Abstract
Objective
To evaluate the clinical significance of the extent of extreme drug resistance (EDR) in in-vitro drug resistance assays in advanced epithelial ovarian, fallopian, and primary peritoneal cancers.
Methods
A retrospective study was conducted using the database for in-vitro drug resistance assay (EDR Assay®, Oncotech, Inc.) results for advanced stage ovarian cancer samples obtained at primary surgery between 1995 and 2009. In-vitro drug resistance assay results were evaluated for thirteen drugs according to the following two groups: platinum and taxane (primary treatment group) vs remaining agents (secondary treatment group). Dual-resistance was then defined as at least one EDR in the primary and secondary treatment groups. Chemotherapy response and survival outcome were correlated with assay results.
Results
There were 253 cases identified. Dual-resistance (n=53, 20.9%) was not associated with chemotherapy response (p=0.62) or survival outcomes (PFS, p=0.52; OS, p=0.11). Only one (0.4%) case exhibited complete EDR to all tested drugs, and 74 (29.4%) cases showed no EDR. There was no statistical correlation between total number of drugs in the EDR range and chemotherapy response (p=0.55), progression-free survival (PFS) (p=0.18), and overall survival (OS) (p=0.87). Proportion of EDR, defined as the ratio of the number of EDR drugs divided by all drugs for an individual patient, was also not related to chemotherapy response (p=0.37), PFS (p=0.13), or OS (p=0.13).
Conclusions
Presence of extreme drug resistance to multiple agents in the in-vitro drug resistance assays was not associated with survival outcomes in advanced stage epithelial ovarian, fallopian, and primary peritoneal cancers.
Keywords: in vitro drug resistance assay, extreme drug resistance, chemotherapy, ovarian cancer
INTRODUCTION
In-vitro drug resistance assays are designed to offer a choice of chemotherapeutic agents determined by the in-vitro response of tumor cells. Such assays have been proposed as an alternative to empiric selection of chemotherapy [1]. This assay, with tumor growth >1 SD above the median, termed extreme drug resistance (EDR), has greater than 99% predictive accuracy in identifying chemotherapeutic agents with no clinical response [2]. Moreover, some studies have reported significantly shorter survival among the patients with EDR to platinum in newly diagnosed ovarian cancer compared to those with low drug resistance (LDR) [3].
We previously examined the survival outcomes of patients with EDR to platinum and/or taxane compared to those without EDR in in-vitro drug resistance assays, and observed somewhat different results [4]. We found that presence of EDR to platinum and/or taxane was not associated with survival compared to non-EDR cases. Chemotherapy response was similar between these two groups, and there was also no difference in progression free survival. Therefore, in-vitro EDR to chemotherapeutic agents as a predictor of patient outcome remains controversial, and prompted us to consider the effects of EDR to other agents on patient outcome. The objective of the current study was to evaluate the clinical significance of the extent of EDR in in-vitro drug resistance assays in advanced epithelial ovarian, fallopian, and primary peritoneal cancers.
STUDY DESIGN
A retrospective study was conducted using a database for in-vitro drug resistance assays (EDR Assay®, Oncotech, Inc., Tustin, CA) performed on specimens obtained at the Mercy Medical Center in Baltimore, Maryland between January 1995 and January 2009. In our facility, specimens for ovarian cancer patients are routinely evaluated for in-vitro drug resistance assays at the time of primary cytoreduction. The surgeons were consistently the same (NRB and DDI), and the standard management of primary ovarian cancer was to achieve maximal cytoreduction followed by postoperative chemotherapy with platinum and taxane chemotherapy. The clinicians did not use the EDR information to determine the postoperative chemotherapy regimen, and these results were only considered at the time of recurrence. Inclusion criteria were FIGO Stage III and IV epithelial ovarian, fallopian, and peritoneal cancers obtained from the initial cytoreductive surgery. Exclusion criteria were neo-adjuvant chemotherapy, co-existence of another malignancy, and tumor of low malignant potential. Patient demographics, clinico-pathologic data, response to chemotherapy, and follow-up outcomes were abstracted from medical records and pathology reports. Survival outcome was correlated with in-vitro drug resistance assay results.
