Abstract
Purpose
This phase I study assessed the safety, tolerability, maximum tolerated dose (MTD), pharmacokinetics, and preliminary antitumor effects of sunitinib combined with modified FOLFOX6 (mFOLFOX6).
Methods
Patients with advanced solid malignancies received mFOLFOX6 in 2-week cycles with escalating sunitinib doses (25, 37.5, and 50 mg/day) on three schedules: 2 weeks on, 2 weeks off (2/2); 4 weeks on, 2 weeks off (4/2); or continuous daily dosing (CDD). Patients received up to 8 treatment cycles (Schedule 2/2 and CDD schedule) or 6 cycles (Schedule 4/2). An expansion cohort enrolled patients with metastatic colorectal cancer at the Schedule 2/2 MTD.
Results
Overall, 53 patients were enrolled, with 43 evaluable for dose-limiting toxicity (DLT). On Schedule 2/2 (n = 18), DLTs occurred in three patients at 50 mg/day (grade 4 neutropenia [n = 1]; grades 3 and 4 thrombocytopenia [n = 2]) and two patients achieved partial responses (PRs). On Schedule 4/2 (n = 13), 37.5 mg/day exceeded the MTD with two DLTs (febrile neutropenia and grade 4 hypokalemia, respectively). On the CDD schedule (n = 12), the MTD was 25 mg/day; one DLT (grade 3 stomatitis) was reported and two patients achieved PRs. The most common adverse events were neutropenia, fatigue, and thrombocytopenia. No clinically significant drug–drug interactions were apparent between sunitinib, its metabolite SU12662, and mFOLFOX6.
Conclusions
Sunitinib combined with mFOLFOX6 had acceptable tolerability. The MTDs were sunitinib 50 mg/day on Schedule 2/2 and 25 mg/day on the CDD schedule. A MTD for Schedule 4/2 was not established.
Keywords: Sunitinib, Tyrosine kinase inhibitor, FOLFOX, Solid tumors, Pharmacokinetics, Phase I
Introduction
Abnormal angiogenesis is important in tumor development, growth, and metastasis [1]. An “angiogenic switch” exists in the tumor microenvironment, characterized by up-regulation of several pro-angiogenic factors, including vascular endothelial growth factors (VEGFs), basic fibroblast growth factor (bFGF), placental growth factors (PlGFs), and platelet-derived growth factors (PDGFs) [2]. These factors trigger endothelial cells to change from quiescent to active, promoting angiogenesis and tumorigenesis (reviewed in Ref. [3]).
Sunitinib is an oral, multitargeted receptor tyrosine kinase inhibitor (TKI) of VEGF receptors (VEGFRs)-1, -2, and -3, PDGF receptors (PDGFRs)-α and -β, stem-cell factor receptor (KIT), FMS-like tyrosine kinase 3 (FLT3), colony-stimulating factor 1 receptor (CSF-1R), and glial cell-line-derived neurotrophic factor receptor (REarranged during Transfection; RET) [4–8]. It is approved multinationally for the treatment of advanced renal cell carcinoma, imatinib-resistant/-intolerant gastrointestinal stromal tumor, and unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumors. In colorectal cancer (CRC), sunitinib was evaluated in a phase II clinical trial of heavily pretreated metastatic CRC (mCRC), in which one patient achieved a partial response (PR) and 13 patients achieved stable disease (SD) lasting ≥ 22 weeks (n = 84) [9].
FOLFOX is a combination regimen of 5-fluorouracil (5-FU; bolus followed by infusional), leucovorin, and oxaliplatin [10]. Compared with other FOLFOX regimens, modified FOLFOX6 (mFOLFOX6) includes a slight dose reduction in oxaliplatin as well as a 46-h 5-FU infusion, rather than two 22-h 5-FU infusions. mFOLFOX6 has been studied extensively in CRC and is often used off label in advanced pancreatic cancer. The most common toxicities are sensory neuropathy, neutropenia, lethargy, and diarrhea [11, 12]. The incidence of grade 3/4 toxicities can be as high as 59 % of patients [12].
The decision to combine sunitinib with chemotherapy was based on studies demonstrating that modulating tumor vasculature improved the efficacy of cytotoxic therapy (reviewed in Ref. [13]). This has been observed clinically, as antiangiogenic agents in combination with cytotoxic chemotherapy regimens showed improved outcomes compared with chemotherapy alone in patients with advanced-stage solid tumors, including non-small cell lung cancer, metastatic breast cancer, and mCRC [14–16]. However, these studies used an antibody to VEGF. As a TKI, sunitinib may have broader activity.
The principal objectives of this study were to evaluate the safety and tolerability of escalating doses of sunitinib in combination with mFOLFOX6; to determine the maximum tolerated dose (MTD) of sunitinib; to evaluate the pharmacokinetics (PK) of sunitinib, oxaliplatin, and 5-FU; and to assess antitumor activity.
Patients and methods
Patient selection
Patients with histologically or cytologically documented solid malignancies not amenable to treatment with curative intent were eligible. Other key eligibility criteria included: age ≥ 18 years; Eastern Cooperative Oncology Group performance status ≤ 1; and adequate hematopoietic, hepatic, and renal function. Exclusion criteria included: previous treatment with high-dose chemotherapy requiring stem-cell rescue, grade ≥ 2 peripheral neuropathy; known hypersensitivity reaction to 5-FU; known brain metastases; grade 3 hemorrhage within 4 weeks of enrollment; uncontrolled hypertension (blood pressure > 150/100 mmHg despite optimal therapy); ongoing cardiac dysrhythmias of grade ≥ 2, atrial fibrillation, or increased QTc interval (>450 ms for males or >470 ms for females); and myocardial infarction, severe/unstable angina, coronary/peripheral artery bypass graft, congestive heart failure, cerebrovascular accident, or pulmonary embolus < 12 months before starting study treatment.
Early in the study, an amendment was added to exclude prior treatment with >6 cycles of alkylating-agent chemotherapy or >2 cycles of carboplatin-based chemotherapy, due to toxicities observed with initial patients.
