Abstract
This review summarizes the potential and also some limitations of using human placentas, or placental cells and structures for toxicology testing. The placenta contains a wide spectrum of cell types and tissues, such as trophoblast cells, immune cells, fibroblasts, stem cells, endothelial cells, vessels, glands, membranes, and many others. It may be expected that in many cases the relevance of results obtained from human placenta will be higher than those from animal models due to species specificity of metabolism and placental structure. For practical and economical reasons, we propose to apply a battery of sequential experiments for analysis of potential toxicants. This should start with using cell lines, followed by testing placenta tissue explants and isolated placenta cells, and finally by application of single and dual side ex vivo placenta perfusion. With each of these steps, the relative workload increases while the number of feasible repeats decreases. Simultaneously, the predictive power enhances by increasing similarity with in vivo human conditions. Toxic effects may be detected by performing proliferation, vitality and cell death assays, analysis of protein and hormone expression, immunohistochemistry or testing functionality of signaling pathways, gene expression, transport mechanisms, and so on. When toxic effects appear at any step, the subsequent assays may be cancelled. Such a system may be useful to reduce costs and increase specificity in testing questionable toxicants. Nonetheless, it requires further standardization and end point definitions for better comparability of results from different toxicants and to estimate the respective in vivo translatability and predictive value.
Keywords: trophoblast, placenta, placenta perfusion, placenta explant, choriocarcinoma, human toxicology
Introduction
The human term placenta offers a broad spectrum for toxicological testing due to its unique function and availability:
Term placentae, in contrast to almost any other human organ or tissue of macroscopic size, can be used for research within less than 15 min after leaving the living human body. Cells and structures (e.g., membranes, vessels) are generally vital and intact. Its weight is approximately 500 g and most university hospitals have far more than 1,000 deliveries per year. The placenta contains a large variety of cell types, including almost all immune cell types, endothelial cells, fibroblasts, trophoblast cells, smooth muscle cells, and others. Ex vivo, under perfusion, complete placenta lobes can be kept vital for up to 24 hr and isolated explants or cells for several days or weeks depending on the respective cell types. Therefore, the term placenta represents an excellent model for toxicology testing on human cells or tissues, even when special regard to pregnancy and reproduction is not requested.
The placenta regulates the transfer of substances into the fetal circulation. Also, toxic agents may be transferred or enriched in the fetal compartment, which can be investigated in placenta perfusion models (Troen and Gordon 1958).
Based on its main functions, alimentation, respiration, and elimination of metabolic wastage of the fetus, many toxic agents tend to accumulate in the placenta (Grabic et al. 2006; Kantola et al. 2000; Reichrtova, Dorociak, and Palkovicova 1998). Therefore, the placenta is, in many cases, more sensitive to toxic influences than other tissues and organs. Due to its pivotal functions, the toxicants that harm the placenta subsequently harm fetal development (Mattison 2010). Therefore, toxicology testing on placental cells or tissues may indicate a risk for pregnancy.
In many aspects, use of human placentae for toxicology studies may provide more relevant information than animal studies because of its human-specific reactions (e.g., specific metabolism) and its unique structure, which differs from almost all other species (Benirschke 2007; Carney et al. 2004; Carter and Enders 2004; Carter and Mess 2010; Cline et al. 2013).
A final important aspect is that human term placenta research is generally well accepted by the donating mothers and worldwide generally approved by ethical committees (Halkoaho et al. 2010). Also first trimester placentae from elective abortions are available in many countries. They may reflect better the situation during pregnancy than term placentae, but they are usually not intact and cannot be used for perfusion studies. The ethical aspect is more complicated than in term placentae. It is very rare that healthy placentae from second pregnancy trimester can be obtained. Therefore, they are not applicable for regular toxicological testing.
The need for setting up and standardizing toxicological test systems including end point definition on placental cells and tissues has been previously highlighted by several authors and must be further pushed forward (Giaginis, Theocharis, and Tsantili-Kakoulidou 2012).
Test Systems
Testing of toxic effects on the placenta can be performed on different levels, which influences the quality and quantity of information, but also the costs and workload. The system allows not only the assessment of transfer of substances across the placental barrier, as previously described (Giaginis, Tsantili-Kakoulidou, and Theocharis 2011), but the placental cells and tissues can vicariously serve as models for investigation of toxic effects on human tissue. Therefore, we recommend a standardized stepwise test system starting at a low-cost screening system and ending with a complex placenta perfusion installation. When toxic effects appear in a lower level assay, the next steps may be cancelled to save resources (rough overview in Table 1). The steps include the following five models, which will be exposed to potential toxicants (in parentheses: functions to be measured; after “—”: test characteristics):
Table 1.
