Abstract
Rationale
Aneurysm and dissection of the ascending thoracic aorta are the main cardiovascular complication of Marfan Syndrome (MFS) resulting in premature death. Studies using mouse models of MFS have shown that activation of transforming growth factor (TGF)-β and the concomitant up-regulation of matrix metalloproteinases (MMPs) contribute to aneurysm development. Our previous study showed that doxycycline delayed aneurysm rupture in a mouse model of MFS, Fbn1mgR/mgR. Losartan has been shown to prevent aneurysms in another mouse model of MFS, Fbn1 C1039G/+, through inhibition of the Erk1/2 pathway. However, the role of MMP-2 in MFS and effect of losartan on the lifespan of MFS mice remain unknown.
Objective
We investigated the role of MMP-2 in MFS and compared the effects of losartan and doxycycline on aortic dilatation and survival in Fbn1mgR/mgR mice.
Methods and Results
By life table analysis, we found that losartan and doxycycline improved the survival of Fbn1mgR/mgR mice. Gelatin zymography and Western blot data showed that only doxycycline inhibited MMP-2 expression while both drugs decreased Erk1/2 phosphorylation. When combined, only one of 9 mice died within the 30 week study; aortic histology and diameter were normalized and the effects on Smad2 phosphorylation was additive. To further explore the role of MMP-2 in MFS, we created MMP-2-deficient Fbn1mgR/mgR mice. MMP-2 deletion inhibited activation of TGF-β and phosphorylation of Erk1/2 and Smad2 and prolonged the lifespan of the mice.
Conclusions
These studies demonstrated that inhibition of MMP-2 by doxycycline delayed the manifestations of MFS, in part, through its ability to decrease active TGF-β and the noncanonical signaling cascade downstream of TGF-β. This study further suggested that targeting TGF-β signaling at different points might be a more effective strategy for inhibiting disease progression.
Keywords: Marfan syndrome, Transforming growth factor-β, Matrix metalloproteinases, Aortic aneurysms, Doxycycline, Losartan
Introduction
Marfan syndrome (MFS) is a dominantly inherited disorder of connective tissue with prominent abnormalities in the ocular, skeletal, and cardiovascular systems. It is caused by mutations in the gene encoding fibrillin-1 (FBN1). FBN1 is the major component of extracellular microfibrils, which acts as a scaffolding protein for elastin deposition and formation of elastic fibers1, 2. The major cause of premature death in patients with MFS is progressive aneurysmal dilatation and rupture of the proximal aorta3. Destruction of elastin, the major component of the lamellar architecture of the aorta, is the sine qua non of aneurysmal disease.
Experimental evidence and biosynthetic considerations originally predicted that FBN1 mutations would reduce tissue integrity by interfering with the normal assembly of microfibrils. The combination of a structurally impaired tissue and chronic cyclic stress was believed to be the cause of the mechanical failure of the aorta4. It was with this understanding of the disease that beta-adrenergic receptor antagonists, which reduce aortic wall stress, became a mainstay of medical therapy for MFS5, 6. A recent meta-analysis has raised questions as to the beneficial effect of β-blocker therapy leaving MFS patients with no proven medical therapy to delay disease progression7, 8. This is particularly unfortunate in this patient population since there is typically a long window of follow-up with serial imaging during which medical therapy could be used.
Studies using mouse models of MFS implicated enhanced TGF-β activation and signaling in the progression of numerous manifestations of the disease including aortic aneurysm formation9–12. Losartan inhibits aortic dilatation by inhibiting TGF-β expression, but recent work suggests that MMP inhibition may also account for this protective effect11, 13. Recent work has demonstrated that the noncanonical Erk signaling pathway is critical for the aortic pathology in Marfan syndrome14. Members of the TGF-β superfamily (TGF-β1-3) are secreted in a large latent complex (LLC) which includes TGF-β, a prodomain that blocks TGF-β activity known as latency-associated peptide (LAP) and latent TGF-β binding protein (LTBP). The activation of TGF-β is a two step process involving release of the LLC from matrix followed by removal of the LAP15. The mutant fibrillin-1 in MFS is believed to interfere with normal matrix binding and sequestration of the LLC9.
Elastolytic matrix metalloproteinases (MMPs) are increased in the aorta of patients affected with MFS and have been presumed to have a direct role in the associated destruction of the structural matrix macromolecules16. However, several of the MMPs, including MMP-2, -3 and -9, are able to effectively perform the second essential step for release of active TGF-β, cleavage of the LAP15, 17. Doxycycline, a nonspecific MMP inhibitor, decreased aortic MMP-2 and -9 protein levels and delayed aneurysm formation and rupture in a murine model of MFS18, 19. Yang et al demonstrated that losartan also inhibited MMP-2 expression in a more chronic model of MFS suggesting an alternative mechanism for losartan’s protective effects13. While MMP-2 and -9 have elastolytic activity, recent work using cells derived from MMP-2 and -9 null mice demonstrates that neither play an important role in the ability of the macrophage to degrade elastin20.
MMP-2 is of particular interest in MFS because it is a product of mesenchymal cells including the smooth muscle cells (SMC) of the aortic media, the cells responsible for synthesis and maintenance of the complex macromolecular structure of the aorta21. In the present study, we find that doxycycline decreases Smad2 and Erk1/2 signaling, the critical noncanonical downstream pathway of TGF-β in MFS that leads to aneurysm formation14, 22. Doxycycline and losartan exhibit similar protective effects on matrix and delay aneurysm formation and rupture; these effects on Erk1/2 signaling are additive when the drugs are combined resulting in significant prolongation of survival. Doxycycline is a nonspecific MMP inhibitor but by creating Marfan mice null for MMP-2, we demonstrate a specific role for MMP-2 in TGF-β signaling and aneurysm formation. SMC derived from the aorta of MMP-2 null mice degrade radiolabeled elastin as efficiently as wild type SMC. Taken together, these studies demonstrate a specific role for MMP-2 in MFS, through its ability to activate latent TGF-β and augment the noncanonical signaling cascade downstream of TGF-β. MMP-2 may represent a useful therapeutic target for MFS.
Methods
Mice
Heterozygous mutant mice (Fbn1mgR/+) in a mixed C57Bl/6J;129 SvEv background were mated to generate homozygous mutant mice (Fbn1mgR/mgR) and wild type littermates18. To create MMP-2−/−/Fbn1mgR/mgR, Fbn1mgR/+ mice were bred with MMP-2 null mice and the offspring bred. Control mice were the littermates, MMP-2+/+/Fbn1mgR/mgR. Genotyping of mice was performed at post-natal day 14 (PD14) by PCR18, 23. For aortic tissue analysis, mice were sacrificed at 8 weeks. This time point was chosen based on our previous observations with this model because advanced aneurysmal changes are present and the mortality from rupture is not excessive thus avoiding a survival bias. All experiments were carried out in accordance with the guidelines of the University of Nebraska Medical Center Animal Care Committee for the use and care of laboratory animals. All mice were maintained in the pathogen free animal facility.
