Supplemental Digital Content is available in the text.
Keywords: attention, attentional engagement, emotion, fMRI
Abstract
The aim of this study was to investigate whether emotion–attention interaction depends on attentional engagement. To investigate emotional modulation of attention network activation, we used a functional MRI paradigm consisting of a visuospatial attention task with either frequent (high-engagement) or infrequent (low-engagement) targets and intermittent emotional or neutral distractors. The attention task recruited a bilateral frontoparietal network with no emotional interference on network activation when the attentional engagement was high. In contrast, when the attentional engagement was low, the unpleasant stimuli interfered with the activation of the frontoparietal attention network, especially in the right hemisphere. This study provides novel evidence for low attentional engagement making attention control network activation susceptible to emotional interference.
Introduction
Humans efficiently select relevant information from a rich multisensory environment to accomplish desired goals. Selective attention permits the selection of goal-relevant sensory stimuli by allocating the limited pool of neural processing resources to the context-relevant stimuli, at the expense of competing stimuli 1. A distributed frontoparietal network is involved in the control of selective attention by exerting top-down modulation of lower-level sensory brain areas 2.
Emotional stimuli occurring either inside or outside the focus of attention readily capture attentional resources for the processing of those stimuli, particularly for aversive events 3. However, attentional and emotional goals can be in conflict requiring attentional resources to be allocated to prioritized actions 4. Competition between bottom-up emotional distractors and attention-demanding task-related targets may lead to diminished activity in the frontoparietal network subserving task performance 5,6. This competition is supported by our previous findings of task-irrelevant emotional stimuli interfering with right hemisphere-dependent processes such as global level visual processing 7 and left visual field stimulus attention 8,9, accompanied by diminished event-related brain potentials to targets in the context of emotional distractors 6,10. This reduction was the greatest over the right frontoparietal region 6. Patients with neglect show similar disruption of right hemisphere-dependent left visual field detection performance, further impaired by preceding unpleasant emotional stimuli 11.
According to the load theory of attention, high perceptual load that exhausts perceptual processing capacity decreases distractor interference 12. Studies on emotion–attention interaction have mainly focused on the impact of task load on the emotional networks, with high task loads tuning down the emotional brain 13–15. However, less is known on the impact of task load on emotion–attention interaction within attentional networks. As opposed to high attention load, low load has been shown to lead to greater emotional interference 12. Thus, low attention load might be expected to show greater interference on task-related attention network activity.
Better understanding of the emotion–attention interaction is clinically relevant as alterations in this interaction are associated with psychiatric disorders such as anxiety 16 and depression 17. In these conditions, there is an excessive tendency to focus selective attention on negative information, which may interfere with cognitive performance. When attentional and executive resources are occupied by negative information, cognitive performance that relies on attentional and executive resources may be compromised 7.
Here, we extended our previous event-related potential studies on emotional influence on frontoparietal attention control networks to explore the effects of varying task engagement on emotion–attention interaction with functional MRI (fMRI). We investigated whether the emotional modulation of attention control network is dependent on task-related attentional demands. To assess this, we used a visuospatial paradigm consisting of target discrimination with some targets preceded by a brief presentation of a neutral or an unpleasant emotional distractor. In different blocks, different proportions of task-related targets were used to produce conditions with either a high or a low attention demand.
Experimental procedure
Fourteen healthy right-handed adults (mean age 32 years; range 20–59; men: five, women: nine) participated in the study. Participants gave their consent according to the University of Berkeley Guidelines and approved by the Ethics Committee of the Institutional Review Board.
Two sets (unpleasant and neutral) of 48 images were chosen from the International Affective Picture System 18. The mean arousal ratings for unpleasant and neutral images were 5.8±0.8 and 3.5±0.6, respectively.