In-vitro drug resistance assay results were evaluated for carboplatin, cisplatin, cyclophosphamide, docetaxel, doxil, doxorubicin, etoposide, fluorouracil, gemcitabine, gemcitabine with cisplatin, ifosfamide, paclitaxel, and topotecan for the tumor obtained at the initial surgery. Relative in-vitro tumor cell proliferation observed in the presence of each drug tested was determined by comparing the control: LDR, less than median; intermediate drug resistance (IDR), median to +1.0 SD; and EDR, +1.0 SD or greater [2,5].
For evaluation of in-vitro drug assay results, total number of chemotherapeutic agents with EDR, and the proportion of EDR was determined for each individual patient. The number of samples with LDR, IDR, or EDR was determined for each of the chemotherapeutic agents tested for each sample. In the in-vitro drug resistance assay, free tumor cells obtained from each individual specimen were placed in the plates that contain different chemotherapeutic drugs in each plate. Test results were then characterized for LDR, IDR, or EDR for each plate. Thus, total number of EDR represents the number of agents with EDR in the tumor from an individual patient. The proportion of EDR per tumor was defined as the ratio of the number of drugs with EDR divided by the number of all drugs for each individual patient. For example, in a tumor with 4 EDR, 4 IDR, and 5 LDR agents, the proportion of individual EDR is 30.8% (4 out of 13).
A dual-resistance model was also evaluated (Table 1). In this model, the 13 tested agents were divided into two groups. The first group (primary treatment group) included platinum (carboplatin and cisplatin) and taxane (paclitaxel and docetaxel) as a marker of the first-line chemotherapy since these agents are routinely used for postoperative chemotherapy for mullerian cancers. The second group (secondary treatment group) included the remaining nine agents. Dual-resistance was then defined as at least one EDR in the primary treatment group and at least one EDR in the secondary treatment group. Non-resistance was defined as absence of EDR in both groups.
Table 1.
Schema of dual-resistance model
| Primary Treatment Group (4 drugs)+ | Secondary Treatment group (9 drugs)++ | ||||
|---|---|---|---|---|---|
| Platinum | Taxane | Gemcitabine | Topotecan | Doxil | |
| Dual-resistance | EDR | LDR | EDR | IDR | LDR |
| Non-resistance | IDR | LDR | IDR | IDR | LDR |
Dual-resistance was defined as the presence of EDR in both groups. Non-resistance was defined as absence of EDR in both groups.
First group included cisplatin, carboplatin, paclitaxel, and docetaxel.
Second group included cyclophosphamide, doxil, doxorubicin, etoposide, fluorouracil, gemcitabine, gemcitabin with cisplatin, ifosamide, and topotecan (schematically 2 and 3 drugs are shown in each group, respectively).
Abbreviations: EDR, extreme drug resistance; IDR, intermediate drug resistance; and LDR, low drug resistance.
Optimal cytoreductive surgery was defined as no residual tumor measuring greater than 1 cm in maximal dimension at the end of the surgical procedure. Date of progression was determined by clinical examination, computed tomography (CT) imaging, and/or CA-125 levels. Platinum resistance was defined as the presence of recurrence within six months from the date of last chemotherapy. Response to chemotherapy was defined by the new Response Evaluation Criteria in Solid Tumours (RECIST) guidelines [6]. Progression-free survival (PFS) was defined as the time interval from the date of initial cytoreductive surgery to the date of documented first recurrence or progression of disease. If there was no recurrence, PFS was determined as the date of last follow-up. Overall survival (OS) was defined as the interval between the initial surgery and the date of death or last follow-up visit.
