Abstract
The addition of adjuvant chemotherapy to hormonal therapy is recommended for patients with estrogen receptor–positive (ER+), node-positive (N+) early breast cancer (EBC). Some of these patients, however, are not likely to benefit from treatment and may, therefore, be overtreated while also incurring unnecessary treatment-related adverse events and health care costs. The 21-gene Recurrence Score assay has been clinically validated and recommended for use in patients with ER+, node-negative (N0) EBC to assess the 10-year risk of distant disease recurrence and predict the likelihood of response to adjuvant chemotherapy. A growing body of evidence from several large phase III clinical trials reports similar findings in patients with ER+, N+ EBC. A systematic review of published literature from key clinical trials that have used the 21-gene breast cancer assay in patients with ER+, N+ EBC was performed. The Recurrence Score has been shown to be an independent predictor of disease-free survival, overall survival, and distant recurrence-free interval in patients with ER+, N+ EBC. Outcomes from decision impact and health economics studies further indicate that the Recurrence Score affects physician treatment recommendations equally in patients with N+ or N0 disease. It also indicates that a reduction in Recurrence Score–directed chemotherapy is cost-effective. There is a large body of evidence to support the use of the 21-gene assay Recurrence Score in patients with N+ EBC. Use of this assay could help guide treatment decisions for patients who are most likely to receive benefit from chemotherapy.
Key Words: gene expression profiling, prognosis, breast cancer, cost-effectiveness, 21-gene recurrence score
There were an estimated 227,000 new cases of breast cancer diagnosed in women in the United States in 2012 and an estimated 39,500 breast cancer deaths that same year.1 In approximately half of the women newly diagnosed with early-stage breast cancer (EBC), the cancer has spread to nearby lymph nodes but has not yet metastasized to more distant parts of the body.2 In treating these patients, the risk of distant recurrence (DR) and the potential benefit obtained from chemotherapy treatment must be balanced against the risk of adverse events associated with its use and the possibility of overtreatment without additional benefit.3 Current guidelines recommend the addition of adjuvant chemotherapy to hormonal therapy for patients with estrogen receptor–positive (ER+), node-positive (N+) EBC,4 yet many of these patients may remain disease-free even if they do not receive chemotherapy.5 Thus, a proportion of patients with N+ EBC may be overtreated, increasing health care costs and exposing patients to the toxic adverse events of chemotherapy, with little additional benefit.
There is a need for improved risk assessment of patients with EBC to guide decisions about the use of surgery, chemotherapy, hormonal therapy, and/or radiation therapy.6,7 EBC is a heterogenous disease, not only in its characteristics and clinical course, but also in its molecular profile. Current methods for assessing disease prognosis, which use clinical and pathologic factors such as age, tumor size, tumor grade, and extent of nodal involvement in their evaluation, are limited in value for estimating the risk of DR8 due to variability in the measure and because the risk estimate is based on an average and is not specific for the individual patient. Genomic profiles such as the 21-gene Recurrence Score provide a quantitative estimate of the 10-year risk of DR, as well as stratifying patients with ER+ EBC into discrete risk categories, and is informative for identifying women who are likely to benefit from adjuvant chemotherapy and women who have a much lower likelihood of benefitting.9,10
A number of prognostic tools have been developed to predict outcome in women with EBC. The past decade has seen the emergence of assays that predict the risk of DR. These new prognostic tools may be based on specific protein expression on the tumor cells (eg, protein-based inmmunohistochemistry [IHC]), or at the molecular level, whether it be assessing gene amplification (eg, fluorescence in situ hybridization) or gene/protein expression (eg, reverse transcription-polymerase chain reaction [RT-PCR]).11 Molecular assays that profile the expression of cancer-related genes more accurately reflect the biological state of the tumor and offer more relevant information on tumor status than anatomic characteristics, such as lymph node involvement, which do not reveal anything about the underlying heterogeneity of the tumor.
Approximately 20% of women diagnosed with EBC will experience recurrence at a distant site within 10 years.5 Thus, it is important that assays differentiate patients with a higher or lower risk of DR. To be most useful, a risk assessment assay needs to demonstrate both clinical validity and clinical utility (relevance for therapeutic decision-making),12 as determined by the level of evidence (LOE; I to IV)13 generated, which depends on the design of the study in which the assay is tested. The highest LOE is level I, and a new designation of level Ib has now been defined, which uses archived tissue specimens from large, randomized, clinical studies with long-term outcomes that prospectively define the endpoints and analyses.13 A robust LOE category provides the evidence and a sound scientific foundation for clinical recommendations, guiding actual change in clinical practice.12 Studies that aim to determine clinical utility are designed differently than validation studies (level I evidence is not generated). However, studies that demonstrate either clinical validity or clinical utility are equally valuable and informative because they reflect the impact of the assay results on treatment decisions and also provide evidence for direct clinical applicability.