The protocol was approved by each institution's Institutional Review Board. All patients provided written informed consent prior to enrollment, according to federal and institutional guidelines.
Study design and drug dosing and administration
This was a multicenter, phase I, open label, dose-escalation study to determine the MTD of sunitinib/mFOLFOX6 combination for three different dosing schedules.
Patients received sunitinib 25, 37.5, or 50 mg/day orally once daily in the morning without regard to meals, in combination with mFOLFOX6 (oxaliplatin 85 mg/m2 + leucovorin 400 mg/m2 as a 2-h intravenous infusion, followed by an intravenous bolus of 5-FU 400 mg/m2 and a 46-h infusion of 5-FU 2,400 mg/m2) on days 1–2 of every 2-week cycle.
Sunitinib dosing was initiated at 37.5 mg/day on Schedule 2/2 (2 weeks on treatment, followed by 2 weeks off treatment), with subsequent dose escalation to 50 mg/day if the regimen was tolerated. If 50 mg/day was tolerated on Schedule 2/2, additional cohorts of patients received either 50 mg/day on Schedule 4/2 (4 weeks on treatment, followed by 2 weeks off treatment) or 37.5 mg/day on the continuous daily dosing (CDD) schedule. Once the MTD was determined for the sunitinib Schedule 2/2 regimen, an expansion cohort of patients with mCRC was enrolled to further evaluate this combination.
The MTD was defined as the dose level at which no more than 1/6 patients in a cohort experienced dose-limiting toxicities (DLTs). If the MTD was not exceeded within the planned dose levels, the maximum dose tested was defined as the MTD. Patients on the 2/2 and CDD schedules continued treatment for up to 8 cycles (16 weeks), while patients on Schedule 4/2 continued treatment for up to 6 cycles (12 weeks).
Toxicity was graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (AEs; version 3.0). DLTs were defined as the following AEs occurring during the first two cycles (4-week cycles) on the 2/2 and CDD schedules, and during the first three cycles (6-week cycles) on Schedule 4/2: grade 4 neutropenia/thrombocytopenia lasting for ≥7 days, febrile neutropenia, neutropenic infection or grade ≥ 3 thrombocytopenia with bleeding, grade ≥ 3 non-hematologic AEs lasting ≥ 7 days, or nausea/vomiting or diarrhea if persistent at grade ≥ 3 despite maximal medical intervention.
Patients were DLT-evaluable if they had received full doses of chemotherapy on days 1 and 2 and ≥85 % of their sunitinib doses (12/14 doses on Schedule 2/2; 24/28 doses on Schedule 4/2 and the CDD schedule). Additional patients were recruited if patients discontinued from the study prior to the completion of the DLT observation time frame for reasons other than treatment-related toxicity.
At study completion, patients deriving clinical benefit were candidates for further sunitinib treatment on a separate continuation protocol.
Dose modifications
Dose reductions in the mFOLFOX6 regimen and/or sunitinib were permitted in order to manage toxicities accordingly. However, patients requiring sunitinib dose reduction beyond 25 mg/day were discontinued from the study. Dose escalation was not permitted.
Patients requiring >4 weeks of dose interruption (including the off-treatment period) were also discontinued from the study.
Safety and antitumor activity assessments
Baseline evaluations were conducted within 21 days of study entry. Laboratory tests were obtained on day 1 of every mFOLFOX6 cycle. Tumor measurements were performed at baseline, at 6- to 8-week intervals during the study depending on dosing schedule and at the end of treatment visit. Tumor response was assessed by investigators according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 [17].
Plasma PK sampling and analysis
Blood specimens for the evaluation of plasma sunitinib (and its active metabolite, SU12662), oxaliplatin, and 5-FU concentrations were collected from patients receiving sunitinib on Schedule 2/2. Administration of sunitinib during cycle 1 was delayed for 2 days to allow for PK evaluation of total and unbound (free) platinum and 5-FU at multiple time points throughout chemotherapy administration, up to the end of the 5-FU infusion. Blood samples for sunitinib and SU12662 analyses were obtained on cycle 1 day 14 and on cycle 2 day 1 pre-dose and at multiple time points up to 24 h post-dose. Plasma sunitinib, SU12662, total and unbound platinum, and 5-FU concentrations were determined using liquid chromatography/mass spectrometry at Bioanalytical Systems Inc. (BASi; West Lafayette, IN). The lower limit of detection was 0.1, 0.1, 1, 2, and 10 ng/mL for sunitinib, SU12662, unbound platinum, total platinum, and 5-FU, respectively. Standard plasma PK parameters were determined by non-compartmental methods using WinNonlin® Version 4.1a (Pharsight Corporation; Mountain View, CA). Some of the PK parameters assessed included maximum plasma concentration (Cmax); area under the plasma concentration–time curve (AUC) from time zero to 24 h post-dose (AUC24), AUC from time zero to 48 h post-dose (AUC48), and AUC from time zero to infinity (AUC∞); steady-state plasma concentration (Css); and steady-state clearance (CLss).
Statistical analysis
The study population for safety analyses included all patients who received at least one dose of study medication. The DLT-evaluable population included all patients who met DLT assessment criteria as described above. The PK-evaluable population included all patients for whom PK sampling was completed on at least 1 day. The efficacy-evaluable population included all patients with measurable disease at baseline. Descriptive statistics were used to summarize patient characteristics, treatment administration/compliance, safety, PK parameters, and efficacy.
Results
Patient characteristics and disposition
Baseline characteristics are shown in Table 1. From September 2005 to August 2008, 53 patients were enrolled in total: 18 on Schedule 2/2, 21 on Schedule 4/2, and 14 on the CDD schedule. Fourteen patients (26.4 %) had CRC. More than two-thirds of patients had previously received chemotherapy for advanced disease (69.8 %; n = 37). Sixteen patients (30.2 %) discontinued the study due to disease progression, five (9.4 %) died on study, four (7.5 %) discontinued due to AEs, two (3.8 %) withdrew consent, and six (11.3 %) withdrew for other reasons. In total, 20 patients (37.7 %) completed the study and enrolled in the continuation protocol.
Table 1.