Continuous variables were assessed for normal distribution by utilizing the Kolmogorov-Smirnov test and expressed either by mean (±SD) or median (range) as appropriate. Categorical variables were evaluated with Fisher’s exact test with odds ratio and 95%CI. Univariate analysis with liner regression test was used to assess all the corrected variables and primary outcome (PFS and OS). For the significant variables in univariate analysis, multivariate analysis with Cox log rank analysis was further performed to determine the difference in survival. Kaplan-Meier analysis was used to estimate survival curves. All statistical tests were two-tailed, and p-values of less than 0.05 were considered statistically significant. The statistical significance of the data was determined by using the Statistical Package for Social Scientists software (SPSS, Inc., version 12.0, Chicago, IL). The study protocol was approved by the Institutional Review Board (IRB) at Mercy Medical Center, Baltimore, Maryland.
RESULTS
There were 265 cases of FIGO Stage III and IV, epithelial ovarian, fallopian, and primary peritoneal cancers identified in the study period. Of those, there were 12 (4.5%) cases that could not be assessed by the in-vitro drug resistance assay. The remaining 253 cases constituted the study set. Patient demographics are shown in Table 2. Mean age was 61.8 ± 11.4. Majority of patients had FIGO stage IIIC (83.4%), serous histology (81.4%), and high grade tumor (76.7%). Most (82.2%) women completed ≥ 4 courses of combination chemotherapy with platinum and taxane after the initial cytoreductive surgery. Of those, 81.0% showed a response to the initial chemotherapy. 77.4% had progression of disease with a median follow-up of 11.4 months. In addition, with a median follow-up of 22.1 months, 50.6% of patients were deceased.
Table 2.
Patient demographics
| Cases | PFS | OS | |
|---|---|---|---|
| Subjects | n=253 | ||
| Age | 61.8 ± 11.4 | 0.002 | 0.001 |
|
| |||
| Type of cancer | |||
| Ovarian cancer | 224 (88.5%) | 0.45 | 0.42 |
| Primary peritoneal cancer | 24 (9.5%) | ||
| Fallopian cancer | 5 (2.0%) | ||
|
| |||
| Lymph nodes metastasis | 99 (39.1%) | 0.54 | 0.57 |
| Lympho-vascular invasion | 92 (36.4%) | 0.006 | 0.001 |
|
| |||
| FIGO Stage | 0.09 | 0.08 | |
| IIIA and IIIB | 7 (2.8%) | ||
| IIIC | 211 (83.4%) | ||
| IV | 35 (13.8%) | ||
|
| |||
| Histologic type | 0.4 | 0.46 | |
| Serous | 206 (81.4%) | ||
| Endometrioid | 9 (3.6%) | ||
| Mucinous | 5 (2.0%) | ||
| Undifferentiated | 5 (2.0%) | ||
| Clear cell | 5 (2.0%) | ||
| Mixed | 21 (8.3%) | ||
| Other | 2 (0.8%) | ||
|
| |||
| Tumor size | 8.5 cm (1.2–24.5) | 0.11 | 0.33 |
| High grade tumor | 194 (76.7%) | 0.72 | 0.12 |
| Preoperative CA-125 | 677 IU/L (8–5620) | 0.006 | 0.03 |
|
| |||
| Optimal surgery | 105 (41.5%) | <0.001 | <0.001 |
| Bowel resection | 88 (34.8%) | 0.023 | 0.017 |
|
| |||
| Type of chemotherapy | |||
| Carboplatin + Paclitaxel | 119 (52.4%) | 0.002 | <0.001 |
| Carboplatin + Docetaxel | 72 (31.7%) | ||
| Cisplatin + Paclitaxel | 34 (15.0%) | ||
| Carboplatin + Cyclophosphamide | 2 (0.9%) | ||
| Number of cycles† | 6 (0–12) | <0.001 | <0.001 |
| Chemotherapy response* | 184 (81.0%) | <0.001 | <0.001 |
|
| |||
| Site of recurrence | 196 (77.4%) | ||
| Extrapelvis | 116 (68.2%) | ||
| Pelvis | 31 (18.2%) | ||
| Chest | 14 (8.2%) | ||
| Other | 9 (5.3%) | ||
Univariate analysis was performed between each variable and survival outcomes (PFS or OS) (P-value is shown). Comparisons of variables were performed for: ovarian vs primary peritoneal vs fallopian cancer for type of cancer; Stage III vs IV for FIGO Stage; serous vs non-serous for histology; and carboplatin + paclitaxel vs carboplatin + docetaxel vs cisplatin + paclitaxel vs carboplatin + cyclophosphamide. Crude tumor size and preoperative CA-125 were compared to survival.