Assays that have been validated for use in patients with EBC include the 5-antibody IHC panel (Mammostrat; Clarient, Aliso Viejo, CA),14–16 the 70-gene signature microarray assay (MammaPrint; Agendia, Irvine, CA),17,18 the PAM50 gene expression signature probe target–based messenger ribonucleic acid (mRNA) (nonamplification) assay (Prosigna; NanoString Technologies, Seattle, WA),19,20 and the Recurrence Score RT-PCR–based assay (Oncotype DX, Genomic Health, Redwood City, CA). According to the analysis by Hornberger et al,12 the 5-antibody IHC panel and the 70-gene signature test satisfy LOE II for predicting the risk of DR and overall survival (OS), whereas the Recurrence Score satisfies LOE I for predicting DR, OS, and response to chemotherapy and LOE II for predicting local recurrence. The analysis by Hornberger and colleagues did not include the PAM50 gene expression signature assay, as it had not yet achieved regulatory approval or been performed in a Clinical Laboratory Improvement Amendment (CLIA)-certified laboratory at the time of the analysis.
In studies that used the 5-antibody IHC panel, the calculated risk (low, medium, or high) of recurrence was found to be independent of tumor stage, grade, or lymph node status in women with hormone receptor–positive EBC.14–16 The 70-gene signature microarray assay was shown to be an independent predictor of 5-year DR (low or high risk), independent of patient age, tumor grade, and tumor size in women with N0 EBC,17 and was significantly superior to traditional prognostic factors in predicting breast cancer–specific survival (BCSS) (P=0.005) in women with 1 to 3 positive lymph nodes.18 The PAM50 risk of recurrence (ROR) score provides prognostic information about 10-year DR (low risk or luminal A subgroup), independent of the clinical treatment score (integrated prognostic information from tumor size, nodal status, histopathologic grade, age, and treatment with anastrozole or tamoxifen) alone (P<0.001), in postmenopausal women with ER+, human epidermal growth factor receptor 2 (HER2) negative, N0 or N+ EBC.19,20
The 21-gene assay Recurrence Score uses RT-PCR to measure the mRNA expression levels of 16 cancer-related genes (Proliferation: Ki67, STK15, survivin, cyclin B1, MYBL2; Invasion: stromolysin 3, cathepsin L2; HER2: GRB7, HER2; Estrogen: ER, PR, Bcl-2, SCUBE2; Other: GSTM1, CD68, BAG1) and 5 reference genes (β-actin, GAPDH, RPLPO, GUS, TFRC).21 The Recurrence Score result, which was clinically validated in the National Surgical Adjuvant Breast and Bowel Project (NSABP) trial B-14,21 provides a quantitative estimate of the 10-year risk of DR in patients with ER+, N0 EBC. Although the recurrence risk estimates based on the 21-gene expression profiles reflect the biology of the tumor and vary on a continuous scale from 0 to 100, patients can also be classified into discrete risk categories of low, intermediate, or high, based on the Recurrence Score result: a score result ≤17 is categorized as low risk, a score result of 18to 30 is considered to be intermediate risk, and a score result ≥31 is categorized as high risk.21
The Recurrence Score result was validated in a second study, using a cohort of patients from the NSABP B-20 trial who were ER+, N0, and showed that patients with a high score result derived benefit from adjuvant chemotherapy, whereas patients with a low score result did not.22 Further validation of the robustness of the Recurrence Score assay was provided by a subsequent population-based study of patients in the Northern California Kaiser Permanente healthcare system, where the Recurrence Score result was found to be associated with a risk of death due to breast cancer in ER+ patients, both those who were treated with tamoxifen (P=0.003) and those who were not (P=0.03).23
On the basis of an increasing understanding of the biology of breast cancer and new genomic-based/molecular-based assays that reveal the underlying biology, efforts to apply similar principles to ER+, N+ EBC patients have been undertaken. The primary question is: What is the stronger and more important driver of the risk of DR—the biology of the disease or the anatomy of the disease (ie, spread to the lymph nodes)?
This review will describe the results from key trials examining the use of the Recurrence Score result in patients with ER+, N+ EBC. The cost-effectiveness of the assay and its effect on real-world clinical decision making (clinical utility) will also be addressed.
MATERIALS AND METHODS
A systematic evaluation of published clinical studies was performed. Validation studies from phase III clinical trials that used the Recurrence Score in patients with ER+, N+ EBC were included. Study results could be published in peer-review medical journals (as accessed through PubMed) or as an abstract in international congresses. Key search terms included: Recurrence Score, N+, early breast cancer, ER-positive, phase III, clinical utility, and cost-effectiveness.
Validation studies were identified that met a preset LOE (Ib) for prognostic and predictive information, including outcomes for DR, disease-free survival (DFS), disease recurrence-free interval (DRFI), OS, and/or BCSS.
RESULTS
Use of the Recurrence Score Result in ER+, Node+ EBC
Evaluation of the published literature revealed several validation studies that met the preset LOE or criteria. These large studies reported results that demonstrated that the Recurrence Score result provides both prognostic and predictive information in patients with ER+, N+ EBC (Table 1).
