In vivo molecular imaging provides the ability to measure expression levels of molecules by quantifying imaging signals generated with the help of contrast agents accumulating at sites of molecular target expression. These contrast agents can be directed to bind various molecular targets in vivo (eg, proteins, DNA, etc), thereby quantifying disease processes at the molecular level in various disease processes. Thus, molecular imaging has the potential to obtain tissue expression profiles in a volumetric manner without invasive tissue sampling procedures and without the limitation of potential sampling errors from biopsies. Emerging research in ultrasound technology and contrast agent design for ultrasound imaging has paved the way for targeted contrast-enhanced ultrasound imaging to be translated to clinical applications in the near future.1–6
Ultrasound imaging is a widely available, inexpensive, and real-time imaging modality that does not expose patients to irradiation. It is already the first-line imaging modality for assessment of many diseases in the abdomen and pelvis. Through the introduction of ultrasound contrast agents (eg, lipid-shelled, gas-filled, 1- to 4-micron-sized microbubbles), the sensitivity and specificity of ultrasound for detection and characterization of for example focal liver lesions7,8 has been substantially improved. Recently, targeted contrast-enhanced ultrasound (molecular ultrasound) imaging has gained great momentum in preclinical research by the introduction of ultrasound contrast microbubbles that are targeted at molecular markers overexpressed on the vasculature of certain diseases (Figure 1A and Supplementary Figure 1). By combining the advantages of ultrasound with the ability to image molecular signatures of diseases, molecular ultrasound has great potential as a highly sensitive and quantitative method that could be used for various clinical applications, including screening for early stage diseases (such as cancer); further characterization of focal lesions and quantitative monitoring of disease processes at the molecular level; assisting in image-guided procedures (eg, biopsy, surgery, or ablation); enhancing drug delivery; and confirming target expression for treatment planning and monitoring (including novel drug candidates in preclinical studies).1,4,6,9,10
Figure 1.
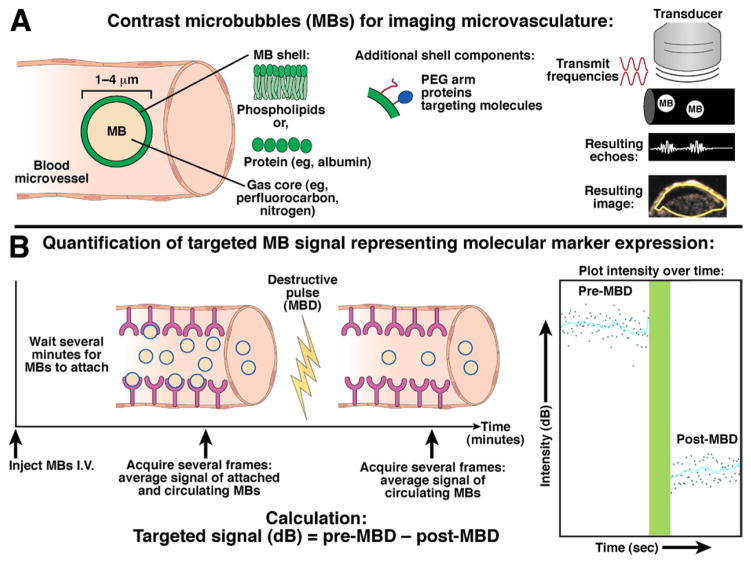
Principles of nontargeted and molecularly targeted contrast-enhanced ultrasound imaging with contrast microbubbles. (A) The gas core of lipid- or protein-shelled microbubbles (left) makes them highly echogenic compared to surrounding tissue and blood. Right, The pulse-inversion technique is commonly used for detection of microbubbles (see Supplementary Material). Two inverse-phase (red) pulses are transmitted through the tissue, and different echoes are reflected back from either microbubbles (MBs; white) or tissue (black). The resulting echo (summation from each pulse) from microbubbles (nonlinear behavior) is a distinct signature (white wave), whereas the waves reflected from the tissue (linear) signal cancel out (white flat line).49 The resulting ultrasound contrast-mode image shows a pixel-by-pixel distribution of the microbubble signal in a subcutaneous human colon cancer xenograft in a mouse. (B) Example of imaging sequence for quantification of molecular ultrasound imaging signal intensities within a region of interest. Please refer to text for more details.
We describe herein concepts and applications in contrast-enhanced and molecular ultrasound imaging with particular focus on imaging cancer and inflammatory diseases in the abdomen and pelvis.
