Abstract
Study objective
We evaluate the cost-effectiveness of polymerase chain reaction (PCR)-based rapid influenza testing and treatment for influenza in adult emergency department (ED) patients who are at high risk for or have evidence of influenza-related complications.
Methods
We developed a cost-utility decision analysis model that assessed adult patients presenting to the ED with symptoms of an acute respiratory infection, who met the Centers for Disease Control and Prevention criteria for recommended antiviral treatment. Analysis was performed from the societal perspective, with incremental comparisons of 4 influenza testing and treatment strategies: treat none, treat according to provider judgment, treat according to results of a PCR-based rapid diagnostic test, and treat all.
Results
Treating no patients with antivirals was dominated by all other strategies that increased in both cost and benefit in the following order: treat according to provider judgment, treat according to results of a PCR-based rapid diagnostic test, and treat all. As influenza prevalence increases, treating all patients eventually dominated all other options.
Conclusion
The economic benefit of incorporating use of rapid PCR-based influenza testing for ED patients at risk of developing influenza-related complications depends on influenza prevalence; treatment guided by physician diagnosis or rapid testing, and treatment of all patients is more effective and less costly than no treatment.
INTRODUCTION
Background
Each year, influenza affects approximate 5% to 20% of the US population, causing more than 200,000 hospitalizations and 3,000 to 49,000 deaths.1–3 Fortunately, the past 15 years has brought both new antiviral medications and increasing evidence of their effectiveness in specific populations. Although the benefit of treatment is questionable in healthy individuals, evidence supports antiviral use for patients considered at increased risk for or those with evidence of existing complications, and routine use in those populations is recommended by the Centers for Disease Control and Prevention (CDC), the World Health Organization, and the Infectious Disease Society of America.4–6 Recent CDC guidelines recommend antiviral treatment specifically for patients with a severe or complicated clinical course, requiring hospitalization, or considered at high risk for influenza complications, including those younger than 2 years or aged 65 years or older, residing in a chronic care facility, with a chronic medical condition, pregnant, or morbidly obese.4 Antiviral medications are currently recommended to be administered within 48 hours of symptom onset and appear to have increased effectiveness when administered closer to symptom onset.7–10 Despite the evidence that decreasing the time between symptom onset and antiviral administration results in improved outcomes, practical ability to diagnose and treat influenza within this 48-hour timeframe is difficult because of timing of patient presentation, medication costs, and lack of reliable rapid diagnostic tools.
In an attempt to fill the need for expediting definitive diagnosis, several rapid influenza tests have been developed. Previous antigen-based assays have been limited by moderate to poor sensitivities, ranging from 10% to 70%, and current CDC guidelines accordingly require additional testing in the setting of a negative rapid influenza test result.11 Given the lack of high-performance tests that yield rapid results, physicians frequently make a presumptive diagnosis of influenza according to clinical presentation. Previous studies that have attempted to validate the use of clinical symptoms to diagnose influenza, however, have demonstrated overall poor sensitivity and specificity. As an example, one of the largest studies ever conducted showed that a combination of fever and cough had a sensitivity of 64% and a specificity of 67%.12 New rapid polymerase chain reaction (PCR)-based influenza tests use PCR-based detection, yield results in 80 minutes, and have recently obtained Food and Drug Administration approval for use in clinical settings. Previous validation studies performed in comparison to a reverse transcriptase PCR (rt-PCR) criterion standard report a sensitivity of 91.2% (95% confidence interval [CI] 85.1% to 95.4%) and specificity of 99.4% (95% CI 96.7% to 100%).13 Although promising, and with significantly improved performance relative to current rapid influenza diagnostic tests observed in clinical settings, PCR-based rapid tests have not yet been integrated into clinical practice, largely because of concerns over the clinical utility of testing relative to existing approaches, and the associated increased cost.
Importance
The majority of the cost-effectiveness analyses of influenza treatment have focused on healthy adults. These studies often conclude that the most cost-effective strategy is to treat all patients with antiviral medications, driven largely by a 1- to 2-day reduction in symptoms and decrease in lost work costs.14,15 Cost-effectiveness studies examining patients at increased risk of influenza complications have more varied outcomes that depend on influenza prevalence. When the prevalence of influenza is low, treating influenza according to the result of an influenza test is often the most cost-effective method; however, with increasing influenza prevalence, treating all patients with suspected influenza without testing becomes most cost-effective.16,17 When considering influenza testing, these studies have considered the accurate but expensive criterion standard rt-PCR testing, or the less expensive but inaccurate older antigen-based rapid testing. The emergence of new accurate rapid PCR-based tests, with a more moderate price and improved accuracy, could potentially shift the cost-utility balance of influenza testing.
Previous cost-effectiveness studies evaluating influenza testing in high-risk patients have restricted evaluation of those patients with influenza-like illness, most commonly defined as fever with cough or sore throat.14–17 Limiting the population of included patients to those with influenza-like illness, which has been shown to have relatively poor sensitivity and specificity for influenza, provides an incomplete analysis because it does not accurately reflect the entire influenza population that may benefit from influenza testing and treatment in practice. A more comprehensive appreciation of the cost-effectiveness of influenza testing and treatment requires inclusion of a population with the broader criterion of acute-onset respiratory or febrile illness to ensure maximal inclusion of influenza patients and reflect the entire population that may benefit from testing or antivirals. Additionally, there remains limited evidence of the cost-effectiveness of influenza testing and treatment in more acute care settings such as the emergency department (ED), where the overall patient acuity mix is higher, with increased rates of hospital admission and hence increased rates of influenza-related complications and death, additionally affecting the balance between influenza testing and treatment.
Goals of This Investigation
We sought to determine the relative cost-effectiveness of influenza testing and treatment strategies for adults who present to the ED with an acute respiratory illness and meet 2011 CDC criteria for recommended influenza treatment. We performed an incremental evaluation of 4 separate influenza testing and antiviral treatment regimens, using a cost-utility– based approach: treat none, treat according to provider judgment, treat according to results of a PCR-based rapid diagnostic test, and treat all.