Doxycycline and losartan treatment and Kaplan-Meier’s survival curve
Beginning on postnatal day (PD)1, Fbn1mgR/+ dams were given plain water or doxycycline (0.5 g/L) (Sigma, St. Louis, MO), or losartan (0.6 g/L)(AK Scientific, Inc, Union City, CA), or a combination of doxycycline (0.5 g/L) and losartan (0.6 g/L) in their drinking water. Water bottles containing doxycycline were covered with foil. The concentration of doxycycline and losartan was chosen based on previous studies of kinetics and treatment effects in mice11, 18, 24. To configure the Kaplan-Meier survival curves, Fbn1mgR/mgR mice with and without treatment, MMP-2+/+/Fbn1mgR/mgR and MMP-2−/−/Fbn1mgR/mgR mice were evaluated daily and survival recorded. Mice were followed up to 30 weeks, at which time, all surviving mice were sacrificed. In addition, one group of wild type littermates (n = 12) and groups of homozygous mice, Fbn1mgR/mgR (n = 12/each group) without or with doxycycline, or losartan, or doxycycline and losartan treatment, and MMP-2+/+/Fbn1mgR/mgR and MMP-2−/−/Fbn1mgR/mgR mice, were sacrificed at 8 weeks of age. The ascending thoracic aortas were perfusion-fixed with 10% neutral buffered formalin and collected for histological studies. Half of the samples were snap frozen in liquid nitrogen for protein extraction.
High frequency ultrasound
Transthoracic ultrasound of wild type littermates and Fbn1mgR/mgR mice with or without treatment, and MMP-2+/+/Fbn1mgR/mgR and MMP-2−/−/Fbn1mgR/mgR mice, were performed with Vevo 770 High Resolution In Vivo Micro-imaging system (VisualSonics, Toronto, Ontario, Canada) equipped with an integrated isoflurane-based anesthesia system. These studies were performed at 5, 8, and 12 weeks of age. Short-axis scans of the ascending aorta were performed using B-mode ultrasonography with the RMV 707 Scanhead. The aortic diameters were measured by M-mode. Three independent measurements were obtained for each mouse. To define user variability, all echocardiographic studies were performed by 2 experienced individuals and results compared for agreement.
Verhoeff-Van Gieson connective tissue staining
After perfusion-fixation with 10% neutral-buffered formalin, mouse ascending aortic tissues were embedded in paraffin and cut into 4-μm sections. The slides were stained with Verhoeff’s solution, ferric chloride, sodium thiosulfate, and Van Gieson’s solution (Poly Scientific, Bay Shore, NY). Each staining cycle alternated between fixing and washing procedures25. The slides were examined and photographed using light microscopy (x40; Nikon).
Isolation of mouse SMC and cell culture
Fbn1mgR/mgR mice and their wild type littermates, MMP-2−/− and their wild type control mice were anesthetized and underwent laparotomy at 7 weeks of age. Mouse thoracic aortas were isolated and minced. SMC isolation was described previously25. The cells were grown to confluence and passed after trypsinization with 0.25% trypsin. Mouse SMC were maintained in F12K media containing 10% FBS. To examine the effect of doxycycline and losartan, cells were incubated with serum-free medium and treated with doxycycline (100 μg/ml) or losartan (100 μg/ml), or doxycycline (100 μg/ml) and losartan (100 μg/ml) for 24 h. Conditioned medium was harvested and analyzed by gelatin zymography. Cells were lysed with 0.5% NP-40 lysis buffer (0.5% NP-40, 50mmol/L Tris (pH7.5), 150mmol/L NaCl, 0.21% NaF, 1mmol/L DTT, 1 mmol/L Na3VO4, 1x protease inhibitor cocktail, 1mmol/L PMSF). Cell lysates were subjected to Western blot analysis.
Elastin preparation and elastolytic assay
Elastin (type E60; Elastin Product Co., St Louis, MO) was reductively labeled (1300 dpm/μg of elastin) as described20. Aortic SMC from wild type and MMP-2−/− mice were isolated as above and activated by incubating with 50 μg/ml ConA for 24 h. Then 1 mg of [3H]elastin was added to each well in the presence of the cysteine protease inhibitor, E64 (20 μM, Sigma), and the serine protease inhibitor, phenylmethylsulfonyl fluoride (1mM), in serum-free F12K at 37°C for 5 days. Solubilized [3H]elastin was quantitated by β-scintillation counting. Results are presented as mean ± SE of three experiments.
Western blot analysis and gelatin zymography
Aortic proteins were extracted as previously described25. The protein concentration of aortic proteins and SMC cell extracts was standardized with a Bio-Rad protein assay. Equal amounts (25 μg) of SMC cell extract or aortic extracts from wild type and Fbn1mgR/mgR mice without treatment, or with doxycycline, losartan, or doxycycline and losartan treatment, and MMP-2+/+/Fbn1mgR/mgR and MMP-2−/−/Fbn1mgR/mgR mice were loaded under reducing conditions onto a 10% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane (Amersham Biosciences, Piscataway, NJ). The membranes were then incubated with rabbit anti-phospho-Smad2 antibody, or rabbit anti-phospho-Erk1/2 antibody, or rabbit anti-TGF-β antibody (Cell Signaling, Danvers, MA). The bound primary antibody was detected with HRP-linked anti-rabbit IgG (Cell Signaling). Immunoreactive bands were visualized by autoradiography using ECL (Amersham Biosciences). Gelatin zymography for aortic tissue extract and SMC conditioned media was performed as described previously by Longo et al.26, with 0.8% gelatin in a 10% SDS-polyacrylamide gel. The molecular sizes were determined using protein standards from Fermentas (Glen Burnie, MA).
Statistical analyses
Data are presented as mean ± SE. Life table analysis was used for the Kaplan-Meier survival curve. Statistical significance (P < .05) for all other variables was determined by analysis of variance (ANOVA).
Results
Doxycycline and losartan inhibit phosphorylation of Erk1/2 and Smad2 while only doxycycline decreases expression of MMP-2 and MMP-9
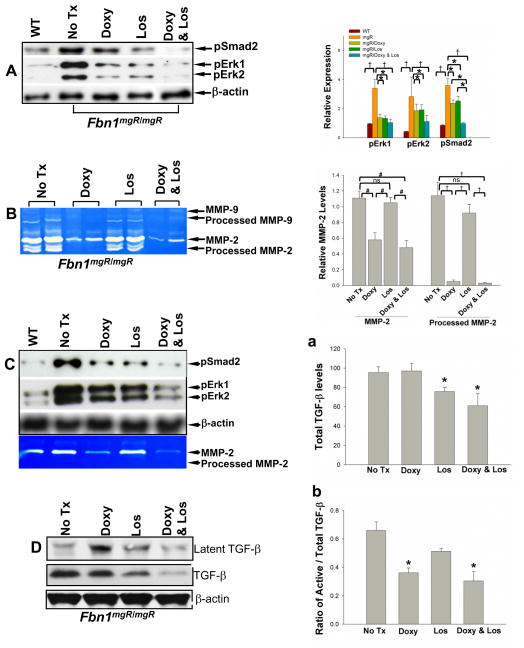
Tissue MMPs are increased in aortic tissue from mouse models of MFS and patients affected with MFS16, 18, 19, 27, 28. We have previously demonstrated that doxycycline, a nonspecific MMP-inhibitor, decreased MMP-2 and -9 expression in the aorta and delayed aneurysm formation and rupture of Fbn1mgR/mgR mice18. In Fbn1mgR/mgR mice, fibrillin-1 is normal but expression is decreased to <25% of levels expressed in wild type mice29. This represents a relatively severe model of disease and since mice typically die of aortic rupture by 12 weeks of age, allows study of both aneurysm progression and rate of rupture18, 29. We concluded in a previous study that the protective effect of doxycycline was secondary to interference with direct elastin degradation by these specific MMPs. Subsequent work by our group has demonstrated that deletion of MMP-2 and -9 had little impact on the ability of macrophages to degrade elastin20, leading us to consider an alternative explanation for the efficacy of doxycycline; that inhibition of MMP-2 and -9 resulted in decreased levels of active TGF-β. The levels of phosphorylated Smad2 and Erk1/2 were assessed in the aorta of the Fbn1mgR/mgR mouse with or without doxycycline treatment using Western blots. Analysis confirmed that both canonical (Smad) and noncanonical (Erk) signaling downstream of TGF-β was increased in the Fbn1mgR/mgR mice compared to wild type controls (Figure 1A). Doxycycline effectively down-regulated both pSmad2 and pErk1/2 (Figure 1A). Losartan is known to decrease TGF-β expression and previous work performed in a less severe murine model of MFS, mice heterozygous for a cysteine