Abstract
Terpenoids constitute a significant fraction of molecules produced by living organisms that have found use in medicine and other industries. Problems associated with their procurement and adaptation for human use can be solved using chemical synthesis, which is an increasingly economical option in the modern era of chemistry. This article documents, by way of individual case studies, strategies for reducing the time and cost of terpene synthesis for drug discovery. A major trend evident in recent syntheses is that complex terpenes are increasingly realistic starting points for both medicinal chemistry campaigns and large-scale syntheses, at least in the context of the academic laboratory, and this trend will likely penetrate the commercial sector in the near future.
Terpenes (or terpenoids or isoprenoids) constitute a major class of organic molecules produced by diverse organisms to perform an assortment of biological functions in varying ecological contexts. Although all terpenes originate from the same five-carbon building blocks (dimethylallyl pyrophosphate and isopentenyl pyrophosphate), the structures and functions of terpenes vary widely, and are highly tailored to the requirements of the source organism’s environmental pressures and resources. As a consequence of the biological functions of terpenes (in humans and other organisms), these molecules have led to six major drug classes over the last century, namely steroids, tocopherols, taxanes, artemisinins, ingenanes and cannabinoids [1]. However, optimization of terpene structures for human use requires economical access to not only the carbon scaffold of the terpene class, but also embedded functional groups that allow specific modification of the molecule through a rational medicinal chemistry campaign. Whether a campaign starts from a complex isolate with an essentially intact scaffold (sometimes called semi-synthesis) or several smaller fragments (sometimes called total synthesis, vide infra), this exploration can be costly and lengthy. In this article, we document several strategies to reduce the cost and time of developing terpene-based therapeutics.
Identifying the problem
The biological role and medical use of terpenes must inform how the problem of chemical synthesis is addressed. Bioactive terpenes are isolated frequently; medically relevant terpenes less so, and distinguishing the latter is not always straightforward prior to clinical validation. One benefit of the isolation literature is the frequent use of phenotypic assays, rather than target-based assays, a preference that is receiving increased interest in the pharmaceuticals industry [2] as it provides important information about potency and selectivity in a cell, rather than on a single macromolecule. The obvious downside is that observation of phenotypic response leaves open the question of the biomolecular basis for that response. Thus, use of a phenotypic versus target-based assay neatly divides medicinally relevant terpenes into two subclasses depending on whether the mechanism of action is known or unknown.
Approximately 35,000 terpenes have been identified and the majority of possible functions of these molecules are unknown [1]. Consequently, synthesis efforts in this ‘naive’ phase of terpene research focus on procuring relatively small amounts of material or affinity-labeled material for target identification. That being said, a disproportionate number of terpenes receive attention in the synthesis literature because they possess known functions of medical importance, which leads to a focus on either scalability of synthesis, or exploration of analogous structures. A chemical synthesis should serve to address real problems associated with the target structure, which are largely dependent on knowledge of the molecule’s mechanism.
So what constitutes the best approach to small molecule synthesis [3–5]? The answer is simple: it’s complicated. For example, a coarse articulation of the ideal synthesis is one that creates homogeneous material in large scale at low cost, which includes low cost of labor, reactants and reagents, solvents, purification and waste disposal. However, even this rudimentary definition does not always hold: what if you do not know the small molecule structure in advance? If not, then these metrics of synthesis disintegrate. In medicinal chemistry, a synthetic route must be diversifiable and modular, and scalability is not a major issue or even a goal. Nor is yield or purification, which can often be performed rapidly on small scale by HPLC. Similarly, metrics that articulate the need for chemical diversity measure diversity only, even though medicinal chemistry is not a random walk into unknown regions of space, but rather a logical exploration of the relationship between structure and function (SAR) [6]. All this is to say that for a given molecule, there can be various goals of synthesis – access to bulk material (process), specific modification of structure (optimization of properties, e.g., medicinal chemistry) or diversification of structure (e.g., combinatorial chemistry) – all of which possess competing agendas rather than a grand, unified vision. This means that a synthesis must be tailored to the problem it is trying to solve, and therefore identification of the actual problem is crucial.