MATERIALS AND METHODS
We constructed a cost-utility decision analysis model with TreeAge Pro (TreeAge Software, Inc, Williamstown, MA) to make an incremental comparison among 4 influenza testing and antiviral treatment strategies: treat none, treat according to provider judgment, treat according to results of a PCR-based rapid diagnostic test, and treat all. This model considered patients presenting to the ED with symptoms of an acute respiratory infection who, if their test results were positive for influenza, would be recommended to receive antiviral treatment according to 2011 CDC guidelines, namely, patients who are at risk or potentially have influenza-related complications. The analysis used a societal perspective. To account for potential differences in mortality between treated and untreated influenza patients, we considered a lifetime horizon and discounted effects at 3%, as recommended by the US Panel on Cost-Effectiveness in Health and Medicine.18
Sensitivity analysis of the assumptions made in the base model were evaluated with a series of 1-way sensitivity analyses displayed in a tornado diagram to highlight the relative effect of potential variation in each of these selected variables. In addition, we explored a range of influenza prevalence because prevalence varies throughout the influenza season and has previously been shown to have a significant effect on cost-utility. Overall robustness of the conclusions based on the model was estimated by a probabilistic sensitivity analysis with a Monte Carlo simulation. This simulation used the stated variable ranges included in the 1-way sensitivity analysis, as well as 95% CIs or interquartile ranges for the included variables as available. To interpret the results of the incremental cost-effectiveness ratios obtained from this analysis, we adhered to the generally accepted willingness-to-pay threshold of $50,000 per quality-adjusted life-year.19
Data Collection and Processing
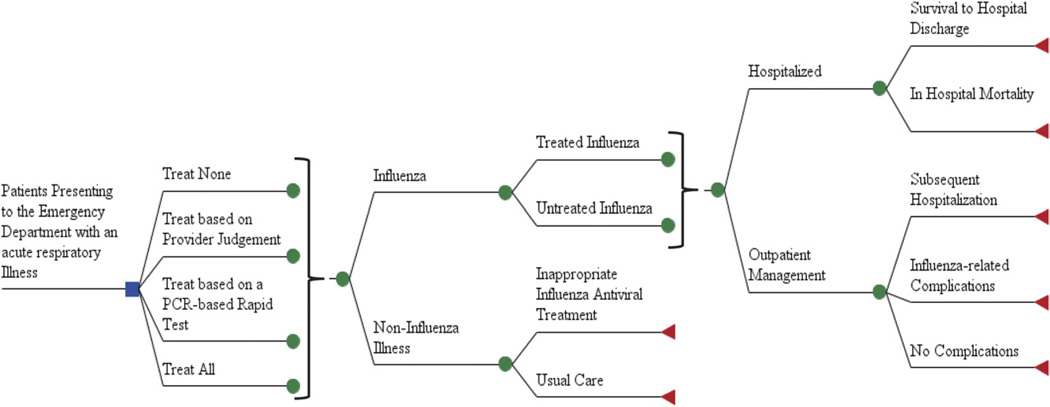
The decision analysis model, as shown in Figure 1, assumed the same influenza prevalence for each of the 4 potential treatment strategies. Although influenza prevalence varies throughout the season, the base prevalence of influenza used in this study (0.20) reflects the average prevalence of influenza among patients presenting to the ED with an acute respiratory infection between January and March.20 Previous cost-effectiveness analyses have used the influenza prevalence in patients presenting with influenza-like illness (fever and cough or sore throat), a more rigorous criterion that increases prevalence but also excludes one third of the patients with influenza.12,17 Thus, a broader definition of acute respiratory virus is likely a more accurate definition of the desired testing population. To fully evaluate the cost-utility of the included testing and treatment options, we performed a secondary analysis over a large range of the potential prevalence of influenza: 0 to 0.6.
Figure 1.
Overview of decision tree.
Patients who did not have influenza were not evaluated further because influenza testing or treatment would have no further effect on their care or outcomes. The only potential difference in the noninfluenza patient’s medical management would be due to adverse effects of the influenza antiviral medications. However, these medications have mild adverse effects that only rarely would require additional medical evaluation or care and hence would not increase costs.21 Therefore, we did not consider adverse effects of antiviral medication in treated patients whether they have influenza or not.
This model assumed that all patients receiving a diagnosis of influenza by either provider diagnosis or rapid test were treated with antivirals. If patients received antiviral therapy, we assumed that therapy was initiated within 48 hours of symptom onset and continued at the dose and length of treatment recommended by the pharmaceutical manufacturer.
For all patients, it was also assumed that the proportion of patients admitted to the hospital from the ED was similar regardless of influenza diagnosis or treatment. For respiratory infections, ED clinicians’ decisionmaking about patient disposition is likely based on the patient’s medical history, appearance, physical examination, and laboratory and radiography results. We assumed that a rapid diagnostic confirmation of influenza would not affect the likelihood to admit a patient to the hospital, nor would administration of antivirals have sufficient time to act and affect the decision to admit to the hospital. As shown in Table 1, the proportion of influenza patients admitted to the hospital from the ED is estimated at 0.13, according to a retrospective evaluation of high-risk patients presenting to the ED with influenza.22 Alternate retrospective evaluations suggest that the rate of admission in high-risk patients could be as high as 0.57, which has been included as the peak range in the sensitivity analysis.23 Subsequent complications after the decision of patient disposition from the ED are influenced by antiviral treatment, and we thus separated patients who have influenza but were not treated with antivirals those with influenza who were treated with antivirals, as displayed in Table 1.
Table 1.