Baseline patient characteristics (ITT population)
| Sunitinib Schedule 2/2 | Sunitinib Schedule 4/2 | Sunitinib CDD schedule | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| 37.5 mg/day (n = 4) | 50 mg/day (n = 9) | 50 mg/day CRC expansion (n = 5) | 37.5 mg/day (n = 12) | 50 mg/day (n = 9) | 25 mg/day (n = 8) | 37.5 mg/day (n = 6) | |
| Median age, years (range) | 61.0 (55–69) | 58.0 (41–72) | 53.0 (43–57) | 59.5 (49–77) | 59.0 (36–72) | 62.5 (59–80) | 56.0 (46–59) |
| Male/female (n) | 3/1 | 5/4 | 3/2 | 6/6 | 8/1 | 5/3 | 4/2 |
| ECOG PS, n (%) | |||||||
| 0 | 1 (25.0) | 1 (11.1) | 1 (20.0) | 1 (8.3) | 1 (11.1) | 5 (62.5) | 1 (16.7) |
| 1 | 3 (75.0) | 8 (88.9) | 4 (80.0) | 11 (91.7) | 8 (88.9) | 3 (37.5) | 5 (83.3) |
| Primary tumor type, n (%) | |||||||
| Breast | – | – | – | – | – | – | 1 (16.7) |
| CRC | 1 (25.0) | 3 (33.3) | 5 (100.0) | 2 (16.7) | 2 (22.2) | – | 1 (16.7) |
| Gastric | – | – | – | – | 1 (11.1) | 1 (12.5) | – |
| Head and neck | 1 (25.0) | – | – | 1 (8.3) | – | – | – |
| Hepatobiliary | – | 1 (11.1) | – | – | 1 (11.1) | – | – |
| Melanoma | 1 (25.0) | – | – | – | – | – | – |
| NSCLC | 1 (25.0) | – | – | – | – | – | – |
| Ovarian | – | 2 (22.2) | – | – | – | – | – |
| Pancreatic | – | 2 (22.2) | – | 5 (41.7) | 2 (22.2) | 3 (37.5) | 2 (33.3) |
| Other | – | 1 (11.1) | – | 4 (33.3) | 3 (33.3) | 4 (50.0) | 2 (33.3) |
| Prior therapy, n (%) | |||||||
| Radiotherapy | 1 (25.0) | 2 (22.2) | 0 | 1 (8.3) | 5 (55.6) | 2 (25.0) | 2 (33.3) |
| Immunotherapy | 1 (25.0) | 2 (22.2) | 1 (20.0) | 0 | 0 | 1 (12.5) | 0 |
| Chemotherapy | |||||||
| Neoadjuvant | 0 | 0 | 0 | 0 | 2 (22.2) | 0 | 0 |
| Adjuvant | 2 (50.0) | 4 (44.4) | 0 | 0 | 0 | 1 (12.5) | 3 (50.0) |
| Advanced | 4 (100.0) | 8 (88.9) | 5 (100.0) | 8 (66.7) | 5 (55.6) | 3 (37.5) | 4 (66.7) |
| Prior systemic therapy, n (%) | |||||||
| Bevacizumab | 3 (75.0) | 2 (22.2) | 3 (60.0) | 2 (16.7) | 2 (22.2) | 0 | 2 (33.3) |
| Capecitabine | 1 (25.0) | 3 (33.3) | 2 (40.0) | 1 (8.3) | 3 (33.3) | 2 (25.0) | 2 (33.3) |
| 5-fluorouracil | 1 (25.0) | 3 (33.3) | 3 (60.0) | 2 (16.7) | 3 (33.3) | 1 (12.5) | 1 (16.7) |
| Irinotecan | 1 (25.0) | 3 (33.3) | 3 (60.0) | 1 (8.3) | 3 (33.3) | 0 | 1 (16.7) |
| Oxaliplatin | 1 (25.0) | 3 (33.3) | 2 (40.0) | 2 (16.7) | 1 (11.1) | 1 (12.5) | 2 (33.3) |
CDD continuous daily dosing, CRC colorectal cancer, ECOG PS Eastern Cooperative Oncology Group performance status, ITT intention-to-treat, NSCLC non-small cell lung cancer
Drug exposure
Table 2 outlines drug exposure for this study. The median number of sunitinib cycles started ranged from 1 to 4, depending on schedule. Sunitinib treatment was temporarily interrupted in at least one patient in each dose group and by all patients on the CDD schedule. Dose reductions were required in at least one patient from each group (except 37.5 mg/day on Schedule 2/2) and were primarily due to hematologic toxicities. The median number of mFOLFOX6 cycles started ranged from 3 to 8. One patient on Schedule 2/2 (sunitinib 50 mg/day) had an interruption of mFOLFOX6 treatment.
Table 2.