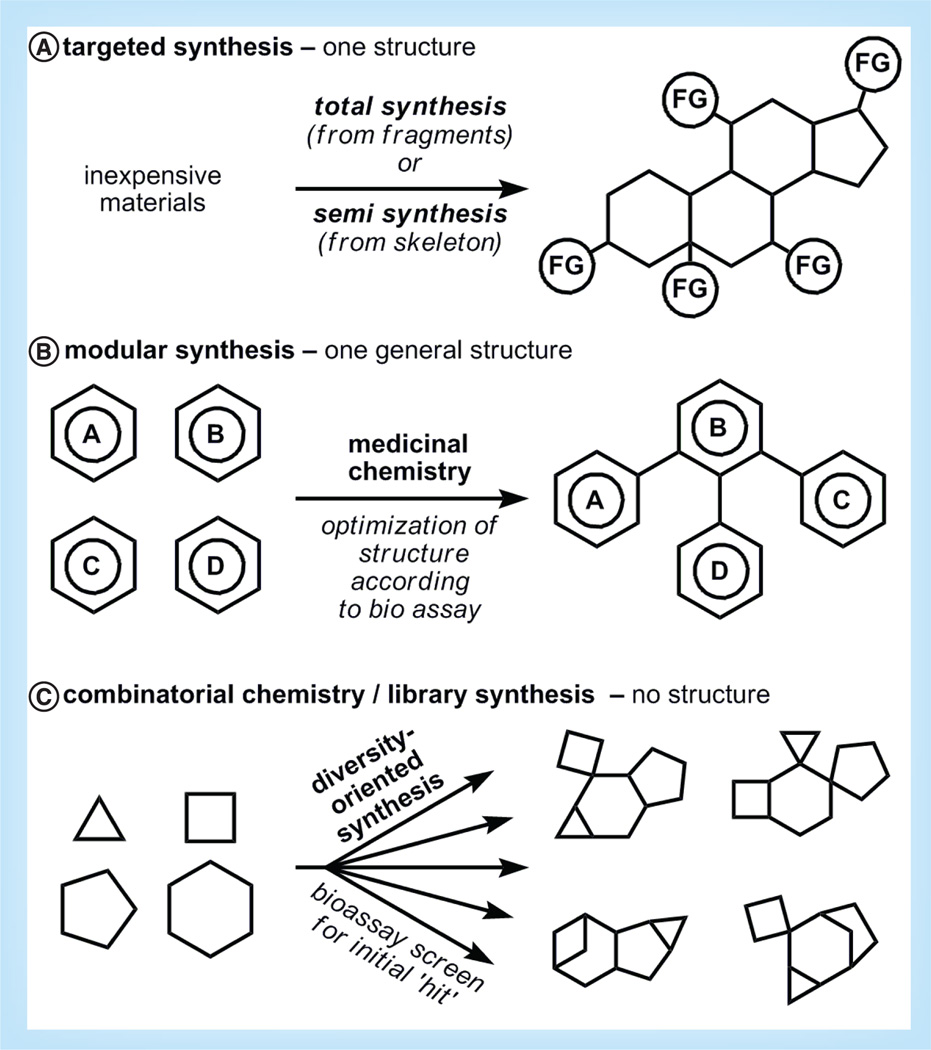
The one unifying feature between the three synthesis goals above (process, optimization and diversification; Figure 1) is the primacy of step count economy [5], which has the potential to minimize costs at multiple levels. Sometimes other features such as modularity requirements or purification problems override the benefits of a short step-count. However, in general, a shorter synthesis is a better synthesis, and therefore in this article we will highlight academic syntheses from the last 5 years where step counts are minimized relative to either prior syntheses or syntheses of similarly complex molecules. Nevertheless, to truly evaluate the quality of a synthesis, it is essential to identify its purpose, (i.e., the problem it is attempting to solve).
Figure 1. Various goals of chemical synthesis.
(A) Targeted synthesis: a single structure is desired. Accessed through ‘total’ or ‘semi’-synthesis.
(B) Modular synthesis: a general structure or scaffold is desired. Accessed by assembling easily diversifiable building blocks. Further optimization of structure is guided by functional assay results. (C) Combinatorial chemistry/library synthesis: no well-defined starting or ending structure. Simple building blocks are assembled in large number. Bioassays are used to determine which compounds provide an initial hit and will be explored further.
FG: Functional group.