Contrast-Enhanced Ultrasound Imaging Techniques and Applications
Contrast-Enhanced Ultrasound Imaging Techniques
Microbubbles (Figure 1A and Supplementary Figure 1) are the most commonly used ultrasound contrast agent, and are defined as microspheres with a lipidic or protein shell and gas core.4,9,11,12 They are usually filled with perfluorocarbon (eg, perfluorobutane) gases that are heavier than air (for stability reasons); this gas filling enables high reflection and scatter of sound in blood for very sensitive ultrasound detection (Figure 1A, right). Because of their micron size of usually between 1 and 4 μm, microbubbles stay confined to the vasculature, and can pass through microcapillaries. The phospholipid shell is highly biocompatible, and can be “decorated” with other biocomponents (eg, polyethylene glycol, proteins) for added stability.4 Microbubbles typically remain in circulation for only a few minutes, and are rapidly cleared through the reticuloendothelial system (in particular the liver and spleen) and the lungs (exhalation of gas core).4,9,13 Generally, microbubbles are safe intravenous contrast agents that are commercially available, and have been used for cardiovascular and body imaging (see below). A major advantage of ultrasound contrast agents compared to iodinated computed tomography contrast agents or gadolinium-based magnetic resonance contrast agents is that they can be used in patients with renal insufficiency and renal function tests are not needed before administration of microbubbles.14
Contrast-enhanced ultrasound imaging with nontargeted microbubbles can be used to assess levels of vascularity in real time (Figure 1A) using the first pass (bolus) of microbubbles into the region of interest with time–intensity curve analysis; maximum intensity projection analysis; or, re-perfusion analysis (see Supplementary Material).
Applications of Contrast-Enhanced Ultrasound Imaging in the Abdomen and Pelvis
Contrast-enhanced ultrasound imaging techniques for assessment of tissue vascularity are increasingly used for clinical imaging of the liver,8,15,16 spleen,17 intestine,18–20 pancreas,21–29 kidneys,30–33 uterus/ovaries,34–37 and testes/prostate.38,39 The use of contrast microbubbles allows for better focal lesion detection, and has been helpful in differentiating between benign and malignant lesions. Additionally, contrast-enhanced ultrasound imaging can provide a more accurate measurement of tumor size and margins, even in the case of avascular tumors (eg, pancreatic ductal adenocarcinomas40). For more information, please refer to a recent review.7
Molecular Ultrasound Imaging: Clinical Translation on the Horizon
Ultrasound Imaging with Molecularly Targeted Microbubbles
Molecular ultrasound imaging utilizes the same contrast-mode detection methods as nontargeted contrast-enhanced ultrasound imaging; however, the quantification of ultrasound signal is performed by using molecularly targeted microbubbles attaching at sites of molecular marker expression. It is challenging to separate the signals of attached microbubbles from background signal associated with freely circulating, unattached microbubbles, and new methods are under development.4 The most common and currently used method measures signal before and after a high-powered, ultrasound pulse that destroys the microbubbles within the beam elevation (Figure 1B). After microbubbles are injected intravenously and allowed to circulate for several minutes to attach to the molecular targets, the average signal intensity is measured over several seconds, representing imaging signals generated from both attached and freely circulating microbubbles. This measurement is followed by application of a high-powered ultrasound pulse for a few seconds to destroy all the attached microbubbles within the beam elevation (microbubble destruction). After a few seconds to allow the freely circulating microbubbles to replenish from outside the beam elevation into the imaging area of consideration, a second measurement records the signal intensity representing only circulating microbubbles. The targeted signal measurement is calculated by the subtraction of the pre-microbubble destruction signal/image minus the post-microbubble destruction signal/image (Figure 1B). Because most ultrasound imaging systems are equipped with contrast mode detection software, the rate-limiting step for clinical translation of molecular ultrasound imaging is design, testing, and approval (eg, by the US Food and Drug Administration) of clinical-grade molecularly targeted microbubble contrast agents.