227 cases with available response.
17 cases, no chemotherapy.
Abbreviations: PFS, progression-free survival; and OS, overall survival.
Results of in-vitro drug resistance assay are shown in Table 3. Among tumors tested for drug resistance against platinum and taxane, paclitaxel showed a significantly higher EDR rate (all, p<0.05). Carboplatin, cisplatin, and gemcitabine showed an EDR proportion of 10% or lower. Additionally, paclitaxel, doxil, doxorubicin, etoposide, fluorouracil, and ifosfamide showed an EDR proportion of 20% or higher. There was only one (0.4%) tumor with complete EDR to all tested drugs.
Table 3.
In-vitro drug assay results
| Carboplatin | Cisplatin | Paclitaxel* | Docetaxel | |
|---|---|---|---|---|
| EDR | 7.9 | 6.9 | 23.2 | 13.6 |
| IDR | 25.7 | 20.7 | 31.4 | 36.7 |
| LDR | 66.4 | 72.3 | 45.3 | 49.7 |
| Cyclophos | Doxil | Doxorubicin | Etoposide | |
|---|---|---|---|---|
| EDR | 18.5 | 32.4 | 44.6 | 31.9 |
| IDR | 30.3 | 36.4 | 24.6 | 38 |
| LDR | 51.3 | 31.3 | 30.8 | 30.1 |
| Fluorouracil | Gemcitabine | Ifosamide | Topotecan | |
|---|---|---|---|---|
| EDR | 25.3 | 7.9 | 22.4 | 17.2 |
| IDR | 28 | 10.9 | 20.9 | 32.5 |
| LDR | 46.7 | 81.2 | 56.7 | 50.2 |
Percentage is shown.
p<0.05 (Paclitaxel EDR vs platinum and docetaxel EDR).
Abbreviations: cyclophos, cyclophosphamide; EDR, extreme drug resistance; IDR, intermittent drug resistance; and LDR, low drug resistance.
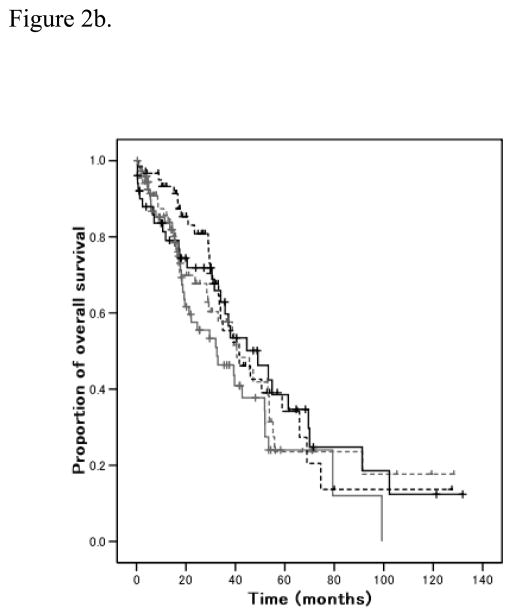
Total number of drugs in EDR range for each patient was evaluated. Over one fourth of patients showed no EDR to tested drugs, which was the most common subpopulation (n=74, 29.2%). Distribution of the total number of individual EDR was skewed right (p<0.001; Figure 1a). Total number of individual EDR was not different between tissue from ovarian and non-ovarian sites (p=0.13), and histological subtypes (p=0.69). There was no statistical correlation between the response to initial chemotherapy and the total number of agents in the EDR range (p=0.55) (Table 4). Univariate analysis showed no statistical significance between total number of EDR chemotherapy agents and PFS (R2 0.007, p=0.18) (Figure 2a). Although total number of EDR drugs and OS showed significance in univariate analysis (R2 0.02, p=0.024), the difference did not remain significant (p=0.87) after adjusting for other significant variables (Figure 2b).