Exposure to sunitinib, 5-fluorouracil, leucovorin, and oxaliplatin (ITT population)
| Sunitinib Schedule 2/2 | Sunitinib Schedule 4/2 | Sunitinib CDD schedule | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| 37.5 mg/day (n = 4) | 50 mg/day (n = 9) | 50 mg/day CRC expansion (n = 5) | 37.5 mg/day (n = 12) | 50 mg/day (n = 9) | 25 mg/day (n = 8) | 37.5 mg/day (n = 6) | |
| Sunitinib | |||||||
| Median number of cycles started (range) | 4 (2–4) | 4 (1–4) | 4 (1–4) | 1 (1–2) | 2 (1–2) | 3 (1–4) | 3 (1–4) |
| Median duration of treatment (range)a, days | 113 (65–147) | 126 (28–166) | 119 (28–160) | 42 (42–112) | 91 (42–106) | 97 (28–133) | 88 (28–147) |
| Patients with treatment interruption (missed dose), n (%) | 1 (25.0) | 4 (44.4) | 2 (40.0) | 8 (66.7) | 6 (66.7) | 8 (100.0) | 6 (100.0) |
| Patients with dose reductions, n (%) | 0 | 3 (33.3) | 2 (40.0) | 2 (16.7) | 4 (44.4) | 1 (12.5) | 3 (50.0) |
| Median average daily dose (mg) | 37.5 | 50.0 | 50.0 | 37.5 | 50.0 | 25.0 | 35.0 |
| 5-fluorouracil | |||||||
| Median number of cycles started (range)b | 5 (3–8) | 8 (2–8) | 8 (1–8) | 3 (1–6) | 5 (2–7) | 5 (1–8) | 4 (1–8) |
| Patients with dosing interruption, n (%) | 0 | 1 (11.1) | 0 | 0 | 0 | 0 | 0 |
| Leucovorin | |||||||
| Median number of cycles started (range)b | 5 (3–8) | 8 (2–8) | 8 (1–8) | 3 (1–6) | 5 (2–7) | 5 (1–8) | 4 (1–8) |
| Patients with dosing interruption, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oxaliplatin | |||||||
| Median number of cycles started (range)b | 5 (3–8) | 7 (2–8) | 8 (1–8) | 3 (1–6) | 5 (2–7) | 5 (1–8) | 4 (1–8) |
| Patients with dosing interruption, n (%) | 0 | 1 (11.1) | 0 | 0 | 0 | 0 | 0 |
CDD continuous daily dosing, CRC colorectal cancer, ITT intention-to-treat
The time period starting from date of first dose and ending at either the termination date or 2 weeks after last dose (whichever was earliest)
Considered to be administered in any cycle if a patient received at least one dose in that cycle
Most patients (≥66.7 % in each group) experienced a delay or modification in dosing due to AEs at some point during the study. Nine patients discontinued sunitinib treatment due to AEs, compared with 18 patients who discontinued mFOLFOX6.
Dose-limiting toxicities
Schedule 2/2
No DLTs were reported at the sunitinib 37.5 mg/day dose level (n = 4). At 50 mg/day, 2/5 initial patients had DLTs (grade 4 thrombocytopenia and grade 3 thrombocytopenia with bleeding, respectively). Both patients were heavily pretreated, leading to a protocol amendment to limit prior alkylating chemotherapy (see “Patient selection” of “Patients and methods”). Among the subsequent four patients enrolled, only one DLT (grade 4 neutropenia) was observed; therefore, the MTD on Schedule 2/2 was defined as sunitinib 50 mg/day.
Schedule 4/2
The sunitinib starting dose for this schedule was the MTD for Schedule 2/2 (50 mg/day). DLTs were reported in 2/6 evaluable patients (persistent grade 3 diarrhea despite maximal medical therapy and grade 3 fatigue ≥ 7 days, respectively). Dose de-escalation occurred to 37.5 mg/day. Among the first four evaluable patients, one patient experienced grade 3 febrile neutropenia, and the cohort was expanded to an additional three patients. A second DLT occurred (grade 4 hypokalemia ≥ 7 days), and therefore, sunitinib 37.5 mg/day on Schedule 4/2 exceeded the MTD. Further dose de-escalation to sunitinib 25 mg/day was not investigated, based upon preclinical data indicating that this schedule would not produce therapeutic levels of inhibition [6]. Hence, a MTD was not established for Schedule 4/2.
CDD schedule
The sunitinib starting dose for the CDD schedule was 37.5 mg/day. DLTs were observed in 2/6 patients (grade 3 neutropenia deemed clinically significant and grade 3 fatigue, respectively; both ≥7 days). Dose de-escalation occurred, and six patients on sunitinib 25 mg/day were DLT-evaluable; one patient experienced a DLT (grade 3 stomatitis lasting ≥ 7 days), and thus, the MTD on the CDD schedule was sunitinib 25 mg/day.
Safety
Overall, the most common treatment-emergent AEs experienced by ≥20 % of patients were neutropenia (86.8 %), fatigue (66.0 %), thrombocytopenia (62.3 %), nausea (60.4 %), vomiting (58.5 %), peripheral neuropathy (50.9 %), and diarrhea (43.4 %). An increased frequency of AEs was observed with higher doses of sunitinib in combination with mFOLFOX6. In the majority of cases, these events were mild to moderate (grades 1–2) in severity (Tables 3, 4).
Table 3.
Sunitinib Schedule 2/2: most common treatment-emergent adverse events by grade (reported in ≥20 % of patients total; safety population)
| Adverse events, n (%) | Sunitinib 37.5 mg/day (n = 4) | Sunitinib 50 mg/day (MTD; n = 9) | Sunitinib 50 mg/day CRC only (n = 5) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Grades 1/2 | Grade 3 | Grade 4 | Grades 1/2 | Grade 3 | Grade 4 | Grades 1/2 | Grade 3 | Grade 4 | |
| Neutropenia | 2 (50.0) | 1 (25.0) | 1 (25.0) | 3 (33.3) | 3 (33.3) | 3 (33.3) | 2 (40.0) | 1 (20.0) | 1 (20.0) |
| Vomiting | 1 (25.0) | 0 | 0 | 6 (66.7) | 1 (11.1) | 0 | 5 (100.0) | 0 | 0 |
| Thrombocytopenia | 2 (50.0) | 1 (25.0) | 0 | 1 (11.1) | 3 (33.3) | 2 (22.2) | 3 (60.0) | 0 | 0 |
| Diarrhea | 1 (25.0) | 0 | 0 | 4 (44.4) | 2 (22.2) | 0 | 5 (100.0) | 0 | 0 |
| Fatigue | 2 (50.0) | 0 | 0 | 6 (66.7) | 0 | 0 | 3 (60.0) | 1 (20.0) | 0 |
| Nausea | 1 (25.0) | 0 | 0 | 6 (66.7) | 0 | 0 | 4 (80.0) | 0 | 0 |
| Anemia | 2 (50.0) | 1 (25.0) | 0 | 7 (77.8) | 0 | 0 | 0 | 0 | 0 |
| Constipation | 2 (50.0) | 0 | 0 | 6 (66.7) | 0 | 0 | 0 | 1 (20.0) | 0 |
| Peripheral neuropathy | 1 (25.0) | 0 | 0 | 6 (66.7) | 0 | 0 | 1 (20.0) | 0 | 0 |
| Abdominal pain | 0 | 0 | 0 | 4 (44.4) | 1 (11.1) | 0 | 1 (20.0) | 0 | 1 (20.0) |
| Decreased appetite | 1 (25.0) | 0 | 0 | 3 (33.3) | 0 | 0 | 3 (60.0) | 0 | 0 |
| Dyspepsia | 2 (50.0) | 0 | 0 | 3 (33.3) | 0 | 0 | 2 (40.0) | 0 | 0 |
| Mucosal inflammation | 2 (50.0) | 0 | 0 | 2 (22.2) | 0 | 0 | 3 (60.0) | 0 | 0 |
| Headache | 0 | 0 | 0 | 5 (55.6) | 0 | 0 | 1 (20.0) | 0 | 0 |
| Dehydration | 1 (25.0) | 0 | 0 | 2 (22.2) | 1 (11.1) | 0 | 0 | 0 | 1 (20.0) |
| Cough | 0 | 0 | 0 | 4 (44.4) | 0 | 0 | 1 (20.0) | 0 | 0 |
| Epistaxis | 1 (25.0) | 0 | 0 | 3 (33.3) | 0 | 0 | 1 (20.0) | 0 | 0 |
| Hiccups | 1 (25.0) | 0 | 0 | 3 (33.3) | 0 | 0 | 0 | 0 | 0 |
| Pyrexia | 1 (25.0) | 0 | 0 | 3 (33.3) | 0 | 0 | 0 | 0 | 0 |
| Dizziness | 1 (25.0) | 0 | 0 | 2 (22.2) | 0 | 0 | 1 (20.0) | 0 | 0 |
CRC colorectal cancer, MTD maximum tolerated dose
Table 4.