TABLE 1.
Results of 21-Gene Recurrence Score Assay in Phase III Trials of Patients With Hormone Receptor–positive, Node-positive Breast Cancer
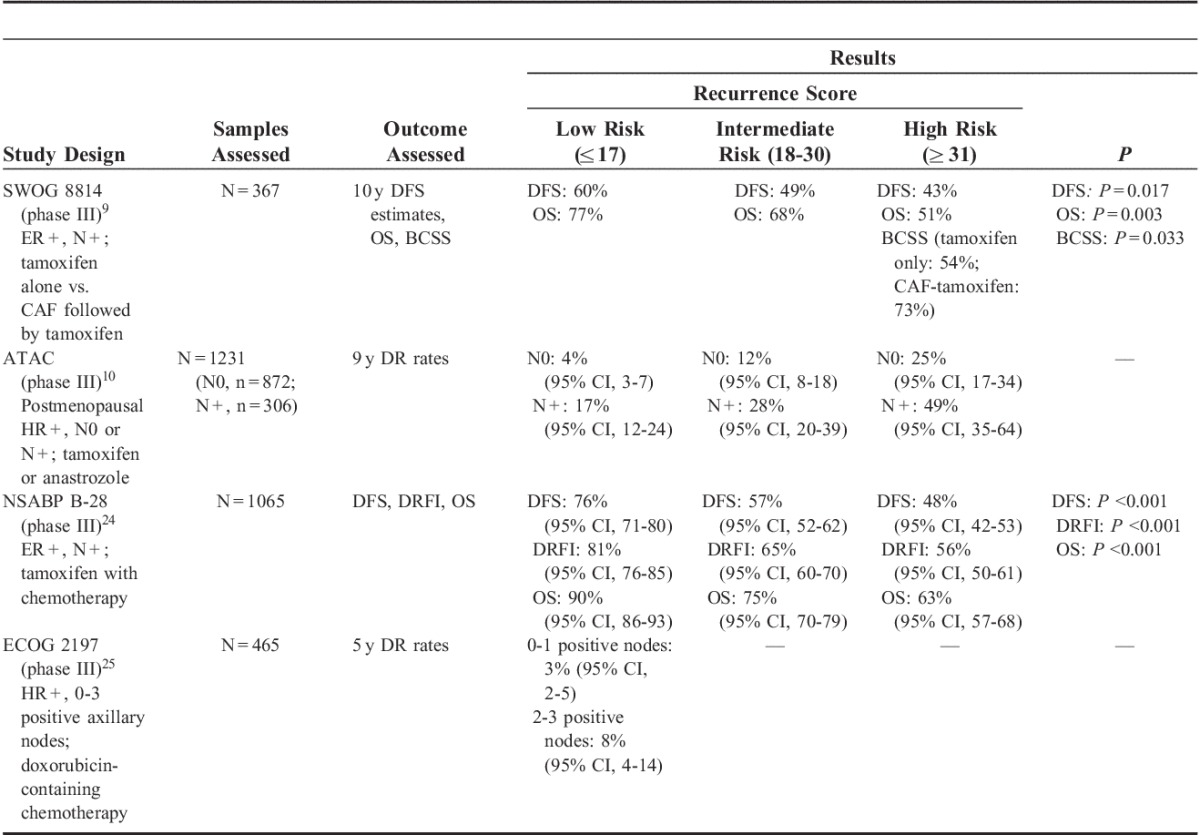
Southwest Oncology Group (SWOG) 8814
SWOG 8814 was a phase III randomized trial in postmenopausal women with ER+, N+ EBC treated with tamoxifen alone (n=148) or cyclophosphamide, doxorubicin, and fluorouracil (CAF) chemotherapy followed by tamoxifen (n=219).9 In the tamoxifen-only arm, the Recurrence Score result was found to be prognostic for 10-year DFS, stratified by the number of positive nodes. Ten-year DFS estimates were 60%, 49%, and 43% for patients with low, intermediate, and high Recurrence Score risk groups, respectively (log-rank stratified P=0.017). Ten-year BCSS estimates (in which all deaths not related to breast cancer were censored) for the high Recurrence Score risk group was 54% for patients treated with tamoxifen and 73% for patients treated with CAF followed by tamoxifen (P=0.033). The Recurrence Score risk category was also found to be prognostic for OS in all risk categories (low, 77%; intermediate, 68%; high, 51%), stratified by the number of positive nodes (log-rank stratified P=0.003). There was no benefit observed for the addition of CAF therapy to tamoxifen in the low Recurrence Score group (hazard ratio, 1.02; 95% confidence interval [CI], 0.54-1.93; log-rank P=0.97), but for the high Recurrence Score group, there was major improvement in DFS associated with the addition of CAF to tamoxifen (hazard ratio, 0.59; 95% CI, 0.35-1.01; log-rank P=0.03). Because of the small sample size in this study, CIs were extremely broad in the low-risk Recurrence Score group; therefore, the possibility of a benefit with CAF in this group cannot be fully ruled out.
