Abstract
Despite decreasing prevalence, new cases of hepatitis C in China are increasing recently with growing percentage of patients who are with advanced disease, aging, or not eligible for interferon-based treatments. Hepatitis C infection represents a serious public health burden. This review was based on expert’s consensus during a medical forum on hepatitis sponsored by the Beijing Wu Jie-Ping Medical Foundation. The literature searches were conducted in PubMed and critical publications in Chinese journals. Data on hepatitis C prevalence, risk factors, viral or host features, and treatment modalities were extracted and reviewed. Recent large-scale surveys reported reducing prevalence of hepatitis C to approximately 0.4% in China, partly because of regulation changes to safer medical practices and illegalizing commercial blood donations. Patient demographics evolved from being dominated by former paid blood donors to include intravenous drug users and others. Although hepatitis C genotype 1 is the most common, other genotypes are emerging in prevalence. The current standard of care is interferon-based without direct acting antivirals. However, many patients failed therapy because of high treatment costs, substantial needs to manage side effects, difficulties with treatment monitoring in the rural areas, and growing populations of elderly and cirrhotic patients. The lack of high efficacy therapies with good safety profile and low disease awareness in China resulted in increasing public burden of advanced hepatitis C disease. Despite significant reduction of hepatitis C prevalence, iatrogenic, nosocomial, and community transmissions are still significant. In addition to promoting disease awareness, interferon-free regimens are needed to reduce the public health burden.
Key Words: disease prevalence, China HCV, standard of care, unmet need, disease burden
Hepatitis C is caused by infection of hepatitis C virus (HCV) primary in the liver and often presents as a chronic disease with nonspecific symptoms. The long-term hepatic impact of HCV infection is highly variable with a spectrum ranging from minimal hepatic inflammation to extensive fibrosis and cirrhosis with or without hepatocellular carcinoma (HCC). Although liver transplantation improves the morbidity and mortality of those with decompensated cirrhosis or HCC, many patients die while on the waiting list because of the shortage of organs. Recent data estimated that up to 25 million individuals in China might be infected by HCV.1 According to the China Ministry of Health annual reports on health statistics, reported cases of hepatitis C have been increasing steadily since 2003 (Fig. 1).2 The trend is expected to continue due in parts to increase of disease awareness, disease progression of the infected populations seeking medical care, increase of new infections, and adopting new generation of HCV testing methods. The risk factors for HCV infections among Chinese patients are unique as the demographics vary according to time periods. As hepatitis C is a curable disease, patients without advanced fibrosis and cirrhosis who receive interferon-based antiviral treatment can achieve sustained viral response (SVR), defined as undetectable HCV RNA at 24 weeks after treatment completion, resulting in the cessation of fibrosis progression in the majority of patients, and minimizing the risk of HCC. However, because of the very low level of disease awareness among Chinese patients, missed opportunities of early diagnosis of HCV infection and linkage-to-care results in patients often presenting with advanced disease and missing the window of opportunity for interferon-based therapy. This article provides a systematic review of current epidemiology of chronic hepatitis C (CHC), risk factors of HCV infection, HCV virologic features in Chinese populations, and current treatment options and their limitations for hepatitis C in China. Proposals to reduce HCV public burden in China are also discussed in this review. The content development is based on the experts’ consensus at a Hepatitis C Forum sponsored by the Wu Jie-Ping Medical Foundation (a not-for-profit organization) in Beijing. In addition, the literature searches were conducted in English language journals in PubMed and critical publications in Chinese journals. References were cited based on availability, large sample sizes, and broad geographic regions.
FIGURE 1.
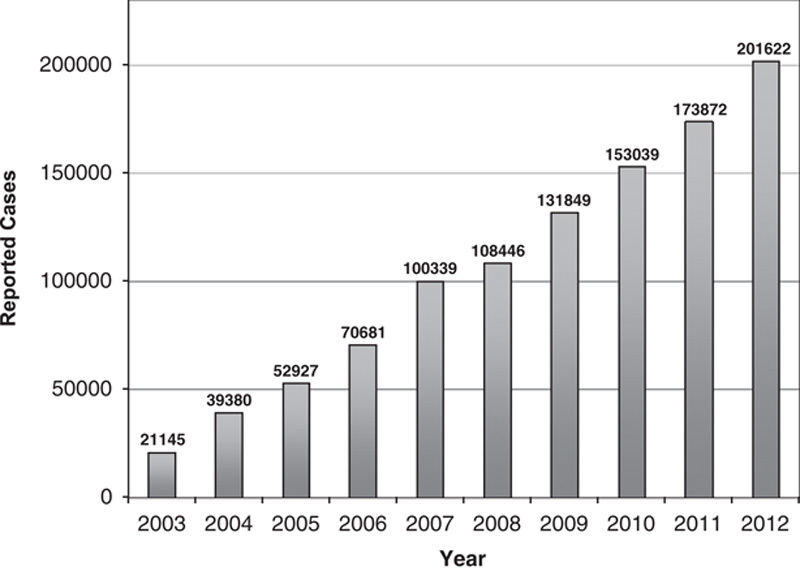
China Ministry of Health annual reports on health statistics showed an increasing number of reported cases of hepatitis C in China from year 2001 to 2012. The data was generated from health care providers and laboratory voluntary report.