The visual stimulus material was delivered using Presentation Software (http//www.neurobs.com). Participants were instructed to keep their eyes on a fixation cross in the middle of the screen throughout the presentation of the stimuli and to discriminate between standard and inverted triangles (target; 150 ms duration) randomly presented in the left or the right visual hemifield. A brief emotional (unpleasant) or neutral picture (150 ms duration) was presented centrally 350 ms before the subsequent target. Participants responded to the orientation of the triangle pressing with different fingers if the triangle was pointing upward or downward. In trials where no picture and/or target was presented, a fixation cross was presented instead.
Each scan session had eight runs consisting of five different blocks, each corresponding to a different experimental condition, separated by a fixation block. Within each session, blocks were presented in a semi-random order. Each block consisted of 16 trials. Within blocks with different trial types, the trials were presented in a semi-random order. The response hand was counterbalanced within participants. The experimental blocks were: targets alone, 14 trials of targets without preceding pictures (TRG) and two extra fixation trials (FIX); emotional images alone, 14 trials of pictures presented alone (unpleasant pictures: NEG; neutral pictures: NEU) and two extra FIX trials; high attentional demand, 12 trials of targets preceded by pictures in all the trials (unpleasant pictures targets: NEG-TRG; neutral pictures targets: NEU-TRG), two trials of pictures alone, and two trials of targets alone; low attentional demand, 12 trials with pictures alone and four trials with targets alone (unpleasant pictures or targets: NEG-trg; neutral pictures or targets: NEU-trg). The fixation block (baseline) between the task blocks consisted of 10 s of presentation of a fixation cross.
Images were acquired with a 4 T Varian INOVA MR scanner (Palo Alto, California, USA, http://www.varian.com). Functional images were obtained using a two-shot gradient-echo-planar sequence with a repetition time of 2 s, echo time of 20 ms, and flip angle of 70°. Each volume consisted of 18.6 mm axial slices with 0.5 mm interslice gap. Each slice was acquired with 24 cm2 field of view with a 64×64 matrix size, giving an in-plane resolution of 3.5×3.5 mm. A total of 170 brain volumes were acquired per scan session.
Images were analyzed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB 12 (Math Works Inc., Natick, Massachusetts, USA). The functional images were temporally aligned, corrected for movement, and spatially normalized to fit to the template created using the Montreal Neurological Institute reference brain based on the Talairach coordinate system. The spatially normalized volumes consisting of 2×2×2 mm3 voxels were smoothed with an 8 mm full-width at half-maximum isotropic Gaussian kernel. No participant was rejected because of excessive head movement (<1 mm).
A canonical hemodynamic response function was used to model task-related activity in a blocked design. Individual linear contrasts were applied to the design to investigate networks associated with each condition with respect to baseline. The design matrix included correction for head movements as regressors of no interest.
To identify the brain areas commonly activated across the whole group, a second-level analysis was carried out, treating participants as a random variable.
To test the effect of emotion and attention load, an analysis of variance (ANOVA) test was performed with main factors: emotion (levels: neutral and negative) and attention load [levels: no response (pictures only), low (pictures with infrequent targets), and high (pictures with frequent targets)]. In regions about which we had a priori hypothesis, a correction for multiple comparisons across a small volume of interest to the P-values in this region was applied 19. Volumes of interest for bilateral middle frontal gyrus, bilateral precentral area, superior temporal gyrus, and inferior and superior parietal lobes (IPL and SPL) were defined from automated anatomical labeling atlas 20.
As a further test, we calculated the percent signal intensity change (%SC) in the anatomical regions of interest (ROIs) 21 extracted from the automated anatomical labeling. The areas studied compose the attentional network as reported in the literature and included bilateral ROIs of frontal inferior, middle, and superior gyrus (IFG, MFG, and SFG), IPL and SPL, precentral area, and middle and superior temporal gyrus (MTG and STG) 2. IFG was in turn divided into anterior, middle, and posterior segments (ant-IFG, mid-IFG, and post-IFG). The %SC from the common precondition baseline was calculated with the Marsbar Region of Interest Toolbox 22 for the different task conditions for each anatomical ROI.