Rough overview of features of placenta-derived toxicological test systems (“workload/n” stands for estimated workload per experiment).
| Exposure time | Availability | Reproducibility | Work load/n | |
|---|---|---|---|---|
| Cell lines | Unlimited | Unlimited | High | Low |
| Placenta explants | A few days | High | Patient-dependent | Low |
| Isolated cells | A few days | limited | Patient-dependent | Medium |
| One side perfusion | 8 hr | 1–2/day | Patient-dependent | Medium |
| Dual perfusion | 8 hr | 3/week | Patient-dependent | High |
Placenta-derived (mainly trophoblastic) cell lines (apoptosis/necrosis, hormone production, intracellular molecules, receptor expression)—high throughput, repeatable under almost identical conditions in different laboratories
Tissue explants (apoptosis, hormone production, immunohistochemistry for cell identification and, depending on size, detection of potential toxicants)—cells remain in their physiological environment, easy to perform
Isolated primary placenta cells (as for 1)—provides information about a concrete specified and relevant cell type, isolation procedure is time consuming
Single maternal side placenta perfusion (as for 2)—intact membranes between mother and fetus not necessary; therefore, high rate of successful experiments
Dual side placenta perfusion (as for 2 plus detection of potential toxicant and its metabolites in the fetal compartment)—barrier between maternal and fetal circulation must be intact, therefore high failure rate, high workload.
The special features of each system are summarized in the following passages.
Cell Lines
Background
Numerous cell lines have been established from choriocarcinoma cells. The most prominent are JEG-3, BeWo, and JAR, which have been developed from brain metastases in the 1970s and are still commercially available. They share numerous characteristics of primary trophoblast cells, but are also different in several aspects due to their malignant transformation (Bilban et al. 2010; Morales-Prieto et al. 2012). To overcome this weakness, primary trophoblast cells have been immortalized, such as the HTR-8/SVneo cell line derived from first trimester extravillous trophoblast cells (Graham et al. 1993), or JEG-3 derivates hybridized with primary first or third trimester trophoblast cells (ACH-3P and AC1-M59 cells, respectively; Hiden et al. 2007). Also, immune cell lines have been established, which are more similar to their placental or decidual homologues than to the respective blood cells. For example, the NK92 cell line shows high expression of CD56, but low expression of CD16, thus resembling the decidual natural killer (NK) cell phenotype, while blood NK cells typically show a high CD16 and medium CD56 expression (Gong, Maki, and Klingemann 1994; Trundley et al. 2006).
Potential Biomarkers
The exposure of cell lines to potential toxicants allows the assessment of numerous biologically relevant parameters. Most of these parameters, as long as they are not cell line–specific, can be measured also in the subsequent more complex systems of freshly isolated cells or tissues. Strong toxic effects may reduce proliferation and increase cell death or apoptosis. Many placental cell lines produce soluble factors, which can be detected in their supernatants after culturing. The release of the following factors can be evaluated: hormones, including estrogens, progesterone, and chorionic gonadotropin, a wide spectrum of cytokines, chemokines, and interleukins, enzymes, matrix metalloproteinases, and immunoregulatory factors, such as Human Leucocyte Antigen-G (HLA-G; Akhter et al. 2012; Bahn et al. 1981; Markert, Morales-Prieto, and Fitzgerald 2011; Schropfer et al. 2010). Also, the whole spectrum of intra-cellular factors, such as signaling molecules or RNA, can be analyzed (Bilban et al. 2010; Fitzgerald et al. 2010; Morales-Prieto et al. 2012). The cells can also be applied in functional assays for metabolism, migration, invasion, chemotaxis, angiogenesis, and many others. It can be expected that some “mild” toxicants do not affect the vitality of the cells, but do affect their functions or their secretion profile. In contrast to the other proposed models, cell lines can be used for analyses of toxic effects during almost unlimited time periods, as for example for mutation assays (Johnson 2012). While cell lines offer a stable easy-to-use system, the disadvantage in toxicological testing is their immortalization or malignant background, which may influence their resistance to toxic agents.
Examples of Toxicology Studies
All most commonly used cell lines have been used also for a variety of toxicological assessments, only a few of which will be listed here as examples. BeWo cells have been exposed to p-Nonylphenol (p-NP), which is a metabolite of alkylphenol ethoxylates used as surfactants in the manufacturing industry with expected estrogenic activity. p-NP stimulates cleavage of caspase-3 much faster than 17β-estradiol (Bechi et al. 2006). In the same cell line, bisphenol A, another estrogen-like chemical, is cytotoxic and, in JEG-3 cells, potentially reduces estrogen synthesis by downregulating aromatase and CYP19 of placental cells (Huang and Leung 2009; Morck et al. 2010). Zearalenone (ZEN), a nonsteroid estrogen mycotoxin produced by numerous strains of Fusarium, which commonly contaminates cereals, is reduced via intestinal and hepatic metabolism to α- and β-zearalenol. Conversely to ZEN, its metabolites do not induce differentiation of BeWo cells. They instead induce significant changes in ABC transporter expression by potential interaction with nuclear receptors (Prouillac et al. 2012).