Arimidex, Tamoxifen, Alone or in Combination (ATAC)
The translational arm of the ATAC (TransATAC) phase III randomized trial compared adjuvant treatment with anastrozole alone versus tamoxifen alone versus anastrozole combined with tamoxifen for 5 years in 9366 postmenopausal women with ER+ or ER(−) EBC.26 The prognostic ability of the Recurrence Score was evaluated in patients with ER+ EBC in the anastrozole-alone or tamoxifen-alone arms only.10 In patients who were N0 (n=872), a low Recurrence Score result was associated with a lower likelihood of DR at 9 years (4%), whereas conversely, a high Recurrence Score result was associated with a higher likelihood of DR (25%). Importantly, these associations were also found to hold true in patients who were N+ (n=306), with low and high Recurrence Score values having 17% and 49% rates of DR, respectively. When adjusted for clinical variables (eg, tumor size, grade, age, and treatment) in patients who were N+, the hazard ratio between high and low Recurrence Score groups was 2.7 (95% CI, 1.5-5.1), and between intermediate and low Recurrence Score groups was 1.8 (95% CI, 1.0-3.2; log-rank P<0.001), with increased risk of DR with higher Recurrence Score results. In a multivariate analysis, the Recurrence Score result was found to be significantly associated with time to DR for both N0 and N+ EBC (P<0.001 and P=0.002, respectively).
NSABP B-28
In the phase III NSABP B-28 trial, 3060 premenopausal and postmenopausal women with ER+ (n=2019) or ER-negative/borderline (n=1041), N+ EBC were treated with tamoxifen plus adjuvant chemotherapy (doxorubicin/cyclophosphamide or doxorubicin/cyclophosphamide followed by paclitaxel); 2687 patients also received concurrent endocrine therapy.27 Patients who were ER+ and had received endocrine therapy were included in an analysis of the Recurrence Score (n=1065).24 In a univariate analysis, the Recurrence Score result was a significant predictor of DFS, DRFI, and OS (P<0.001 for all 3 endpoints) (Table 1). In a multivariate analysis, the Recurrence Score result was found to be an independent prognostic factor for DFS, DRFI, and OS (P<0.001). These results demonstrate that the Recurrence Score result can stratify ER+, N+ patients for the residual risk of DR. Furthermore, for patients with low-risk disease, the addition of a taxane to an anthracycline-based regimen did not seem to have any benefit, suggesting that aggressive chemotherapy may not be warranted in patients with a low Recurrence Score result.28
Eastern Cooperative Oncology Group (ECOG) 2197
In the phase III ECOG 2197 trial, 2185 premenopausal and postmenopausal women with ER+, N+ (1 to 3 positive axillary nodes) or N0 EBC were treated with doxorubicin-containing chemotherapy or docetaxel plus endocrine therapy. The Recurrence Score result was evaluated in patients who were treated with doxorubicin-containing chemotherapy (n=465).25 The Recurrence Score result was found to be a significant prognostic marker for DR. When evaluated as a continuous variable up to 40, a significant correlation was seen between the Recurrence Score result and the rate of actual recurrence at 5 years (P<0.001 for both N0 and N+ groups). When evaluated as a categorical variable, the Recurrence Score result was found to stratify patients with N+ disease for recurrence risk. For patients with a low Recurrence Score result (46%), those with 0 to 1 positive nodes had <3% ROR at 5 years, whereas those with 2 to 3 positive nodes had an 8% risk at 5 years. As noted by the investigators, however, as both arms included chemotherapy, it is unclear whether the favorable outcomes in patients with low Recurrence Scores results were related to chemotherapy benefit, good prognosis, or a combination of both.25 These findings suggest that in patients with a low Recurrence Score result, chemotherapy regimens such as those used in ECOG 2197 can yield excellent 5-year disease-free intervals in patients who are ER+ and N0 or are N+ with up to 3 positive nodes, whereas patients with high Recurrence Score results may derive benefit from more aggressive treatment regimens. These results were further confirmed in a follow-up analysis at 10 years, which found that the Recurrence Score continued to be a highly significant prognostic marker for recurrence in both N0 and N+ EBC (P<0.0001).29
Consistent results across multiple studies support the Recurrence Score as a prognostic indicator for DR for tamoxifen-treated patients with N+ EBC. Similarly, results from the SWOG 8814 trial suggest that the Recurrence Score could predict which postmenopausal patients may derive benefit from the addition of chemotherapy to tamoxifen therapy and which patients are unlikely to benefit from the addition of chemotherapy.
In summary, there is now a large body of evidence supporting the utility of the Recurrence Score result in patients with ER+, N+ EBC. By utilizing the Recurrence Score, clinicians may be able to identify a subset of patients with N+ disease who are at the highest risk for recurrence and could potentially benefit from chemotherapy treatment, as well as patients who are low risk for DR and may not derive much, if any, benefit from chemotherapy. The cumulative results from these studies help to support a greater understanding of each individual patient’s tumor biology as reflected in the Recurrence Score result, and may help guide treatment decisions so that patients can benefit from all available information regarding their individual tumor, beyond just nodal status (patients with nodal spread have a higher risk overall, compared with patients without positive nodes).