Molecularly Targeted Microbubble Design and Steps Toward Clinical Translation
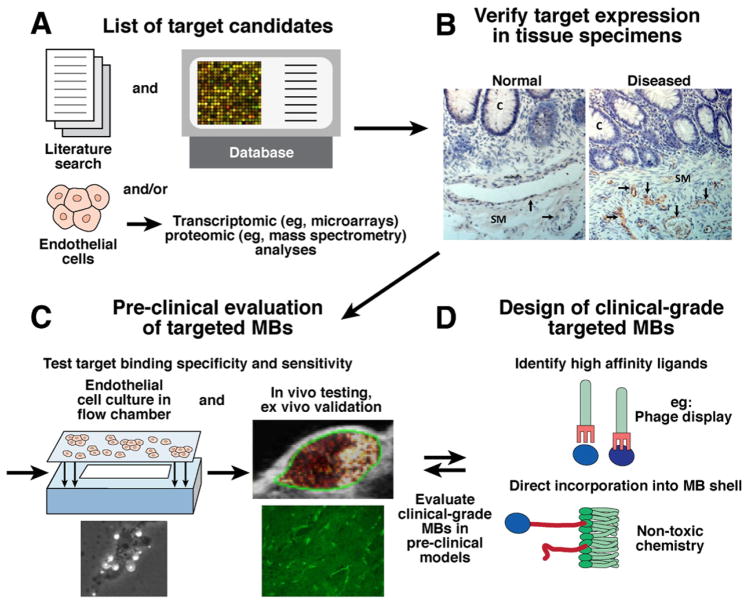
Microbubbles are molecularly targeted by coating with molecules such as antibodies, peptides, proteins, or small molecules that can bind to endothelial cell surface proteins expressed on the vessel wall (Figure 1A and Supplementary Figure 1). Identification of specific molecular markers for microbubble targeting is of utmost importance in design consideration, and must be verified for both clinical (human) and preclinical (small and large animal) imaging. The process for identifying new, disease-specific markers, and designing and testing microbubbles targeted to these markers is shown in Figure 2. First, extensive biocomputational mining of literature reports and publicly available databases can be used for discovery of potential molecular imaging targets that are taken alone or as a group characteristic for certain diseases.10,41 For contrast microbubble design, it is important to (1) ensure that targets are expressed on the surface of vascular endothelial cells, (2) characterize the extent and duration of target expression (eg, temporal variability during the course of a disease or during a certain treatment plan), and (3) verify that the molecular targets are expressed in human diseases, and can be tested in well-characterized animal models of those disease. An alternative approach for target discovery is direct differential expression analysis on tissue samples. In this case, endothelial cells can be extracted (eg, by laser capture microdissection) from normal and diseased human tissue, and used to obtain proteomic (eg, mass spectrometry) and/or transcriptomic (eg, DNA microarrays) expression profiles.41 Expression patterns are compared for differences between normal and diseased tissue, and a list of potential targets is generated. The second step is to validate target expression on endothelial vessels associated with the disease process in human tissues, for example, by using immunohistochemistry analysis (Figure 2B, black arrows).
Figure 2.
Possible approaches for molecular target discovery (step 1; A) and validation (step 2; B), testing of targeted contrast microbubbles (step 3; C), and clinical-grade contrast microbubble design (step 4; D). See text for more details. Example micrographs for target validation are immunohistochemically stained normal and diseased colon (C, crypt; SM, submucosa) tissues with hematoxylin-stained cell nuclei (blue) and target-stained (brown) vascular endothelial cells (black arrows); note that in this example, imaging targets are only expressed on vascular endothelial cells in diseased but not in normal tissue. Targeted microbubble can then be tested preclinically for binding specificity both in cell culture with flow chamber (schematic; brightfield micrograph [original magnification, ×100] shows white microbubbles attaching to cells2) and in animal models in vivo in a subcutaneous human colon cancer xenograft (green outline) in a mouse. Target expression is also verified ex vivo by immunostaining; a 100-× micrograph shows brightly stained green blood vessels. Example of clinical-grade targeted microbubble design shows direct incorporation of binding ligand (eg, peptides identified by phage display; see also Supplementary Figure 1) into the microbubble shell.
Once a specific target has been discovered and validated as a differentially expressed marker on endothelial cells in human diseased tissue, the third step is to test the target for molecular ultrasound imaging in a preclinical model in vivo. For this purpose, microbubbles are functionalized with ligands that bind to the identified molecular target (Supplementary Figure 1 and Deshpande et al4). These targeted microbubbles are then tested for binding affinity and specificity both in cell culture and in vivo animal models.9 Several preclinical studies have executed these first three steps to characterize microbubbles targeted to vascular endothelial growth factor receptor type 242,43 (human analog: kinase domain insert receptor [KDR]2), integrin αVβ3,5,42 or endoglin in various cancer models including ovarian, prostate, and pancreatic cancers.5,42– 44 Molecular markers of inflammation, including mucosal addressin cellular adhesion molecule45 and P-selectin,46 have been used for molecular ultrasound imaging of inflammation in mouse models of inflammatory bowel disease.18
After extensive characterization and verification of target specificity, the fourth step is to design a microbubble to bind to the target of interest and be safe to use in human patients. Recently, a clinical-grade KDR-targeted microbubble (BR55) was designed and tested preclinically in subcutaneous/orthotopic mouse models of breast,47 colon2 (Figure 3A and Supplementary Figure 1) and prostate cancer.3 Before approval for the first clinical trials, the clinical-grade, molecularly targeted microbubbles have to be tested for toxicity, side effects, and binding specificity for the intended clinical application.1,10
Figure 3.