EPIDEMIOLOGY OF HEPATITIS C IN CHINA
Recent review of data estimated that 1% to 1.9% (13 to 25 million) individuals in China might be infected by HCV.1 More recent studies reported lower prevalence. Testing of blood samples previously collected for the 2006 National Hepatitis B Sero-survey and using third generation assays for detection with improved specificity, the China Center for Disease Control and Prevention (CCDC) determined that the overall prevalence rate of anti-HCV was 0.43% among the population of ages ranging from 1 to 59.3 However, prevalence rates varied in different geography regions. Anti-HCV-positive rates were higher in the central (0.67%) and northern (0.53%) regions when compared with those in the eastern (0.37%), western (0.31%), and southern (0.29%) regions. The rates were similar between rural and urban areas and sexes.3 In a smaller population study sponsored by CCDC and conducted by the National Institute for Viral Disease Control and Prevention, serum samples (n=9538) collected from 6 regions including Beijing, Heilongjiang, Shandong, Ningxia, Gansu, and Sichuan, between 2006 and 2008, were tested. The overall rate of anti-HCV positivity was 0.39%.4 The prevalence was higher in older age groups. A recent meta-analysis was conducted on 265 studies, which included >4.5 million first-time blood donors across China mainland.5 The analysis reported that, from 1990 to 2010, the prevalence of HCV infection decreased from >13.42% to 0.36% (Fig. 2).5 These recently reported prevalence rates of approximately 0.4% are much decreased from the 1992 reported rate of 3.2%. This could be attributed, in parts, to providing treatment to infected individuals, new regulations to promote no payment for blood donations, no reuse of unsterilized needles for medical injections, increased health education, and more accurate diagnostic technologies in recent years.
FIGURE 2.
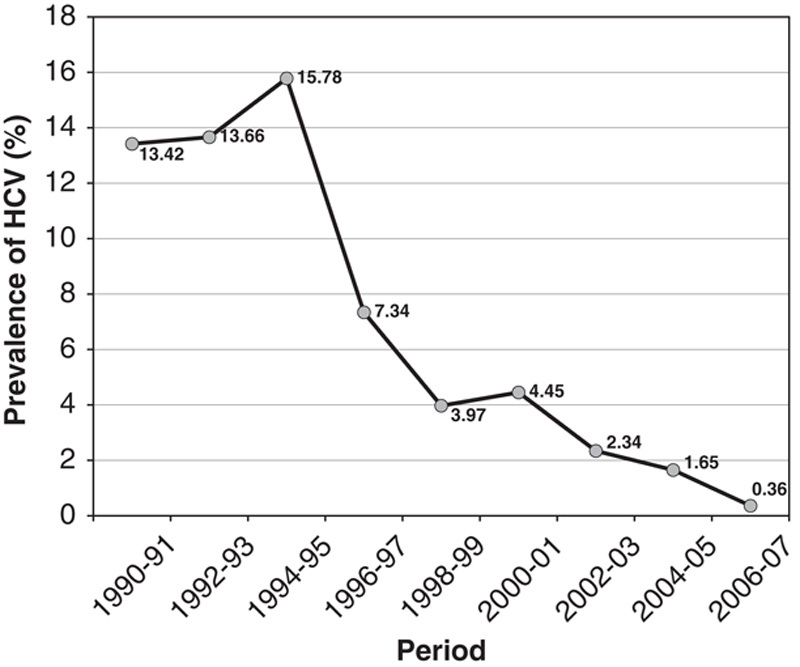
The prevalence of hepatitis C virus (HCV) infection among blood donors decreased from 1990 to 2010. New regulations to promote no payment for blood donations significantly attributed the decline in recent years.
RISK FACTORS FOR HEPATITIS C IN CHINESE PATIENTS
The risk factors for HCV infection in China are unique compared with developed countries wherein the majority of patients were infected through injections of intravenous drug (IVD) with contaminated needles. In addition to IVD users, Chinese patients who are at-risk for CHC included former paid plasma donors, patients who received transfusions of contaminated blood products, injectable medication(s) with reused unsterilized (or inappropriate-sterilized) syringe/needles or hemodialysis with contaminated devices, and individuals with unsafe sexual practices, especially those with sexually transmitted disease.
The dominant risk factors have changed over time. During the 1980s and early 1990s, unsafe medical practices during plasma donation led to the spread of HCV infection in the country. In contrast, a majority of reported outbreaks of hepatitis C between 2000 and 2010 were in hemodialysis patients and patients who received blood transfusions or injections with reused syringes. For example, hepatitis C outbreak during 2011 in the rural boundary border region of Anhui and Henan affected >100 patients in 2 townships. The cause was thought to be reuse of unsterilized or inappropriately sterilized needles.6 Additional outbreaks of hepatitis C because of iatrogenic factors were reported to affect hundreds of residents in rural areas of Anhui, Guangdong, and Liaoning provinces.7–9
Personal behaviors and economic development in different regions also contributed to the geographic variation of risk factors for HCV infection. Patients with HCV in rural areas were mostly former commercial blood donors who received payments for blood donations, or infected with HCV from blood transfusions. A recent cross-sectional study investigated residents in the village of Hebei province, wherein many former commercial blood donors lived.10 All residents were invited for a questionnaire interview and testing for anti-HCV. Of the 520 villagers who participated, 236 (45.4%) reported a history of selling whole blood or plasma. HCV seropositivity was confirmed in 148/520 (28.5%) interviewees and 101/236 (42.8%) former commercial plasma and blood donors. Selling plasma was the strongest independent predictor of HCV seropositivity (P=0.004). Past history of an operation was also independently associated with HCV infection (P=0.027) in this village.10 Another study investigated risk factors for hepatitis C in 5187 villagers in rural areas of Henan province.11 Multivariate analysis showed that iatrogenic causes, such as intravenous injections or infusions and history of receiving endoscopic procedures, were associated with HCV infection.