We should briefly note that a decreasing number of syntheses are undertaken just to show how a biologically produced molecule (natural product) might be synthesized in the laboratory by chemical means. This is primarily due to funding pressures, despite the inherent value of this pure science endeavor. The justifications for these syntheses are not necessarily biological/medicinal in nature, but are structural/chemical, and often result in innovations in reaction invention or strategies to access a general structure. This is certainly not to say that all complex molecule syntheses are justified or contribute to progress in chemistry. But rather, the decline of exercises in the pure science of synthesis as ends in themselves will likely parallel a decline in organic chemistry as a whole, and negatively impact the development of new drugs.
Below, we analyze several recent examples of terpene syntheses from the academic literature, illustrate how they solve problems associated with the target molecule, and in some cases discuss what problems remain to be solved. The syntheses of these terpenes are grouped according to the primary ‘higher-level strategy’ employed, although in most cases the syntheses utilize multiple strategies. Along the way, we discuss changes in focus that have accrued over time within the synthesis field, and attempt to project these trends into the future. One major trend evident in these recent efforts is that complex terpenes are increasingly realistic starting points for both medicinal chemistry campaigns and large-scale syntheses, at least in the context of the academic laboratory, and this trend will likely penetrate the commercial sector in the near future. Imitation of these syntheses’ strengths and repudiation of their weaknesses will, in a general way, reduce the cost of developing terpene-based therapeutics.
Stereochemistry-based strategies: the power of substrate relay
One defining feature of terpenoid metabolites is their stereochemical complexity. In order to arrive at an efficient synthesis, the number of stereogenic steps must be minimized, and the level of stereocontrol at each of these steps must be maximized. A logical way to achieve these goals is to prioritize retrosynthetic transforms that efficiently set multiple stereocenters at once (see also ‘Pumilaside aglycon’ section). Furthermore, stereochemical relay from the substrate can be an effective tool to rapidly increase complexity, as long as the appropriate choreography of bond disconnections can be determined. Below, we document several recent examples of terpenoid synthesis and we focus on the stereochemical decisions made during retrosynthetic analysis. In many cases, the actual stereocontrol achieved is not perfect, even though the logic is good and reduces the length of the route. The lessons learned from these syntheses should inform future work in the same or related molecules.
Englerin A
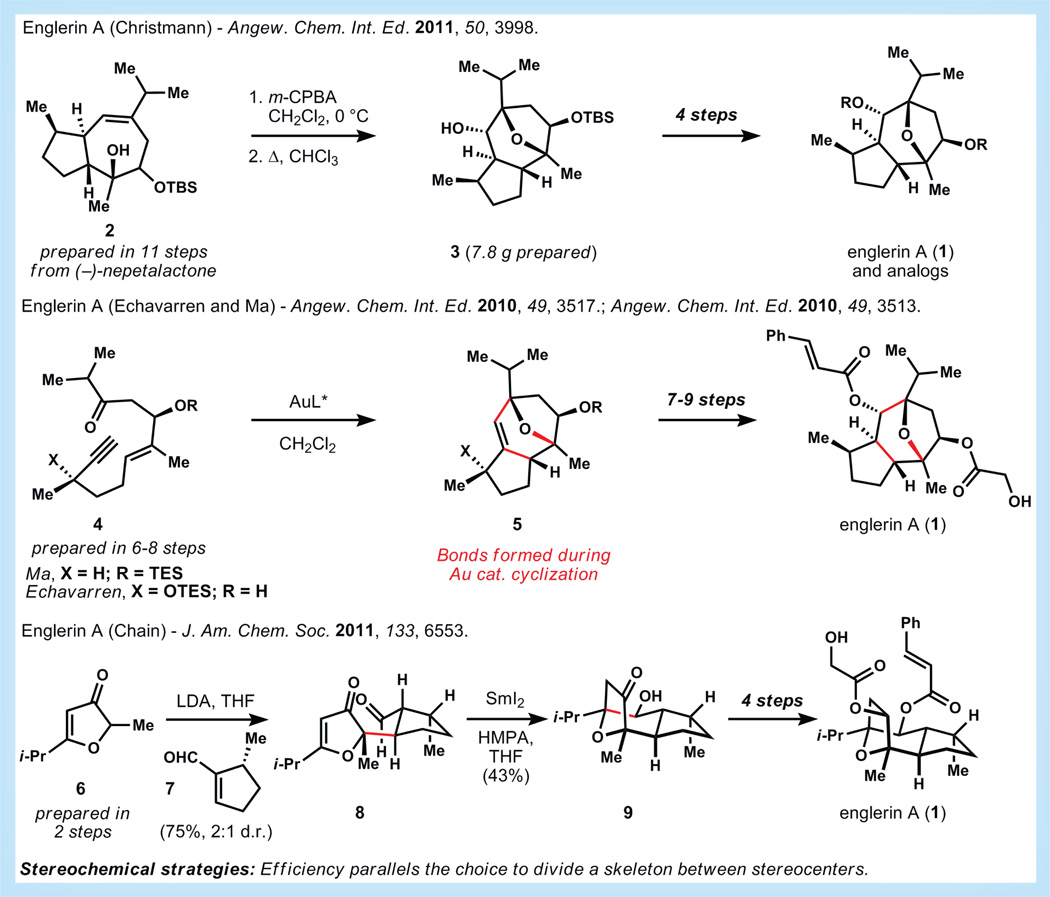