substitution at position 1039 (Fbn1C1039G/+ mice), has demonstrated decreased phosphorylation of Erk1/2 and Smad211, 14. We corroborated those findings in the Fbn1mgR/mgR mouse, demonstrating that losartan had an effect similar in magnitude to that observed with doxycycline treatment (Figure 1A)22. The inhibitory mechanisms through which doxycycline (decreased release of active TGF-β from LAP by MMP-2 and -9) and losartan (decreased expression of TGF-β) are distinct but both result in less TGF-β signaling. Considering that both pathways converge at TGF-β, it would be difficult to predict whether both drugs together would have an additive effect. We find that the combination of losartan and doxycycline results in a significant decrease of TGF-β signaling. In comparison to the effects of doxycycline or losartan alone post-hoc groupwise comparison demonstrates that the combination resulted in greater decrease in phosphorylation of Smad2 than either drug alone. A similar trend in phosphorylation of Erk1/2 was observed although this difference did not reach significance (Figure 1A). We next examined the effects of doxycycline and losartan on expression of MMP-2 and MMP-9, in part, because of previous reports suggesting that losartan might also impact MMP expression and considering that MMP expression is a downstream target of TGF-β. Gelatin zymogram, in which latent MMPs are chemically activated such that band intensity is proportional to protein content30, demonstrated increased MMP-2 expression with minimal expression of MMP-9 in the aorta of Fbn1mgR/mgR mice (Figure 1B). Doxycycline decreased MMP-2 levels while losartan-treated Fbn1mgR/mgR mouse aortas demonstrated MMP-2 levels that did not differ from untreated controls. As further validation of the results seen in the tissue, we isolated smooth muscle cells (SMC) from the aorta of Fbn1mgR/mgR mice. Cells were treated without or with doxycycline (100μg/ml), losartan (100 μg/ml), or a combination of doxycycline (100μg/ml) and losartan (100 μg/ml). Phosphorylation of Erk1/2 and Smad2 levels in the cells were examined by Western blot (Figure 1C). The culture studies confirm that while both doxycycline and losartan inhibit Smad2 and Erk1/2 phosphorylation, only doxycycline decreases MMP expression (Figure 1C, lower panel). The consistency of the results seen in culture and tissue of treated mice suggest a stable treatment effect over time.
Figure 1.
Phosphorylation of Smad2 and Erk1/2 in the aortic tissues and smooth muscle cells from Fbn1mgR/mgR mice and wild type (WT) littermate controls analyzed by Western blotting (A&C) and MMP-2 and -9 levels analyzed by gelatin zymography (B&C). At the age of 8 weeks, mouse thoracic aortas were harvested from WT control and Fbn1mgR/mgR mice treated without, or with doxycycline (Doxy)(0.5 g/L), or losartan (Los)(0.6 g/L), or doxycycline (0.5 g/L) and losartan (0.6 g/L) added to the drinking water. Aortic proteins were extracted. A) Representative Western blot showing the immunoreactivity to antibodies for phosphorylated smad2 and Erk1/2 (left panel) and relative expression was quantified (right panel) using 5 or more aortic specimens per group; B) A representative gelatin zymogram of MMP-2 and -9 expression using 10 μg of aortic protein per lane with quantification of relative MMP-2 levels in the aortas (n=4) (right panel). C) Aortic smooth muscle cells from wild type and Fbn1mgR/mgR mice were isolated and received no treatment (none), doxycycline (100 μg/ml), losartan (100 μg/ml), or combination of doxycycline (100 μg/ml) and losartan (100 μg/ml). Cellular proteins (25 μg) were analyzed for phosphorylation of Smad2 and Erk1/2 using Western blot (upper panel); MMP-2 expression in cell conditioned media were analyzed by zymography (lower panel). D) TGF-β levels in the aortic tissue of Fbn1mgR/mgR mice with or without treatment were analyzed by Western blotting. Total TGF-β and the ratio active TGF-β to total TGF-β were quantified (n=4) (a&b). Western blots and zymograms are representatives of 3–5 separate experiments. β-actin expression served as internal loading control, * p < 0.05; # p < 0.01; † p < 0.001.
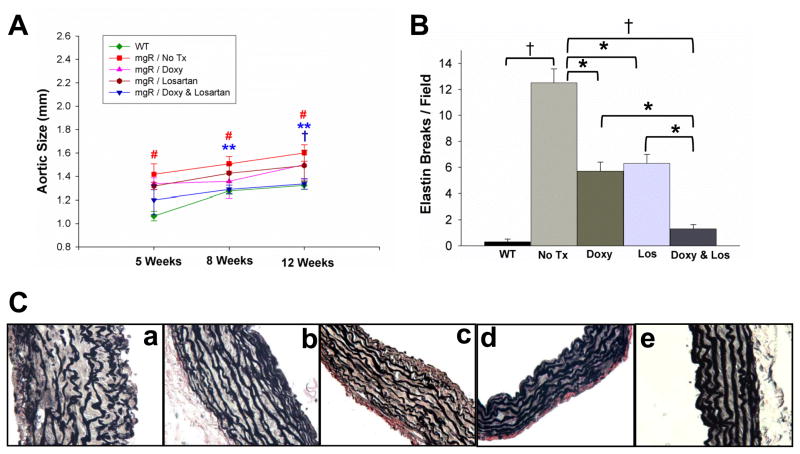
We further examined the effect of doxycycline and losartan on TGF-β expression and activation using Western blots (Figure 1D). The effects of losartan and doxycycline were distinct: losartan decreased total TGF-β levels (Figure 1Da) while doxycycline decreased the ratio of active to total TGF-β (Figure 1Db). The findings regarding losartan are confirmatory31 while the observation related to doxycycline suggests an alternative mechanism to a presumed direct role for MMP-2 in the elastin degradation seen during aneurysm formation. Treatment of doxycycline, losartan, and a combination of doxycycline and losartan prevent elastic fiber degradation and inhibit aortic dilatation in Fbn1mgR/mgR mice. Disruption of the orderly elastic lamellae, progressive dilatation and rupture represent the natural history of MFS in patients without surgical intervention. The Fbn1mgR/mgR mice demonstrate the same progression of events. Based on the trend suggesting a greater decrease in Erk1/2 phosphorylation seen when doxycycline and losartan were combined, we considered whether combination therapy could result in greater preservation of elastin fiber integrity and inhibition of aneurysmal dilatation. The ascending aortic diameters of wild type or Fbn1mgR/mgR mice receiving no treatment or treatment with doxycycline, losartan, or doxycycline and losartan were measured with high frequency ultrasound at 5, 8, and 12 weeks of age. The aortic diameter increased with age in all groups. The diameters of wild type mice were significantly smaller than untreated Fbn1mgR/mgR mice at all time points (Figure 2A). Either doxycycline or losartan inhibited dilatation while the combination showed significantly greater inhibition such that the diameter of the combined treatment group did not differ from the wild type aortic diameter at the 8 and 12 week time points (Figure 2A, Table 1). At the 12 weeks-time-point, the combination of doxycycline and losartan further inhibited aortic growth compared to doxycycline or losartan alone (Figure 2A, Table 1). Previous studies of the Fbn1mgR/mgR mouse aorta demonstrate medial thickening with progressive fragmentation of aortic elastic lamellae18, 29. We studied the effects of each drug or the combination on aortic histology. Untreated Fbn1mgR/mgR mice demonstrate medial hypertrophy and fragmentation of the elastin lamellae (Figure 2Ca). Doxycycline- or losartan-treated Fbn1mgR/mgR mice demonstrate a lesser degree of medial hypertrophy and elastin fragmentation (Figure 2Cb & c), a finding consistent with previous observations in Fbn1C1039G/+ mice11, 18. The aortic elastic lamella in Fbn1mgR/mgR mice receiving combined treatment with doxycycline and losartan (Figure 2Cd) was similar in thickness and elastin morphology to wild type control mice (Figure 2Ce). Elastin breaks were quantitated. Elastin degradation was significantly less with doxycycline or losartan treatment than untreated Fbn1mgR/mgR mice. Elastin integrity is further improved with doxycycline and losartan in combination (Figure 2B). These data demonstrate that both doxycycline and losartan have an impact on preservation of aortic fiber integrity and aortic growth and that combination therapy is more effective than either drug alone.