SWOG S1007/RxPONDER
In January 2011, the randomized phase III Treatment for Positive Node, Endocrine Responsiveness breast cancer trial (RxPONDER; NCT01272037) was initiated to evaluate the use of the Recurrence Score in the decision-making process of whether to administer chemotherapy to patients with N+ disease who are receiving endocrine therapy.30,31 Approximately 4000 patients with ER+ and HER2-negative EBC involving 1 to 3 lymph nodes with a Recurrence Score result of ≤25 will be enrolled. Patients will be stratified by the Recurrence Score (0 to 13 vs. 14 to 25) and randomized to receive hormone therapy alone or hormone therapy plus chemotherapy. Study enrollment is expected to be completed in late 2016.32 The RxPONDER trial aims to validate the results of SWOG 8814 (that patients with a low Recurrence Score result do not benefit from the addition of chemotherapy9) using current chemotherapy regimens in patients with a low to intermediate Recurrence Score results. Another objective is to identify a Recurrence Score threshold to guide treatment decisions regarding the administration of chemotherapy in this patient population.
Decision Impact Data
In addition to the clinical validation studies, the clinical utility of the Recurrence Score has been demonstrated in several decision impact studies.
A recent German study evaluated the impact of the Recurrence Score on treatment decisions in 366 patients with EBC, 244 of whom were N0 and 122 of whom were N+.3 After receiving the Recurrence Score result, treatment recommendations were changed in 30% and 39% of patients with N0 and N+ EBC, respectively, with an overall reduction in the recommendation for chemotherapy. Overall, for those patients with an initial chemotherapy recommendation, a high Recurrence Score resulted in increased (27%) use of chemotherapy, whereas a low or intermediate Recurrence Score resulted in decreased (73% and 3%, respectively) use of chemotherapy.
As reported by questionnaire, physician confidence in their treatment recommendations increased in 45% of all cases (P <0.001),3 including in 45% of patients with N0 and 46% of patients with N+ EBC.33 Patient decisional conflict decreased by 6% overall (P=0.028).3
These results are consistent with those reported in other studies, including an Australian study of patients with ER+, HER2-negative EBC.34 In this study of 151 patients with N0 (n=101) and N+ (n=50) EBC, data show that initial treatment recommendations were changed for 36 (24%) patients post–Recurrence Score assay, including 23% (23/101) and 26% (13/50) of patients with N0 and N+ disease, respectively. The majority of changes were from initial recommendations of chemotherapy plus endocrine therapy to a recommendation post–Recurrence Score of endocrine therapy alone; for 15.9% of patients (24/151), treatment recommendation changed from chemotherapy plus endocrine therapy to endocrine therapy alone. For 7.9% of patients (12/151) who were initially recommended endocrine therapy alone, treatment recommendation was changed to chemotherapy plus endocrine therapy.
A total of 160 medical oncologists who ordered the Recurrence Score for patients with hormone receptor–positive, N+ EBC responded to a study survey regarding the impact of assay results on their treatment recommendations.35 Of the 138 patients who received a treatment recommendation before their Recurrence Score was obtained, 70 (51%) had their recommendation changed after receiving their Recurrence Score result. Forty-six (33%) patients had their treatment recommendation changed to eliminate chemotherapy. Thirteen (9%) patients had their recommended treatment intensity increased to include chemotherapy in addition to hormone therapy.
Overall, these studies showed that physicians used the Recurrence Score assay result to reassess patient ROR, and in a large percentage of cases (26% to 51% of N+ cases), changed their treatment recommendation following receipt of the Recurrence Score. In the majority of N0 and N+ cases (60% to 66%), changes in treatment recommendations led to the elimination of chemotherapy.3,34,35 Use of the Recurrence Score also led to increased physician confidence in their treatment recommendations.
Health Economics
A proportion of patients with N+ EBC may be at risk of overtreatment, thereby increasing health care costs and also exposing patients to chemotherapy-related adverse events with little additional benefit. Reduction in chemotherapy usage from Recurrence Score–directed therapy decisions has been shown to be cost-effective. In the United States, an economic analysis based on the NSABP B-14 study data estimated that reclassifying patients who were defined by National Comprehensive Cancer Network (NCCN) guidelines to be low risk as intermediate/high risk as defined by the Recurrence Score was projected to increase costs (by about $25,000 [2005 US Dollars (USD)], with an average gain in OS of 1.86 y per reclassified patient). In contrast, reclassifying NCCN-defined high-risk patients as low risk based on Recurrence Score was expected to be cost-saving (by approximately $9000 [2005 USD] in lifetime costs).36 Among a hypothetical cohort group of 100 patients that were based on NSABP B-14 data (where >90% were defined as high risk by NCCN guidelines), Recurrence Score–guided therapy was estimated to increase quality-adjusted life years (QALY) by 8.6 years. Use of Recurrence Score was predicted to lead to reclassification of 2 patients from low to intermediate/high risk and 45 patients from high to low risk, leading to improvements in quality-adjusted survival and reducing overall costs by $202,828 (2005 USD).