Figure 1.
Figure 1a. Number of extreme drug resistance
Y-axis represents the number of patients with 0–6 individual EDR. Inversed distribution of EDR is shown (p<0.001). EDR 0 was the majority of subgroups (n=74, 29.2%).
Figure 1b. Proportion of extreme drug resistance
Inversed distribution of EDR is shown (p<0.001). Proportions of EDR at 0%, 20%, 40%, and 60% were 29.2%, 16.6%, 5.1%, and 2.4%, respectively.
Table 4.
Number of extreme drug resistance and chemotherapy response
| No. of EDR | Cases | Response |
|---|---|---|
| 0 | 63 (28.5%) | 80.9% |
| 1 | 60 (27.1%) | 80.0% |
| 2 | 40 (18.1%) | 82.5% |
| 3 | 35 (15.8%) | 88.6% |
| 4 | 13 (5.9%) | 69.2% |
| 5 | 7 (3.2%) | 85.7% |
| 6 | 3 (1.4%) | 100% |
Among the 227 patients who received postoperative chemotherapy treatment, 225 patients received combination chemotherapy with platinum and taxane. Four patients developed allergic reaction to taxane and in total 221 patients were available for chemotherapy response. Fisher’s exact test. P=0.76.
Figure 2.
Figure 2a. Progression-free survival and number of EDR
Univariate analysis (p=0.18). Gray, number of EDR 0; gray dash, EDR 1; black, EDR 2; and black dash, EDR 3–6.
Figure 2b. Overall survival and number of EDR
Cox log rank analysis (p=0.87). Gray, number of EDR 0; gray dash, EDR 1; black, EDR 2; and black dash, EDR 3–6.
The proportion of drug resistance per tumor was evaluated. Proportion of EDR was similar to the distribution of total number of individual EDR (Figure 1b). There was no statistical significance between proportion of EDR and chemotherapy response (R2 0.003, p=0.37), PFS (R2 0.009, p=0.13), or OS (adjusted, p=0.13).
Next, a dual-resistance model was evaluated using crude in-vitro drug resistance assay results (Table 1). In this model, dual-resistance was observed in 53 (20.9%) cases. Of those, cases with EDR to both platinum and taxane as well as presence of at least one EDR among the second group of remaining nine agents was seen in 9 (3.6%) cases. Presence of EDR in only one of the two groups was seen in 126 (49.8%) cases. Non-resistance was seen in 74 (29.2%) cases. Presence of EDR to platinum and/or taxane was not associated with chemotherapy response or survival outcomes. All tested variables including chemotherapy response were similar among the two groups (78% and 81% response rates in the dual-, and non-resistance groups, respectively, p=0.62). Chemotherapy response was also similar (84.3%) when EDR was noted in only one of the two groups. Similarly, survival outcome showed no statistical difference among the three groups: PFS, R2 0.002, p=0.52 (median PFS, 13.0, 12.8, and 12.2 months, respectively); and OS, R2 0.01, p=0.11 (5-year OS ratio, 37.8%, 31.3%, and 38.9%, respectively).
DISCUSSION
The key findings from our study are that presence of extreme drug resistance to multiple agents in the in-vitro drug resistance assays was not associated with survival outcomes in advanced stage epithelial ovarian, fallopian, and primary peritoneal cancers. This information is valuable with respect to the feedback given to the patient during counseling when the assay results reveal dual-resistance.