Figure 2.
Doxycycline and losartan effects on aneurysm expansion and elastin degradation. A) Aortic diameters for wild type littermate and Fbn1mgR/mgR (mgR) mice receiving no treatment (No Tx) or doxycycline (Doxy)(0.5 g/L), losartan (Los) (0.6 g/L) or a combination were measured by high frequency ultrasound at 5, 8, and 12 weeks of ages. The aortic diameter of untreated Fbn1mgR/mgR mice were significantly enlarged compared with wild type control at all time points, # p < 0.002. Doxycycline- and losartan-treatment significantly inhibited aortic enlargement of Fbn1mgR/mgR mice at 8 and 12 weeks-time-points as compared with untreated Fbn1mgR/mgR mice (** p = 0.0054 and p = 0.0060, respectively). At 12 weeks-time-point, combination of doxycycline and losartan further inhibited aortic growth compared to doxycycline and losartan alone, † p<0.05. C) Verhoeff-van Gieson staining of elastic fibers of the ascending aorta of wild type and Fbn1mgR/mgR mice treated with or without of doxycycline and losartan examined at 8 weeks of age; Fbn1mgR/mgR mice without treatment (a), doxycycline (0.5 g/L) treatment (b), losartan (0.6 g/L) treatment (c), or combination of doxycycline (0.5 g/L) and losartan (0.6 g/L) (d). Wild type littermate (e). B) Elastin breaks per field under 40x magnification in wild type (WT) and Fbn1mgR/mgR mice: No Tx, or Doxy, or Los, or Doxy &Los (n= 3–5 aortas/group). * p < 0.05; † p < 0.001.
Table 1.
The ascending aortic diameter changes of WT and Fbn1mgR/mgR mice treated with or without doxycycline (Doxy) and losartan (Los) measured with echocardiogram
| Age(weeks) | Mouse | Treatment | Aortic diameter (mm) | Number (n) |
|---|---|---|---|---|
| 5 | WT | None | 1.06 ± 0.04 | 11 |
| Fbn1mgR/mgR | None | 1.41 ± 0.09 | 17 | |
| Fbn1mgR/mgR | Doxy | 1.33 ± 0.05 | 19 | |
| Fbn1mgR/mgR | Los | 1.34 ± 0.03 | 6 | |
| Fbn1mgR/mgR | Doxy&Los | 1.20 ± 0.12 | 6 | |
|
| ||||
| 8 | WT | None | 1.27 ± 0.03 | 15 |
| Fbn1mgR/mgR | None | 1.51 ± 0.06 | 19 | |
| Fbn1mgR/mgR | Doxy | 1.35 ± 0.14 | 8 | |
| Fbn1mgR/mgR | Los | 1.43 ± 0.08 | 6 | |
| Fbn1mgR/mgR | Doxy&Los | 1.29 ± 0.04 | 8 | |
|
| ||||
| 12 | WT | None | 1.32 ± 0.03 | 21 |
| Fbn1mgR/mgR | None | 1.60 ± 0.07 | 9 | |
| Fbn1mgR/mgR | Doxy | 1.50 ± 0.12 | 12 | |
| Fbn1mgR/mgR | Los | 1.49 ± 0.13 | 6 | |
| Fbn1mgR/mgR | Doxy&Los | 1.34 ± 0.04 | 5 | |
Treatment of Fbn1mgR/mgR mice with doxycycline, losartan, and combination of doxycycline and losartan delays aortic rupture
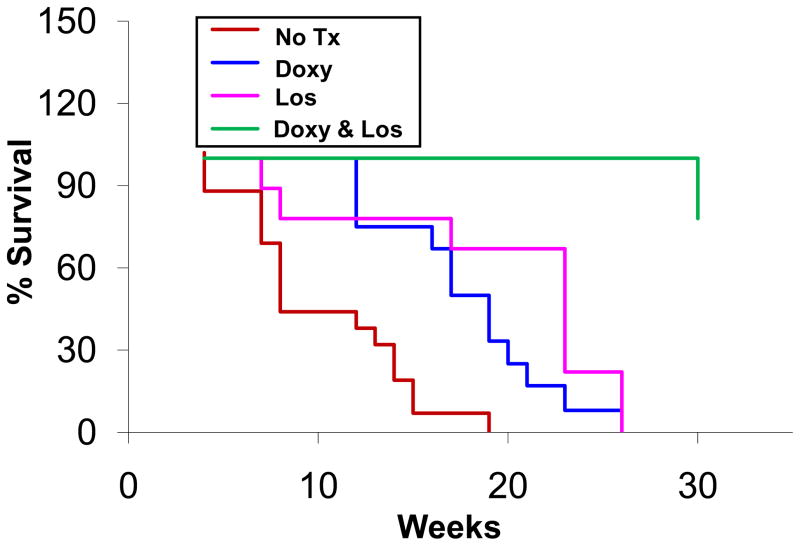
Our previous studies have shown that doxycycline prolongs the lifespan of Fbn1mgR/mgR mice18. Other groups have investigated the effects of losartan or doxycycline using the Fbn1 C1039G/+ mice, a murine model of MFS that is less severely affected and typically does not progress to rupture13. In these studies, preservation of aortic histology inferred protection from the primary clinical endpoint, aortic rupture, but this could not be verified. Furthermore, we noted that the combination of doxycycline and losartan appears to decrease Smad2 and Erk1/2 signaling to a greater extent than either drug alone (Figure 1A). Survival studies were done in Fbn1mgR/mgR mice without treatment or with doxycycline, losartan, or combination of doxycycline and losartan administered at the same dosages as described in the previous sections. The mice were followed until death or out to 30 weeks. Autopsy was performed on 50% of the mice dying during the study and each was found to have an ascending aortic aneurysm with associated hemorrhage. Mice surviving to 30 weeks were sacrificed. The mean survival of untreated Fbn1mgR/mgR mice (n=16) and those treated with doxycycline (n=12), or losartan (n=9) was 80 ± 6.3 days, 134 ± 8.9 days and 131 ± 17.3 days, respectively (Figure 3). The mean survival for the mice treated with combination therapy could not be calculated since only one mouse died before 30 weeks. By life table analysis, the combined therapy resulted in significant prolongation of life.
Figure 3.
The effect of doxycycline and losartan on the survival of Fbn1mgR/mgR mice. Fbn1mgR/mgR mice were untreated (No Tx) or treated with doxycycline (Doxy) (0.5 g/L), or losartan (Los) (0.6 g/L), or combination of doxycycline (0.5 g/L) and losartan (0.6 g/L), in their drinking water. Life table analysis shows improved survival in mice with doxycycline (p <0.001), losartan (p < 0.01), and doxycycline & losartan (p < 0.001) compared to untreated Fbn1mgR/mgR mice. Compared with single drug treatment, combination of doxycycline and losartan further extends the life span of Fbn1mgR/mgR mice, p < 0.001.