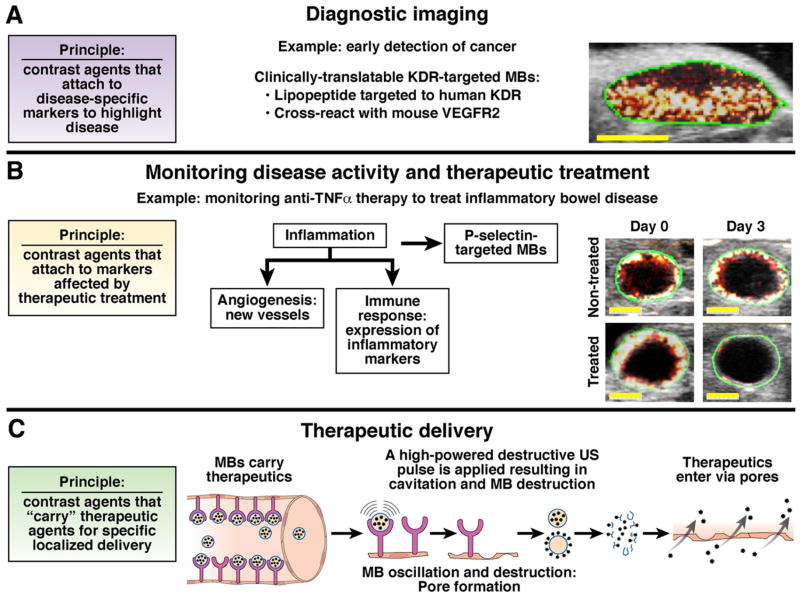
Nontargeted and molecularly targeted contrast-enhanced ultrasound imaging techniques can be used for several applications: Primary diagnostic imaging (detection and characterization of disease foci), monitoring disease activity and therapeutic treatment, and highly focused therapeutic delivery. (A) Early detection of cancer in a subcutaneous mouse xenograft by visualizing KDR, a marker of tumor angiogenesis expressed at early tumor stages (few mm of size; yellow bar, 3 mm), using KDR-targeted BR55 microbubbles.2 (B) Transverse ultrasound images show inflamed mouse colon (green region of interest around colon wall) visualized with contrast microbubbles (red and white colormetric map overlaid on B-mode image) targeted at inflammatory marker P-selectin, which is over-expressed in inflammatory bowel disease.46 (C) Nontargeted and/or disease-targeted (more focused delivery) microbubbles carrying therapeutics combined with ultrasound can be used to enhance therapeutic delivery to highly localized anatomical regions. Delivery process is described in text (also see Tinkov et al48).
Taken together, design and characterization of molecularly targeted microbubbles involves several steps. Recent advances in target discovery (eg, bioinformatics and highly sensitive, small-sample analysis [eg, proteomics]41), ligand chemistries, and ligand incorporation into microbubbles will enable clinical translation of molecular ultrasound imaging of disease-specific targets with molecularly targeted microbubbles.
Applications of Molecular Ultrasound Imaging
Molecular ultrasound imaging can be used for several applications, including primary diagnostics with improved lesion detection, characterization of focal lesions, monitoring disease activity and therapeutic treatment at the molecular level, and for improved delivery of drugs (Figure 3). For example, vascular endothelial growth factor receptor type 2 is often over-expressed on angiogenic vessels during tumorigenesis of many cancer types; therefore, it could be used for detecting cancer at an early, potentially still curable stage (with a tumor size of only few millimeters) when angiogenesis is necessary to promote tumor growth (Figure 3A).9
Once a diagnosis is established, molecular ultrasound allows monitoring of diseases at the molecular level over time, as well as in response to therapeutic treatments. For example, early prediction of therapeutic efficacy of molecularly targeted drugs in cancer (eg, anti-angiogenic drugs) through quantification of molecular marker expression levels directly affected by therapy can weigh in on treatment regimens early on (within a few days and not only after several weeks when side effects and costs may have already accumulated). Molecular ultrasound could also help the monitoring of chronic, relapsing inflammatory diseases (eg, inflammatory bowel disease) to properly account for effective therapies and the duration of treatment. For example, P-selectin is an endothelial surface protein that plays an important role in the inflammatory response (ie, leukocyte rolling and attachment), and can be used as a target for molecular ultrasound imaging to measure the inflammatory reaction activity of inflammatory bowel disease.46 Figure 3B shows the molecular ultrasound signal of P-selectin–targeted microbubbles in inflamed colon (chemically induced model of colitis) of mice that did not (untreated) or did receive anti-inflammatory therapy; notably, P-selectin marker expression (ie, ultrasound signal associated with P-selectin–targeted microbubbles) decreased after therapeutic treatment, but was sustained in nontreated mice.46