Due to the reduced rate of drug metabolism, in-vitro tumor exposure to chemotherapy agents is 5 to 80 times greater than in-vivo [5]. Thus, presence of EDR means that tumor growth is significantly increased even under such a high drug concentration. Nevertheless, extent of EDR and survival outcome was not correlated. Various explanations could be raised. In-vitro drug resistance assays most likely reflect intrinsic drug resistance at the time of initial diagnosis. Generally, after initial cytoreduction, most ovarian cancer patients undergo treatment with multiple chemotherapy agents, which possibly alters drug resistance profiles (acquired resistance). For example, paclitaxel resistance seems more common after previous exposure to paclitaxel [7,8]. Thus, assay results from initial surgery may not fit the in-vitro drug resistance results in the setting of recurrent cancer. Drug resistance profiles of tumors seem to change in various circumstances such as prior chemotherapy exposure, radiation, or surgery. Obtaining tumor tissue may be required every time a selective choice of chemotherapeutic agents is considered for the patient.
Host factors such as vascularization of the tumor, expression of enzymes that detoxify the drug, and protein binding in the circulation, may also interfere with response under in-vitro circumstance. Tumor hetererogeneity of drug resistance in primary and metastatic sites could also explain our results. In a previous study of human ovarian and breast cancer, there was discordance in drug resistance levels between primary and metastatic sites in synchronous tumor (cisplatin 4%, paclitaxel 13%, and doxorubicin 13%, respectively) [9]. The profile of in-vitro drug resistance in our study, thus, may differ between the tumor tissue from ovarian and non-ovarian sites, which could mislead the assay results. However, our results demonstrated no obvious differences in assay results between ovarian and non-ovarian sites. Furthermore, in a larger study of in-vitro drug resistance assays, in-vitro drug resistance results were similar in primary and metastatic sites among synchronous tumors [10].
There are two types of in-vitro testing, chemoresistance assays and chemosensitivity assays [11]. Chemoresistance assays and chemosensitivity assays are not equivalent tests. The former represents tumor cell growth inhibition while the latter represents cell-death via drug induced apoptosis [11]. Chemoresistance assays are used to exclude agents that are less likely to be effective rather than to select an agent that would result in clinical response [12]. Compared to chemoresistance assays, chemosensitivity offers relatively low positive predictive accuracy and no survival advantage has been shown [13,14]. One concern regarding these previous in-vitro drug testing assays is that the sample size was too small to identify statistical differences in chemotherapy response or survival outcome [4]. In our previous estimation, at least 68 subjects exhibiting EDR are needed to have adequate power for assessing chemotherapy response in postoperative combination chemotherapy with platinum and taxane for ovarian cancer patients [4]. However, as shown in Table 3, the proportion of EDR to platinum is only less than 10%, and at least 680 subjects may be needed to obtain an adequate sample size. For such a large sample size, a multicenter study would be required to definitively address some of the controversies surrounding in-vitro drug testing.
A strength of our study is that we first evaluated dual-resistance in in-vitro drug resistance assays in advanced mullerian cancers. However, some limitations must also be considered. The retrospective nature of the study may miss accounting for some confounding factors. In addition, correlating in-vitro drug sensitivity or resistance testing to survival is a very difficult process. Many patients will receive more than one regimen of chemotherapy throughout their treatment course. In our study, we defined dual-resistance as presence of at least one EDR in the primary and secondary treatment groups. Additional studies with larger numbers of cases may be instructive in evaluating the significance of dual-resistance in clinical settings.
In summary, our study demonstrates that dual-resistance in in-vitro drug resistance assays was not associated with response to initial chemotherapy and survival outcomes in advanced epithelial ovarian, fallopian, and peritoneal cancers. Although correlating in-vitro and in-vivo drug resistance can be difficult, this interesting topic of drug resistance merits further investigation.
Acknowledgments
Authors thank William A. Ricketts, Ph.D., Exiqon, Inc. for technical advice in the discussion.