MMP-2-deficiency results in decreased Erk1/2 phosphorylation, preserved aortic lamellar integrity and prolonged survival of Fbn1mgR/mgR mice
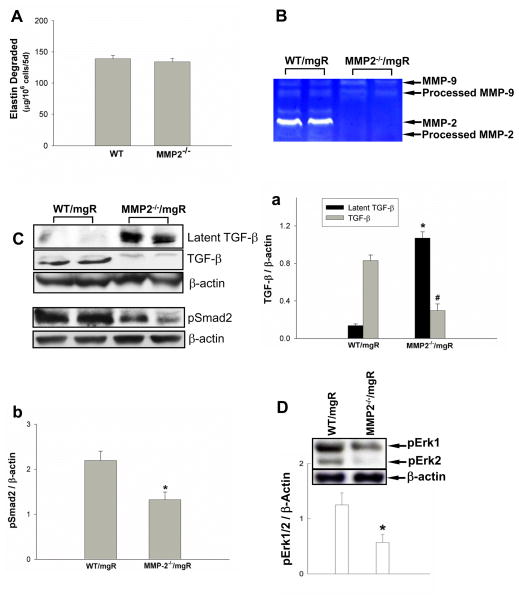
Doxycycline is a nonspecific MMP inhibitor so one cannot make conclusions regarding the role of specific MMPs when it is used. The role of MMP-2 in MFS is of particular interest since it is the primary gelatinolytic MMP secreted from aortic SMC. We examined the role of MMP-2 in elastin degradation by comparing the elastolytic activities between wild type and MMP-2 deficient aortic SMC. We found that aortic SMC elastolytic activities were not affected by deletion of MMP-2 (Figure 4A). This finding is similar to results reported in macrophages and suggests MMP-2 does not have a primary role in the elastin degradation in MFS. It has been shown in cell culture that MMP-2 can efficiently release TGF-β by cleavage of the latency-associated peptide (LAP)15. This second step in the activation of TGF-β can only occur after the LLC is released from matrix, as occurs in the presence of mutant fibrillin-19. In order to assess the specific role of MMP-2, we developed MMP-2 null (MMP-2−/−) Fbn1mgR/mgR mice as described in the methods section. Aortic MMP-9 expression was not affected by the deletion of MMP-2 (Figure 4B). We hypothesized that MMP-2 deficiency might affect activation of latent TGF-β complexes. We assessed the abundance of LAP-TGF-β and mature TGF-β in the mouse aortic tissues. We observed higher immunoreactivity for LAP-TGF-β and lower active TGF-β in MMP-2-deficicent Fbn1mgR/mgR mice compared with Fbn1mgR/mgR mice expressing wild type MMP-2 (Figure 4C upper panel & Ca), demonstrating the important role that MMP-2 plays in activation of TGF-β in Fbn1mgR/mgR mouse aorta. To determine MMP-2 effect on TGF-β signaling in vivo, we assessed the levels of phosphorylated Smad2 and Erk1/2. Western blot data revealed decreased levels of phosphorylated Smad2 and Erk1/2 in MMP-2-deficient Fbn1mgR/mgR mice (Figure 4C upper panel&b & 4D).
Figure 4.
The effects of MMP-2-deletion on elastolytic activities and TGF-β signaling. A) SMC from wild type (WT) and MMP-2−/− mice were co-cultured with [3H]elastin and incubated with 50 μg/ml ConA. The elastolytic activities were quantified. Results are expressed as μg elastin degraded/106 cells for 5 days (mean ± SE; n=3). B) MMP-2 and MMP-9 expression in the aortas of MMP-2+/+/fbn1mgR/mgR (WT/mgR) and MMP-2−/−/fbn1mgR/mgR (MMP-2−/−/mgR) mice at 8 weeks of age were analyzed by zymography. WT/mgR and MMP-2−/−/mgR mouse aortic tissues expressed similar levels of MMP-9. C) TGF-β levels and Smad2 phosphorylation in the aortic tissue of WT/mgR and MMP-2−/−/mgR mice were analyzed by Western blotting; latent and active TGF-β (n = 4) (a) and pSmad2 (n =4) (b) were quantified. D) Representative Western blot analysis of Erk1/2 phosphorylation in the aorta of WT/mgR and MMP-2−/−/mgR mice (bar graph represents 6/group). * p < 0.05; # p < 0.01.
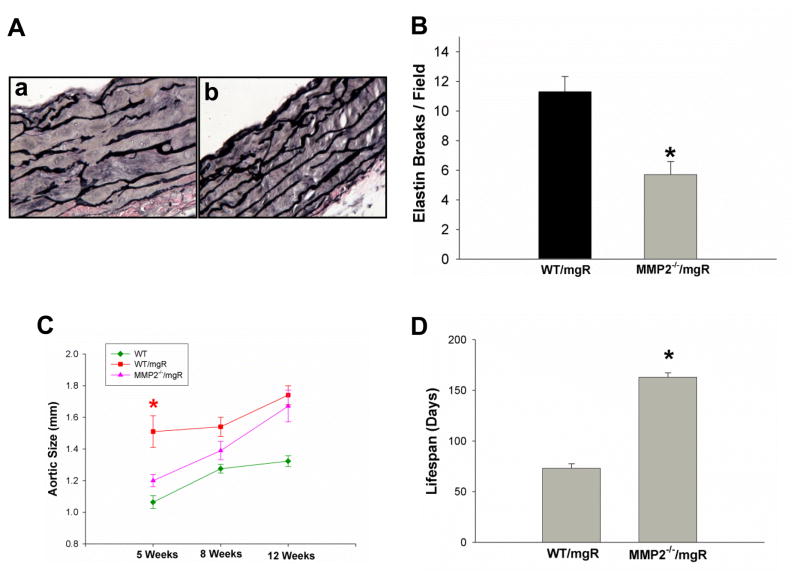
To determine whether reduced TGF-β activation and signaling contributed to aortic lamellar preservation and extension of lifespan in MMP-2-deficient Fbn1mgR/mgR mice, we examined histological changes in mouse aorta and recorded lifespan of MMP-2-deficient Fbn1mgR/mgR mice. Study of the ascending aorta at 8 weeks of age demonstrated elastin degradation or medial hypertrophy (Figure 5Ab & B) was significantly less than their controls, WT/Fbn1mgR/mgR mice (Figure5Aa & B). Using high frequency ultrasound, the diameter of the ascending aorta was found to be significantly less than that of the Fbn1mgR/mgR mice at 5 weeks with the difference becoming progressively less at 8 and 12 weeks (Figure 5C). The MMP-2−/−/Fbn1mgR/mgR mice lived significantly longer (163.4 ± 18.5 days, n=36) than littermate control mice expressing MMP-2 (MMP-2+/+Fbn1mgR/mgR) (77 ± 4.6 days, n=36) (Figure 5D). Taken together, these data demonstrate a specific role for MMP-2 through the activation of TGF-β and its downstream signaling pathways, particularly Erk1/2, the critical pathway for aortic pathology in MFS.
Figure 5.