Toxic effects of the environmental organophosphate insecticides phosmet and chlorpyrifos on JEG-3 cells viability, proliferation, cell cycle, and inflammatory molecule production have been reported (Guinazu et al. 2012). In the same cell line, an acute low dose of cadmium reduces cortisol production and increases 11β-HSD2 expression, probably by affecting the methylation status of some target genes (Ronco et al. 2010). Thirty pesticides have been investigated for their ability to modulate aromatase activity in JEG-3 cells, 4 of which inhibit and 9 induce aromatase activity; another 4 induce CYP19 gene expression (Laville et al. 2006). Jeg-3 cells have been used to demonstrate increased human choriogonadotropin (HCG) production when epidermal growth factor (EGF)–stimulated cells are additionally exposed to ethanol (Wimalasena, Beams, and Caudle 1994).
Exposure of JAR cells to the endocrine disruptor 2,3,7,8-tetrachlorodibenzo-p-dioxin results in impairment of cell viability, increased generation of reactive oxygen species generation, oxidative damage, decreased mitochondrial DNA copy number, and ATP content, while mitochondrial DNA mutations and protein levels of p53, Bax, Bcl2, cytochrome c, and caspase 3 increased (Chen et al. 2010).
Placenta Explants
Background
First and third trimester tissue explants can be obtained from several structures, which are macroscopically distinguishable, such as the placental villi, decidua, membranes, or blood vessels. A diameter of 1 to 2 mm allows sufficient nutrition in cell culture medium for several days. Explants can be obtained very easily and without a long separation procedure. The simple preparation reduces cell isolation stress and allows for analysis of cells within their physiological environment. The scientific potential of placenta explants has been summarized previously (Miller et al. 2005).
Potential Biomarkers
In explants, similar parameters can be measured as in cell lines. Additionally, toxic effects on tissue structures can be observed by histological and histochemical methods (de Oliveira Gomes et al. 2011). The reaction of cells within their tissue on external stressors is more physiologically relevant than that of cell lines.
Examples of Toxicology Studies
Bisphenol A exposure significantly increased β-hCG secretion and caspase-3 expression in first trimester placental explants at an environmentally relevant concentration of 1 nM (Morck et al. 2010). Explant cultures have been treated with p-NP and 17β-E2, which significantly increases β-hCG secretion and cell apoptosis. After 72 hr of exposure, hormone release is significantly higher in p-NP- than 17β-E2-treated explant cultures. By this time, cleavage of caspase-3 occurs in cultures treated with 17β-E2 and p-nonylphenol, which leads the authors to “raise considerable concern about the implications of exposure to this chemical for the fetus and pregnancy” (Bechi et al. 2006).
Isolated Primary Placenta Cells
Background
Different cell types can be isolated from placental or decidual tissue. The most frequently isolated cells are cytotrophoblast cells, different subtypes of immune cells (Kammerer, von Wolff, and Markert 2004) and endothelial cells (mainly from umbilical/fetal vessels; Male, Gardner, and Moffett 2012; Solder et al. 2012). Working with isolated cells allows the specific observation of one cell type, but in contrast to explant tissue culture, the isolation process is stressful for the cells. Thereafter in culture, the loss of the physiological cellular and soluble environment induces additional stress and may guide the cells away from their physiological into an artificial behavior.
Potential Biomarkers
Assays can be performed as for cell lines, but especially isolated trophoblast cells practically do not proliferate ex vivo and other placental cell types (e.g., endothelium) survive only a low number of passages (Potgens et al. 2001).
Examples of Toxicology Studies
TNF and IFN-gamma are cytotoxic on isolated trophoblast cells whereas fibronectin increases their viability, although not completely abolishing their cytotoxic action. EGF exerts protective effects that may be related to stimulation of fibronectin secretion in trophoblast cells (Pijnenborg et al. 2000).
Cultures of isolated and purified trophoblast cells of first-trimester placental villi of nonsmoker donors were exposed to polynuclear aromatic hydrocarbon agents (major components of cigarette smoke). Their metabolism was increased, which may lead to production of genotoxic metabolites deleterious to conceptus development (Sanyal et al. 1993).
Term trophoblast cells have been exposed to Cd or Zn. Both metals, in particular Cd, induce increase in the cellular concentration of metallothione. Cells pretreated with low doses of Cd and then challenged with toxic concentrations of Cd have higher levels of metallothione and develop higher resistance (Lehman and Poisner 1984).
Single Maternal Side Placenta Perfusion
Background
Installation of a single maternal side placenta perfusion system is technically easy. Term placenta cotyledons can be installed within 10 to 20 min and maintained vital for several hours. For this approach, 4 to 10 tubes are infixed into the intervillous space of 2 separated cotyledons, each within 2 separated perfusion systems. One cotyledon can be perfused with a control medium and the second cotyledon with the test substance. The perfusion with oxygenized and warmed (37°C) medium maintains the tissue vital for more than 8 hr. The system can be used to test the deposition of instilled substances or cells in the placental tissue and the potential toxic effects therein (Heinzelmann et al. 2009).