SPSS software (SPSS Inc., Chicago, Illinois, USA) was used for statistical analysis. Repeated-measures ANOVA was performed on reaction times (RT), accuracy, and %SC values, and Greenhause–Geisser correction for sphericity was applied. Main effects of emotion (two levels: neutral and negative) and attention load and their interactions were investigated. For RT and accuracy, the factor attention load had two levels: pictures with high task engagement and pictures with low task engagement. For %SC, the factor attention load had three levels: pictures, pictures with high task engagement, and pictures with low task engagement. Significance level was set at P value of 0.05. Post-hoc analysis was carried out with paired t-tests.
Results
Behavioral results
RT presented significant emotion and attention load main effects (P=0.044, F=5.07 and P<0.001, F=26.29, respectively) and a significant emotion–demand interaction (P=0.006, F=10.8). Post-hoc comparison showed that, in high task-engagement conditions, RTs were slower to targets preceded by negative pictures (696±36 ms) compared with targets preceded by neutral pictures (683±36 ms). In the case of low task engagement, targets preceded by both neutral (730±48 ms) and negative (729±41 ms) stimuli were associated with significantly longer RT than those of high task engagement (P<0.001, t=4.79) but did not show differences regarding an emotional factor. Accuracy in target detection was not affected by either the presence or valence of the preceding pictures.
Imaging results
In experimental blocks with only targets (TRG), brain activity associated with the visuospatial task was found in the medial frontal areas and bilateral frontal eye fields (FEF), IFG, MFG, and IPL (Supplementary Table 1, Supplemental digital content 1, http://links.lww.com/WNR/A292).
To study the effect of emotional distractors on this attention network, brain responses were examined in the conditions where different proportions of targets were presented – that is, when different task engagement was required – in the presence of unpleasant or neutral images. The ANOVA analysis showed an increased involvement of the bilateral MFG and FEF, bilateral IPL, and right STG with increased attention load (Fig. 1a; Supplementary Table 2, Supplemental digital content 2, http://links.lww.com/WNR/A293). In addition, there was a main effect of emotion on bilateral insula and STG. A significant interaction effect of task engagement by emotion was seen in bilateral SPL, right MFG, and left FEF. Post-hoc contrast showed significantly greater activation in context of neutral compared with emotional distractors in the low task-engagement condition (NEU-trg>NEG-trg) in the right hemisphere in MFG and STG and in bilateral IPL and SPL (Fig. 1b; Supplementary Table 2, Supplemental digital content 2, http://links.lww.com/WNR/A293). In contrast, there was no significant activation in the neutral over negative comparisons. These results indicate that the presentation of unpleasant images affected the activation of brain areas subserving attention control when targets were presented sparsely within the block (i.e., task engagement was low) but not when they appeared in every trial (i.e., task engagement was high).
Fig. 1.
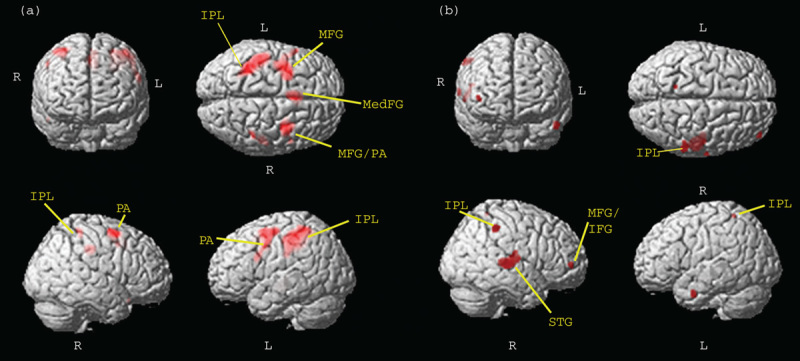
(a) Main effect of attention load. Regions belonging to the dorsal frontoparietal attention network show greater activation with higher attention load (P<0.05 FWE); (b) emotional modulation of attention network during low attentional load. NEU>NEG contrast in low attention-load condition (P<0.0001 uncorrected for visualization). IFG, inferior frontal gyrus; IPL, inferior parietal lobe; L, left; MFG, middle frontal gyrus; PA, precentral area; R, right; STG, superior temporal gyrus.
