulation, tPA, carotid endarterectomy, neurocritical care, and neurorehabilitation may be inaccessible to many or all patients. Given that more detailed epidemiologic data on cerebrovascular disease in LMIC are not currently available, we used the best available data and accounted for inherent limitations in these data through sensitivity analyses. Second, while much of the data used in the model comes from meta-analyses,15,17 data on the effects of aspirin use after ICH are limited to large cohort studies, which may be less robust.13,14 In particular, data on the effect of aspirin on the RR of IS after initial ICH suggest that aspirin actually affords greater RR reduction for subsequent IS after initial ICH than after initial IS,13 and the CI for the aspirin-associated RR of IS after ICH crosses 1 (table 1). However, in sensitivity analysis, the predicted benefit of aspirin was maintained up to a threshold value of 1.49 for this RR (table 3). In addition, when we repeated the base case analysis with RR of IS after ICH increased greater than 3-fold to be equivalent to RR of IS after IS, aspirin therapy remained the preferred treatment strategy up to a proportion of initial strokes due to ICH of 54.6%. Third, our model assessed aspirin's effects on secondary stroke prevention but did not incorporate the potential benefits of aspirin in prevention of ischemic heart disease and peripheral vascular disease. Thus, our model may underestimate the benefits of aspirin in patients with stroke of undetermined etiology, since ischemic heart disease and peripheral vascular disease share risk factors with both IS and ICH. Finally, data for outcomes after ICH used in this decision analysis include only patients who survived for 1 month after ICH. Since mortality due to ICH is 40% at 1 month even in high-resource settings,33 our base case analysis may overestimate the proportion of patients with ICH who survive to be candidates for long-term secondary prevention, because data utilized for the proportion of strokes due to ICH were drawn from a study of first-ever strokes.24 However, this overestimation of the proportion of strokes caused by ICH would lead to an underestimation of the benefit of aspirin in patients whose stroke type is unknown.
Aspirin is only one component of a comprehensive secondary stroke prevention strategy that includes screening for and control of risk factors such as hypertension, diabetes, smoking, hypercholesterolemia, and atrial fibrillation. However, aspirin represents a widely available, inexpensive, and cost-effective secondary prevention therapy for cerebrovascular disease that is underutilized in areas of greatest stroke-related disability and mortality.3,7 Taken together with our previous decision analysis evaluating the risks and benefits of aspirin administration during the period of initial hospitalization after acute stroke of undetermined etiology,9 our models predict that the potential benefits of aspirin as both an acute and long-term secondary prevention strategy may outweigh the perceived risks when neuroimaging is unavailable to distinguish IS from ICH. In the absence of a clinical trial to test this approach empirically, clinical decisions still require patient-specific assessment of risk and benefit. However, the results of our decision analysis suggest that increased use of aspirin therapy after stroke of undetermined etiology could lead to decreased stroke recurrence and mortality in the areas of the world where the burden of cerebrovascular disease is highest.
ACKNOWLEDGMENT
Dr. Berkowitz would like to thank Dr. Wendell Blaise of Hôpital St. Boniface in Fond-des-Blancs, Haiti and Dr. Kerling Israel of Hôpital St. Nicolas in St. Marc, Haiti for posing this clinical conundrum. All authors would like to thank Dr. Steven M. Greenberg and Dr. Steven Feske for helpful comments on earlier versions of this manuscript.
GLOSSARY
- CI
confidence interval
- DALY
disability-adjusted life-year
- ICH
intracerebral hemorrhage
- IS
ischemic stroke
- LMIC
low- and middle-income countries
- mRS
modified Rankin Scale
- QALY
quality-adjusted life-year
- RR
relative risk
- SAH
subarachnoid hemorrhage
- tPA
tissue plasminogen activator
AUTHOR CONTRIBUTIONS
Aaron L. Berkowitz: study design, data collection, data analysis, data interpretation, literature search, figures, drafting and revising of the manuscript. M. Brandon Westover: study design, data analysis, data interpretation, figures, manuscript revision. Matt T. Bianchi: study design, data analysis, data interpretation, manuscript revision. Sherry H-Y. Chou: study design, data analysis, data interpretation, manuscript revision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
A. Berkowitz receives royalties from Clinical Pathophysiology Made Ridiculously Simple (Medmaster, Inc.) and The Improvising Mind (Oxford University Press). M. Brandon Westover and M. Bianchi report no disclosures relevant to the manuscript. S. Chou has been funded by the American Heart Association and the NIH; is currently funded by the National Institute of Neurological Disorders and Stroke; and serves on the endpoint committee of a clinical trial funded by Novartis. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Global Health 2013;1:e259–e281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brainin M, Teuschl T, Kalra L. Acute treatment and long-term management of stroke in developing countries. Lancet Neurol 2007;6:553–561 [DOI] [PubMed] [Google Scholar]