Estimates of model parameters.
| Variable | Baseline Value | Sensitivity Range | Source |
|---|---|---|---|
| Influenza variables | |||
| Probability of influenza in ED patient with acute respiratory illness | 0.20 | 0–0.60 | 20 |
| Proportion of ED patients admitted | 0.13 | 0.13–0.57 | 22,23 |
| Untreated influenza | |||
| Probability of death in hospitalized patients | 0.10 | 0.06–0.14 | 24 |
| Proportion with complication requiring repeated PCP/ED visit | 0.46 | 0.30–0.62 | 25,26 |
| Proportion with complication requiring antibiotics | 0.38 | 0.23–0.53 | 25,26 |
| Proportion rehospitalized after discharge | 0.032 | 0.015–0.049 | 33 |
| Length of influenza illness, days | 7.5 | 3.5–14.5 | 25,26 |
| Missed work, days | 10.0 | 5.5–20.5 | 25 |
| Treated influenza | |||
| Probability of death in hospitalized patients | 0.039 | 0.002–0.078 | 24 |
| Proportion with complication requiring repeated PCP/ED visit | 0.14 | 0.03–0.25 | 25,26 |
| Proportion with complication requiring antibiotics | 0.14 | 0.03–0.25 | 25,26 |
| Proportion rehospitalized after discharge | 0.016 | 0.003–0.029 | 33 |
| Length of influenza illness, days | 5.0 | 3.0–9.0 | 25,26 |
| Missed work, days | 7.0 | 4.0–16.0 | 25 |
| QALYs gained by antiviral treatment | |||
| QALY gained for improvement of symptoms with antiviral use | 0.006 | ||
| QALY gained per hospitalized patient because of decreased mortality | 0.75 | 0.61–1.83 | |
| Rapid influenza test characteristics | |||
| Sensitivity | 0.91 | 0.85–0.95 | 34 |
| Specificity | 0.99 | 0.97–1.00 | 34 |
| Provider decisionmaking | |||
| Sensitivity | 0.67 | 0.29–0.67 | 20 |
| Specificity | 0.92 | 0.92–0.96 | 20 |
| Costs, $US | |||
| Antiviral (full treatment course) | 100.60 | 72.95–100.60 | 30 |
| Antibiotic (full treatment course) | 3.69 | 3.69–68.91 | 30 |
| Rapid diagnostic test | 53 | Cepheid | |
| Repeated visit, PCP/ED | 303.87 | 72.77–303.87 | 23,35 |
| Hospitalization (with survival) | 31,970 | 31,541–32,399 | 28 |
| Hospitalization (with mortality) | 52,646 | 50,572–54,717 | 28 |
| Mean hourly wage | 22.02 | 31 |
QALY, Quality-adjusted life-year; PCP, primary care physician; ED, emergency department.
Hospitalized influenza patients can either die in the hospital or survive to discharge. Hospitalized influenza patients who are treated with an antiviral have a lower risk of death (4%) compared with those who are not treated with an antiviral (10%).24 The mortality benefit of antivirals was explored in the sensitivity analysis, ranging from no benefit (0% difference in mortality between treated and not treated) to 12% difference in mortality between treated and nontreated individuals. After discharge from inpatient hospitalization, we assumed that the patient incurred no additional complications or expenses and that influenza resolved without further effects.
Patients initially discharged from the ED continued with no complications, had complications that required a repeat provider visit, or were subsequently hospitalized for influenza. Previous studies have shown that antivirals reduce the rates of complications.25,26 It was thus assumed that patients with subsequent complications had a repeated provider visit to address the complication and that no patients died at home. Several influenza complications require antibiotics, such as pneumonia, sinusitis, and otitis media, which have also been included in the analysis. Proportions of all complications and those requiring antibiotics are listed in Table 1 for treated and untreated influenza patients.
Several studies have demonstrated that antiviral medications reduce the duration of symptoms by 1.5 to 2.5 days in both healthy and at-risk individuals.26 One retrospective evaluation of the quality of life during a typical influenza illness, using the EuroQol instrument, found that influenza resulted in a 0.883 reduction in health-state compared with baseline, which was used to calculate the quality-adjusted life-years gained from reducing days of symptoms.27 The quality-adjusted life-years gained from preventing a death depends on the life expectancy. The adult population at high risk of influenza complications consists of a wide array of risk variables, including aged older than 65 years and having chronic or acute medical illness. It was assumed for the purposes of this analysis that the life expectancy of these patients was 15 years, an estimate based on the age distribution of patients considered to be at high risk for influenza complications in previous studies.28 However, a range of life expectancy from 10 to 30 years was included in the sensitivity analysis to evaluate the effect of a range of potential values for this assumption. Using 3% discounting, 12.3 discounted quality-adjusted life-years are gained from preventing a death. The estimated quality-adjusted life-years used for this analysis are listed in Table 1.
The clinical diagnosis of influenza is challenging to make despite numerous attempts to define a clear syndrome associated with influenza. The most commonly used set of symptoms is fever with cough or sore throat, which is only 64% sensitive.12 In an undifferentiated population with an acute respiratory illness, provider decisionmaking has a poor sensitivity (0.29) and specificity (0.92).20 During the initial 2 days of symptoms, when antivirals are most effective, provider sensitivity increases to 0.67. Thus, 0.67 was used as our base case sensitivity for provider decisionmaking, but a range down to 0.29 was included in our sensitivity analysis. The new rapid PCR-based diagnostic tests have a far superior sensitivity (0.91) and specificity (0.99).13
Costs were estimated from a societal perspective and are in 2011 US dollars. Costs not initially found in 2011 US dollars were converted to 2011 US dollars with the Medical Care Consumer Price Index.29 All costs occur within the first year of diagnosis and treatment as influenza is an acute disease; therefore, no discounting was performed on costs. The cost of the initial ED visit was not included because this occurred before any specific treatment and testing; however, the cost of testing and antiviral treatment was included for each patient receiving either.