To further examine these differences, we performed a ROI analysis in circumscribed anatomical regions associated with attention processes and extracted the mean %SC values. The results revealed that several areas of the right hemisphere presented statistically significant interactions of task engagement by emotion (P<0.05): right IPL, MFG, IFG, and STG (Fig. 2a). Marginal means comparisons showed that, in all these ROIs, the activity was low in conditions with images alone irrespective of their emotional valence but increased because of the attentional task in NEU-TRG, NEG-TRG, and NEU-trg. Notably, the activity did not increase relative to baseline in the NEG-trg condition in which there were few target trials within the block, thus evidencing an interaction between emotion and attention processes (right IPL is depicted as an example in Fig. 2b).
Fig. 2.
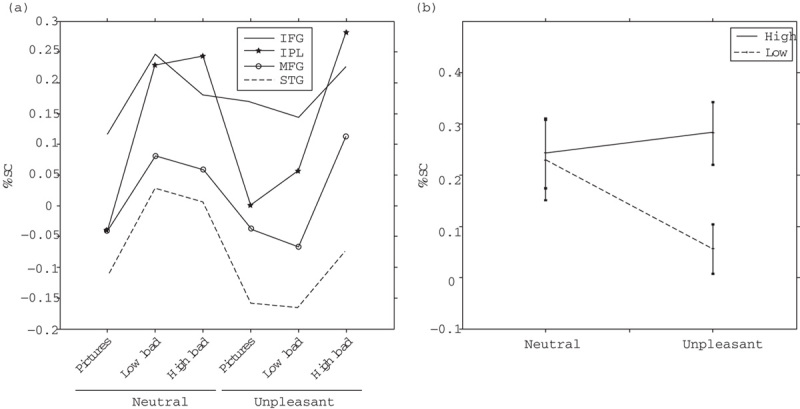
(a) Average values of percent signal change (%SC) in the different task conditions for the following regions of interest: right superior temporal gyrus (STG R); right inferior parietal lobe (IPL R); right inferior frontal gyrus (IFG R), and right middle frontal gyrus (MFG R). (b) %SC values of IPL R as in Fig. 2a are depicted for the conditions with targets and distractors.
Discussion
Two interacting attention systems have been proposed based on neuroimaging studies. The dorsal network includes frontal eye fields and intraparietal sulcus/SPL and is involved in voluntary top-down attention. The ventral right lateralized frontoparietal network includes IFG and MFG as well as IPL and STG in the temporoparietal junction, and it is involved in orienting attention to salient stimuli or to infrequent targets 2,23. In the current study, we found areas from the dorsal and ventral attention networks recruited by the task, as previously reported in fMRI studies of attention in the context of emotion (for a review, see Viviani 24). Importantly, we demonstrate that low attention load predisposes the ventral attention network to emotional interference. In the low attention-load condition, activation in IPL/STG and MFG was reduced by emotional distractors in comparison with neutral distractors. In accordance with the load theory of attention 12, lower attentional demands due to infrequent targets lead to greater bottom-up emotional interference with task-related attention network activation.
MFG has been suggested as the link between the ventral and dorsal attention networks, allowing for flexible interaction of the two attention systems when top-down and bottom-up attentional demands meet 23. Suppression of MFG activation due to emotional distractors has been previously reported with fMRI by Yamasaki et al. 5. It has been suggested that frontoparietal control recruited by a highly demanding task may facilitate the disengagement from emotional distraction 25. Reduced frontoparietal network activation due to unpleasant emotional stimuli in the low load condition may be related to greater challenges in disengaging attention from emotional distractors back to the task during low attention load. This facilitation of attentional disengagement from emotional distractors due to greater frontoparietal control might explain why no emotion effect on attention network activity was observed in high attention-load condition. When targets were sparser, disengaging attention from distractors back to the task may be less efficient due to weaker top-down control. Weaker or less consistent top-down control of attentional resources to the task was reflected in overall slowed response speed and lower frontoparietal attention network activation due to low load. Weaker top-down control of attentional resources allowed for greater bottom-up influence of emotional distractors, reducing task-related attention network activity. In the current study, the low attention load with more time off task most likely allowed for repeated negative emotional stimuli to induce negative moods, mind wandering, and reduced shifting of attention to the task-related processes (i.e., stimulus expectation, response selection). Negative moods are associated with more self-centered thoughts and mind wandering 26.