It is estimated that roughly 65,000 people were affected by renal cancer in 2013 and a further 13,500 would die because of it [7]. Englerin A (1, Figure 2), a guaiane sesquiterpene, was isolated in 2008 from the extract of Phyllanthusengleri as a result of a positive hit in an National Cancer Institute 60-cell screen of natural product extracts that exhibit preferential selectivity for renal tumor cells [8]. Indeed, against several renal cancer cells lines (1) possesses nanomolar potency and in many cases, it was more potent than paclitaxel. Recent work has shown that englerin A activates PKCθ in renal cancer cells, which appears to have a two-pronged effect [9]. First, activation of PKCθ causes phosphorylation of IRS-1 and decreases insulin receptor-mediated glucose uptake. Second, PKCθ activation causes production of HSF1, which enhances the glucose dependence of the cells. Since englerin A decreases cellular glucose uptake while increasing cellular glucose dependence, the cancer cell is starved of energy. With a possible mechanism of action in hand, the goal for chemists should be to increase access to (1) within the cancer community, synthesize derivatives and develop SARs, all of which can be facilitated by an efficient synthesis.
Figure 2. Syntheses of englerin A by Christmann [11], Echavarren [13], Ma [14], and Chain [15].
cat.: Catalytic; d.r.: Diastereomeric ratio; HMPA: Hexamethylphosphoramide; LDA: Lithium diisopropylamide; m-CPBA: meta-Chloroperoxybenzoic acid; THF: Tetrahydrofuran.
As of this publication, there have been 13 syntheses of (−)-englerin A or its derivatives. Christmann and co-workers recorded the first synthesis and confirmed the absolute configuration of ent-(+)-englerin A in 2009. These authors published a follow-up synthesis of the natural enantiomer along with several analogs in 2011 [10,11]. Bicycle 2 is synthesized in 11 steps from (−)-nepetalactone and can be engaged in a transannular epoxide opening to generate the bridging ether with high regioselectivity. Christmann and co-workers prepared over 7 g of intermediate 3 and were able to make dozens of analogs, several of which had greater potency than englerin A itself.
In 2006, Echavarren and co-workers developed a novel gold (I)-catalyzed Prins cyclization cascade to synthesize (+)-orientalol F and other molecules that possess essentially the same tricyclic core as englerin A [12]. In 2010, both Echavarren and Ma recognized this relationship, targeted a similar core substructure, and independently published syntheses using similar synthetic routes (see 4→5) [13,14]. From the cascade cyclization product 5, several functional group interconversions are necessary to then elaborate this core substructure to englerin A (1).
But perhaps the most elegant and efficient synthesis to date was accomplished by Chain and co-workers in 2011 [15]. Using simple carbonyl chemistry, these authors synthesize englerin A in only eight linear steps. Realization that the esters of the cyclic core are nascent ketones in a 1,4 and 1,5 relationship prompts Chain to disconnect the tricyclic core by a Michael addition and an umpolung carbonyl-alkene radical cyclization. These disconnections are highly simplifying because they divide the molecule into two simple cyclopentenes, and the two targeted bonds lie between vicinal stereocenters, thus removing four chiral centers in just two steps. The logic behind this strategy is both powerful and general.
Chain executes his strategy by first reacting the enolate of 6 with citronellal-derived enal 7. A modest 2:1 ratio of 8 to all other isomers is obtained, but this mixture can be subjected to samarium (II) iodide in hexamethylphosphoramide to effect carbonyl-alkene cyclization in 43% yield (maximum 66% yield based on 2:1 diastereomeric mixture of starting material). Keto-alcohol 9 is then elaborated to englerin A in four steps, completing a synthesis of only eight steps from commercial materials in a remarkable 20% overall yield.