It was assumed that patients treated with antivirals received a full treatment course with oseltamivir, which is estimated to cost $100.60.30 Oseltamivir is the most commonly used antiviral; however, zanamivir is slightly cheaper and can also be used. Thus, these 2 prices included the sensitivity range for antiviral cost. We did not include costs for the amantadines because they are currently no longer recommended for influenza treatment due to high rates of resistance. Some patients require antibiotics, and amoxicillin was selected as the representative antibiotic. The majority of subsequent infections include sinus infections, ear infections, and pneumonias, all of which can be treated with amoxicillin. Pneumonias in particular are often treated with more expensive antibiotics such as azithromycin or moxifloxacin for their added atypical bacterial coverage, and patients admitted to the hospital would likely require additional antibiotics to amoxicillin. These more expensive antibiotics were considered in the sensitivity analysis. Details on medication costs are listed in Table 1.
The expense associated with the rapid diagnostic test was estimated according to one of the new rapid influenza tests: Cepheid’s Xpert Flu assay (Cepheid, Sunnyvale, CA). The test is performed on a platform that is used for several other purposes in the hospital and hence does not require specific purchasing for this purpose. This cost-utility analysis assumed an ED setting in a moderately sized hospital that would therefore carry this platform. The overall price for the test included $50 for the test cartridge as stated by the manufacturer and $3 per test for labor according to an estimate from the hospital laboratory.
In the event of complications, the patient is likely to visit a medical professional either by returning to the ED or consulting a primary care physician. If patients are initially evaluated in the ED, they are likely to return to the ED for follow-up care for the same reasons they originally sought care in the ED: they have no primary care physician, are unable to make an appointment to see their primary care physician, or believe that their illness requires the higher level of care available in the ED. Therefore, for the base case, the repeated visit was assumed to be in the ED. A cost of a repeated ED visit is estimated at $304 per a previously performed cost analysis study evaluating the cost of treating influenza patients in the ED, which estimated the true cost of medical care, including medications, supplies, and health professional fees. From this analysis, we extracted the costs for patients older than 65 years as a representative of our high-risk patient population.23 The cost of a primary care physicians visit is substantially less, $72.11, as estimated by the American Medical Association. To account for potential variability, we used these 2 figures in the sensitivity analysis.
Estimates of hospitalization costs were determined according to a previous cost analysis of the effect of seasonal influenza. This cost analysis used a probabilistic model to estimate costs.28 To estimate hospitalization costs from this cost analysis, we used a weighted average of all medical care costs for all high-risk adults who were hospitalized with influenza. This average was weighted by the number of patients in each age group who were hospitalized.
The population in question includes the elderly and those with chronic illnesses, so employment rates are likely to be lower than that of the general population. To that end, it is assumed that 50% of this population is employed and works 8 hours a day at the national mean hourly wage of $22.02.31 The actual proportion of employment and wage varies by location, so analysis was performed with and without estimates of this indirect cost by allowing the sensitivity analysis range for this variable to decrease to 0. Days of missed work were estimated to be 10 days in patients without influenza treatment and 7 days in those who receive antivirals according to the time to return to normal activities used in previous studies.25
RESULTS
In the base case analysis, treating none was clearly dominated by the other alternatives because it had the greatest cost ($1,260; 95% CI $537 to $4,915) and least effectiveness (0 quality-adjusted life-years saved by definition). Because there are other cheaper and more effective options, dominated alternatives, such as treating no patients, are not considered further as potentially cost-effective treatment options. Treatment based on provider judgment had the lowest cost ($1,153; 95% CI $528 to $4,727) but also a low effectiveness (0.014 quality-adjusted life-year saved; 95% CI 0.009 to 0.209 quality-adjusted life-year saved). This was followed by treatment based on a PCR-based rapid diagnostic test with a slightly higher cost and effectiveness, and treating all patients, which had the highest cost but also the highest effectiveness. As shown in Table 2, the results for each of these options are expressed as a cost-utility ratio, which demonstrates the cost to gain 1 quality-adjusted life-year in that particular testing and treatment arm. Additionally, nondominated alternatives are evaluated by the incremental cost-effectiveness ratio, which allows for comparison between 2 treatment arms, one of which has greater cost but also greater effectiveness. The incremental cost-effectiveness ratio demonstrates the additional cost for each additional quality-adjusted life-year saved by moving from the lower-cost to the higher-cost option. The incremental cost-effectiveness ratio between treatment according to provider judgment and treatment according to PCR-based rapid diagnostic testing was $1,389 per quality-adjusted life-year saved, and the incremental cost-effectiveness ratio between treatment based on PCR-based rapid diagnostic testing and treating all was $6,249 per quality-adjusted life-year saved. Both of these incremental cost-effectiveness ratios are below the $50,000 per quality-adjusted life-year willingness-to-pay threshold generally accepted in the United States.
Table 2.
Base case cost-utility ratios with 95% CIs of estimates based on probabilistic sensitivity analysis.
| 95% CI | ||||
|---|---|---|---|---|
| Testing and Treatment Strategy | Cost (2011 $US) | QALYs Gained | Cost:Utility Ratio ($/QALY) | ICER ($/QALY) |
| Provider judgment | 1,153 (528–4,727) | 0.014 (0.009–0.209) | 84,376 | |
| Rapid diagnostic test | 1,160 (558–4,664) | 0.019 (0.015–0.347) | 62,490 | 1,389 |
| Treat all | 1,171 (594–4,653) | 0.020 (0.017–0.382) | 57,428 | 6,246 |
| Treat none | 1,260 (537–4,915) | 0 | >100,000 | Dominated |
ICER, Incremental cost-effectiveness ratio.