Behavioral data showed significantly faster RT in response to targets during high attention load blocks with respect to low attention load blocks. In the former case, participants expected targets in every trial, and consequently reacted faster than when the targets occurred unexpectedly, as was the case in the low attention load. Interestingly, RTs in the context of unpleasant distractors were longer in comparison with neutral distractors in the high attention-load condition. In contrast, in the low attention load, additional slowing of response speed due to emotional distraction was not observed. With only few targets in the low attention-load task, there are not enough data to reliably assess possible emotional interference on speed or accuracy of responding. The emotional interference observed as slowed RTs in the high attention-load condition suggests that emotional stimuli were processed at least initially to an extent that they differentially influenced response speed. Thus, low attention load led to greater effect of emotional distractors on attention network activation; however, high attention load did not prevent emotional stimuli from being processed.
We conclude that emotional distractors were processed in both high and low attention-load conditions as indicated by emotional modulation of response speed in the high attention-load condition and emotional modulation of frontoparietal attention network activation in the low attention-load condition. Furthermore, there was greater activation of insula and STG during attention task with emotional distractors independent of load, supporting orienting of attention to salient emotional distractors in both conditions. In addition, insula and STG activation modulated by emotional distractors during attention task points toward the previously suggested role of these regions in integrating emotional and attentional demands.
The insula is also considered to be part of the ventral attention network mediating bottom-up control of visuospatial attention and has been assigned roles in emotion and attention regulation 27. Specifically, insula is thought to allow for detection of salient stimuli 28. In concordance with these functions, we found attention task with emotional distractors to activate insula to a greater extent than task with neutral distractors.
As shown in the current study and previously in healthy individuals, vulnerability of the right hemisphere attention network to emotional capture of resources 6,7 can lead to further deterioration in patients with compromised right hemisphere attention performance due to neglect 11. Better understanding of emotion–attention interaction in healthy individuals provides insights into its alterations in affective disorders, in attentional deficits, and in treatments that directly influence limbic and associative circuitries behind this interaction, such as deep brain stimulation 29. The current study points to the intricate interplay between emotion and attention with clinical relevance in treatment and rehabilitation of patients with emotion–attention dysfunction.
Conclusion
Our study demonstrates that the frontoparietal attention network, especially the right lateralized ventral network, is vulnerable to emotional interference. Furthermore, this interference effect depends on the level of task-related attentional engagement. Low level of task engagement allows for a greater influence of emotion. The current study contributes to the literature on emotion–attention interaction with novel evidence for greater influence of emotional stimuli on attention network when top-down attentional control is weak or inconsistent.
Acknowledgements
This work was supported by the Academy of Finland, Competitive Research Funding of Pirkanmaa Hospital District, and NINDS Grant (NS21135).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.neuroreport.com).