Within the same geography region, the frequency of self-reported independent risk factors also varied. In a study to estimate the prevalence of anti- HCV positivity among blood donors from Chengdu, anti-HCV test was performed in 119,518 volunteer donors between 2006 and 2007.12 Risk factors were identified based on 305 (prevalence rate 0.25%) unique HCV seropositive donors versus 610 seronegative donors matched for age and sex. Multivariate model identified the major independent HCV risk factors were razor sharing [odds ratio (OR)=29.16; 95% confidence interval (CI), 12.89-66.00], blood transfusion (OR=20.84; 95% CI, 3.76-115.45), and acupuncture (OR=8.01; 95% CI, 3.16-20.30). Additional risk factors included history of hospitalization, dental treatment, and ear piercing.12
The incidence rate of HCV infection among patients on hemodialysis was significantly high. A cross-sectional survey was performed on HCV infection and related factors among patients undergoing continuous hemodialysis in 423 hospitals that voluntarily participated in the survey during a 1-month period in 2010. A total of 21,918 patients were surveyed, and 1506 patients were positive (7.01%).13 HCV infection rates were higher at hospitals performing more dialysis, in patients receiving dialysis for longer durations, and from multiple hospitals (P<0.01 for each).13
IVD users are at high risks for HCV infection. A study at a drug rehabilitation center in the Huiyang district (Huizhou, Guangdong province) showed that 368/500 (73.6%) IVD users were found to be anti-HCV-positive.14 Investigators in Yunnan province, which is a high prevalence area of illicit drugs use, tested >2000 IVD users in 5 regions of the province and found that 77.7% had HCV infection.15
HCV VIROLOGIC AND HOST FEATURES AMONG CHINESE PATIENTS
HCV genotypes 1, 2, 3, and 6 are distributed among patients in distinct geographic locations in China.1 A recent study by Wei et al16 reports on a first country-wide survey with 1012 HCV-infected patients from 28 university hospitals across China. Substantial regional variation in the HCV genotype distribution was observed (Fig. 3). Genotype 1b was the most common with overall prevalence of 56.8%, and it is the predominant genotype in the east (71.8%). The less common genotype 6 was found mainly in the south (20.4%) and the west (6.9%). The south displayed the greatest diversity of the HCV genotypes.16 In a sera-prevalence study among approximately 560,000 first-time volunteer blood donors in the Guangzhou Blood Center, Guangdong province, 0.34% (n=1877) were positive for anti-HCV.17 A subgroup of anti-HCV-positive donors were randomly selected for HCV RNA detection, and those who were positive were subjected to further detailed HCV genotype analyses (n=236). Genotype 6a was detected in 82 of 236 patients analyzed, wherein, remarkably, 72 of the 82 were from Guangdong province. Among the group of donors from Guangdong province, nearly 50% had genotype 6a.17
FIGURE 3.
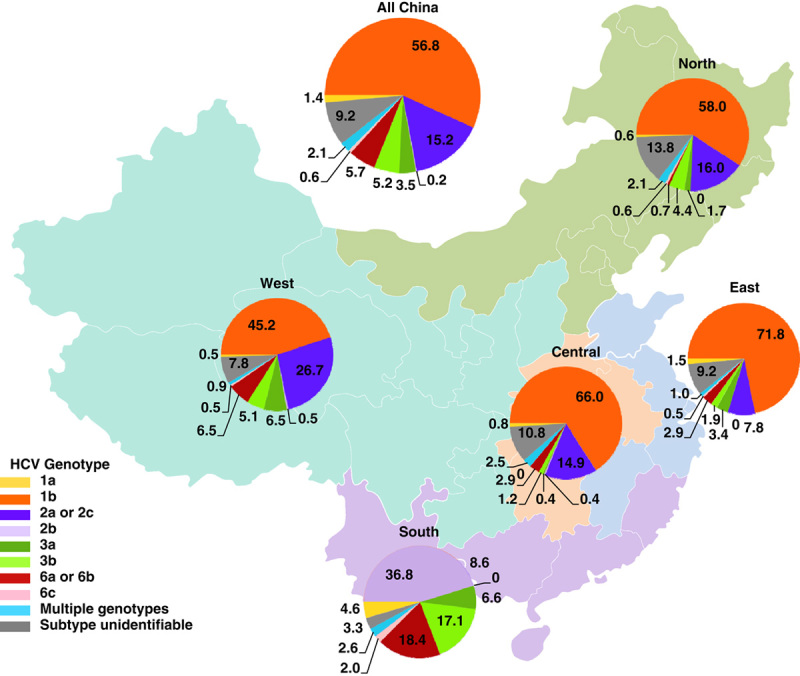
The prevalence of hepatitis C, the distribution of hepatitis C virus (HCV) genotypes, and host IL28B in different regions of China. Adapted with permission from Wei et al.16
There is a trend that patients with genotype 1 and 2 were older than those with genotype 3 and 6. This age divergence reflected the evolving demographics according to modes of transmission. In a study with 483 anti-HCV-positive patients from 10 provinces in China, the mean age of patients with genotype 1 and 2 was significantly older than those with genotype 3 and 6 (P<0.05).18 The distribution of HCV genotypes in relation to the mode of HCV transmission was remarkable (P<0.001), wherein the main mode of transmission was through transfusion of blood or blood products. Most genotype 1 infection (53.1%) was found in the group with duration of HCV infection of 10 to 20 years and genotype 1b was independently associated with age (P=0.001).18 In a retrospective study of 1,208 patients in southwest China, individuals older than 40 years of age were mainly infected with genotypes 1b or 2a, whereas younger patients were predominantly infected with genotypes 3 or 6. Subtypes 1b and 2a were observed more frequently among patients with a history of invasive operations. Subtypes 3b and 6a constituted the majority of HCV infections among IVD users.19 It was noted that age 40 years or below and infection by IVD use were independently associated with genotypes 3 and 6 in southwest China.20
The rs12979860 polymorphism near the human interleukin-28B (IL28B) gene is associated with interferon responsiveness. The IL28B-CC genotype, which is associated with favorable response to interferon, predominates in China with an overall prevalence of 84.1%.16 Unlike the HCV genotypes, the host IL28B genotypes were distributed rather uniformly across the country.