- 4.Chen ZM, Sandercock P, Pan HC, Counell C, Collins R, Liu LS. Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40,000 randomized patients from the Chinese acute stroke trial and the international stroke trial. Stroke 2000;31:1240–1249 [DOI] [PubMed] [Google Scholar]
- 5.Johnson ES, Lanes S, Wentworth C, Satterfield M, Abebe BL, Dicker LW. A metaregression analysis of the dose-response effect of aspirin on stroke. Arch Intern Med 1999;159:1248–1253 [DOI] [PubMed] [Google Scholar]
- 6.He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: a meta-analysis of randomized controlled trials. JAMA 1998;280:1930–1935 [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Islam S, Chow CK, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet 2011;378:1231–1243 [DOI] [PubMed] [Google Scholar]
- 8.Runchey S, McGee S. Does this patient have a hemorrhagic stroke?: clinical findings distinguishing hemorrhagic stroke from ischemic stroke. JAMA 2010;303:2280–2286 [DOI] [PubMed] [Google Scholar]
- 9.Berkowitz AL, Westover MB, Bianchi MT, Chou SHY. Aspirin for acute stroke of unknown etiology in resource-limited settings: a decision analysis. Neurology 2014;83:787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandian JD, Padma V, Vijaya P, Sylaja PN, Murthy JMK. Stroke and thrombolysis in developing countries. Int J Stroke 2007;2:17–26 [DOI] [PubMed] [Google Scholar]
- 11.Berkowitz AL, Mittal M, Mclane HC, et al. Worldwide reported use of IV tissue plasminogen activator for acute ischemic stroke. Int J Stroke 2014;9:349–355 [DOI] [PubMed] [Google Scholar]
- 12.Feigin VL, Lawes CMM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review Lancet Neurol 2009;8:355–69 [DOI] [PubMed] [Google Scholar]
- 13.Flynn R, MacDonald TM, Murray GD, MacWalter RS, Doney ASF. Prescribing antiplatelet medicine and subsequent events after intracerebral hemorrhage. Stroke 2010;41:2606–2611 [DOI] [PubMed] [Google Scholar]
- 14.Huhtakangas J, Lopponen P, Tetri S, Juvela S, Saloheimo P, Bode MK. Predictors for recurrent primary intracerebral hemorrhage: a retrospective population-based study. Stroke 2013;44:585–590 [DOI] [PubMed] [Google Scholar]
- 15.Antithrombotic Trialists' (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong KS; for the Asian Acute Stroke Advisory Panel. Risk factors for early death in acute ischemic stroke and intracerebral hemorrhage: A prospective hospital-based study in Asia. Stroke 1999;30:2326–2330 [DOI] [PubMed] [Google Scholar]
- 17.Thompson BB, Béjot Y, Caso V, et al. Prior antiplatelet therapy and outcome following intracerebral hemorrhage: a systematic review. Neurology 2010;75:1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375:1695–1703 [DOI] [PubMed] [Google Scholar]
- 19.Anderson CS, Heeley R, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013;368:2355–2365 [DOI] [PubMed] [Google Scholar]
- 20.Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2003;34:1710–1716 [DOI] [PubMed] [Google Scholar]
- 21.Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999;159:677–685 [DOI] [PubMed] [Google Scholar]
- 22.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin and warfarin on quality of life. Arch Intern Med 1996;156:1829–1836 [PubMed] [Google Scholar]
- 23.National Center for Health Statistics. Vital Statistics in the United States, Volume II: Mortality. Washington, DC: Public Health Service, US Government Printing Office; 1988. Part A [Google Scholar]
- 24.O'Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010;376:112–123 [DOI] [PubMed] [Google Scholar]
- 25.Zenebe G, Alemayehu M, Asmera J. Characteristics and outcomes of stroke at Tikur Anbessa Teaching Hospital, Ethiopia. Ethiop Med J 2005;43:251–259 [PubMed] [Google Scholar]
- 26.Matuja W, Janabi M, Kazema R, Mashuke D. Stroke subtypes in Black Tanzanians: a retrospective study of computerized tomography scan diagnoses at Muhimbili National Hospital, Dar es Salaam. Trop Doct 2004;34:144–146 [DOI] [PubMed] [Google Scholar]
- 27.Nyame PK, Jumah KB, Adjei S. Computerised tomographic scan of the head in evaluation of stroke in Ghanians. East Afr Med J 1998;75:637–639 [PubMed] [Google Scholar]
- 28.Silberberg D, Katabira E. Neurological disorders. In: Jamison DT, Feachem RG, Makgoba MW, et al., editors. Disease and Mortality in Sub-Saharan Africa, 2nd ed Washington, DC: World Bank; 2006. Chapter 23 Available at: http://www.ncbi.nlm.nih.gov/books/NBK2295/. Accessed February 17, 2014 [Google Scholar]
- 29.Ehrhardt S, Meyer CG. Transfer of evidence-based medical guidelines to low- and middle-income countries. Trop Med Int Health 2012;17:144–146 [DOI] [PubMed] [Google Scholar]
- 30.Chinnock P, Siegfried N, Clarke M. Is evidence-based medicine relevant to the developing world? PLoS Med 2005;2:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pauker SG, Kassirer JP. Decision analysis. N Engl J Med 1987;316:250–258 [DOI] [PubMed] [Google Scholar]
- 32.Monette J, Gurwitz JH, Rochon PA, Avorn J. Physician attitudes concerning warfarin for stroke prevention in atrial fibrillation: results of a survey of long-term care practitioners. J Am Geriatr Soc 1997;45:1060–1065 [DOI] [PubMed] [Google Scholar]
- 33.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–76 [DOI] [PubMed] [Google Scholar]