The effects of MMP-2-deletion on Fbn1mgR/mgR mice. A) Verhoeff-van Gieson staining of elastic fibers of thoracic ascending aortas of MMP-2+/+/fbn1mgR/mgR (WT/mgR)(a) and MMP-2−/−/fbn1mgR/mgR (MMP-2−/−/mgR)(b) mice at 8 weeks of age, representative images from 3–5 independent experiments. B) Elastin breaks per field under 40x magnification in WT/mgR and MMP-2−/−/mgR mice (n= 3–5 aortas/group); C) Ascending thoracic aorta diameter measured by high frequency ultrasound in WT/mgR and MMP-2−/−/mgR mice at 5, 8, and 12 weeks of age (* p = 0.0283); D) comparison of average lifespan of WT/mgR with MMP-2−/−/mgR, *p < 0.0001.
Discussion
The most deadly feature of Marfan syndrome (MFS) is dilatation of the aortic root that, untreated, leads to rupture or dissection and sudden death3. When rupture risk is deemed to exceed risk of intervention, surgical repair of the aortic valve and ascending aorta is undertaken and has been shown to improve survival32. An effective medical therapy that could impact the natural history of the disease would be valuable, not only during the window of observation before repair, but lifelong as the disease is progressive, leading to pathologic changes in other vascular beds and organ systems. β-adrenergic blockers have been a mainstay of cardiovascular therapy for MFS but clinical data are not entirely consistent in showing benefit and, even if effective, there remains a significant margin for improvement7, 8. Transgenic mouse models that alter the expression or sequence of the fibrillin-1 gene in combination with the identification of receptor mutations that increase TGF-β signaling in Marfan-like syndromes have led to our current understanding of a central role for the TGF-β signaling axis in the pathogenesis of MFS33, 34. In the present study, we demonstrate a specific role for MMP-2 in MFS, not through its elastolytic activity, but rather, through its ability to activate TGF-β and augment TGF-β signaling. Doxycycline decreases MMP-2 expression, Smad2 and Erk1/2 phosphorylation and delays aneurysm rupture. Combination drug therapy with agents directed against two distinct targets in the TGF-β axis is more effective in inhibiting phosphorylation of Erk1/2 and disease progression than single drug therapy alone at the concentration used for this study. The concentration of doxycycline used for this study has been shown to achieve comparable plasma levels to those in aneurysm patients taking a standard therapeutic dose of doxycycline24. Our study does not exclude the possibility that by titrating one or the other drug to a higher level might achieve the same effect although using concentrations considered outside the normal therapeutic range raises concerns of toxicity and translational value.
Matrix metalloproteinases are capable of degrading all of the component macromolecules of the aorta and have been implicated by their presence in surgical specimens from MFS patients16, 28. Their concentration and location become more variable in large aneurysms because of SMC apoptosis and cellular areas of cystic medial necrosis16, 35. Factors responsible for increased MMP expression are not entirely clear although fibrillin-1 fragments with the RGD binding motif or elastin-binding-protein GxxPG consensus sequences upregulate MMP expression in culture36, 37. MMPs are also a downstream product of TGF-β38. Among the MMPs in Marfan tissue, MMP-2 is of interest as a product of the medial SMCs that is capable of cleaving an unusually wide array of matrix proteins including the most abundant aortic proteins, type I collagen, and elastin21, 39. In two murine models of MFS, MMP-2 expression is markedly increased29, 35, 40. The tetracyclines are nonspecific MMP inhibitors that have been shown, at various concentrations, to affect mRNA levels, protein expression and activity of the MMPs41–43. They have clinical efficacy in a number of conditions where MMP over-expression causes tissue injury42, 44, 45. We have previously demonstrated that doxycycline inhibited MMP-2 and MMP-9 in the Fbn1mgR/mgR mouse model of MFS18, a finding corroborated in a subsequent study by Chung et al using a more chronic MFS model, the Fbn1C1039G/+mouse19. Serial aortic measurements obtained with high frequency ultrasound in the present study provide some new insight in demonstrating that doxycycline inhibits expansion until eight weeks of age. Thereafter, the aorta expands at a rate similar to untreated Marfan mice. This is consistent with survival data demonstrating that rupture is not prevented, but only delayed, by doxycycline. This protective effect correlates with decreased MMP-2, but since the array of molecular targets for doxycycline is not well defined, these studies cannot define a causal role for MMP-2.
Murine models of MFS have provided new insights into aortic disease progression9, 12, 29, implicating increased TGF-β signaling in the pathogenesis of the aortic dilatation46–48. Active TGF-β is difficult to measure directly but both TGF-β blocking antibodies and losartan, an angiotensin II receptor blocker that decreases TGF-β expression, inhibit Smad2 phosphorylation, the canonical signaling pathway of TGF-β; this restores, to an extent, aortic architecture and root dimension11. More recent work has demonstrated that noncanonical Erk1/2 phosphorylation is the critical pathway for aortic pathogenesis14, 22. In the present study, we demonstrate a novel effect of doxycycline, its ability to decrease Erk1/2 phosphorylation. When combined with losartan, Erk1/2 phosphorylation is additively decreased resulting in complete preservation of aortic lamellar structure, prevention of dilatation and improvement in survival. Again, the serial ultrasound measurement were informative in demonstrating that the combined treatment exhibits both an early and prolonged effect such that by 12 weeks of age, the aortic diameter of treated mice does not differ from the diameter of age-matched, wild type mice. Only a single mouse treated with both drugs died by the end of the study (30 wks).
The tetracyclines are used therapeutically as MMP inhibitors. We have shown previously that doxycycline decreases MMP-2 mRNA in SMC culture but whether this is a direct effect is unclear43. If doxycycline inhibited the TGF-β axis through an alternative pathway, MMPs, as a downstream product of TGF-β, would be decreased. Alternatively, decreased Erk1/2 phosphorylation could result from decreased MMP expression. Yu and Stamenkovic were the first to demonstrate the ability of MMP-2 to cleave and activate TGF-β1-349. The stepwise process of TGF-β activation was further delineated by Ge and Greenspan who found that the initial step requires release of the LLC from matrix15. Only after this can the latent associated peptide (LAP), that covers the active site of TGF-β, be cleaved. MMP-2, but not MMP-3 or -9 siRNA, blocked removal of the LAP and release of biologically active TGF-β. Since the first step in this process is facilitated by mutations in the fibrillin-1 gene to which the LLC binds, MMP-2 would be expected to have an exaggerated role in the Marfan aorta.
MMP-2 can degrade elastin but, in vitro, activated MMP-2 has minimal activity relative to pancreatic elastase (data not shown) and we have previously demonstrated that the ability of murine macrophages to degrade radiolabeled elastin was not impacted by a deficiency of MMP-2 or MMP-920. These findings suggest the gelatinases are indirectly involved in matrix degradation. In order to better understand the role that MMP-2 plays in MFS, we created Fbn1mgR/mgR mice that were null for MMP-2. MMP-2 deletion had a significant protective effect. Based on serial ultrasound imaging, the benefit was the greatest before 5 weeks, with dilatation progressing at a rate similar to MMP-2+/+ Fbn1mgR/mgR littermate controls thereafter. As was observed with doxycycline or losartan alone, MMP-2 deletion delayed but did not prevent aortic dilatation and eventual rupture. Importantly, this protective effect was associated with decreased phosphorylation of Erk1/2 demonstrating a specific role for MMP-2 in the critical signaling pathway involved in the aortic pathology of MFS.