DISEASE BURDEN, TREATMENT OPTIONS, AND CHALLENGES
Viral hepatitis-associated diseases such as liver cirrhosis and HCC continue to emerge as a major burden and serious public health challenge. As the implementation of the infant hepatitis B virus (HBV) vaccination program by the China Ministry of Health in 1992, the HBV surface antigen (HBsAg) carrier rate has decreased from 9.75% to 7.18% in 2006.21,22 The incidence of hepatitis B has also been decreasing steadily during the past decade because of hepatitis B vaccination program across the country. In contrast, the reported incidence of hepatitis C in China has increased >10-fold from 2003 to reach 219,110 cases in 2012 (Fig. 1).2 Cases reported in 2010 are highest in Henan (>20,000), followed by Xinjiang, Guangdong, and Guangxi (10,001 to 20,000 each; China CDC). The trend is expected to continue, in part, due to better diagnosis, disease progression of the aging infected populations, as well as new infections.
Public awareness of hepatitis C is much lower than hepatitis B in China. On the basis of a survey in 2007 reported by the Chinese Foundation for Hepatitis Prevention and Control, only 1% of the public participants were reported to have some knowledge about HCV transmission and prevention, and 5% had been tested for HCV infection.23 Another recent survey of 1362 physicians (nonspecialists for hepatitis C) in over 200 cities in 30 provinces showed physicians had very limited understanding of hepatitis C such as diagnosis, treatment, and prevention.24 For example, 58% did not test patients for HCV before invasive procedures, 5% tested themselves when accidently injured at the hospital, 11% did not understand the routes of transmission of HCV, and nearly 50% thought there were no effective therapies. Only about half of the physicians would refer their anti-HCV-positive patients to infectious or liver disease specialists.24 Thus, it is critical to expand education to health care professionals to diagnose at-risk populations, implement safer medical practices, so risks for iatrogenic transmissions could be minimized or eliminated, and to refer infected patients for early treatment and appropriate care. In addition, there appears to be a need to increase public awareness about HCV; therefore, risky behaviors and life style factors could be modified to avoid transmission, which include sharing shaving razors, practicing unsafe sex, and using nonsterile needles for recreational drugs.
Because of the aging of populations and delays in diagnosis of hepatitis C because of low public awareness of the disease, many Chinese patients seen in clinics are presented with advanced liver disease. The proportion of patients with cirrhosis has increased in recent years. A recent observation about patients who have cirrhosis within the Beijing Medical Insurance System showed that those with cirrhosis secondary to hepatitis C have increased from 6.34% in 2004 to 9.33% in 2009 (Unpublished data provided by L.W.). Alcohol-related cirrhosis also increased and those with hepatitis B decreased from 65.85% to 40.51% during the same period. In the recent country-wide survey of HCV infection, 152 (15%) among the study population of 1012 were diagnosed with liver-related complications.16 Of the 101 patients with cirrhosis, 59.4% were Child-Turcotte-Pugh category A, 33.7% B, and 6.9% C.16 As hepatitis C is a curable disease, earlier diagnosis and access to treatment would improve outcome of patients with hepatitis C and might relief burden on the public health system in China. The frequent diagnosis of advanced liver disease in Chinese population points to the lack of effective management during early disease stage for patients, which may be because of barriers from patients, physicians, and health care systems.