Sunitinib Schedule 4/2 and CDD schedule: most common treatment-emergent adverse events by grade (reported in ≥20 % of patients total; safety population)
| Adverse events, n (%) | Sunitinib Schedule 4/2 | Sunitinib CDD schedule | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| 37.5 mg/day (n = 12)a | 50 mg/day (n = 9) | 25 mg/day (MTD; n = 8) | 37.5 mg/day (n = 6) | |||||||||
|
|
|
|
|
|||||||||
| G1/2 | G3 | G4b | G1/2 | G3 | G4 | G1/2 | G3 | G4b | G1/2 | G3 | G4b | |
| Neutropenia | 2 (16.7) | 5 (25.0) | 1 (8.3) | 1 (11.1) | 4 (44.4) | 3 (33.3) | 3 (37.5) | 4 (50.0) | 2 (25.0) | 0 | 2 (33.3) | 2 (33.3) |
| Fatigue | 4 (33.3) | 2 (16.7) | 0 | 4 (44.4) | 3 (33.3) | 0 | 4 (50.0) | 1 (12.5) | 0 | 4 (66.7) | 1 (16.7) | 0 |
| Thrombocytopenia | 6 (50.0) | 1 (8.3) | 0 | 5 (55.6) | 1 (11.1) | 0 | 3 (37.5) | 0 | 2 (25.0) | 3 (50.0) | 0 | 0 |
| Nausea | 6 (50.0) | 1 (8.3) | 0 | 5 (55.6) | 0 | 0 | 5 (62.5) | 0 | 0 | 4 (66.7) | 0 | 0 |
| Peripheral neuropathy | 5 (41.7) | 0 | 0 | 7 (77.8) | 0 | 0 | 3 (37.5) | 0 | 0 | 4 (66.7) | 0 | 0 |
| Vomiting | 4 (33.3) | 1 (8.3) | 0 | 5 (55.6) | 0 | 0 | 3 (37.5) | 0 | 0 | 4 (66.7) | 0 | 1 (16.7) |
| Anemia | 5 (41.7) | 1 (8.3) | 0 | 3 (33.3) | 0 | 0 | 1 (12.5) | 3 (37.5) | 0 | 2 (33.3) | 0 | 0 |
| Mucosal inflammation | 1 (8.3) | 2 (16.7) | 0 | 5 (55.6) | 0 | 0 | 2 (25.0) | 1 (12.5) | 0 | 2 (33.3) | 2 (33.3) | 0 |
| Abdominal pain | 0 | 3 (25.0) | 0 | 5 (55.6) | 0 | 0 | 2 (25.0) | 2 (25.0) | 0 | 2 (33.3) | 0 | 1 (16.7) |
| Pyrexia | 4 (44.4) | 1 (8.3) | 0 | 4 (44.4) | 0 | 0 | 3 (37.5) | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 2 (16.7) | 0 | 0 | 3 (33.3) | 2 (22.2) | 0 | 2 (25.0) | 0 | 0 | 0 | 1 (16.7) | 1 (16.7) |
| Decreased appetite | 2 (16.7) | 0 | 0 | 3 (33.3) | 1 (11.1) | 0 | 1 (12.5) | 1 (12.5) | 0 | 1 (16.7) | 2 (33.3) | 0 |
| Leukopenia | 3 (25.0) | 1 (8.3) | 0 | 1 (11.1) | 0 | 1 (11.1) | 2 (25.0) | 1 (12.5) | 0 | 0 | 1 (16.7) | 0 |
| Dysgeusia | 4 (33.3) | 0 | 0 | 2 (22.2) | 0 | 0 | 3 (37.5) | 0 | 0 | 1 (16.7) | 0 | 0 |
| Constipation | 5 (41.7) | 0 | 0 | 2 (22.2) | 0 | 0 | 1 (12.5) | 0 | 0 | 1 (16.7) | 0 | 0 |
| Dyspepsia | 5 (41.7) | 0 | 0 | 2 (22.2) | 0 | 0 | 0 | 0 | 0 | 2 (33.3) | 0 | 0 |
| Peripheral edema | 3 (25.0) | 0 | 0 | 3 (33.3) | 0 | 0 | 0 | 0 | 0 | 2 (33.3) | 0 | 0 |
| Epistaxis | 1 (8.3) | 0 | 0 | 3 (33.3) | 0 | 0 | 2 (25.0) | 1 (12.5) | 0 | 0 | 0 | 0 |
CDD continuous daily dosing, G grade, MTD maximum tolerated dose
MTD exceeded at 37.5 mg/day
Five grade 5 events were reported during the study and follow-up period due to disease progression (n = 1, sunitinib 37.5 mg/day Schedule 4/2; n = 2, sunitinib 37.5 mg/day CDD schedule); sepsis (n = 1, sunitinib 37.5 mg/day Schedule 4/2); and acute liver and renal failure secondary to disease progression (n = 1, sunitinib 25 mg/day CDD schedule). None of these events were assessed as related to treatment
Serious adverse events (SAEs) were reported for 20 patients across all dosing regimens, but the 37.5 mg/day on Schedule 4/2 had the highest incidence with 7/12 patients (58.3 %) experiencing 21 SAEs. The most common SAEs were pyrexia (7.5 %) and dehydration, disease progression, febrile neutropenia, pulmonary embolism, and sepsis (all 5.7 %).