In summary, combination therapy with doxycycline and losartan was more effective than either drug alone in the Fbn1mgR/mgR model of MFS resulting in normalization of aortic histology and diameter at 8 and 12 weeks, respectively. Yang et al also observed improved aortic histology with combined therapy in Fbn1C1039G/+ mice, a more chronic model that does not typically progress to rupture13. By creating transgenic MMP-2 deficient Marfan mice, we were able to demonstrate that MMP-2 has a key role in MFS and that this is independent of its direct elastolytic activity. In this setting of MFS, where binding of the LLC to matrix is compromised, MMP-2 would be expected to have a greater impact and, considering that MMP-2 is a downstream product of TGF-β, a positive feedback loop could be established. Thus, MMP-2 appears to be a potentially important target. Observations made in acute murine models must be interpreted carefully and only translated to patients in controlled trials. Losartan is being prospectively evaluated in a number of clinical trials. Developing the infrastructure and recruiting patients to these trials is costly. If the losartan proves to have less benefit than hoped for, extending the current trials to evaluate additional therapies, including doxycycline, should be considered.
Supplementary Material
Novelty and Significance.
What Is Known?
Marfan syndrome (MFS) is caused by mutations in the fibrillin 1 gene.
Aneurysms in MFS are caused by increased TGF-β signaling.
Matrix metalloproteinases (MMPs) have an important role in aneurysm development in MFS.
What New Information Does This Article Contribute?
MMP-2 deletion or antagonism delays aneurysm development and rupture in mice with MFS.
MMP-2 works primarily through release of active TFG-β from the large latent complex.
Clinical evaluation of the combination of doxycycline and losartan should be considered in patients with MFS.
Marfan syndrome is a genetic disorder associated with abnormalities in multiple organ systems; aneurysm development is the most lethal of its complications. Beta-blockade may have some minor impact on progression and losartan has not yet shown benefit despite many ongoing trials. Doxycycline delays aneurysm formation in mice with MFS but its efficacy compared to losartan and precise mechanism is unknown. The present study demonstrated that losartan and doxycycline were equally effective in delaying, but not preventing, aneurysm formation and rupture. Doxycycline exerted its positive effects by inhibiting MMP-2. MMP-2 is responsible for release of active TGF-β from the large latent complex and a subsequent increase in downstream Erk 1/2 signaling. Combination therapy with doxycycline and losartan additively reduced Erk 1/2 signaling, delaying aneurysm formation and rupture. The study suggests that 1) MMP-2 may be an important target in delaying disease progression in patients with Marfan syndrome and 2) combination therapy with losartan and doxycycline may be more effective than either drug alone.
Acknowledgments
The Fbn1mgR/mgR mice were provided by the laboratory of Francesco Ramirez, The Mount Sinai School of Medicine. Anna M. Baxter assisted with preparation of the figures. This work was supported by the National Institutes of Health NHLBI grant 974015N (to B.T.B) and the National Marfan Foundation (to W.X).
Abbreviations
- MFS
Marfan syndrome
- TGF-β
transforming growth factor-beta
- MMPs
Matrix metalloproteinases
- FBN1
fibrillin-1
- SMC
smooth muscle cells
Footnotes
Disclosures
None.
References
- 1.Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 2.Dietz HC, Pyeritz RE. Mutations in the human gene for fibrillin-1 (fbn1) in the marfan syndrome and related disorders. Hum Mol Genet. 1995;4(Spec No):1799–1809. doi: 10.1093/hmg/4.suppl_1.1799. [DOI] [PubMed] [Google Scholar]
- 3.Murdoch JL, Walker BA, Halpern BL, Kuzma JW, McKusick VA. Life expectancy and causes of death in the marfan syndrome. N Engl J Med. 1972;286:804–808. doi: 10.1056/NEJM197204132861502. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez F, Dietz HC. Marfan syndrome: From molecular pathogenesis to clinical treatment. Curr Opin Genet Dev. 2007 doi: 10.1016/j.gde.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Prokop EK, Palmer RF, Wheat MW., Jr Hydrodynamic forces in dissecting aneurysms. In-vitro studies in a tygon model and in dog aortas. Circulation research. 1970;27:121–127. doi: 10.1161/01.res.27.1.121. [DOI] [PubMed] [Google Scholar]
- 6.Engelfriet P, Mulder B. Is there benefit of beta-blocking agents in the treatment of patients with the marfan syndrome? International journal of cardiology. 2007;114:300–302. doi: 10.1016/j.ijcard.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Gersony DR, McClaughlin MA, Jin Z, Gersony WM. The effect of beta-blocker therapy on clinical outcome in patients with marfan’s syndrome: A meta-analysis. International journal of cardiology. 2007;114:303–308. doi: 10.1016/j.ijcard.2005.11.116. [DOI] [PubMed] [Google Scholar]
- 8.Selamet Tierney ES, Feingold B, Printz BF, Park SC, Graham D, Kleinman CS, Mahnke CB, Timchak DM, Neches WH, Gersony WM. Beta-blocker therapy does not alter the rate of aortic root dilation in pediatric patients with marfan syndrome. The Journal of pediatrics. 2007;150:77–82. doi: 10.1016/j.jpeds.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of tgf-beta activation contributes to pathogenesis in marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 10.Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC. Tgf-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an at1 antagonist, prevents aortic aneurysm in a mouse model of marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of marfan syndrome. J Clin Invest. 2004;114:172–181. doi: 10.1172/JCI20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang HH, Kim JM, Chum E, van Breemen C, Chung AW. Effectiveness of combination of losartan potassium and doxycycline versus single-drug treatments in the secondary prevention of thoracic aortic aneurysm in marfan syndrome. The Journal of thoracic and cardiovascular surgery. 2010;140:305–312. e302. doi: 10.1016/j.jtcvs.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, Loeys BL, Thomas CJ, Patnaik S, Marugan JJ, Judge DP, Dietz HC. Noncanonical tgfbeta signaling contributes to aortic aneurysm progression in marfan syndrome mice. Science. 2011;332:358–361. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge G, Greenspan DS. Bmp1 controls tgfbeta1 activation via cleavage of latent tgfbeta-binding protein. J Cell Biol. 2006;175:111–120. doi: 10.1083/jcb.200606058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, Zeeshan A, Bavaria JE, Gorman JH, 3rd, Spinale FG, Gorman RC. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with marfan syndrome. Circulation. 2006;114:I365–370. doi: 10.1161/CIRCULATIONAHA.105.000810. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, Monticone R, Lakatta EG. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (tgf-beta1) and tgf-beta1-type ii receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26:1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 18.Xiong W, Knispel RA, Dietz HC, Ramirez F, Baxter BT. Doxycycline delays aneurysm rupture in a mouse model of marfan syndrome. J Vasc Surg. 2008;47:166–172. doi: 10.1016/j.jvs.2007.09.016. discussion 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung AW, Yang HH, Radomski MW, van Breemen C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circulation research. 2008;102:e73–85. doi: 10.1161/CIRCRESAHA.108.174367. [DOI] [PubMed] [Google Scholar]