Sensitivity Analyses
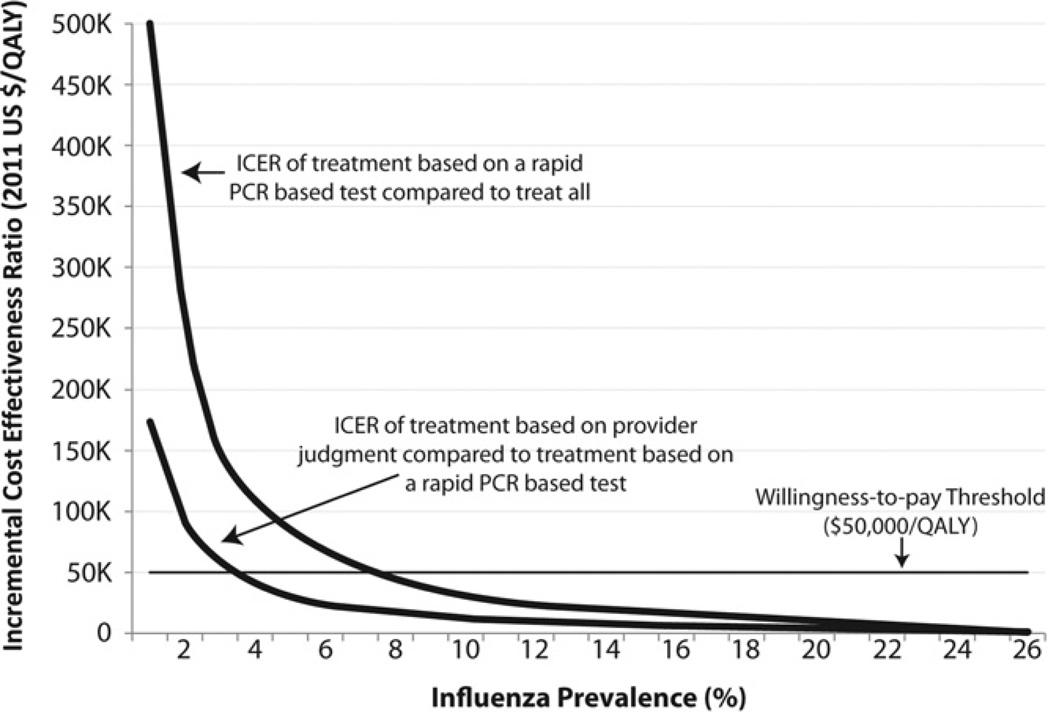
A 1-way sensitivity analysis of influenza prevalence demonstrated the significant effect of prevalence on cost-utility. At any potential prevalence greater than 0%, treating none was dominated by the other alternatives. At a prevalence above 23%, rapid diagnostic testing dominated provider judgment. At a prevalence above 26%, treating all dominated both provider judgment and rapid diagnostic testing. Thus, between 1% and 23% prevalence, provider judgment, rapid diagnostic testing, and treating all (in order of increasing cost) were potential options. Below a prevalence of approximately 3%, the incremental cost-effectiveness ratio between provider judgment and rapid PCR-based diagnostic testing was greater than the $50,000 per quality-adjusted life-year threshold. Between 3% and 7% prevalence, the incremental cost-effectiveness ratio between rapid diagnostic testing and treating all was greater than the $50,000 per quality-adjusted life-year threshold. Between 7% and 23% prevalence, the incremental cost-effectiveness ratios between provider judgment and rapid PCR-based diagnostic testing, and rapid PCR-based diagnostic testing and treating all, remained below the $50,000 per quality-adjusted life-year threshold. Adhering to the $50,000 per quality-adjusted life-year threshold, the most cost-effective options are to treat according to provider diagnosis from 0% to 3% prevalence, treat according to the results of rapid PCR-based testing from 3% to 7% prevalence, and treat all above 7% prevalence. Figure 2 demonstrates the change in the incremental cost-effectiveness ratio between treatment according to provider judgment and rapid PCR-based diagnostic testing and the incremental cost-effectiveness ratio between treatment according to rapid PCR-based diagnostic testing and treat all, both compared to the willingness-to-pay threshold over the range of 1% prevalence until treat all dominates all alternatives at 26%.
Figure 2.
One-way sensitivity analysis of influenza prevalence. ICER comparing treatment according to provider judgment with treatment according to a rapid PCR test and treatment according to provider judgment to treat all, compared with the accepted willingness-to-pay threshold of $50,000 per QALY.
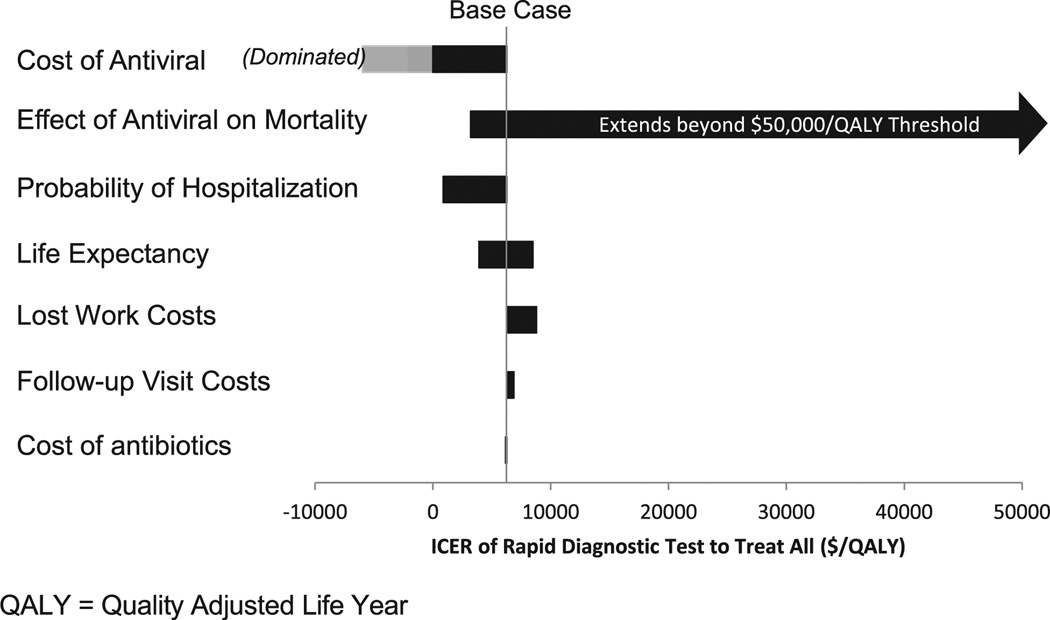
Several 1-way sensitivity analyses were performed to evaluate the effect of particular variables and assumptions on the incremental cost-effectiveness ratio between treatment based on PCR-based rapid diagnostic testing and treating all. As seen in Figure 3, a tornado diagram was performed to evaluate sensitivity analysis of the remaining variables indicated in the “Materials and Methods” section. A tornado diagram displays the results in order of potential influence on outcome, with the variables with the greatest potential effect on the top and least on the bottom. The greatest effect came from the cost of antiviral treatment, in which a cost of less than approximately $78 led to the treat-all option dominating over the treat according to a PCR-based rapid diagnostic test. Also with a significant effect on the outcome, if antiviral medications caused no improvement in mortality in admitted patients, then the incremental cost-effectiveness ratio between treatment based on a PCR-based rapid test and treating all was greater than the $50,000 per quality-adjusted life-year threshold, indicating a potential benefit to treatment according to PCR-based rapid influenza testing. However, a mortality benefit of even 0.5% in admitted patients receiving antiviral medications reduces the incremental cost-effectiveness ratio to below the $50,000 per quality-adjusted life-year threshold. The remaining variables, probability of hospitalization, life expectancy, lost work costs, follow-up visit costs, and cost of antibiotics, did not have substantial influence on the most cost-effective option.