Artemisinin
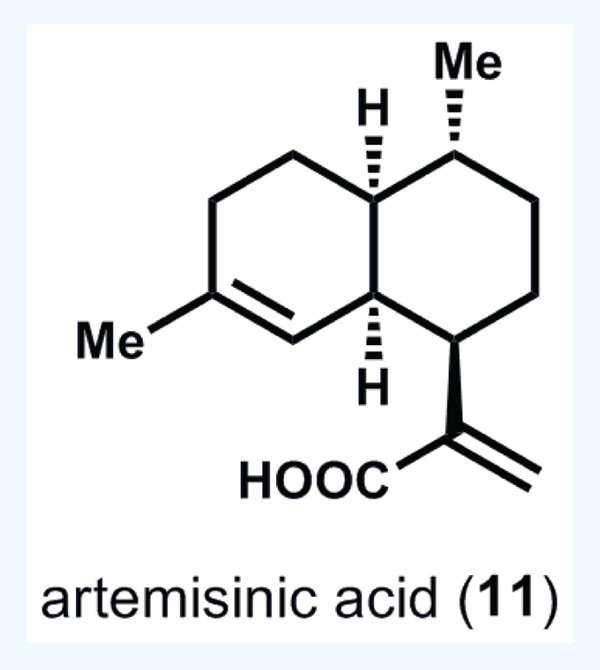
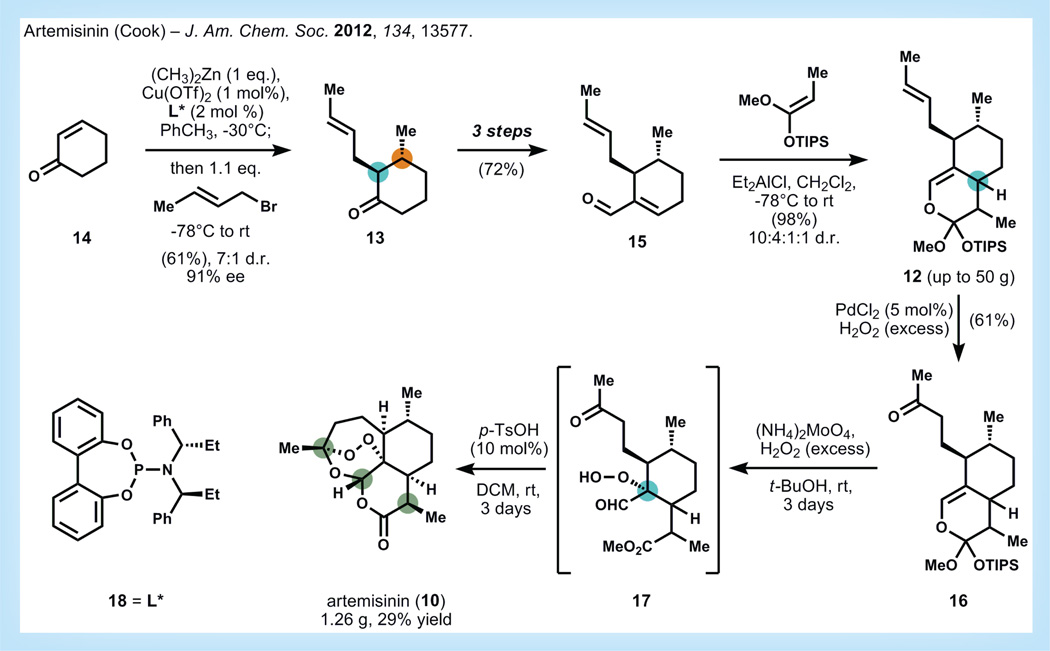
One of the more well-known and clinically validated terpenes for the treatment of an infectious disease is artemisinin (10, Figure 3), a plant-derived antimalarial that exhibits rapid clearance rates of Plasmodium parasites from infected patients [16]. Since malaria affects 300–500 million people per year, primarily in low-income countries, there is an ongoing search for means to provide bulk material at very low cost. Extraction of 10 from Artemesia annua is a time-tested successful strategy for procuring bulk material, but the low yield of this process and the vagaries of crop production have not allowed extraction to keep pace with demand [17]. Efforts in selective breeding of higher yielding Artemesia plants have not proven successful, and conversion of the more abundant metabolite artemisinic acid (11, see Figure 4) to 10 generally suffers from poor yields [18]. The combined approach of engineering the plant biosynthetic pathway of 11 into Saccharomyces cerevisiae followed by semi-synthetic conversion to 10 has been pioneered by the Keasling group, and may serve to stabilize the market price of artemisinin [19]. However, the ultimate cost of production using this route is still an open question, although early estimates are promising with Sanofi (Paris, France) reporting artemisinin production in 370 kg batches (60 tons estimated for 2014) [20].