References
- 1.Samson DJ, Seidenfeld J, Ziegler K, Aronson N. Chemotherapy sensitivity and resistance assays: a systematic review. J Clin Oncol. 2004;22:3618–30. doi: 10.1200/JCO.2004.04.077. [DOI] [PubMed] [Google Scholar]
- 2.Kern DH, Weisenthal LM. Highly specific prediction of antineoplastic drug resistance with an in vitro assay using suprapharmacologic drug exposures. J Natl Cancer Inst. 1990;82:582–8. doi: 10.1093/jnci/82.7.582. [DOI] [PubMed] [Google Scholar]
- 3.Holloway RW, Mehta RS, Finkler NJ, Li KT, McLaren CE, Parker RJ, Fruehauf JP. Association between in vitro platinum resistance in the EDR assay and clinical outcomes for ovarian cancer patients. Gynecol Oncol. 2002;87:8–16. doi: 10.1006/gyno.2002.6797. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo K, Bond VK, Eno ML, Im DD, Rosenshein NB. Low drug resistance to both platinum and taxane chemotherapy on an in-vitro drug resistance assay predicts improved survival in patients with advanced epithelial ovarian, fallopian, and peritoneal cancer. Int J Cancer. 2009 Jun 15; doi: 10.1002/ijc.24654. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Oncotech. [as of May 6, 2009];EDR Assay®. http://www.exiqon.com/dxps/Documents/edr_4_pager.pdf.
- 6.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 7.McAlpine JN, Eisenkop SM, Spirtos NM. Tumor heterogeneity in ovarian cancer as demonstrated by in vitro chemoresistance assays. Gynecol Oncol. 2008;110:360–4. doi: 10.1016/j.ygyno.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo K, Eno ML, Im DD, Rosenshein NB. Chemotherapy time interval and development of platinum and taxane resistance in ovarian, fallopian, and peritoneal carcinomas. Arch Gynecol Obstet. 2009 doi: 10.1007/s00404-009-1121-1. Epub Ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Kern DH. Heterogeneity of drug resistance in human breast and ovarian cancers. Cancer J Sci Am. 1998;4:41–5. [PubMed] [Google Scholar]
- 10.Tewari KS, Mehta RS, Burger RA, Yu IR, Kyshtoobayeva AS, Monk BJ, Manetta A, Berman ML, Disaia PJ, Fruehauf JP. Conservation of in vitro drug resistance patterns in epithelial ovarian carcinoma. Gynecol Oncol. 2005;98:360–8. doi: 10.1016/j.ygyno.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Samson DJ, Seidenfeld J, Ziegler K, Aronson N. Chemotherapy sensitivity and resistance assays: a systematic review. J Clin Oncol. 2004;22:3618–30. doi: 10.1200/JCO.2004.04.077. [DOI] [PubMed] [Google Scholar]
- 12.Loizzi V, Chan JK, Osann K, Cappuccini F, DiSaia PJ, Berman ML. Survival outcomes in patients with recurrent ovarian cancer who were treated with chemoresistance assay-guided chemotherapy. Am J Obstet Gynecol. 2003;189:1301–7. doi: 10.1067/s0002-9378(03)00629-x. [DOI] [PubMed] [Google Scholar]
- 13.Eltabbakh GH, Piver MS, Hempling RE, Recio FO, Lele SB, Marchetti DL, Baker TR, Blumenson LE. Correlation between extreme drug resistance assay and response to primary paclitaxel and cisplatin in patients with epithelial ovarian cancer. Gynecol Oncol. 1998;70:392–7. doi: 10.1006/gyno.1998.5109. [DOI] [PubMed] [Google Scholar]
- 14.Mäenpää JU, Heinonen E, Hinkka SM, Karnani P, Klemi PJ, Korpijaakko TA, Kuoppala TA, Laine AM, Lähde MA, Nuoranne EK, et al. The subrenal capsule assay in selecting chemotherapy for ovarian cancer: a prospective randomized trial. Gynecol Oncol. 1995;57:294–8. doi: 10.1006/gyno.1995.1145. [DOI] [PubMed] [Google Scholar]