In addition to therapeutic monitoring, therapeutics (eg, small molecule drugs; or, plasmids for gene expression of toxic or therapeutic proteins) can be delivered to a diseased location using ultrasound and microbubbles via sonoporation (Figure 3C). Although sonoporation can also occur with nontargeted microbubbles, use of molecularly targeted microbubbles may provide improved specificity for localized therapeutic delivery to the region of interest by accumulation of molecularly targeted microbubbles. Therapeutics can be attached (either covalently or non-covalently bonded) to the surface of microbubbles, or they can be enclosed in the core of the microbubbles.48 The process of sonoporation is shown in Figure 3C. First, drug-carrying targeted microbubbles are injected intravenously, and allowed several minutes to bind to their targets (as in Figure 1B for targeted signal quantification). Second, application of high-powered ultrasound pulses (on/off) allows for microbubbles to oscillate and create shear stresses/forces that result in cavitation, or formation of pores in cell membranes; and then to destroy the microbubbles to release the therapeutics in close proximity to cells. Finally, therapeutics can cross the cell membranes via the pores created with cavitation. Ultrasound has also been shown to create separations between neighboring cells, thereby enabling therapeutics to reach multiple layers of tissue. Thus, molecular ultrasound with molecularly targeted microbubbles can provide quantification of disease-specific endothelial marker expression levels for diagnosing, therapeutic monitoring, and for localized delivery of therapeutics.
Conclusion and Future Directions
Contrast-enhanced ultrasound imaging plays an increasing role in the clinical arena. Emerging strategies can expand the current morphological and functional imaging capabilities of ultrasound to molecular imaging to obtain quantitative measures of molecular signatures in various diseases. This becomes increasingly important with the introduction and clinical testing of novel molecularly targeted drugs that often have minimal or delayed effects on tissue morphology or size; therefore, molecular imaging surrogate read-outs that allow quantification of therapeutic effects at the molecular level are warranted. As a relatively inexpensive, real-time, high-throughput, and safe modality, molecular ultrasound has great potential for assessing treatment effects early on after treatment initiation in future clinical trials (eg, in cancer patients as early as after the first few doses of therapy). Further improvements such as introduction of large-field, 3-dimensional transducers and/or co-registration with other cross-sectional imaging techniques (such as magnetic resonance imaging or computed tomography) are expected. These improvements will make ultrasound a more reliable and reproducible imaging modality, which will help to standardize the anatomical/spatial visualization of molecular ultrasound signal for treatment planning and monitoring. Another exciting potential indication for molecular ultrasound includes cancer screening since ultrasound does not involve irradiation, is a relatively low cost examination, and is already considered among the first-line imaging modalities for various organs where earlier detection of cancer may have a substantial influence on patient survival (eg, ovarian or pancreatic as well as breast cancer). Ongoing research explores how molecular ultrasound could be integrated into a screening algorithm in combination with, for example, blood biomarker tests (ie, to first enrich the screening population by identifying high-risk patients through family history and blood biomarker tests, and then to perform molecular ultrasound imaging examinations to confirm the diagnosis made with the blood biomarker test). After the recent preclinical introduction of clinical-grade molecularly targeted contrast agents, first-ever clinical trials exploring the potential of molecular ultrasound in patients with various diseases are expected in the near future.
Supplementary Material
Acknowledgments
Funding
RSNA Seed grant RSD0809 (JKW), the Howard S. Stern Research Grant of the Society of Gastrointestinal Radiologists (JKW), NIH R21 CA139279 (JKW), the National Pancreas Foundation (JKW), the Canary Foundation (JKW), and the Stanford Molecular Imaging Scholars Program NIH/NCI R25 CA11868 (MAP).