In addition, a joint study of the NSABP B-14 and B-20 data found that Recurrence Score–guided treatment decisions resulted in greater efficacy (a gain of 2.2 y in life expectancy) with acceptable cost-effectiveness ratios ($1944 [2007 USD]/life-year saved) compared with tamoxifen alone, and similar efficacy and lower costs compared with chemotherapy plus tamoxifen ($3385 [2007 USD]/life-year saved).37
Further data from a decision-analytic model study predicted that, among 2 million patients from a managed care plan with N+ ER+, HER2-negative EBC, adoption of the Recurrence Score would improve health outcomes with no added incremental cost (net gains: 4.44 QALYs/y; savings: $13,476 [2011 USD]/y).38
From a health care payer perspective, a cost-effectiveness analysis by Eiermann et al3 that included patients with both N0 and N+ EBC projected that the use of the Recurrence Score would save approximately €561 (2012 Euros) per patient, in part due to the reduced overall use of chemotherapy typically associated with Recurrence Score utilization.
Finally, an economic analysis of 925 women with N0 EBC enrolled with the insurance program Humana (Louisville, KY) assessed the real-world cost-effectiveness of treatment directed by Recurrence Score.39 Following Recurrence Score testing, adjuvant chemotherapy was administered to 10% of low-risk, 36% of intermediate-risk, and 72% of high-risk Recurrence Score patients. On the basis of a meta-analysis of chemotherapy reduction post–Recurrence Score assay, a validated Markov model estimated an average net savings of $1160 per patient, based on savings for chemotherapy drugs ($1885), supportive care ($2578), adverse event management ($472), and prevention of DR ($199), after the cost of the test itself ($3975) (2011 USD for all). On the basis of claim data from Humana, a health insurance provider in the United States, Recurrence Score testing of these 925 women over a 4-year period decreased their overall total plan cost by >$1 million dollars (2011 USD), while also projecting QALY gains of 2 to 3 months for the tested patients. Together, these studies demonstrate that the Recurrence Score could be cost-effective and may be cost-saving in patients with N0 EBC.
Although limited research has been conducted thus far on the cost-effectiveness of Recurrence Score testing in patients with N+ EBC, a study based in the United Kingdom has evaluated the cost-effectiveness of Recurrence Score–directed chemotherapy compared with chemotherapy for all eligible patients with ER+, N+ EBC, using recurrence rates from the SWOG 8814 study.40 Recurrence Score–guided chemotherapy resulted in a relatively modest increase of 0.16 QALY, but with an incremental cost-effectiveness ratio of £5529 (2011 UK pounds [GBP]) per QALY (using a cutoff of Recurrence Score >18). The probability that Recurrence Score–directed chemotherapy is cost-effective was found to be 0.61 at a willingness-to-pay threshold of £30,000 (2011 GBP) per QALY.
Although these results demonstrated that the Recurrence Score has the potential to be cost-effective if adopted in the United Kingdom for N+ EBC, further research is needed. However, if results are similar to those seen in patients with N0 EBC, there is the potential for improved utilization of health care resources through the use of the Recurrence Score.
DISCUSSION
Data from large, randomized trials indicate that the Recurrence Score is an independent predictor of outcomes in patients with N+ EBC, which is reflective of the biology of the disease, above and beyond nodal status. Forthcoming results from the RxPONDER trial (SWOG 1007) may confirm the findings from the SWOG 8814 study, which demonstrated that patients with low Recurrence Score results do not derive benefit from chemotherapy. However, in the absence of the RxPONDER data at this point in time, multiple clinical utility studies indicate that utilization of the Recurrence Score assay for N+ EBC could be a rational option for this patient population. These studies have shown that, in patients with N+ EBC, the Recurrence Score result impacts treatment decisions with a reduction in chemotherapy utilization.