Pharmacokinetics
Plasma concentration profiles of sunitinib, SU12662, free platinum, and 5-FU, for sunitinib or mFOLFOX6 alone and in combination, are shown in Fig. 1 and indicate no significant changes in PK parameters when all drugs were given in combination. Similarly, geometric mean ratios for Cmax and AUC (sunitinib plus mFOLFOX6 to sunitinib or mFOLFOX6 alone) ranged from 0.98 to 1.24 and from 0.96 to 1.19, respectively, for sunitinib, SU12662, and free and total platinum (Online resource 1). For 5-FU, the geometric mean ratios for Css and CLss were 1.84 and 0.61, respectively.
Fig. 1.
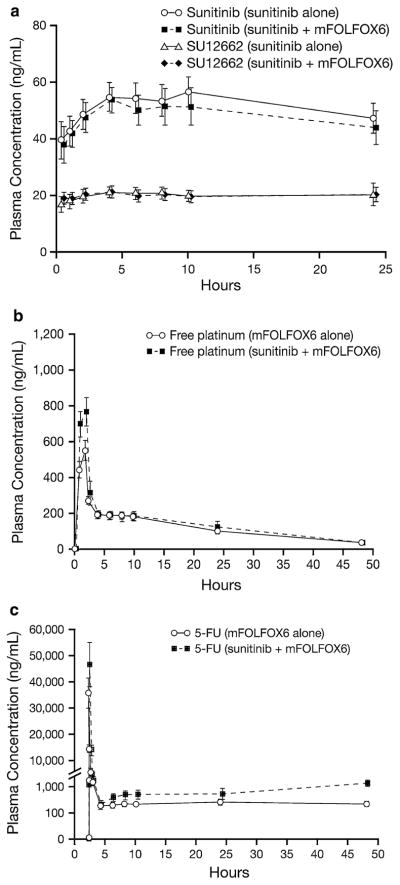
Mean (±SD) plasma concentration–time profiles for a sunitinib and SU12662 following administration of sunitinib (50 mg/day on Schedule 2/2) with or without mFOLFOX6, b free platinum following administration of mFOLFOX6 with or without sunitinib, c 5-FU following administration of mFOLFOX6 with or without sunitinib
Antitumor effect
Table 5 summarizes the best overall tumor responses in 47 patients who had evaluable disease. Online resource 2 displays the maximum percentage change in target lesion size for each patient.
Table 5.
Best overall response (efficacy-evaluable population)
| Best response, n (%) | Sunitinib Schedule 2/2 | Sunitinib Schedule 4/2 | Sunitinib CDD schedule | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| 37.5 mg/day (n = 3) | 50 mg/day (n = 9) | 50 mg/day CRC expansion (n = 4) | 37.5 mg/day (n = 11) | 50 mg/day (n = 8) | 37.5 mg/day (n = 5) | 25 mg/day (n = 7) | |
| Partial response | 0 | 2 (22.2)a | 0 | 0 | 0 | 0 | 2 (28.6)b |
| Stable disease | 3 (100.0) | 4 (44.4) | 1 (25.0) | 4 (36.4) | 3 (37.5) | 1 (20.0) | 1 (14.3) |
| Progressive disease | 0 | 3 (33.3) | 2 (50.0) | 3 (27.3) | 3 (37.5) | 2 (40.0) | 0 |
| Non-evaluable | 0 | 0 | 0 | 2 (18.2) | 1 (12.5) | 1 (20.0) | 2 (28.6) |
| Missing | 0 | 0 | 1 (25.0) | 2 (18.2) | 1 (12.5) | 1 (20.0) | 2 (28.6) |
CDD continuous daily dosing, CRC colorectal cancer
Partial responses in one patient with primary diagnosis of ovarian cancer (cycles 4/5/8) and one patient with pancreatic cancer (cycles 4/6)
Partial responses in one patient with primary diagnosis of duodenal cancer (cycles 2/6/8) and one patient with pancreatic cancer (cycles 6/8)
With regard to preliminary antitumor effects, there were no complete responses. However, four patients achieved PRs. Two of these patients were treated with sunitinib 50 mg/day on Schedule 2/2: a 42-year-old woman with metastatic ovarian cancer previously treated with paclitaxel/carboplatin, doxorubicin, docetaxel/carboplatin, gemcitabine, and erlotinib; and a 58-year-old man with metastatic pancreatic adenocarcinoma who received gemcitabine in the adjuvant setting. Both patients completed 8 treatment cycles per protocol and transitioned to the continuation study with sustained PRs. They exhibited clinical benefit (PR or SD) for 9 and 7 months, respectively. The other two patients with PRs received sunitinib 25 mg/day on the CDD schedule: a 67-year-old man who had received no prior treatment for metastatic duodenal cancer (8 months of clinical benefit) and a 59-year-old man with metastatic pancreatic adenocarcinoma previously treated with capecitabine in the adjuvant setting (>31 months of clinical benefit).
An additional 17 patients exhibited a best response of SD, including five with mCRC. All 12 patients with other tumor types were stable for ≥12 weeks and transitioned to the continuation protocol. Four of these patients had pancreatic adenocarcinoma and had prolonged clinical benefit of >48, 36, 7, and 4 months, respectively. Sustained tumor stability was also seen in medullary thyroid cancer (17 months) and adrenal carcinoma (13 months).