Figure 3.
Tornado diagram displaying ICER between treatment according to rapid PCR-based influenza testing and treat all algorithms.
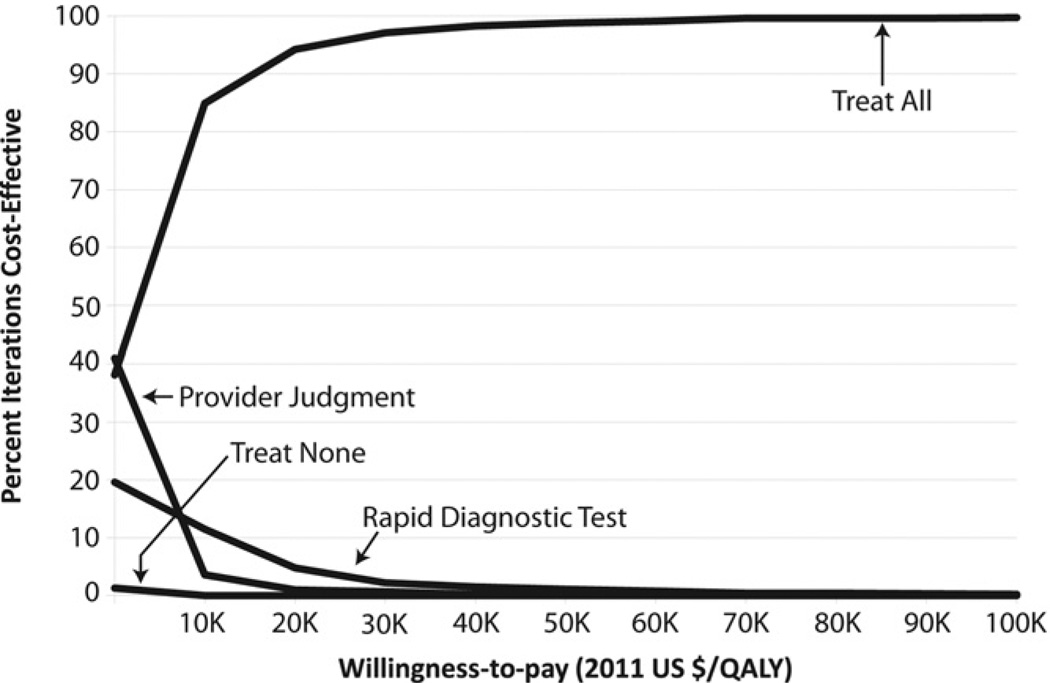
The cost-effectiveness acceptability curve shown in Figure 4 summarizes the repeated Monte Carlo simulations. If a quality-adjusted life-year had no economic value (willingness to pay equals zero), treatment according to provider judgment was the most economically preferred (saved the most money) in approximately 40% of repeated simulations, followed closely by treat all, which was the most economically preferred in 38% of repeated simulations. This was followed by treatment according to rapid PCR-based diagnostic treating, which was most efficient in 20% of the simulations. As the willingness to pay for quality-adjusted life-years increased to the commonly used $50,000 per quality-adjusted life-year, treating all was most economically preferred in almost all the simulations, whereas the remaining options were rarely most economically preferred. Treating none was rarely the most cost-effective at any willingness-to-pay value.
Figure 4.
Cost-effectiveness acceptability curve of Monte Carlo simulation results across a range of willingness-to-pay thresholds.
LIMITATIONS
True to any cost-effectiveness analysis, the results are limited by the assumptions used to create the model. This model applies only to patients treated within 48 hours because that is the commonly used time cutoff in much of the pertinent literature. This time limit thus decreases the generalizability of this analysis in patients who present or are potentially treated later in their disease course. Additionally, this analysis assumes that hospital admission and ED resource use is based on the patient’s clinical presentation and would not be affected positively or negatively by a diagnosis of influenza, whether by clinical judgment or rapid test. Finally, this study uses a willingness-to-pay threshold of $50,000 per quality-adjusted life-year. The exact monetary value of a quality-adjusted life-year is subjective, and although we use this standard threshold to aid in interpretation of the data, consideration of the actual monetary value of 1 quality-adjusted life-year spent should be considered.
This analysis evaluates all adults at high risk of influenza-related complications, which is a varied group including those at risk because of age (>65 years), chronic illnesses, pregnancy, obesity, and those presenting with complications. It is likely that the cost-effectiveness of the 4 testing and treatment regimens examined here vary within the above-referenced risk groups according to age, medical history, and severity of illness. However, given the lack of literature describing influenza by specific subgroups, it is challenging to further reduce the population for specific subpopulation calculations.