Clinical care for patients with HCV-related liver disease has advanced in recent years. The current international standard of preferred therapy for HCV infection is interferon-based therapy together with first-generation direct antiviral agent (DAA) for genotype 1 and without DAA for other genotypes. These regimens achieve approximately 80% SVR for genotype 1, but they are not indicated for genotypes 2 or 3. However, DAA is currently not available in China; thus, the standard of care in China is the combination therapy of pegylated interferon and ribavirin (P/R), which achieves SVR of varying proportions in patients depending on the HCV and host genotypes. Subsequent to the landmark genome-wide association studies publications in 2009,25–27 there is ample evidence, including meta-analysis of 46 studies,28 to verify that the polymorphisms at rs12979860 and rs8099917 near the IL28 gene were associated with P/R treatment outcomes. The rs12979860 IL28-CC genotype is strongly associated with higher SVR in genotype 1 patients treated with P/R, irrespective of ethnicity and other factors. This IL28-CC genotype was also shown to be predictive of higher SVR in Chinese Han patients.29 In addition, the rs8099917 IL28 TT genotype was shown to be associated with poor SVR in Chinese patients treated with P/R.30 During recent years, SVR rates from treatment of P/R varying from 44% to 83% have been reported from a number of studies in China. Most of these studies enrolled a majority of genotype 1b Chinese patients.29–36 However, it is difficult to determine the overall SVR rate unequivocally from these reports because the definition of SVR varied from study to study, the HCV genotype inclusion were heterogenous, and assay methods were not uniform. Nevertheless, the apparent higher SVR in the Chinese studies relative to global studies might be explained by the fortunately high prevalence of IL28B-CC genotype among Chinese patients.
Patient adherence and tolerability to the current therapy are serious challenges in treating hepatitis C in China. A predominant portion of Chinese patients are infected with the more difficult-to-treat genotype 1b HCV, which have lower therapeutic response to P/R compared with patients with genotype 2 or 3. Interferon/ribavirin combination treatment is known to cause severe adverse effects. Use of P/R requires intensive monitoring, and some patients may require hospitalization to manage adverse effects (eg, blood transfusion for severe anemia). Because of the less than optimal treatment response coupled with significant side effects during the therapy, substantial proportion of patients are not able to tolerate the medications, thus leading to treatment failures. Studies in China had shown that the undesirable side effects are even more severe in elderly patients. It was shown that patients over 60 or 65 years of age responded to P/R with lower SVR than younger patients.37,38 High percentage of Chinese patients had severe disease at initial presentation because of the poor public awareness of HCV disease. These patients had limited treatment options because SVR is reduced in cirrhotic patients and interferon is contraindicated in patients with decompensated cirrhosis. It is unfortunate that the challenging patient populations in China are increasing, particularly those who are older, have liver cirrhosis, experienced relapse posttreatment, and have partial or no response to, are noneligible for, or do not tolerate interferon-based treatment. In addition, significant portion of patients are not willing to receive or afford P/R treatment. P/R is becoming less adequate to address the hepatitis C challenge.
DISCUSSION AND FUTURE DIRECTIONS
To reduce the HCV public burden in China, early diagnosis of hepatitis C infection followed by more effective treatment are the key elements to combat HCV. Screening for HCV by targeting individuals at-risk should be promoted among health care providers. However, unlike the screening models in the United States, there is no sufficient evidence to support a screening strategy with age-based cohort in China as iatrogenic, nosocomial, and community transmissions are still significant and dominant. Thus, risk factors for HCV infection should remain the requirement for initial screening in China.
The extended pool of unidentified HCV-infected population puts health care workers at-risk for transmission and also impact nosocomial transmissions. To that end, the Chinese Preventive Medicine Association issued guidelines in 2013 on the control and prevention of hepatitis C transmission in Chinese hospitals. The guidelines outline policy aimed to increase protection of health care workers and to decrease transmission in hospitals. Community education on HCV disease state should be implemented to promote disease awareness in the public. Direct to patients education could be considered by faith-based organizations, community social networks, or work-related settings. Early linkage-to-care and treatment initiation may slow down HCV disease progression and minimize the incidence of HCC for individual infected patients and also may control the spread of the infection in the communities.
Most Han Chinese patients are infected with a more difficult-to-treat genotype 1 HCV. The 2 first-generation DAAs, telaprevir (TVR), and boceprevir (BOC), both target genotype 1 HCV protease NS3. When used in combination with P/R, each protease inhibitor can significantly increase SVR in patients infected with genotype 1 HCV and have non-CC IL28B genotypes. In patients with IL28-CC genotype, SVR rates are similar or slightly higher in the triple combinations than P/R.39,40 As the majority of Chinese patients have the favorable CC genotype, the addition of a first-generation protease inhibitor to standard of care P/R is expected to have marginal improvement of the SVR rate in Chinese patients with genotype 1 HCV who have no contraindication to P/R. For those with advanced histology, the triple therapy with protease inhibitor is unlikely to improve the SVR significantly. No HCV protease inhibitors are licensed in China, and clinical data in Chinese patients are not available. Nevertheless, the potentials of applying these first-generation drugs to patients in China with genotype 1 HCV warrant consideration.
In considering different options for genotype 1 patients in China, modifications on regimens with protease inhibitors have not been explored. For example, therapy could initiate with standard of care P/R as a lead-in phase and based on rapid virologic response (undetectable HCV RNA at week 4), delineate the difficult-to-treat patients. For patients who failed to achieve undetectable HCV RNA at week 4 or 8 with P/R, protease inhibitor as an add-on regimen may be considered. However, prospective, randomized, and control trials are needed to prove the concept. Recent study in genotype 1 patients in Japan showed that TVR in combination with P/R demonstrated favorable SVR in relapsers or nonresponders to previous P/R. However, adverse effects were very significant.41 The triple therapy might be a future potential consideration for Chinese genotype 1 patients who failed P/R treatment.