Potential Biomarkers
The placenta perfusate can be used for testing substances secreted into the maternal circulation. It provides a potpourri obtained from all cell types. The way of exposure to toxicants and washing out of secretions via perfusion is more natural than in explant cultures. Histological assessment allows a wider overview than that of explants (Heinzelmann et al. 2009). Accumulation of toxicants or their fragments in the tissue or in defined compartments or cell types can be perceived depending on the nature of the toxic agent and the available detection systems. The relatively large size of perfused tissue also allows, at the end of perfusion, isolation of useful amounts of specific cell types, which can be used for further analyses, as for intracellular molecules
Examples of Toxicology Studies
Since most placenta perfusion experiments are focused on the analysis of passage through the placenta barrier, the option of performing single-side perfusion for toxicology assays seems to be widely neglected and underestimated. Therefore, we were not able to find related publications. The comparably simple equipment and training needed for performing single-side perfusion should encourage more groups to use this system. The technique has successfully been used at the Placenta laboratory in Jena to analyze adhesion and homing of autologous lymphocytes perfused through the placenta (Heinzelmann et al. 2009; Schamberger et al. 2012).
Dual Side Placenta Perfusion
Background
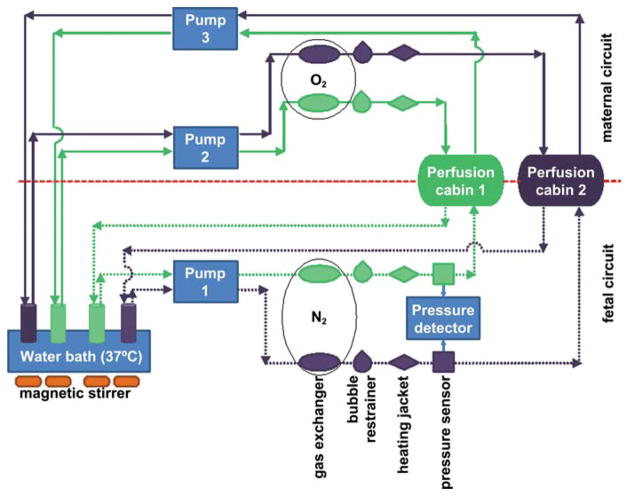
The dual side perfusion of term placentae is principally similar to the single-side perfusion, but additionally, a second circulation system is connected to the respective fetal blood vessels of the perfused cotyledons (Figure 1; Reiber et al. 1990). A human placenta has approximately 15 to 25 cotyledons, which are separated by septa within the intervillous space and which contain a villous tree that has its individual vessel connection to major arms of the cord vessels. In addition to tissue analysis, this system allows the analysis of transfer or passage of substances from one circulation into the other (Hutson et al. 2011; Schniewind 1960; Troen and Gordon 1958; Vahakangas and Myllynen 2009). For dual side perfusion experiments, healthy, intact term placentas are used. Placentas from elective abortions are usually damaged and do not have an intact feto-maternal barrier. Term placentas have the advantage of performing the highest transfer activity in both directions and of having the lowest distance from the maternal to the fetal blood vessels. Therefore, term placentas offer a more sensitive system for toxicant transfer studies than preterm placentas could do (if they were intact and available). Generally, placentas from sections and from vaginal deliveries can be used.
Figure 1.
Scheme of a dual side ex vivo placenta perfusion on two cotyledons (in cabin 1 and 2, respectively) for testing transfer of substances through the human term placenta. One cotyledon can be used for perfusion with a test substance, the other one for control. On the fetal side, tubules are connected to arterial and venous blood vessels. Only one pump is necessary to produce sufficient pressure for influx and outflow. On the maternal side, tubules are instilled into the intervillous space from where perfusion medium drops out into a collector recipient. Here, two pumps are necessary to maintain permanent flow. Gas exchangers are used to enrich perfusion medium with oxygen on the maternal and nitrogen on the fetal side. The pressure is detected on the fetal side. A rapidly decreasing pressure indicates a leakage between the maternal and fetal circuit. The system can be simplified using only one cotyledon. For a single-side perfusion, the fetal circuit can be omitted. One side perfusion can be used to analyze substance accumulation or tissue toxicity.
Antipyrin, supplemented into the maternal circulation, diffuses passively through the intact barrier and can be used to calculate the relative clearance of substances from the maternal compartment (Mose et al. 2012; Schneider, Panigel, and Dancis 1972). Several markers, such as dextran, are available to test the integrity of the placental barrier (Balakrishnan et al. 2011). The permanent achievement of the flow pressure in the fetal system helps to detect leakages (Kovo et al. 2008). A placental tissue damage leading to such leakage occurs relatively frequently during delivery or during installation of the perfusion tubes into the intervillous space. The subsequent disruption of the maternofetal barrier is often not visible and can be detected only after a certain, variable time of perfusion. Due to the necessary intensive cleaning and decontamination of the system, it will usually not be available more than once per day. A systematic evaluation of published results from the placenta perfusion model demonstrated that the maternal-to-fetal drug concentration ratios matched well between placental perfusion experiments and in vivo samples taken at the time of delivery of the infant (Hutson et al. 2011).