Abbreviations used in this paper
- KDR
kinase domain insert receptor
Footnotes
Note: The first 5 references associated with this article are available below in print. The remaining references accompanying this article are available online only with the electronic version of the article. To access the remaining references, as well as additional online-only data, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2011.01.027.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: current status and emerging strategies. Clin Radiol. 2010;65:500–516. doi: 10.1016/j.crad.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pysz MA, Foygel K, Rosenberg J, et al. Antiangiogenic cancer therapy: monitoring with molecular US and a clinically translatable contrast agent (BR55) Radiology. 2010;256:519–527. doi: 10.1148/radiol.10091858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tardy I, Pochon S, Theraulaz M, et al. Ultrasound molecular imaging of VEGFR2 in a rat prostate tumor model using BR55. Invest Radiol. 2010;45:573–578. doi: 10.1097/RLI.0b013e3181ee8b83. [DOI] [PubMed] [Google Scholar]
- 4.Deshpande N, Needles A, Willmann JK. Molecular ultrasound imaging: current status and future directions. Clin Radiol. 2010;65:567–581. doi: 10.1016/j.crad.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willmann JK, Kimura RH, Deshpande N, et al. Targeted contrast-enhanced ultrasound imaging of tumor angiogenesis with contrast microbubbles conjugated to integrin-binding knottin peptides. J Nucl Med. 2010;51:433–440. doi: 10.2967/jnumed.109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiessling F. Science to practice: the dawn of molecular US imaging for clinical cancer imaging. Radiology. 2010;256:331–333. doi: 10.1148/radiol.100717. [DOI] [PubMed] [Google Scholar]
- 7.Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: what role? Radiology. 2010;257:24–39. doi: 10.1148/radiol.10091210. [DOI] [PubMed] [Google Scholar]
- 8.Wilson SR, Jang HJ, Kim TK, et al. Real-time temporal maximum-intensity-projection imaging of hepatic lesions with contrast-enhanced sonography. AJR Am J Roentgenol. 2008;190:691–695. doi: 10.2214/AJR.07.3116. [DOI] [PubMed] [Google Scholar]
- 9.Deshpande N, Pysz MA, Willmann JK. Molecular ultrasound assessment of tumor angiogenesis. Angiogenesis. 2010;13:175–188. doi: 10.1007/s10456-010-9175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willmann JK, van Bruggen N, Dinkelborg LM, et al. Molecular imaging in drug development. Nat Rev Drug Discov. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 11.Klibanov AL. Ligand-carrying gas-filled microbubbles: ultrasound contrast agents for targeted molecular imaging. Bioconjug Chem. 2005;16:9–17. doi: 10.1021/bc049898y. [DOI] [PubMed] [Google Scholar]
- 12.Klibanov AL. Preparation of targeted microbubbles: ultrasound contrast agents for molecular imaging. Med Biol Eng Comput. 2009;47:875–882. doi: 10.1007/s11517-009-0498-0. [DOI] [PubMed] [Google Scholar]
- 13.Willmann JK, Cheng Z, Davis C, et al. Targeted microbubbles for imaging tumor angiogenesis: assessment of whole-body biodistribution with dynamic micro-PET in mice. Radiology. 2008;249:212–219. doi: 10.1148/radiol.2491072050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blomley M, Claudon M, Cosgrove D. WFUMB Safety Symposium on Ultrasound Contrast Agents: clinical applications and safety concerns. Ultrasound Med Biol. 2007;33:180–186. doi: 10.1016/j.ultrasmedbio.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Bhayana D, Kim TK, Jang HJ, et al. Hypervascular liver masses on contrast-enhanced ultrasound: the importance of washout. AJR Am J Roentgenol. 2010;194:977–983. doi: 10.2214/AJR.09.3375. [DOI] [PubMed] [Google Scholar]
- 16.Brannigan M, Burns PN, Wilson SR. Blood flow patterns in focal liver lesions at microbubble-enhanced US. Radiographics. 2004;24:921–935. doi: 10.1148/rg.244035158. [DOI] [PubMed] [Google Scholar]
- 17.Gorg C. The forgotten organ: contrast enhanced sonography of the spleen. Eur J Radiol. 2007;64:189–201. doi: 10.1016/j.ejrad.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 18.Nylund K, Hausken T, Gilja OH. Ultrasound and inflammatory bowel disease. Ultrasound Q. 2010;26:3–15. doi: 10.1097/RUQ.0b013e3181ce0929. [DOI] [PubMed] [Google Scholar]
- 19.Nylund K, Odegaard S, Hausken T, et al. Sonography of the small intestine. World J Gastroenterol. 2009;15:1319–1330. doi: 10.3748/wjg.15.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doust BD. The use of ultrasound in the diagnosis of gastroenterological disease. Gastroenterology. 1976;70:602–610. [PubMed] [Google Scholar]