Both TVR and BOC are associated with significant side effects and patients undergoing the triple combination therapy suffer side effects from all 3 medications. Both exhibit drug-drug interactions with many drugs.42,43 In addition, both drugs require dosing 3 times daily at 8-hour intervals and intensive monitoring during the treatment; these requirements are likely to decrease patient adherence to the treatment. Treatment cost is a barrier for patients. We might presume that the protease inhibitor add-on P/R therapy in China, if available, would be significantly higher than P/R, based on the US pricing structure. In addition, the cost would increase further as the management of adverse events may require frequent doctor’s visit or even blood transfusion. Therefore, it is very unlikely for protease inhibitor add-on P/R therapy to be cost-effective in China. Taken together, the benefit of SVR versus risk of side effects and potential high costs of the first-generation protease inhibitors in interferon-based regimen is questionable.
At present, several anti-HCV oral agents in development clearly demonstrate the possibilities of removing interferon and shortening treatment durations in achieving high SVR in patients across several HCV genotypes.44 A pan-genotypic nucleotide polymerase inhibitor in combination with P/R for 12 weeks showed high SVR in genotype 1, 4, 5, and 6 patients.45 Interferon-free regimens are possible for genotype 2 and 3 patients, including interferon-experienced or interferon-unable, where high SVR can be achieved with the nucleotide inhibitor plus ribavirin without interferon for 12 and 16 weeks, respectively.45,46 Recent study in Japan with an all-oral regimen of HCV NS5A and protease inhibitors without ribavirin showed high SVR in patients with HCV genotype 1b who were interferon-ineligible or intolerable, or nonresponsive (more than half were null responders).47 The interferon-unable populations have high prevalence of IL28-CC genotype, similar to populations in China. The nonresponders were mainly IL28-CT genotype, also similar to the Chinese patients. It was observed that relatively higher percentage of patients in Japan than in non-Asian countries had elevated alanine transaminase leading to study discontinuation. These new interferon-free regimens are favorable advances for Chinese patients who are interferon-nonresponsive or interferon-unable, especially those who are cirrhotic and have very limited options currently.
Besides providing effective treatment to patients with advanced HCV disease, a practical reason to adopt interferon-free therapy is to fulfill the unmet need in rural areas where P/R therapy has very low adherence rate because of the requirement of intense monitoring and additional needs to take care of potential adverse effects. Treatments without interferon would provide much advantage for patients who have poor access to health care providers. In addition, interferon-free regimens would significantly advance the care for patients with cirrhosis, relapses, treatment failure, or who are intolerant to or unwilling to receive interferon. This population is increasing in numbers in China. There is an important need for new DAAs that can be used without interferon, with shortened durations of treatment and good safety profile. Patients in China are diverse based on HCV genotypes, simplification of treatment by a pan-genotypic, all-oral regimen for all genotypes would be ideal. The disease prevalence and unique IL28 genotype distribution among Chinese patients point to the direction of adopting all-oral regimens rather than interferon-based triple therapy with first-generation protease inhibitors. When all-oral and pan-genotypic regimen for HCV are available in China, physicians who manage patients with HCV infection may consider some of the options showed on Figure 4.
FIGURE 4.
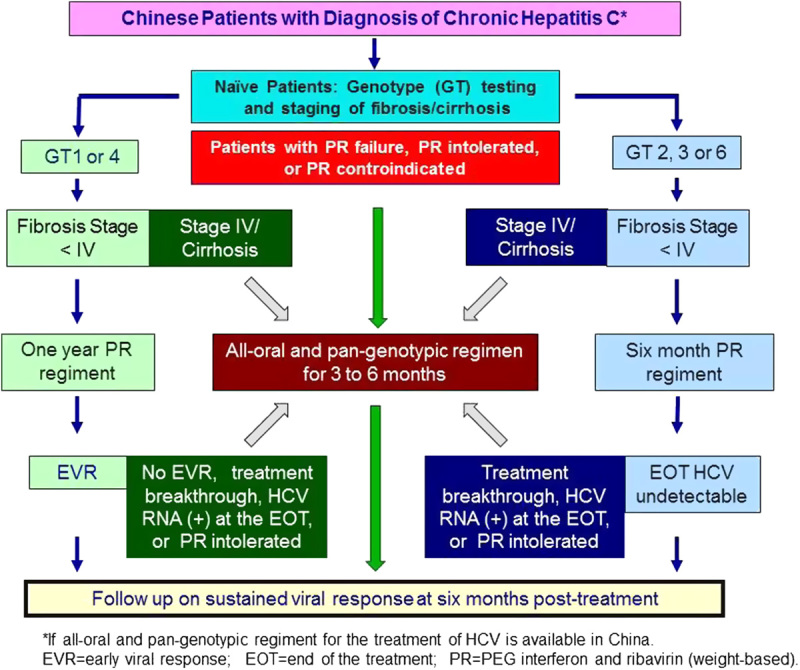
Algorithm proposed by our expert consensus group to manage patients with hepatitis C virus (HCV) infection when all-oral and pan-genotypic regiment for HCV are available in China.
In summary, despite marked reduction of HCV prevalence in China since the 1990s, newly diagnosed patients are often presented with advanced disease because of poor public disease awareness and delays in seeking medical care. There is an urgent need to conduct clinical studies of the new investigational agents in China. Clinical development on interferon-based triple therapy with DAAs is unlikely to advance the care for the growing populations of patients who are cirrhotic or ineligible, unwilling, cannot tolerate, or have failed interferon regimens. Therefore, swift regulatory review and approval on next-generation DAAs with all-oral regimens is warranted. Only new all-oral regimens without interferon that are pan-genotypic and with reduced prices would allow us to address the public health challenges caused by hepatitis C in China.