This study additionally assumes that any patient who received a diagnosis of influenza, whether by rapid test or clinical evaluation, received antiviral treatment because we included only patients who were recommended to receive treatment according to CDC guidelines. In reality, this is not the case because only 50% of high-risk patients who received a diagnosis of influenza in the ED receive antivirals.22 Previous studies have shown that use of a rapid influenza test increases antiviral prescription rate, likely because of increased physician confidence in the diagnosis of influenza.32 Hence, the simplified assumption that all patients receiving a diagnosis will be treated likely places rapid diagnostic testing at a disadvantage compared with how each of the testing and treatment arms would likely be implemented in a real clinical setting. Additional work is thus needed to evaluate the actual clinical utility of rapid testing and of provider diagnosis and the corresponding rates of antiviral prescription in high-risk populations.
Finally, this model provides a cost-utility analysis to provide information on a population level; however, there are additional factors that may affect ultimate decisionmaking. For example, this model does not attempt to model antiviral resistance patterns over time. Some of the considered treatment algorithms, such as treat all, would likely lead to increased selection for resistant variants, which may, along with other unmeasured factors, ultimately affect clinical decisionmaking.
DISCUSSION
To our knowledge, this incremental cost-utility analysis is the first ever evaluation of influenza testing and treatment in patients with a high risk of influenza complications presenting to the ED with an acute respiratory illness in the era of new highly sensitive rapid diagnostics. As demonstrated in previous analyses, the optimal method of influenza testing and treatment is highly dependent on influenza prevalence, which changes rapidly throughout the influenza season. Assuming a $50,000 per quality-adjusted life-year willingness-to-pay threshold, the most cost-effective treatment option is treatment according to provider judgment from 0% to 3% prevalence, treatment according to a PCR-based rapid influenza test from 3% to 7% prevalence, and treating all at greater than 7% prevalence. These prevalences are based on a population presenting with a broadly defined respiratory illness, whereas previous studies have based their prevalence estimates on patients presenting with influenza-like illness, which is defined as fever and cough or sore throat.17 Although using the stricter criterion of influenza-like illness increases the prevalence of influenza in the testing population, it is also relatively insensitive because it results in up to a third of influenza patients’ being left untreated. Hence, the prevalence levels referenced in this analysis are likely to be lower than the corresponding influenza prevalence among a more select group of patients with influenza-like illness.
In considering the base case analysis, the 3 undominated treatment protocols, treat according to provider judgment, treat according to the results of a rapid PCR-based test, and treat all, do not differ substantially in terms of cost or effect and are all superior to the treat none approach. Thus, in patients with high risk or current influenza complications who present to the ED in less than 48 hours, treatment with antivirals, whether based on provider judgment, rapid test, or treating all, results in decreased costs and increased benefit compared with not treating with antivirals. Which of the 3 treatment options is most cost-effective depends on prevalence and other individual and societal factors. In all but the lowest prevalences, treatment according to rapid PCR-based testing results in improved outcomes compared with provider judgment, with a minimal additional cost of $1,389 per quality-adjusted life-year in the base case scenario. The treatment according to rapid PCR-based testing option has the added benefit of influenza testing and hence information about influenza prevalence to inform future decisionmaking. From a strict cost-effectiveness perspective, the treat all option is similarly favorable at a prevalence above 7%, with an incremental cost-effectiveness ratio of $6,246 per quality-adjusted life-year in the base case analysis. However, the treat all option raises some concerns about subsequent development of antiviral resistance because of heavy antiviral use. Although this analysis was taken from a societal perspective, we did not attempt to estimate how the rates of antiviral treatment would affect developing viral resistance for the 2 remaining effective antiviral medications, oseltamivir and zanamivir. The optimal method of administering antiviral treatment to high-risk influenza patients is likely influenced by additional considerations not modeled in this analysis, such as antiviral resistance and individual patient evaluation and preferences.
Overall, the most cost-effective method of influenza testing and treatment in high-risk ED patients depends on local influenza prevalence; however, with any active influenza, antiviral treatment of any kind is superior to no treatment. Given the rapidly changing prevalence, the costs and effects of each treatment algorithm vary throughout the influenza season, and the most efficient ED policy may change throughout the influenza season. From a practical standpoint, providers in acute care settings would thus benefit from having real-time estimates of the prevalence of disease in their community or locale to make the most cost-effective decisions for evaluating and treating patients who may have influenza. Although promising methods are being developed for real-time influenza monitoring, additional research combining surveillance with influenza treatment strategies is required to optimize an effective approach to clinical practice.
Editor’s Capsule Summary.
What is already known on this topic
Antiviral treatment benefits some influenza patients, but clinical diagnosis is inaccurate and rapid antigen tests are not always reliable. Newer rapid polymerase chain reaction (PCR) tests are more accurate.
What question this study addressed
A cost analysis model examined 4 strategies for antiviral treatment of influenza patients at risk for complications: treat none, according to clinical diagnosis, according to rapid PCR result, or treat all possible influenza cases.
What this study adds to our knowledge
Though limited by the model assumptions, at influenza prevalence of 3% to 7% it is cost-effective to use rapid PCR result, and at a prevalence greater than 7% it is more cost-effective to treat all.
How this is relevant to clinical practice
Rapid PCR-based influenza tests could be used to guide antiviral treatment decisions and gauge local prevalence of influenza.
Acknowledgments
Funding and support: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist. This work was supported by Johns Hopkins Clinical Research Scholars (5KL2RR025006), Department of Homeland Security (PACER: National Center for Study of Preparedness and Response [2010-ST-061-PA0001]), and Middle Atlantic RCE Program (NIAID/NIH 2 U54 AI057168).
Footnotes
Author contributions: AFD, CAG, and RER conceived of the study and obtained funding. AFD, SC, CAG, and KDF designed the study. AFD and SC collected the required data. AFD, SC, and KDF performed statistical analysis. AFD drafted the article, and all authors contributed substantially to its revision. AFD takes responsibility for the paper as a whole.