- 21.Fusaroli P, Spada A, Mancino MG, et al. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629–634. doi: 10.1016/j.cgh.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Helmstaedter L, Riemann JF. Pancreatic cancer—EUS and early diagnosis. Langenbecks Arch Surg. 2008;393:923–927. doi: 10.1007/s00423-007-0275-1. [DOI] [PubMed] [Google Scholar]
- 23.Hohl C, Schmidt T, Honnef D, et al. Ultrasonography of the pancreas. 2. Harmonic imaging. Abdom Imaging. 2007;32:150–160. doi: 10.1007/s00261-006-9017-z. [DOI] [PubMed] [Google Scholar]
- 24.Kinney TP, Freeman ML. Pancreatic imaging: current state of the art. Gastroenterology. 2009;136:776–779. doi: 10.1053/j.gastro.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 25.Nair RJ, Lawler L, Miller MR. Chronic pancreatitis. Am Fam Physician. 2007;76:1679–1688. [PubMed] [Google Scholar]
- 26.Rickes S, Monkemuller K, Malfertheiner P. Acute severe pancreatitis: contrast-enhanced sonography. Abdom Imaging. 2007;32:362–364. doi: 10.1007/s00261-007-9250-0. [DOI] [PubMed] [Google Scholar]
- 27.Rickes S, Rauh P, Uhle C, et al. Contrast-enhanced sonography in pancreatic diseases. Eur J Radiol. 2007;64:183–188. doi: 10.1016/j.ejrad.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 28.Saftoiu A, Vilmann P. Role of endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. J Clin Ultrasound. 2009;37:1–17. doi: 10.1002/jcu.20534. [DOI] [PubMed] [Google Scholar]
- 29.Seicean A, Badea R, Stan-Iuga R, et al. The added value of real-time harmonics contrast-enhanced endoscopic ultrasonography for the characterisation of pancreatic diseases in routine practice. J Gastrointest Liver Dis. 2010;19:99–104. [PubMed] [Google Scholar]
- 30.Lassau N, Koscielny S, Albiges L, et al. Metastatic renal cell carcinoma treated with sunitinib: early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin Cancer Res. 2010;16:1216–1225. doi: 10.1158/1078-0432.CCR-09-2175. [DOI] [PubMed] [Google Scholar]
- 31.Correas JM, Claudon M, Tranquart F, et al. The kidney: imaging with microbubble contrast agents. Ultrasound Q. 2006;22:53–66. [PubMed] [Google Scholar]
- 32.Hoeffel C, Pousset M, Timsit MO, et al. Radiofrequency ablation of renal tumours: diagnostic accuracy of contrast-enhanced ultrasound for early detection of residual tumour. Eur Radiol. 2010;20:1812–1821. doi: 10.1007/s00330-010-1742-6. [DOI] [PubMed] [Google Scholar]
- 33.Xu ZF, Xu HX, Xie XY, et al. Renal cell carcinoma and renal angio-myolipoma: differential diagnosis with real-time contrast-enhanced ultrasonography. J Ultrasound Med. 2010;29:709–717. doi: 10.7863/jum.2010.29.5.709. [DOI] [PubMed] [Google Scholar]
- 34.DePriest PD, DeSimone CP. Ultrasound screening for the early detection of ovarian cancer. J Clin Oncol. 2003;21:194s–199s. doi: 10.1200/JCO.2003.02.054. [DOI] [PubMed] [Google Scholar]
- 35.Fleischer AC, Lyshchik A, Andreotti RF, et al. Advances in sonographic detection of ovarian cancer: depiction of tumor neovascularity with microbubbles. AJR Am J Roentgenol. 2010;194:343–348. doi: 10.2214/AJR.09.3446. [DOI] [PubMed] [Google Scholar]
- 36.Fleischer AC, Lyshchik A, Jones HW, et al. Diagnostic parameters to differentiate benign from malignant ovarian masses with contrast-enhanced transvaginal sonography. J Ultrasound Med. 2009;28:1273–1280. doi: 10.7863/jum.2009.28.10.1273. [DOI] [PubMed] [Google Scholar]
- 37.Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med. 2009;361:170–177. doi: 10.1056/NEJMcp0901926. [DOI] [PubMed] [Google Scholar]
- 38.Aigner F, Mitterberger M, Rehder P, et al. Status of transrectal ultrasound imaging of the prostate. J Endourol. 2010;24:685–691. doi: 10.1089/end.2009.0640. [DOI] [PubMed] [Google Scholar]
- 39.Aigner F, Pallwein L, Mitterberger M, et al. Contrast-enhanced ultrasonography using cadence-contrast pulse sequencing technology for targeted biopsy of the prostate. BJU Int. 2009;103:458–463. doi: 10.1111/j.1464-410X.2008.08038.x. [DOI] [PubMed] [Google Scholar]
- 40.Faccioli N, Crippa S, Bassi C, et al. Contrast-enhanced ultrasonography of the pancreas. Pancreatology. 2009;9:560–566. doi: 10.1159/000225960. [DOI] [PubMed] [Google Scholar]