Potential Biomarkers
The dual perfusion permits all analyses as the single-side perfusion does. In addition, the questionable toxicants or their degradation fragments can be assessed in the fetal circulation. Also concentration changes of biological substances produced in the placenta, such as inflammation markers, can be detected. Permanent measurement of glucose consumption and lactate production as well as β-hCG, placental lactogen, or placenta alkaline phosphatase (PLAP) release helps to estimate the metabolic activity of the perfused tissue (Cannell et al. 1988; Partanen et al. 2012).
Examples of Toxicology Studies
Numerous placenta perfusion studies for analysis of transfer of toxicants have been published since the late 1950s. The presented examples are a small selection representing different classes of substances.
In an above-mentioned study on bisphenol A, which has also used BeWo cells and placenta explants, a rapid transfer of this substance across the term placenta has been reported (Morck et al. 2010).
Mercuric chloride (HgCl2) decreases placental amino acid, but not glucose transfer as determined by the use of their nonmetabolizable radioactive analogues, aminoisobutyric acid (AIB) and 3-o-methyl glucose (3MG), respectively. It also decreases the placental oxygen consumption rate (Urbach et al. 1992).
The anti-flu preparation oseltamivir (Tamiflu) phosphate is extensively metabolized in the ex vivo human placenta model, and the transplacental passage of the active metabolite oseltamivir carboxylate is incomplete (Worley, Roberts, and Bawdon 2008).
Human placentas have been perfused to reveal the toxicokinetics of ochratoxin, one of the most frequent mycotoxins detected in human blood, using concentrations found in serum of pregnant women. In this study, the transfer of ochratoxin through term human placenta was barely detectable, in contrast to epidemiological studies reporting higher ochratoxin levels in fetal than in maternal circulation in vivo (Woo et al. 2012). This contradictive finding may be due to the longer lasting exposure in vivo than ex vivo.
Placentas have been perfused with different medications known for their vasodilating effects in the periphery. They induced similar effects on placental vessels. Aminophylline and nitrangin were the strongest vasodilators while prostaglandin F2 alpha provoked potent vascular contraction (Noschel, Reiber, and Schroder 1982).
Conclusion
Human placenta tissue and placenta-derived cells and cell lines have been used for toxicological analyses for more than 5 decades. Several authors have used cascades of assays, but the system needs further improvements and standardization, based on current and innovative methods, for better comparability of results from different toxicants and to understand the respective in vivo translatability and predictive values and to reduce costs for more routine applications.
Acknowledgments
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: CG is supported by a research grant from the German Ministry of Research (BMBF).
Abbreviations
- AIB
aminoisobutyric acid
- EGF
epidermal growth factor
- HCG
human choriogonadotropin
- HgCl2
mercuric chloride
- NK
natural killer
- PLAP
placenta alkaline phosphatase
- p-NP
p-nonylphenol
- ZEN
zearalenone
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
For reprints and permissions queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav.
References
- Akhter A, Das V, Naik S, Faridi RM, Pandey A, Agrawal S. Upregulation of HLA-G in JEG-3 cells by dexamethasone and hydrocortisone. Arch Gynecol Obstet. 2012;285:7–14. doi: 10.1007/s00404-011-1880-3. [DOI] [PubMed] [Google Scholar]
- Bahn RS, Worsham A, Speeg KV, Jr, Ascoli M, Rabin D. Characterization of steroid production in cultured human choriocarcinoma cells. J Clin Endocrinol Metab. 1981;52:447–50. doi: 10.1210/jcem-52-3-447. [DOI] [PubMed] [Google Scholar]
- Balakrishnan B, Thorstensen E, Ponnampalam A, Mitchell MD. Passage of 4-nonylphenol across the human placenta. Placenta. 2011;32:788–92. doi: 10.1016/j.placenta.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Bechi N, Ietta F, Romagnoli R, Focardi S, Corsi I, Buffi C, Paulesu L. Estrogen-like response to p-nonylphenol in human first trimester placenta and BeWo choriocarcinoma cells. Toxicol Sci. 2006;93:75–81. doi: 10.1093/toxsci/kfl043. [DOI] [PubMed] [Google Scholar]
- Benirschke K. [Accessed March 9, 2013];Comparative placentation. 2007 http://placentation.ucsd.edu/