REFERENCES
- 1.Centers for Disease Control and Prevention. Seasonal influenza. [Accessed March 12, 2013];2011 Available at: http://www.cdc.gov/flu/about/qa/disease.htm.
- 2.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 4.Fiore AE, Fry A, Shay D, et al. Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Surveill Summ. 2011;60:1–24. [PubMed] [Google Scholar]
- 5.Harper SA, Bradley JS, Englund JA, et al. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1003–1032. doi: 10.1086/604670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO recommendations on the use of rapid testing for influenza diagnosis. [Accessed March 12, 2013]; Available at: http://www.who.int/influenza/resources/documents/rapid_testing/en/index.html.
- 7.Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 8.Monto AS, Fleming DM, Henry D, et al. Efficacy and safety of the neuraminidase inhibitor zanamivirin the treatment of influenza A and B virus infections. J Infect Dis. 1999;180:254–261. doi: 10.1086/314904. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet. 2000;355:1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 10.Heinonen S, Silvennoinen H, Lehtinen P, et al. Early oseltamivir treatment of influenza in children 1–3 years of age: a randomized controlled trial. Clin Infect Dis. 2010;51:887–894. doi: 10.1086/656408. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) Virus–United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:826–829. [PubMed] [Google Scholar]
- 12.Monto AS, Gravenstein S, Elliott M, et al. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–3247. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 13.Sambol AR, Iwena PC, Pieretti M, et al. Validation of the Cepheid Xpert Flu: a real time RT-PCR detection panel for emergency use authorization. J Clin Virol. 2010;48:234–238. doi: 10.1016/j.jcv.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Rothberg MB, He S, Rose DN. Management of influenza symptoms in healthy adults. J Gen Intern Med. 2003;18:808–815. doi: 10.1046/j.1525-1497.2003.20822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talbird SE, Brogan AJ, Winiarski AP, et al. Cost-effectiveness of treating influenzalike illness with oseltamivir in the United States. Am J Health Syst Pharm. 2009;66:469–480. doi: 10.2146/ajhp080296. [DOI] [PubMed] [Google Scholar]
- 16.Lee BY, McGlone SM, Bailey RR, et al. To test or to treat? an analysis of influenza testing and antiviral treatment strategies using economic computer modeling. PLoS One. 2010;5:E11284. doi: 10.1371/journal.pone.0011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothberg MB, Bellantonio S, Rose DN. Management of influenza in adults older than 65 years of age: cost-effectiveness of rapid testing and antiviral therapy. Ann Intern Med. 2003;139(5 pt 1):321–329. doi: 10.7326/0003-4819-139-5_part_1-200309020-00007. [DOI] [PubMed] [Google Scholar]
- 18.Siegel JE, Weinstein MC, Russell LB, et al. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339–1341. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 19.Braithwaite RS, Meltzer DO, King JTJ, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 20.Stein J, Louie J, Flanders S, et al. Performance characteristics of clinical diagnosis, a clinical decision rule, and a rapid influenza test in the detection of influenza infection in a community sample of adults. Ann Emerg Med. 2005;46:412–419. doi: 10.1016/j.annemergmed.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Donner B, Bader-Weder S, Schwarz R, et al. Safety profile of oseltamivir during the 2009 influenza pandemic. Pharmacoepidemiol Drug Saf. 2009;20:532–543. doi: 10.1002/pds.2136. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh Y-H, Kelen GD, Dugas AF, et al. Emergency department management of adult patients with a clinical diagnosis of influenza and provider’s adherence to CDC guidance during the 2009 H1N1 Pandemic. West J Emerg Med. doi: 10.5811/westjem.2012.11.12246. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox FM, Cobb MM, Chua WQ, et al. Cost of treating influenza in emergency department and hospital settings. Am J Manag Care. 2000;6:205–214. [PubMed] [Google Scholar]
- 24.McGeer A, Green KA, Plevneshi A, et al. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis. 2007;45:1568–1575. doi: 10.1086/523584. [DOI] [PubMed] [Google Scholar]
- 25.Lalezari J, Campion K, Keene O, et al. Zanamivir for the treatment of influenza A and B infection in high-risk patients: a pooled analysis of randomized controlled trials. Arch Intern Med. 2001;161:212–217. doi: 10.1001/archinte.161.2.212. [DOI] [PubMed] [Google Scholar]
- 26.MIST (Management of Influenza in the Southern Hemisphere Trialists) Study Group. Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet. 1998;352:1877–1881. [PubMed] [Google Scholar]
- 27.Griffin AD, Perry AS, Fleming DM. Cost-effectiveness analysis of inhaled zanamivir in the treatment of influenza A and B in high-risk patients. Pharmacoeconomics. 2001;19:293–301. doi: 10.2165/00019053-200119030-00007. [DOI] [PubMed] [Google Scholar]
- 28.Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 29.United States Department of Labor. Consumer Price Index. [Accessed March 12, 2013]; Available at: ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt.
- 30.Drug Topics Red Book. Montvale, NY: Thomson Healthcare; 2011. [Google Scholar]
- 31.United States Department of Labor. Occupational Employment Statistics. [Accessed March 12, 2013];2010 Available at: http://www.bls.gov/oes/current/ oes_nat.htm#00-0000.
- 32.Noyola DE, Demmler GJ. Effect of rapid diagnosis on management of influenza A infections. Pediatr Infect Dis J. 2000;19:303–307. doi: 10.1097/00006454-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Kaiser L, Wat C, Mills T, et al. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med. 2003;163:1667–1671. doi: 10.1001/archinte.163.14.1667. [DOI] [PubMed] [Google Scholar]
- 34.Popowitch EB, Rogers E, Miller MB. Retrospective and prospective verification of the Cepheid Xpert influenza virus assay. J Clin Microbiol. 2011;49:3368–3369. doi: 10.1128/JCM.01162-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Medical Association. Current Procedural Terminology code: 99213. [Accessed March 12, 2013];2011 Available at: https://ocm.ama-assn.org/OCM/CPTRelativeValueSearchResults.do?locality_39&keyword_99213. [Google Scholar]