- 20.Xiong W, Knispel R, MacTaggart J, Greiner TC, Weiss SJ, Baxter BT. Membrane-type 1 matrix metalloproteinase regulates macrophage-dependent elastolytic activity and aneurysm formation in vivo. J Biol Chem. 2009;284:1765–1771. doi: 10.1074/jbc.M806239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadar A, Bihari-Varga M, Csonka E. The synthesis, transport and excretion of connective tissue macromolecules by smooth muscle cells. Connect Tissue Res. 1981;8:175–180. doi: 10.3109/03008208109152370. [DOI] [PubMed] [Google Scholar]
- 22.Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. Angiotensin ii type 2 receptor signaling attenuates aortic aneurysm in mice through erk antagonism. Science. 2011;332:361–365. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato T, Kure T, Chang JH, Gabison EE, Itoh T, Itohara S, Azar DT. Diminished corneal angiogenesis in gelatinase a-deficient mice. FEBS Lett. 2001;508:187–190. doi: 10.1016/s0014-5793(01)02897-6. [DOI] [PubMed] [Google Scholar]
- 24.Prall AK, Longo GM, Mayhan WG, Waltke EA, Fleckten B, Thompson RW, Baxter BT. Doxycycline in patients with abdominal aortic aneurysms and in mice: Comparison of serum levels and effect on aneurysm growth in mice. J Vasc Surg. 2002;35:923–929. doi: 10.1067/mva.2002.123757. [DOI] [PubMed] [Google Scholar]
- 25.Xiong W, MacTaggart J, Knispel R, Worth J, Persidsky Y, Baxter BT. Blocking tnf-alpha attenuates aneurysm formation in a murine model. J Immunol. 2009;183:2741–2746. doi: 10.4049/jimmunol.0803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nataatmadja M, West M, West J, Summers K, Walker P, Nagata M, Watanabe T. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108(Suppl 1):II329–334. doi: 10.1161/01.cir.0000087660.82721.15. [DOI] [PubMed] [Google Scholar]
- 28.Segura AM, Luna RE, Horiba K, Stetler-Stevenson WG, McAllister HA, Jr, Willerson JT, Ferrans VJ. Immunohistochemistry of matrix metalloproteinases and their inhibitors in thoracic aortic aneurysms and aortic valves of patients with marfan’s syndrome. Circulation. 1998;98:II331–337. discussion II337–338. [PubMed] [Google Scholar]
- 29.Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, Biery NJ, Dietz HC, Sakai LY, Ramirez F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci U S A. 1999;96:3819–3823. doi: 10.1073/pnas.96.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleiner DE, Stetler-Stevenson WG. Quantitative zymography: Detection of picogram quantities of gelatinases. Analytical biochemistry. 1994;218:325–329. doi: 10.1006/abio.1994.1186. [DOI] [PubMed] [Google Scholar]
- 31.Campistol JM, Inigo P, Jimenez W, Lario S, Clesca PH, Oppenheimer F, Rivera F. Losartan decreases plasma levels of tgf-beta1 in transplant patients with chronic allograft nephropathy. Kidney international. 1999;56:714–719. doi: 10.1046/j.1523-1755.1999.00597.x. [DOI] [PubMed] [Google Scholar]
- 32.Schwill S, Kallenbach K, Beller CJ, Karck M. An alternative surgical approach for the combined treatment of pectus excavatum and acute aortic dissection type-a in marfan syndrome. Interact Cardiovasc Thorac Surg. 2011;12:526–528. doi: 10.1510/icvts.2010.256123. [DOI] [PubMed] [Google Scholar]
- 33.Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S, Manouvrier S, Roberts AE, Faravelli F, Greco MA, Pyeritz RE, Milewicz DM, Coucke PJ, Cameron DE, Braverman AC, Byers PH, De Paepe AM, Dietz HC. Aneurysm syndromes caused by mutations in the tgf-beta receptor. N Engl J Med. 2006;355:788–798. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 34.Akutsu K, Morisaki H, Takeshita S, Sakamoto S, Tamori Y, Yoshimuta T, Yokoyama N, Nonogi H, Ogino H, Morisaki T. Phenotypic heterogeneity of marfan-like connective tissue disorders associated with mutations in the transforming growth factor-beta receptor genes. Circ J. 2007;71:1305–1309. doi: 10.1253/circj.71.1305. [DOI] [PubMed] [Google Scholar]
- 35.Nataatmadja M, West J, West M. Overexpression of transforming growth factor-beta is associated with increased hyaluronan content and impairment of repair in marfan syndrome aortic aneurysm. Circulation. 2006;114:I371–377. doi: 10.1161/CIRCULATIONAHA.105.000927. [DOI] [PubMed] [Google Scholar]
- 36.Booms P, Ney A, Barthel F, Moroy G, Counsell D, Gille C, Guo G, Pregla R, Mundlos S, Alix AJ, Robinson PN. A fibrillin-1-fragment containing the elastin-binding-protein gxxpg consensus sequence upregulates matrix metalloproteinase-1: Biochemical and computational analysis. J Mol Cell Cardiol. 2006;40:234–246. doi: 10.1016/j.yjmcc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Booms P, Pregla R, Ney A, Barthel F, Reinhardt DP, Pletschacher A, Mundlos S, Robinson PN. Rgd-containing fibrillin-1 fragments upregulate matrix metalloproteinase expression in cell culture: A potential factor in the pathogenesis of the marfan syndrome. Human genetics. 2005;116:51–61. doi: 10.1007/s00439-004-1194-7. [DOI] [PubMed] [Google Scholar]
- 38.Kim HS, Shang T, Chen Z, Pflugfelder SC, Li DQ. Tgf-beta1 stimulates production of gelatinase (mmp-9), collagenases (mmp-1, -13) and stromelysins (mmp-3, -10, -11) by human corneal epithelial cells. Exp Eye Res. 2004;79:263–274. doi: 10.1016/j.exer.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park JM, Kim A, Oh JH, Chung AS. Methylseleninic acid inhibits pma-stimulated pro-mmp-2 activation mediated by mt1-mmp expression and further tumor invasion through suppression of nf-kappab activation. Carcinogenesis. 2007;28:837–847. doi: 10.1093/carcin/bgl203. [DOI] [PubMed] [Google Scholar]
- 41.Vidal A, Sabatini M, Rolland-Valognes G, Renard P, Madelmont JC, Mounetou E. Synthesis and in vitro evaluation of targeted tetracycline derivatives: Effects on inhibition of matrix metalloproteinases. Bioorg Med Chem. 2007;15:2368–2374. doi: 10.1016/j.bmc.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Morlandt AB, Xu X, Carnes DL, Jr, Chen Z, Steffensen B. Tetracycline at subcytotoxic levels inhibits matrix metalloproteinase-2 and -9 but does not remove the smear layer. J Periodontol. 2005;76:1129–1139. doi: 10.1902/jop.2005.76.7.1129. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Xiong W, Baca-Regen L, Nagase H, Baxter BT. Mechanism of inhibition of matrix metalloproteinase-2 expression by doxycycline in human aortic smooth muscle cells. J Vasc Surg. 2003;38:1376–1383. doi: 10.1016/s0741-5214(03)01022-x. [DOI] [PubMed] [Google Scholar]
- 44.Carmeli E, Kodesh E, Nemcovsky C. Tetracycline therapy for muscle atrophy due to immobilization. J Musculoskelet Neuronal Interact. 2009;9:81–88. [PubMed] [Google Scholar]
- 45.Franklin IJ, Harley SL, Greenhalgh RM, Powell JT. Uptake of tetracycline by aortic aneurysm wall and its effect on inflammation and proteolysis. Br J Surg. 1999;86:771–775. doi: 10.1046/j.1365-2168.1999.01137.x. [DOI] [PubMed] [Google Scholar]
- 46.Dietz HC, Loeys B, Carta L, Ramirez F. Recent progress towards a molecular understanding of marfan syndrome. American journal of medical genetics. 2005;139C:4–9. doi: 10.1002/ajmg.c.30068. [DOI] [PubMed] [Google Scholar]
- 47.Boileau C, Jondeau G, Mizuguchi T, Matsumoto N. Molecular genetics of marfan syndrome. Current opinion in cardiology. 2005;20:194–200. doi: 10.1097/01.hco.0000162398.21972.cd. [DOI] [PubMed] [Google Scholar]
- 48.Kaartinen V, Warburton D. Fibrillin controls tgf-beta activation. Nat Genet. 2003;33:331–332. doi: 10.1038/ng0303-331. [DOI] [PubMed] [Google Scholar]
- 49.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates tgf-beta and promotes tumor invasion and angiogenesis. Genes & development. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.