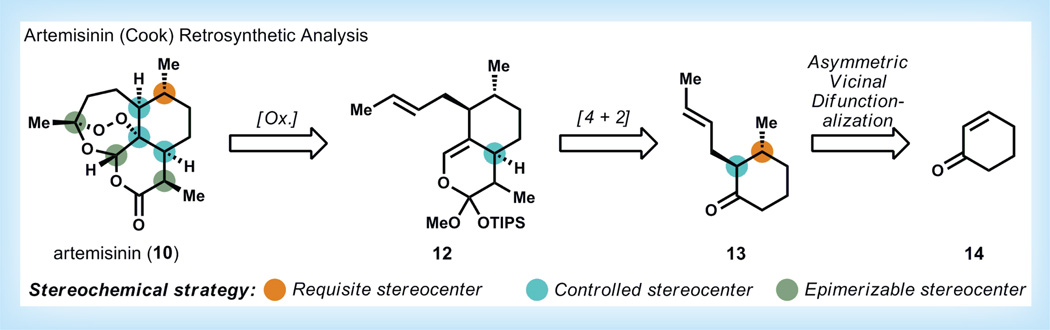
Figure 3. Retrosynthetic analysis of artemisinin [21].
OTIPS: Triisopropylsiloxy; Ox: Retrosynthetic oxidation transform.
Figure 4. Artemisinic acid.
Chemical synthesis may prove an effective strategy to provide 10 in large quantity and at low cost. A general ‘blueprint’ of a potential route to produce 10 from inexpensive building blocks was recently developed by the Cook laboratory at Indiana University (IN, USA) [21]. Based on knowledge gleaned from the semi-synthetic conversion of 11 to 10, the Cook group realized that three stereocenters (in green) of the stereochemically complex artemisinin core could be immediately cleared to achiral or racemic precursor carbons (Figure 3). Therefore, the only stereocenter necessary to control in 10 is the tert-alkyl endoperoxide (in blue), which would be favored based on the concavity of octalin 12. This concavity imparted by the ring-fused carbon of 12 (in blue) could be generated by a stereocontrolled annulation of cyclohexanone 13, which in turn might be formed by vicinal difunctionalization of the chemical feedstock, 2-cyclohexenone (14). Thus, seven stereocenters of artemisinin 10 could be relayed from a single stereocenter derived from asymmetric conjugate addition into 14.
Utilization of the Cook group’s previous investigations into asymmetric vicinal difunctionalization reactions enables the synthesis of 13 in 61% yield and in very good diastereo- and enantio-selectivity (Figure 5) [22,23]. Ketone 13 is then elaborated in three steps to enal 15, which is engaged in a [4+2] ring annulation with a silyl ketene acetal to set an additional nonepimerizable stereocenter. After experimentation with several Lewis acids, dialkylaluminum chlorides were found to promote formation of adduct 12 in 95% yield and in preference to other side reactions (aldol, Michael addition, [2+2]). It is noteworthy that this reaction can be scaled to produce large quantities (up to 50 g) of 12, albeit as a 10:4:1:1 mixture of diastereomers. The authors suggest that the targeted diastereomer 12 is the major component of this mixture, but a rigorous assignment of stereoisomers has not been published. A subsequent Wacker oxidation of 12 with catalytic palladium chloride and aqueous hydrogen peroxide affords ketone 16 as a mixture of diastereomers in 61% yield. To convert 16 to artemisinin, various oxidations were attempted but proved fruitless. Eventually, it was found that generation of singlet oxygen with a molybdate catalyst and hydrogen peroxide allows for the selective oxidation of the enol ether olefin. Previous studies on the conversion of dihydroartemisinic acid to artemisinin have found that the efficiency of peroxidation is dependent on the ability to populate similar enol tautomers, so intermediate 12 may be useful for follow-up study [24]. Treatment of the mixture of oxidized products (17) with mild acid provides artemisinin (up to 1.26 g) in 29–42% yield after crystallization.