In patients with mCRC (n = 14), five patients achieved SD as their best response as noted above, with two successfully completing 8 treatment cycles and enrolling in the continuation protocol.
Discussion
The use of angiogenesis inhibitors is no longer a new concept. Angiogenesis involves signaling via receptor tyrosine kinases that include the VEGFRs and PDGFRs [18, 19]. Expression of the VEGF ligand has been associated with tumor growth, tumor metastasis, and poor survival in several tumor types, including colorectal, gastric, pancreatic, breast, prostate, lung, and melanoma [19]. Additionally, growing evidence suggests that inhibitors modulating tumor vasculature improve the efficacy of cytotoxic therapy (reviewed in Ref. [13]), as reflected in the Food and Drug Administration approval of bevacizumab plus chemotherapy for CRC therapy regimens [14, 20]. Sunitinib is an oral, multitargeted receptor TKI against several receptors, including VEGFR and PDGFR. In preclinical models, sunitinib in combination with chemotherapy has shown increased efficacy compared with chemotherapy alone [21–23].
In this phase I study, the most common AEs reported with sunitinib combined with mFOLFOX6 were neutropenia, fatigue, thrombocytopenia, nausea, vomiting, peripheral neuropathy, and diarrhea. These are expected side effects of sunitinib, but there was a higher incidence of grade 3 and 4 hematologic toxicities when compared with single-agent sunitinib [24]. Although the incidence of some AEs was greater at higher dose levels, dose-dependent cumulative toxicity was not apparent on Schedule 4/2 and the CDD schedule. Many patients in this study were heavily pretreated, possibly leading to increased susceptibility to myelosuppression. The prophylactic administration of granulocyte-colony-stimulating factor was not allowed; such use in future studies could potentially decrease the incidence and/or severity of neutropenia. The higher incidence of peripheral neuropathy in this study may also be attributed to the co-administration of mFOLFOX6.
It is also noteworthy that angiogenesis inhibitors do not all have similar AE profiles. Bevacizumab in combination with FOLFOX4 demonstrated an increase in hypertension, bleeding, vomiting, proteinuria, neuropathy, and thromboembolism compared with FOLFOX4 alone [20]. This difference in toxicity profiles may be important for patients who have a contraindication to one antiangiogenic agent and potentially allow the use of another drug in this class.
There were no apparent PK-mediated drug–drug interactions between sunitinib and mFOLFOX6. Even though there was an apparent increase in infusional 5-FU Css on cycle 2 day 1 during co-administration of sunitinib and mFOLFOX6, this increase was consistent with prior reports in which 5-FU was shown to have disproportionately higher plasma exposures after multiple dosing (e.g., cycle 2 day 1) as compared with single dosing (e.g., cycle 1 day 1), referred to as time dependence [25]. Therefore, the higher exposure was not caused by co-administration with sunitinib, but was most likely due to the time-dependent PK of 5-FU.
The preliminary antitumor activity with this drug combination is encouraging, with several patients exhibiting clinical benefit. However, since mFOLFOX6 is an active chemotherapy regimen in some of these tumors, the additional benefit of sunitinib in this non-randomized clinical trial is unclear. Recently, results of a randomized phase IIb study of sunitinib 37.5 mg/day (Schedule 4/2) plus mFOLFOX6 versus bevacizumab plus mFOLFOX6 as first-line treatment for mCRC demonstrated similar anti-tumor activity in both groups [26].
Interestingly, the combination of mFOLFOX6 and sunitinib in this study demonstrated activity in pancreatic cancer with some clinical benefit (two PRs and six patients with SD). Three patients had remarkable sustained clinical benefit (>48,>31, and 30 months). Furthermore, antitumor activity was observed in several other tumor types.
In summary, the combination of sunitinib and mFOLFOX6 was tolerated. A MTD was identified for two of the three schedules. Preliminary evidence of antitumor activity was observed, especially in patients with pancreatic cancer. Further evaluation of this combination in pancreatic cancer should be considered.
Supplementary Material
Acknowledgments
We would like to thank the patients and their families, as well as the investigators, research nurses, study coordinators, and operations staff. This study was sponsored by Pfizer Inc. The study used the Vanderbilt GCRC resources and therefore was supported in part by the Vanderbilt CTSA grant UL1 RR024975 from NCRR/NIH. Authors drafted the manuscript, with medical writing support from Molly Heitz at ACUMED®, Tytherington, UK that was funded by Pfizer Inc.
Footnotes
Previous presentations: ECCO 14—the European Cancer Conference, Barcelona, Spain, September 23–27, 2007 (abstr. P-716).
2007 Gastrointestinal Cancers Symposium: Multidisciplinary Approaches to the Prevention, Diagnosis, and Therapy of GI Cancers, January 19–21, 2007, Orlando, FL, USA (abstr. 285).
2007 World Congress on Gastrointestinal Cancer, June 27–30, Barcelona, Spain (abstr. P-0358).
Electronic supplementary material The online version of this article (doi:10.1007/s00280-012-1880-4) contains supplementary material, which is available to authorized users.
Conflict of interest RK, XL, ECM are/were employees of Pfizer. SGE and DRC have acted as consultants or in an advisory role for Pfizer, while EC has done so for Pfizer, Sanofi-Aventis, Genentech, Amgen, Imclone, Bristol-Myers Squibb, and Celegene. RK, XL, and ECM all own Pfizer stock. SL, SGE, EC, WAM, and ACL have all received research funding from Pfizer. EC has also received research funding from Genentech, Amgen, Idera, Merck, Bristol-Myers Squibb, Lilly, Imclone, and MethylGene, while ACL has received additional funding from Allos, Amgen, Bayer, Cephalon, Imclone/Lilly, Merck, Millennium, Novartis, Sanofi-Aventis, and Zenyaku. JS has received research funding from Roche Canada and Sanofi-Aventis Canada. SL has received travel support from Pfizer to attend meetings. SD has no conflicts of interest to disclose.