Footnotes
Z.D., J.-D.J., J.H., L.L., H.T., X.Y.X., L.W., H.Z., and C.Q.P. contributed to the content of this review. C.Q.P. provided the outline and the first draft and performed the final review and communicated with the journal. He further revised the manuscript with inputs from all authors. L.L. assisted on editorial work.
The authors declare that they have nothing to disclose.
REFERENCES
- 1.Sievert W, Altraif I, Razavi HA, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31suppl 261–80 [DOI] [PubMed] [Google Scholar]
- 2.China Center for Disease Control and Prevention, Ministry of Health.National official infectious disease reports, 2003—2012. Available at: http://www.china.cdc.cn/tjsj/fdcrbbg/. Accessed December 10, 2013
- 3.Chen YS, Li L, Cui FQ, et al. A sero-epidemiological study on hepatitis C in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2011;32:888–891 [PubMed] [Google Scholar]
- 4.Lu J, Jiang YQ, Zhao HL, et al. Hepatitis C viruses infection situation in the human population of six provinces in China. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2011;25:448–449 [PubMed] [Google Scholar]
- 5.Gao X, Cui Q, Shi X, et al. Prevalence and trend of hepatitis C virus infection among blood donors in Chinese mainland: a systematic review and meta-analysis. BMC Infect Dis. 2011;11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. News article in China Daily. China probes outbreak of hepatitis C, unclean needles blamed, December 1, 2011. Available at: http://www.chinadaily.com.cn/china/2011-12/01/content141199115.html. Accessed June 1, 2013.
- 7. News article in The Epoch Times. Hepatitis Outbreak Covered up by Chinese Authorities, December 25, 2012. Available at: http://www.theepochtimes.com/n2/china-news/hepatitis-outbreak-covered-up-by-chinese-authorities-3238523.htm. Accessed December 10, 2013.
- 8. News article in Huffpost Healthy Living. Hepatitis C outbreak in southern Chinese Province, February 27, 2012. Available at: http://www.huffingtonpost.com/2012/02/27/hepatitis-c-china-guangdong n 1304040.html. Accessed December 10, 2013.
- 9. News article in China Daily. Clinic closed following outbreak of hepatitis C, February 6, 2013. Available at: http://europe.chinadaily.com.cn/china/2013-02/06/content16205359.htm. Accessed December 10, 2013.
- 10.Huang C, Qiu F, Guo M, et al. Prevalence and risk factors of hepatitis C among former blood donors in rural China. Int J Infect Dis. 2012;16:e731–e734 [DOI] [PubMed] [Google Scholar]
- 11.Guo YH, Fan JX, Wang Z, et al. Sero-prevalence and associated risk factors on hepatitis C in Maqiao township, Henan province of China. Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33:722–725 [PubMed] [Google Scholar]
- 12.He Y, Zhang J, Zhong L, et al. Prevalence of and risk factors for hepatitis C virus infection among blood donors in Chengdu, China. J Med Virol. 2011;83:616–621 [DOI] [PubMed] [Google Scholar]
- 13.Ren NWX, Wu AH. Hepatitis C virus infection in patients undergoing continuous hemodialysis: an investigation from China National Nosocomial Infection Surveillance System. Chin J Infect Control. 2011;10:412–415 [Google Scholar]
- 14.Su L, Lin D. 2009-2010 Regional hepatitis C prevalence survey in drug addicts. Int J Lab Med. 2011;32:2236–2237 [Google Scholar]
- 15.Zhou YH, Yao ZH, Liu FL, et al. High prevalence of HIV, HCV, HBV and co-infection and associated risk factors among injecting drug users in Yunnan province, China. PLoS One. 2012;7:e42937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei L, Lopez-Talavera J, Roa H, et al. Prevalence of HCV viral and host IL28B genotypes in China. Hepatology. 2011;54:563A–564A [Google Scholar]
- 17.Fu Y, Wang Y, Xia W, et al. New trends of HCV infection in China revealed by genetic analysis of viral sequences determined from first-time volunteer blood donors. J Viral Hepat. 2011;18:42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong ZX, Zhou HJ, Wang JH, et al. Distribution of hepatitis C virus genotypes in Chinese patients with chronic hepatitis C: correlation with patients’ characteristics and clinical parameters. J Dig Dis. 2012;13:564–570 [DOI] [PubMed] [Google Scholar]
- 19.Yan Z, Fan K, Wang Y, et al. Changing pattern of clinical epidemiology on hepatitis C virus infection in southwest china. Hepat Mon. 2012;12:196–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Wang X, Mao Q, et al. Changes in modes of hepatitis C infection acquisition and genotypes in southwest China. J Clin Virol. 2009;46:230–233 [DOI] [PubMed] [Google Scholar]
- 21.Chinese Center for Disease Control and Prevention.The national hepatitis B sero-epidemiological survey results. 2008. Available at: http://www.china.cdc.cn/dcbg/200804/t20080423 34870.htm. Accessed December 10, 2013 [DOI] [PMC free article] [PubMed]
- 22.Liang X, Bi S, Yang W, et al. Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550–6557 [DOI] [PubMed] [Google Scholar]
- 23.Xinhua News Agency. About 38 mil Chinese carry hepatitis C virus, November 23, 2007. Available at: http://www.chinaorg.cn/english/health/232872.htm. Accessed December 10, 2013.