Recently, other molecular assays have reported results of studies in patients with N+ EBC, assessing the performance of their assay to prognosticate and potentially identify patients that are likely to benefit from chemotherapy. The 70-gene assay showed in a cohort of 144 N+ patients that the probability of distant metastasis-free survival (DMFS) and OS at 5 years was 94.5 (95% CI, 88.7-100) and 98.2 (95% CI, 94.7-100), respectively, for patients stratified as low risk, and 64.7 (95% CI, 55.3-75.8) and 76.9 (95% CI, 68.5-86.3), respectively, for patients stratified as high risk.41 The difference between low risk and high risk were significant for both DMFS (P=0.0004) and OS (P=0.03). Data from the PAM50 ROR assay showed that among 431 patients with N+ EBC, the probability of DMFS at 10 years was 100% for patients stratified as low risk, 93.6% (95% CI, 86.9-79.0) for patients stratified as intermediate risk, and 76.1 (95% CI, 69.9-81.2) for patients stratified as high risk (the difference between high risk and low risk for 10-year DRFS was noted as significant; however, P-values were not indicated in the publication).42
CONCLUSIONS
The advent of molecular profiling assays has moved the field forward by providing greater insight into individual patients’ tumor biology and identifying which patients may or may not benefit from the addition of chemotherapy to adjuvant hormone therapy. This shift in patients away from receiving chemotherapy with N(−) EBC has been substantial, and over the past 10 years the rate of chemotherapy use had decreased dramatically. The shift in patients with N+ EBC has not yet been as remarkable, but assays such as the 21-gene Recurrence Score are beginning to have an impact. Treatment decisions based on the individual patient and their unique tumor biology will likely result in better outcomes overall by making sure the right patient receives the right therapy. With improved treatment selection (with or without chemotherapy), there will be improved quality of life, more efficient use of resources, and reduced direct and indirect costs.
ACKNOWLEDGMENT
The authors thank KnowledgePoint360 for providing writing, editorial, and graphics support for the development of this manuscript; their participation was funded by Genomic Health.
Footnotes
A.M.B. has received consulting fees from Genomic Health.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A.Cancer statistics, 2012.CA Cancer J Clin. 2012;62:10–29 [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations). 2011Bethesda, MD:National Cancer Institute; Available at: http://seer.cancer.gov/csr/1975_2009_pops09/. Accessed November 1, 2013 [Google Scholar]
- 3.Eiermann W, Rezai M, Kümmel S, et al. The 21-gene Recurrence Score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use.Ann Oncol. 2013;24:618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines)—Breast Cancer, v. 2.2013. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed November 1, 2013
- 5.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG).Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials.Lancet. 2005;365:1687–1717 [DOI] [PubMed] [Google Scholar]
- 6.Wood WC, Anderson M, Lyles RH, et al. Can we select which patients with small breast cancers should receive adjuvant chemotherapy?Ann Surg. 2002;235:859–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes DF, Isaacs C, Stearns V.Prognostic factors in breast cancer: Current and new predictors of metastasis.J Mammary Gland Biol Neoplasia. 2001;6:375–392 [DOI] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG).Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials.Lancet. 2012;379:432–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene Recurrence Score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial.Lancet Oncol. 2010;11:55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene Recurrence Score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study.J Clin Oncol. 2010;28:1829–1834 [DOI] [PubMed] [Google Scholar]
- 11.Ross JS, Hatzis C, Symmans WF, et al. Commercialized multigene predictors of clinical outcome for breast cancer.Oncologist. 2008;13:477–493 [DOI] [PubMed] [Google Scholar]
- 12.Hornberger J, Alvarado MD, Rebecca C, et al. Clinical validity/utility, change in practice patterns, and economic implications of risk stratifiers to predict outcomes for early-stage breast cancer: a systematic review.J Natl Cancer Inst. 2012;104:1068–1079 [DOI] [PubMed] [Google Scholar]
- 13.Simon RM, Paik S, Hayes DF.Use of archived specimens in evaluation of prognostic and predictive biomarkers.J Natl Cancer Inst. 2009;101:1446–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross DT, Kim CY, Tang G, et al. Chemosensitivity and stratification by a five monoclonal antibody immunohistochemistry test in the NSABP B14 and B20 trials.Clin Cancer Res. 2008;14:6602–6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartlett JM, Thomas J, Ross DT, et al. Mammostrat as a tool to stratify breast cancer patients at risk of recurrence during endocrine therapy.Breast Cancer Res. 2010;12:R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ring BZ, Seitz RS, Beck R, et al. Novel prognostic immunohistochemical biomarker panel for estrogen receptor-positive breast cancer.J Clin Oncol. 2006;24:3039–3047 [DOI] [PubMed] [Google Scholar]
- 17.Buyse M, Loi S, van’t Veer L, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer.J Natl Cancer Inst. 2006;98:1183–1192 [DOI] [PubMed] [Google Scholar]