- 41.Dudley JT, Butte AJ. Biomarker and drug discovery for gastroenterology through translational bioinformatics. Gastroenterology. 2010;139:735–741. doi: 10.1053/j.gastro.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 42.Willmann JK, Lutz AM, Paulmurugan R, et al. Dual-targeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology. 2008;248:936–944. doi: 10.1148/radiol.2483072231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willmann JK, Paulmurugan R, Chen K, et al. US imaging of tumor angiogenesis with microbubbles targeted to vascular endothelial growth factor receptor type 2 in mice. Radiology. 2008;246:508–518. doi: 10.1148/radiol.2462070536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deshpande N, Ren Y, Foygel K, et al. Tumor angiogenic marker expression levels during tumor growth: longitudinal assessment with molecularly targeted microbubbles and US imaging. Radiology. 2011;258:804–811. doi: 10.1148/radiol.10101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bachmann C, Klibanov AL, Olson TS, et al. Targeting mucosal addressin cellular adhesion molecule (MAdCAM)-1 to noninvasively image experimental Crohn’s disease. Gastroenterology. 2006;130:8–16. doi: 10.1053/j.gastro.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Deshpande N, Ren Y, Foygel K, et al. Quantification of inflammation in inflammatory bowel disease by molecular ultrasound imaging. World Molecular Imaging Congress; Kyoto, Japan. 2010. Abstract # 0188. [Google Scholar]
- 47.Pochon S, Tardy I, Bussat P, et al. BR55: a lipopeptide-based VEGFR2-targeted ultrasound contrast agent for molecular imaging of angiogenesis. Invest Radiol. 2010;45:89–95. doi: 10.1097/RLI.0b013e3181c5927c. [DOI] [PubMed] [Google Scholar]
- 48.Tinkov S, Bekeredjian R, Winter G, et al. Microbubbles as ultrasound triggered drug carriers. J Pharm Sci. 2009;98:1935–1961. doi: 10.1002/jps.21571. [DOI] [PubMed] [Google Scholar]
- 49.Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 50.Ignee A, Jedrejczyk M, Schuessler G, et al. Quantitative contrast enhanced ultrasound of the liver for time intensity curves—reliability and potential sources of errors. Eur J Radiol. 2010;73:153–158. doi: 10.1016/j.ejrad.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 51.Lamuraglia M, Bridal SL, Santin M, et al. Clinical relevance of contrast-enhanced ultrasound in monitoring anti-angiogenic therapy of cancer: current status and perspectives. Crit Rev Oncol Hematol. 2010;73:202–212. doi: 10.1016/j.critrevonc.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Pysz MA, Foygel K, Panje CM, et al. Assessment and monitoring tumor vascularity with contrast-enhanced ultrasound maximum intensity persistence imaging. Invest Radiol. 2011;46:187–195. doi: 10.1097/RLI.0b013e3181f9202d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palmowski M, Lederle W, Gaetjens J, et al. Comparison of conventional time-intensity curves vs. maximum intensity over time for post-processing of dynamic contrast-enhanced ultrasound. Eur J Radiol. 2010;75:e149–153. doi: 10.1016/j.ejrad.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 54.Wei K, Jayaweera AR, Firoozan S, et al. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 55.Arditi M, Frinking PJ, Zhou X, et al. A new formalism for the quantification of tissue perfusion by the destruction-replenishment method in contrast ultrasound imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2006;53:1118–1129. doi: 10.1109/tuffc.2006.1642510. [DOI] [PubMed] [Google Scholar]
- 56.Gao J, Huang S, Li M, et al. GM-CSF-surface-modified B16. F10 melanoma cell vaccine. Vaccine. 2006;24:5265–5268. doi: 10.1016/j.vaccine.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 57.Marshall D, Pedley RB, Boden JA, et al. Polyethylene glycol modification of a galactosylated streptavidin clearing agent: effects on immunogenicity and clearance of a biotinylated anti-tumour antibody. Br J Cancer. 1996;73:565–572. doi: 10.1038/bjc.1996.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyer DL, Schultz J, Lin Y, et al. Reduced antibody response to streptavidin through site-directed mutagenesis. Protein Sci. 2001;10:491–503. doi: 10.1110/ps.19901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pillai R, Marinelli ER, Fan H, et al. A phospholipid-PEG2000 conjugate of a vascular endothelial growth factor receptor 2 (VEGFR2)-targeting heterodimer peptide for contrast-enhanced ultrasound imaging of angiogenesis. Bioconjug Chem. 2010;21:556–562. doi: 10.1021/bc9005688. [DOI] [PubMed] [Google Scholar]
- 60.Pillai R, Marinelli ER, Swenson RE. A flexible method for preparation of peptide homo- and heterodimers functionalized with affinity probes, chelating ligands, and latent conjugating groups. Biopolymers. 2006;84:576–585. doi: 10.1002/bip.20570. [DOI] [PubMed] [Google Scholar]
- 61.Shrivastava A, von Wronski MA, et al. A distinct strategy to generate high-affinity peptide binders to receptor tyrosine kinases. Protein Eng Des Sel. 2005;18:417–424. doi: 10.1093/protein/gzi049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.