Contributor Information
S. Leong, Email: stephen.leong@ucdenver.edu, Medical Oncology, Developmental Therapeutics Program/GI Malignancies, University of Colorado Cancer Center, University of Colorado at Denver, Mail Stop 8117, 12801 E 17th Ave, Room 8120, Aurora, CO 80045, USA
S. G. Eckhardt, Medical Oncology, Developmental Therapeutics Program/GI Malignancies, University of Colorado Cancer Center, University of Colorado at Denver, Mail Stop 8117, 12801 E 17th Ave, Room 8120, Aurora, CO 80045, USA
E. Chan, Vanderbilt University Medical Center, Nashville, TN, USA
W. A. Messersmith, Medical Oncology, Developmental Therapeutics Program/GI Malignancies, University of Colorado Cancer Center, University of Colorado at Denver, Mail Stop 8117, 12801 E 17th Ave, Room 8120, Aurora, CO 80045, USA
J. Spratlin, Medical Oncology, Developmental Therapeutics Program/GI Malignancies, University of Colorado Cancer Center, University of Colorado at Denver, Mail Stop 8117, 12801 E 17th Ave, Room 8120, Aurora, CO 80045, USA
D. R. Camidge, Medical Oncology, Developmental Therapeutics Program/GI Malignancies, University of Colorado Cancer Center, University of Colorado at Denver, Mail Stop 8117, 12801 E 17th Ave, Room 8120, Aurora, CO 80045, USA
S. Diab, Medical Oncology, Developmental Therapeutics Program/GI Malignancies, University of Colorado Cancer Center, University of Colorado at Denver, Mail Stop 8117, 12801 E 17th Ave, Room 8120, Aurora, CO 80045, USA
R. Khosravan, Pfizer Oncology, La Jolla, CA, USA
X. Lin, Pfizer Oncology, La Jolla, CA, USA
E. Chow Maneval, Pfizer Oncology, La Jolla, CA, USA.
A. C. Lockhart, Washington University, St. Louis, MO, USA
References
- 1.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 3.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 4.Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2:471–478. [PubMed] [Google Scholar]
- 5.Kim DW, Jo YS, Jung HS, Chung HK, Song JH, Park KC, Park SH, Hwang JH, Rha SY, Kweon GR, Lee SJ, Jo KW, Shong M. An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. J Clin Endocrinol Metab. 2006;91:4070–4076. doi: 10.1210/jc.2005-2845. [DOI] [PubMed] [Google Scholar]
- 6.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 7.Murray LJ, Abrams TJ, Long KR, Ngai TJ, Olson LM, Hong W, Keast PK, Brassard JA, O'Farrell AM, Cherrington JM, Pryer NK. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis. 2003;20:757–766. doi: 10.1023/b:clin.0000006873.65590.68. [DOI] [PubMed] [Google Scholar]
- 8.O'Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong LM, Hong W, Lee LB, Town A, Smolich BD, Manning WC, Murray LJ, Heinrich MC, Cherrington JM. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–3605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 9.Saltz LB, Rosen LS, Marshall JL, Belt RJ, Hurwitz HI, Eckhardt SG, Bergsland EK, Haller DG, Lockhart AC, Rocha Lima CM, Huang X, DePrimo SE, Chow-Maneval E, Chao RC, Lenz HJ. Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol. 2007;25:4793–4799. doi: 10.1200/JCO.2007.12.8637. [DOI] [PubMed] [Google Scholar]
- 10.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 11.Cheeseman SL, Joel SP, Chester JD, Wilson G, Dent JT, Richards FJ, Seymour MT. A ‘modified de Gramont’ regimen of fluorouracil, alone and with oxaliplatin, for advanced colorectal cancer. Br J Cancer. 2002;87:393–399. doi: 10.1038/sj.bjc.6600467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hochster HS, Hart LL, Ramanathan RK, Hainsworth JD, Hedrick EE, Childs BH. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 13.Maity A, Bernhard EJ. Modulating tumor vasculature through signaling inhibition to improve cytotoxic therapy. Cancer Res. 2010;70:2141–2145. doi: 10.1158/0008-5472.CAN-09-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 15.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Eng J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 16.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Board R, Jayson GC. Platelet-derived growth factor receptor (PDGFR): a target for anticancer therapeutics. Drug Resist Updat. 2005;8:75–83. doi: 10.1016/j.drup.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 20.Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB, III Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 21.Abrams TJ, Murray LJ, Pesenti E, Holway VW, Colombo T, Lee LB, Cherrington JM, Pryer NK. Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with “standard of care” therapeutic agents for the treatment of breast cancer. Mol Cancer Ther. 2003;2:1011–1021. [PubMed] [Google Scholar]
- 22.Blansfield JA, Caragacianu D, Alexander HR, III, Tangrea MA, Morita SY, Lorang D, Schafer P, Muller G, Stirling D, Royal RE, Libutti SK. Combining agents that target the tumor microenvironment improves the efficacy of anticancer therapy. Clin Cancer Res. 2008;14:270–280. doi: 10.1158/1078-0432.CCR-07-1562. [DOI] [PubMed] [Google Scholar]
- 23.Guerin O, Formento P, Lo NC, Guérin O, Formento P, Lo Nigro C, Hofman P, Fischel JL, Etienne-Grimaldi MC, Merlano M, Ferrero JM, Milano G. Supra-additive antitumor effect of sunitinib malate (SU11248, Sutent) combined with docetaxel. A new therapeutic perspective in hormone refractory prostate cancer. J Cancer Res Clin Oncol. 2008;134:51–57. doi: 10.1007/s00432-007-0247-4. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 25.Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet. 2001;40:85–104. doi: 10.2165/00003088-200140020-00002. [DOI] [PubMed] [Google Scholar]
- 26.Hecht JR, Yoshino T, Mitchell EP, Dees MS, III, Countouriotis AM, Maneval EC, Kretzschmar A. A randomized, phase IIb study of sunitinib plus 5-fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) versus bevacizumab plus mFOLFOX6 as first-line treatment for metastatic colorectal cancer (mCRC): Interim results. J Clin Oncol. 2010;28 (suppl abstract 3532) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