References
- 1.Duncan J, Humphreys G, Ward R. Competitive brain activity in visual attention. Curr Opin Neurobiol 1997;7:255–261 [DOI] [PubMed] [Google Scholar]
- 2.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002;3:201–215 [DOI] [PubMed] [Google Scholar]
- 3.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron 2001;30:829–841 [DOI] [PubMed] [Google Scholar]
- 4.Fichtenholtz HM, Labar KS.Mangun George. Emotional influences on visuospatial attention. Neuroscience of attention. Attentional control and selection 2012Oxford:Oxford University Press; p. 250 [Google Scholar]
- 5.Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci USA 2002;99:11447–11451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartikainen KM, Ogawa KH, Soltani M, Knight RT. Emotionally arousing stimuli compete for attention with left hemispace. Neuroreport 2007;18:1929–1933 [DOI] [PubMed] [Google Scholar]
- 7.Hartikainen KM, Ogawa KH, Knight RT. Trees over forest: unpleasant stimuli compete for attention with global features. Neuroreport 2010; 21:344–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartikainen KM, Ogawa KH, Knight RT. Transient interference of right hemispheric function due to automatic emotional processing. Neuropsychologia 2000;38:1576–1580 [DOI] [PubMed] [Google Scholar]
- 9.Gupta R, Raymond JE. Emotional distraction unbalances visual processing. Psychon Bull Rev 2012;19:184–189 [DOI] [PubMed] [Google Scholar]
- 10.Weinberg A, Hajcak G. The late positive potential predicts subsequent interference with target processing. J Cogn Neurosci 2011;23:2994–3007 [DOI] [PubMed] [Google Scholar]
- 11.Oren N, Soroker N, Deouell LY. Immediate effects of exposure to positive and negative emotional stimuli on visual search characteristics in patients with unilateral neglect. Neuropsychologia 2013;51:2729–2739 [DOI] [PubMed] [Google Scholar]
- 12.Lavie N, Beck DM, Konstantinou N. Blinded by the load: attention, awareness and the role of perceptual load. Philos Trans R Soc Lond B Biol Sci 2014;369:20130205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pessoa L. To what extent are emotional visual stimuli processed without attention and awareness?Curr Opin Neurobiol 2005;15:188–196 [DOI] [PubMed] [Google Scholar]
- 14.Morawetz C, Baudewig J, Treue S, Dechent P. Diverting attention suppresses human amygdala responses to faces. Front Hum Neurosci 2010; 4:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Dillen LF, Papies EK, Hofmann W. Turning a blind eye to temptation: how task load can facilitate self-regulation. J Pers Soc Psychol 2013;104:427–443 [DOI] [PubMed] [Google Scholar]
- 16.Matthews G, Wells A. Attentions, automaticity and affective disorder. Behav Modif 2000;24:69–93 [DOI] [PubMed] [Google Scholar]
- 17.Liotti M, Mayberg HS. The role of functional neuroimaging in the neuropsychology of depression. J Clin Exp Neuropsychol 2001;23:121–136 [DOI] [PubMed] [Google Scholar]
- 18.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): technical manual and affective ratings 1997Gainesville, FL:NIMH Center for the Study of Emotion and Attention, University of Florida [Google Scholar]
- 19.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 1996;4:58–73 [DOI] [PubMed] [Google Scholar]
- 20.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–289 [DOI] [PubMed] [Google Scholar]
- 21.Poldrack RA. Regions of interest analysis for fMRI. Soc Cogn Affect Neurosci 2007;2:67–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain; 2–6 June 2002, Sendai, Japan.
- 23.Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist 2014;20:150–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viviani R. Emotion regulation, attention to emotion, and the ventral attentional network. Front Hum Neurosci 2013;7:746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke PJ, MacLeod C, Guastella AJ. Assessing the role of spatial engagement and disengagement of attention in anxiety-linked attentional bias: a critique of current paradigms and suggestions for future research directions. Anxiety Stress Coping 2013;26:1–19 [DOI] [PubMed] [Google Scholar]
- 26.Smallwood J, Fitzgerald A, Miles LK, Phillips LH. Shifting moods, wandering minds: negative moods lead the mind to wander. Emotion 2009;9:271–276 [DOI] [PubMed] [Google Scholar]
- 27.Hahn B, Ross TJ, Stein EA. Neuroanatomical dissociation between bottom-up and top-down processes of visuospatial selective attention. Neuroimage 2006;32:842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 2010; 2145–6655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartikainen K, Sun L, Polvivaara M, Brause M, Lehtimäki K, Haapasalo J, et al. Immediate effects of deep brain stimulation of anterior thalamic nuclei on executive functions and emotion-attention interaction in humans. J Clin Exp Neuropsychol 2014;36:540–550 [DOI] [PMC free article] [PubMed] [Google Scholar]