- 18.Mook S, Schmidt MK, Viale G, et al. The 70-gene prognosis-signature predicts disease outcome in breast cancer patients with 1-3 positive lymph nodes in an independent validation study.Breast Cancer Res Treat. 2009;116:295–302 [DOI] [PubMed] [Google Scholar]
- 19.Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy.J Clin Oncol. 2013;31:2783–2790 [DOI] [PubMed] [Google Scholar]
- 20.Gnant M, Dowsett M, Filipits M, et al. Identifying clinically relevant prognostic subgroups in node-positive postmenopausal HR+ early breast cancer patients treated with endocrine therapy: a combined analysis of 2485 patients from ABCSG-8 and ATAC using the PAM50 risk of recurrence (ROR) score and intrinsic subtype.J Clin Oncol. 2013;31suppl 15abstract 506 [DOI] [PubMed] [Google Scholar]
- 21.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer.N Engl J Med. 2004;351:2817–2826 [DOI] [PubMed] [Google Scholar]
- 22.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer.J Clin Oncol. 2006;24:3726–3734 [DOI] [PubMed] [Google Scholar]
- 23.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients.Breast Cancer Res. 2006;8:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mamounas EP, Tang G, Paik S, et al. Prognostic impact of the 21-gene Recurrence Score (RS) on disease-free and overall survival of node-positive, ER-positive breast cancer patients (pts) treated with adjuvant chemotherapy: Results from NSABP B-28.J Clin Oncol. 2012;30suppl 27Abstract 1 [Google Scholar]
- 25.Goldstein LJ, Gray R, Badve S, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features.J Clin Oncol. 2008;26:4063–4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial.Lancet. 2002;359:2131–2139 [DOI] [PubMed] [Google Scholar]
- 27.Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28.J Clin Oncol. 2005;23:3686–3696 [DOI] [PubMed] [Google Scholar]
- 28.Mamounas EP, Tang G, Paik S, et al. Association between the 21-Gene Recurrence Score (RS) and benefit from adjuvant paclitaxel (Pac) in node-positive (N+), ER-positive breast cancer patients (pts): results from NSABP B-28.Cancer Res. 2012;72suppl 24abstract S1–S10 [Google Scholar]
- 29.Sparano JA, O’Neill A, Gray RJ, et al. 10-year update of E2197: phase III doxorubicin/docetaxel (AT) versus doxorubicin/cyclophosphamide (AC) adjuvant treatment of LN+ and high-risk LN- breast cancer and the comparison of the prognostic utility of the 21-gene Recurrence Score (RS) with clinicopathologic features.J Clin Oncol. 2012;30Suppl 15abstract 1021 [Google Scholar]
- 30.Gonzalez-Angulo AM, Barlow WE, Gralow J, et al. SWOG S1007: A phase III randomized clinical trial of standard adjuvant endocrine therapy with or without chemotherapy in patients with one to three positive nodes, hormone receptor (HR)-positive, and HER2-negative breast cancer with Recurrence Score (RS) of 25 or less.J Clin Oncol. 2011;29Suppl 15abstract TPS104 [Google Scholar]
- 31.Ramsey SD, Barlow WE, Gonzalez-Angulo AM, et al. Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating Oncotype DX-guided management for women with breast cancer involving lymph nodes.Contemp Clin Trials. 2012;34:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clinicaltrials.gov.Tamoxifen citrate, letrozole, anastrozole, or exemestane with or without chemotherapy in treating patients with invasive RxPONDER breast cancer. Clinicaltrials.gov identifier NCT01272037. Available at: http://clinicaltrials.gov/show/NCT01272037. Accessed November 1, 2013
- 33.Rezai M, Eiermann W, Kümmel S, et al. Impact of the Recurrence Score on adjuvant decision-making in ER-positive early breast cancer—results of a large prospective multicentre decision impact study in node negative and node positive disease.Cancer Res. 2011;71suppl 24abstract P2-12-26 [Google Scholar]
- 34.de Boer RH, Baker C, Speakman D, et al. Australian Decision Impact Study: the impact of Oncotype DX Recurrence Score (RS) on adjuvant treatment decisions in hormone receptor positive (HR+), node negative (N0) and node positive (N+) early stage breast cancer (ESBC) in the multidisciplinary clinic (MDC).Cancer Res. 2011;71suppl 24abstract P4-09-18 [Google Scholar]
- 35.Oratz R, Kim B, Chao C, et al. Physician survey of the effect of the 21-gene Recurrence Score assay results on treatment recommendations for patients with lymph node-positive, estrogen receptor-positive breast cancer.J Oncol Pract. 2011;7:94–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hornberger J, Cosler LE, Lyman GH.Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer.Am J Manag Care. 2005;11:313–324 [PubMed] [Google Scholar]
- 37.Lyman GH, Cosler LE, Kuderer NM, et al. Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: an economic analysis based on prognostic and predictive validation studies.Cancer. 2007;109:1011–1018 [DOI] [PubMed] [Google Scholar]
- 38.Vanderlaan BF, Broder MS, Chang EY, et al. Cost-effectiveness of 21-gene assay in node-positive, early-stage breast cancer.Am J Manag Care. 2011;17:455–464 [PubMed] [Google Scholar]
- 39.Hornberger J, Chien R, Krebs K, et al. US insurance program’s experience with a multigene assay for early-stage breast cancer.J Oncol Pract. 2011;7:e38s–e45s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall PS, McCabe C, Stein RC, et al. Economic evaluation of genomic test-directed chemotherapy for early-stage lymph node-positive breast cancer.J Natl Cancer Inst. 2012;104:56–66 [DOI] [PubMed] [Google Scholar]
- 41.Drukker CA, van Tinteren H, Schmidt MK, et al. Long-term impact of the 70-gene signature on breast cancer outcome.Breast Cancer Res Treat. 2014;143:587–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone.Ann Oncol. 2014;25:339–345 [DOI] [PubMed] [Google Scholar]