- Bilban M, Tauber S, Haslinger P, Pollheimer J, Saleh L, Pehamberger H, Wagner O, Knofler M. Trophoblast invasion: Assessment of cellular models using gene expression signatures. Placenta. 2010;31:989–96. doi: 10.1016/j.placenta.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Cannell GR, Kluck RM, Hamilton SE, Mortimer RH, Hooper WD, Dickinson RG. Markers of physical integrity and metabolic viability of the perfused human placental lobule. Clin Exp Pharmacol Physiol. 1988;15:837–44. doi: 10.1111/j.1440-1681.1988.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Carney EW, Scialli AR, Watson RE, DeSesso JM. Mechanisms regulating toxicant disposition to the embryo during early pregnancy: An interspecies comparison. Birth Defects Res Part C, Embryo Today. 2004;72:345–60. doi: 10.1002/bdrc.20027. [DOI] [PubMed] [Google Scholar]
- Carter AM, Enders AC. Comparative aspects of trophoblast development and placentation. Reprod Biol Endocrinol. 2004;2:46. doi: 10.1186/1477-7827-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AM, Mess A. Hans Strahl’s pioneering studies in comparative placentation. Placenta. 2010;31:848–52. doi: 10.1016/j.placenta.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Chen SC, Liao TL, Wei YH, Tzeng CR, Kao SH. Endocrine disruptor, dioxin (TCDD)-induced mitochondrial dysfunction and apoptosis in human trophoblast-like JAR cells. Mol Human Reprod. 2010;16:361–72. doi: 10.1093/molehr/gaq004. [DOI] [PubMed] [Google Scholar]
- Cline JM, Dixon D, Faas M, Göhner C, Häger JD, Markert UR, Pfarrer C, Ernerudh J, Svensson J, Buse E. The placenta in toxicology. Part V. Pathologic assessment of the placenta. Toxicol Pathol. doi: 10.1177/0192623313482207. (In Press) [DOI] [PubMed] [Google Scholar]
- de Oliveira Gomes A, de Oliveira Silva DA, Silva NM, de Freitas Barbosa B, Franco PS, Angeloni MB, Fermino ML, Roque-Barreira MC, Bechi N, Paulesu LR, Dos Santos MC, Mineo JR, Ferro EA. Effect of macrophage migration inhibitory factor (MIF) in human placental explants infected with Toxoplasma gondii depends on gestational age. Am J Pathol. 2011;178:2792–801. doi: 10.1016/j.ajpath.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JS, Germeyer A, Huppertz B, Jeschke U, Knofler M, Moser G, Scholz C, Sonderegger S, Toth B, Markert UR. Governing the invasive trophoblast: Current aspects on intra- and extracellular regulation. Am J Reprod Immunol. 2010;63:492–505. doi: 10.1111/j.1600-0897.2010.00824.x. [DOI] [PubMed] [Google Scholar]
- Giaginis C, Theocharis S, Tsantili-Kakoulidou A. Current toxicological aspects on drug and chemical transport and metabolism across the human placental barrier. Expert Opin Drug Metabol Toxicol. 2012;8:1263–75. doi: 10.1517/17425255.2012.699041. [DOI] [PubMed] [Google Scholar]
- Giaginis C, Tsantili-Kakoulidou A, Theocharis S. Assessing drug transport across the human placental barrier: From in vivo and in vitro measurements to the ex vivo perfusion method and in silico techniques. Curr Pharmaceut Biotechnol. 2011;12:804–13. doi: 10.2174/138920111795470930. [DOI] [PubMed] [Google Scholar]
- Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–8. [PubMed] [Google Scholar]
- Grabic R, Hansen LG, Ptak A, Crhova S, Gregoraszczuk EL. Differential accumulation of low-chlorinated (Delor 103) and high-chlorinated (Delor 106) biphenyls in human placental tissue and opposite effects on conversion of DHEA to E2. Chemosphere. 2006;62:573–80. doi: 10.1016/j.chemosphere.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–11. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- Guinazu N, Rena V, Genti-Raimondi S, Rivero V, Magnarelli G. Effects of the organophosphate insecticides phosmet and chlorpyrifos on trophoblast JEG-3 cell death, proliferation and inflammatory molecule production. Toxicol In Vitro. 2012;26:406–13. doi: 10.1016/j.tiv.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Halkoaho A, Pietila AM, Dumez B, Van Damme K, Heinonen S, Vahakangas K. Ethical aspects of human placental perfusion: Interview of the mothers donating placenta. Placenta. 2010;31:686–90. doi: 10.1016/j.placenta.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Heinzelmann J, Enke U, Seyfarth L, Schleussner E, Malek A, Markert UR. Development of a human model to study homing behavior of immune cells into decidua and placental villi under ex vivo conditions. Am J Reprod Immunol. 2009;61:19–25. doi: 10.1111/j.1600-0897.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- Hiden U, Wadsack C, Prutsch N, Gauster M, Weiss U, Frank HG, Schmitz U, Fast-Hirsch C, Hengstschlager M, Potgens A, Ruben A, Knofler M, Haslinger P, Huppertz B, Bilban M, Kaufmann P, Desoye G. The first trimester human trophoblast cell line ACH-3P: A novel tool to study autocrine/paracrine regulatory loops of human trophoblast subpopulations–TNF-alpha stimulates MMP15 expression. BMC Dev Biol. 2007;7:137. doi: 10.1186/1471-213X-7-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Leung LK. Bisphenol A downregulates CYP19 transcription in JEG-3 cells. Toxicol Lett. 2009;189:248–52. doi: 10.1016/j.toxlet.2009.06.853. [DOI] [PubMed] [Google Scholar]