- 24.Feng B, Zhang J, Wei L. Inadequate awareness of hepatitis C among nonspecialist physicians in China. Adv Med Ed Pract. 2011;2:209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109 [DOI] [PubMed] [Google Scholar]
- 26.Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104 [DOI] [PubMed] [Google Scholar]
- 27.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401 [DOI] [PubMed] [Google Scholar]
- 28.Jia Z, Ding Y, Tian S, et al. Test of IL28B polymorphisms in chronic hepatitis C patients treated with PegIFN and ribavirin depends on HCV genotypes: results from a meta-analysis. PLoS One. 2012;7:e45698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao XW, Ling Y, Li XH, et al. Association of genetic variation in IL28B with hepatitis C treatment-induced viral clearance in the Chinese Han population. Antivir Ther. 2011;16:141–147 [DOI] [PubMed] [Google Scholar]
- 30.Guo X, Zhao Z, Xie J, et al. Prediction of response to pegylated-interferon-alpha and ribavirin therapy in Chinese patients infected with different hepatitis C virus genotype. Virol J. 2012;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He LL, Chen Z, Chen Y, et al. Association between the influential factors and the effectiveness of pegylated interferon alpha-2a plus ribavirin as a combination treatment for chronic hepatitis C patients. Zhonghua Gan Zang Bing Za Zhi. 2011;19:34–37 [DOI] [PubMed] [Google Scholar]
- 32.Ma LN, Chen XY, Chen J, et al. Efficacy, influencing factors and safety of PEG-INF alpha-2a (PEG-INF-2a) in the treatment of chronic hepatitis C: analysis of 89 patients. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2006;20:42–45 [PubMed] [Google Scholar]
- 33.Tsang OT, Zee JS, Chan JM, et al. Chronic hepatitis C genotype 6 responds better to pegylated interferon and ribavirin combination therapy than genotype 1. J Gastroenterol Hepatol. 2010;25:766–771 [DOI] [PubMed] [Google Scholar]
- 34.Yu JW, Wang GQ, Sun LJ, et al. Predictive value of rapid virological response and early virological response on sustained virological response in HCV patients treated with pegylated interferon alpha-2a and ribavirin. J Gastroenterol Hepatol. 2007;22:832–836 [DOI] [PubMed] [Google Scholar]
- 35.Zhou BT, Fan YM, Li TM, et al. Effectiveness of combined therapies using two types of peginterferon and ribavirin in treating chronic hepatitis C virus genotypes 1b/6a infections. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2010;32:320–323 [DOI] [PubMed] [Google Scholar]
- 36.Chen GF, Chen XY, Shao Q, et al. Analysis of factors tonferiat impact efficacy of pegylated interferon plus ribnavirin in the treatment of chronic hepatitis C [in Chinese]. Infect Dis Info . 2010;23:236–238 [Google Scholar]
- 37.Zheng YY, Fan XH, Wang LF, et al. Efficacy of pegylated interferon-alpha-2a plus ribavirin for patients aged at least 60 years with chronic hepatitis C. Chin Med J (Engl). 2012;125:1852–1856 [PubMed] [Google Scholar]
- 38.Yu JW, Sun LJ, Kang P, et al. Efficacy and factors influencing treatment with peginterferon alpha-2a and ribavirin in elderly patients with chronic hepatitis C. Hepatobiliary Pancreat Dis Int. 2012;11:185–192 [DOI] [PubMed] [Google Scholar]
- 39.Poordad F, Bronowicki JP, Gordon SC, et al. Factors that predict response of patients with hepatitis C virus infection to boceprevir. Gastroenterology. 2012;143:e1–e5 [DOI] [PubMed] [Google Scholar]
- 40.Jacobson IM, Catlett I, Marcellin P, et al. Telaprevir substantially improved SVR rates across all IL 28B genotypes in the advance trial. J Hepatol. 2011;54:S535–S545 [Google Scholar]
- 41.Hayashi N, Okanoue T, Tsubouchi H, et al. Efficacy and safety of telaprevir, a new protease inhibitor, for difficult-to-treat patients with genotype 1 chronic hepatitis C. J Viral Hepat. 2012;19:e134–e142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vertex Pharmaceuticals. Incivek (telaprevir). US prescribing information, December 2012. Available at: http://pi.vrtx.com/files/uspi telaprevir.pdf. Accessed December 10, 2013.
- 43.Merck & Co. Victrelis (boceprevir). US prescribing information, February 2003. Available at: http://www.merck.com/product/usa/pi circulars/v/victrelis/victrelis pi.pdf. Accessed December 10, 2013.
- 44.Barreiro P, Vispo E, Poveda E, et al. Hepatitis C therapy: highlights from the 2012 annual meeting of the European Association for the Study of the Liver. Clin Infect Dis. 2013;56:560–566 [DOI] [PubMed] [Google Scholar]
- 45.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887 [DOI] [PubMed] [Google Scholar]
- 46.Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–1877 [DOI] [PubMed] [Google Scholar]
- 47.Chayama K, Suzuki Y, Ikeda K, et al. All-oral combination of daclatasvir plus asunaprevir in interferon ineligible naive/intolerant and nonresponder Japanese patients chronically infected with HCV genotype 1b: Results from a phase 3 trial. Hepatology. 2013;58S186A23348596 [Google Scholar]