- Hutson JR, Garcia-Bournissen F, Davis A, Koren G. The human placental perfusion model: A systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin Pharmacol Ther. 2011;90:67–76. doi: 10.1038/clpt.2011.66. [DOI] [PubMed] [Google Scholar]
- Johnson GE. Mammalian cell HPRT gene mutation assay: Test methods. Methods Mol Biol. 2012;817:55–67. doi: 10.1007/978-1-61779-421-6_4. [DOI] [PubMed] [Google Scholar]
- Kammerer U, von Wolff M, Markert UR. Immunology of human endometrium. Immunobiology. 2004;209:569–74. doi: 10.1016/j.imbio.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Kantola M, Purkunen R, Kroger P, Tooming A, Juravskaja J, Pasanen M, Saarikoski S, Vartiainen T. Accumulation of cadmium, zinc, and copper in maternal blood and developmental placental tissue: Differences between Finland, Estonia, and St. Petersburg. Environ Res. 2000;83:54–66. doi: 10.1006/enrs.1999.4043. [DOI] [PubMed] [Google Scholar]
- Kovo M, Haroutiunian S, Feldman N, Hoffman A, Glezerman M. Determination of metformin transfer across the human placenta using a dually perfused ex vivo placental cotyledon model. Eur J Obstet Gynecol Reprod Biol. 2008;136:29–33. doi: 10.1016/j.ejogrb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Laville N, Balaguer P, Brion F, Hinfray N, Casellas C, Porcher JM, Ait-Aissa S. Modulation of aromatase activity and mRNA by various selected pesticides in the human choriocarcinoma JEG-3 cell line. Toxicology. 2006;228:98–108. doi: 10.1016/j.tox.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Lehman LD, Poisner AM. Induction of metallothionein synthesis in cultured human trophoblasts by cadmium and zinc. J Toxicol Environ Health. 1984;14:419–32. doi: 10.1080/15287398409530590. [DOI] [PubMed] [Google Scholar]
- Male V, Gardner L, Moffett A. In: Isolation of cells from the feto-maternal interface. Current Protocols in Immunology. Unit 7. Coligan John E, et al., editors. Chapter 7. Vol. 40. John Wiley and Sons; Hoboken, NJ: 2012. pp. 1–11. [DOI] [PubMed] [Google Scholar]
- Markert UR, Morales-Prieto DM, Fitzgerald JS. Understanding the link between the IL-6 cytokine family and pregnancy: Implications for future therapeutics. Expet Rev Clin Immunol. 2011;7:603–9. doi: 10.1586/eci.11.60. [DOI] [PubMed] [Google Scholar]
- Mattison DR. Environmental exposures and development. Curr Opin Pediat. 2010;22:208–18. doi: 10.1097/MOP.0b013e32833779bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, Genbacev O, Turner MA, Aplin JD, Caniggia I, Huppertz B. Human placental explants in culture: Approaches and assessments. Placenta. 2005;26:439–48. doi: 10.1016/j.placenta.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Morales-Prieto DM, Chaiwangyen W, Ospina-Prieto S, Schneider U, Herrmann J, Gruhn B, Markert UR. MicroRNA expression profiles of trophoblastic cells. Placenta. 2012;33:725–34. doi: 10.1016/j.placenta.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Morck TJ, Sorda G, Bechi N, Rasmussen BS, Nielsen JB, Ietta F, Rytting E, Mathiesen L, Paulesu L, Knudsen LE. Placental transport and in vitro effects of Bisphenol A. Reprod Toxicol. 2010;30:131–7. doi: 10.1016/j.reprotox.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Mose T, Mathiesen L, Karttunen V, Nielsen JK, Sieppi E, Kummu M, Morck TA, Myohanen K, Partanen H, Vahakangas K, Knudsen LE, Myllynen P. Meta-analysis of data from human ex vivo placental perfusion studies on genotoxic and immunotoxic agents within the integrated European project NewGeneris. Placenta. 2012;33:433–9. doi: 10.1016/j.placenta.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Noschel H, Reiber W, Schroder S. Drug modification of contractions of fetoplacental vessels of bilaterally in vitro perfused human placentas. Zentralblatt fur Gynakologie. 1982;104:1149–54. [PubMed] [Google Scholar]
- Partanen H, Vahakangas K, Woo CS, Auriola S, Veid J, Chen Y, Myllynen P, El Nezami H. Transplacental transfer of melamine. Placenta. 2012;33:60–66. doi: 10.1016/j.placenta.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Luyten C, Vercruysse L, Keith JC, Jr, Van Assche FA. Cytotoxic effects of tumour necrosis factor (TNF)-alpha and interferon-gamma on cultured human trophoblast are modulated by fibro-nectin. Mol Human Reprod. 2000;6:635–41. doi: 10.1093/molehr/6.7.635. [DOI] [PubMed] [Google Scholar]
- Potgens AJ, Gaus G, Frank HG, Kaufmann P. Characterization of trophoblast cell isolations by a modified flow cytometry assay. Placenta. 2001;22:251–5. doi: 10.1053/plac.2000.0597. [DOI] [PubMed] [Google Scholar]
- Prouillac C, Koraichi F, Videmann B, Mazallon M, Rodriguez F, Baltas M, Lecoeur S. In vitro toxicological effects of estrogenic mycotoxins on human placental cells: Structure activity relationships. Toxicol Appl Pharmacol. 2012;259:366–75. doi: 10.1016/j.taap.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Reiber W, Noschel H, Schroder S, Muller B, Gross W, Michels W. Effects of various oxygen concentrations on metabolic performance of in vitro perfused human placentas. Zentralblatt fur Gynakologie. 1990;112:207–14. [PubMed] [Google Scholar]
- Reichrtova E, Dorociak F, Palkovicova L. Sites of lead and nickel accumulation in the placental tissue. Hum Exp Toxicol. 1998;17:176–81. doi: 10.1177/096032719801700309. [DOI] [PubMed] [Google Scholar]
- Ronco AM, Llaguno E, Epunan MJ, Llanos MN. Effect of cadmium on cortisol production and 11beta-hydroxysteroid dehydrogenase 2 expression by cultured human choriocarcinoma cells (JEG-3) Toxicol In Vitro. 2010;24:1532–7. doi: 10.1016/j.tiv.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Sanyal MK, Li YL, Biggers WJ, Satish J, Barnea ER. Augmentation of polynuclear aromatic hydrocarbon metabolism of human placental tissues of first-trimester pregnancy by cigarette smoke exposure. Am J Obstet Gynecol. 1993;168:1587–97. doi: 10.1016/s0002-9378(11)90803-5. [DOI] [PubMed] [Google Scholar]
- Schamberger S, Weber M, Sonnemann J, Markert UR. Establishment of a one-sided ex vivo human placenta perfusion model to assess adhesion and invasion behavior of T cell leukemia cell lines. Leuk Lymphoma. 2013 doi: 10.3109/10428194.2012.758844. submitted epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Schneider H, Panigel M, Dancis J. Transfer across the perfused human placenta of antipyrine, sodium and leucine. Am J Obstet Gynecol. 1972;114:822–28. doi: 10.1016/0002-9378(72)90909-x. [DOI] [PubMed] [Google Scholar]
- Schniewind H. An experimental apparatus for perfusion of the human placenta vessels. Zeitschrift fur die gesamte experimentelle Medizin. 1960;132:577–84. [PubMed] [Google Scholar]
- Schropfer A, Kammerer U, Kapp M, Dietl J, Feix S, Anacker J. Expression pattern of matrix metalloproteinases in human gynecological cancer cell lines. BMC Cancer. 2010;10:553. doi: 10.1186/1471-2407-10-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solder E, Bockle BC, Nguyen VA, Furhapter C, Obexer P, Erdel M, Stossel H, Romani N, Sepp NT. Isolation and characterization of CD133+CD34+VEGFR-2+CD45− fetal endothelial cells from human term placenta. Microvasc Res. 2012;84:65–73. doi: 10.1016/j.mvr.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Troen P, Gordon EE. Perfusion studies of the human placenta. I. Effect of estradiol and human chorionic gonadotropin on citric acid metabolism. J Clin Invest. 1958;37:1516–23. doi: 10.1172/JCI103743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trundley AE, Hiby SE, Chang C, Sharkey AM, Santourlidis S, Uhrberg M, Trowsdale J, Moffett A. Molecular characterization of KIR3DL3. Immunogenetics. 2006;57:904–16. doi: 10.1007/s00251-005-0060-7. [DOI] [PubMed] [Google Scholar]
- Urbach J, Boadi W, Brandes JM, Kerner H, Yannai S. Effect of inorganic mercury on in vitro placental nutrient transfer and oxygen consumption. Reprod Toxicol. 1992;6:69–75. doi: 10.1016/0890-6238(92)90023-m. [DOI] [PubMed] [Google Scholar]
- Vahakangas K, Myllynen P. Drug transporters in the human blood-placental barrier. Br J Pharmacol. 2009;158:665–78. doi: 10.1111/j.1476-5381.2009.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalasena J, Beams F, Caudle MR. Ethanol modulates the hormone secretory responses induced by epidermal growth factor in choriocarcinoma cells. Alcohol Clin Exp Res. 1994;18:1448–55. doi: 10.1111/j.1530-0277.1994.tb01449.x. [DOI] [PubMed] [Google Scholar]
- Woo CS, Partanen H, Myllynen P, Vahakangas K, El-Nezami H. Fate of the teratogenic and carcinogenic ochratoxin A in human perfused placenta. Toxicol Lett. 2012;208:92–9. doi: 10.1016/j.toxlet.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Worley KC, Roberts SW, Bawdon RE. The metabolism and transplacental transfer of oseltamivir in the ex vivo human model. Infect Dis Obstet Gynecol. 2008;2008:927574. doi: 10.1155/2008/927574. [DOI] [PMC free article] [PubMed] [Google Scholar]