Figure 5. Synthesis of artemisinin by Zhu and Cook [21].
DCM: Dichloromethane; d.r.: Diastereomeric ratio; ee: Enantiomeric excess; eq.: Equivalent; OTIPS: Triisopropylsiloxy; rt: Room temperature.
This proof-of-principle synthesis of artemisinin from chemical feedstocks in an academic laboratory highlights the increasing potential of chemical synthesis to provide meaning quantities of highly complex molecules. Key to the success of this approach is the judicious use of a stereochemical relay in the initial retrosynthetic analysis. Further reports on the stereochemical outcome of the key annulation reaction are crucial to evaluate the long-term and large-scale prospects of this approach.
It should be noted that chemical synthesis has the potential to contribute in a more deep-seated way to the artemisinin story. Unlike most small molecule therapeutics, artemisinin does not bind to a macromolecule target, but instead reacts with heme, a byproduct of hemoglobin metabolism in blood-stage malaria. Simple 1,2,4-trioxanes can be used to mimic this biological reactivity of artemisinin and can be procured by synthesis quite easily on large scale [25–27]. It will take time for these unnatural analogs to progress through the clinic, but they appear to be viable replacements for artemisinin therapy in the future. In the meantime, the lives saved every day by the current application of artemisinin against malaria still justify the continued investigation of its economical production.
Structure-goal strategies: the power of ′semi-synthesis′
Historically, there have been three major terms to characterize the chemical synthesis of a natural product: total synthesis, semi-synthesis and formal synthesis [28]. The term total synthesis arose from early justifications for undertaking the synthesis of a natural product: rigorous structural assignment, proof-of-concept for constructing ‘biological’ molecules by chemical means, or understanding basic reactivity of organic compounds, all of which can be thought of as the chemical analog of a mathematical proof. The term ‘total’ can also be taken to mean chemical synthesis starting from molecules so simple that they can be easily derived from the pure elements (C, H2, O2, N2 and so on), and therefore the possibility of synthesizing the target in a general way is proven. Currently, such rationale for synthesis is infrequently invoked: there is seldom any justification for synthesizing a complex molecule from elemental carbon. Instead, a natural product synthesis is now usually undertaken because of the relevance of the target molecule to biology, medicine and the drug discovery process. Given the increased ability of organic chemistry to procure these molecules on large scale for drug discovery or production, this justification is projected to only increase in the future.
The term ‘total synthesis’ is now most frequently used to connote a synthesis that begins from commercially available materials that do not contain the full, partial or rearranged skeleton of the target molecule (as opposed to ‘semi-synthesis’; see below) [29]. However, commercially available materials vary widely in their cost and complexity, and often constitute an ‘outsourcing’ of the starting materials rather than an economical basis of the synthesis. To achieve the total synthesis of a molecule is not necessarily to produce it on a large scale or economically. If access to bulk material is a major problem, total synthesis is only one strategy among many.
The alternative approach to procuring a molecule chemically is semi-synthesis, where most of the molecular skeleton of the target is already intact. This approach can occasionally limit drug development since it inherently limits the number and diversity of analogs that can be easily accessed. However, semi-synthesis has a proven track record within the steroid class of terpenes, which outnumber all other terpenes among validated therapeutics. As a testament to its power, the semi-synthesis of progesterone by Russell Marker was responsible for reshaping all of society: it led to the development of the birth control pill, which partly fueled the sexual revolution of the 1960s [30]. Clearly, semi-synthesis has been the most useful means to access diverse analogs of some natural product classes for use in drug development.
It may be prescient to consider phasing-out the terms ‘total’ and ‘semi-synthesis’ in the current and future eras of chemistry, where advances in the field increasingly deal with practicality, economy and interface with the therapeutic sciences. These authors favor ‘chemical synthesis’ as a catch-all term to describe the construction of a molecule using largely nonenzymatic methods. The benefit of such a revision of common usage is that it hopefully encourages practitioners of chemical synthesis to focus on solving the specific problems possessed by a molecule, rather than becoming fixated on achieving feats of technical success. ‘Total’ synthesis does not totally solve a problem any more than ‘semi-’ synthesis partially solves it. Rather, the unique structural, biological, physical, ecological and economical problems associated with a given molecule dictate the tools that may best be applied, and chemists should think carefully about what approach should be taken.
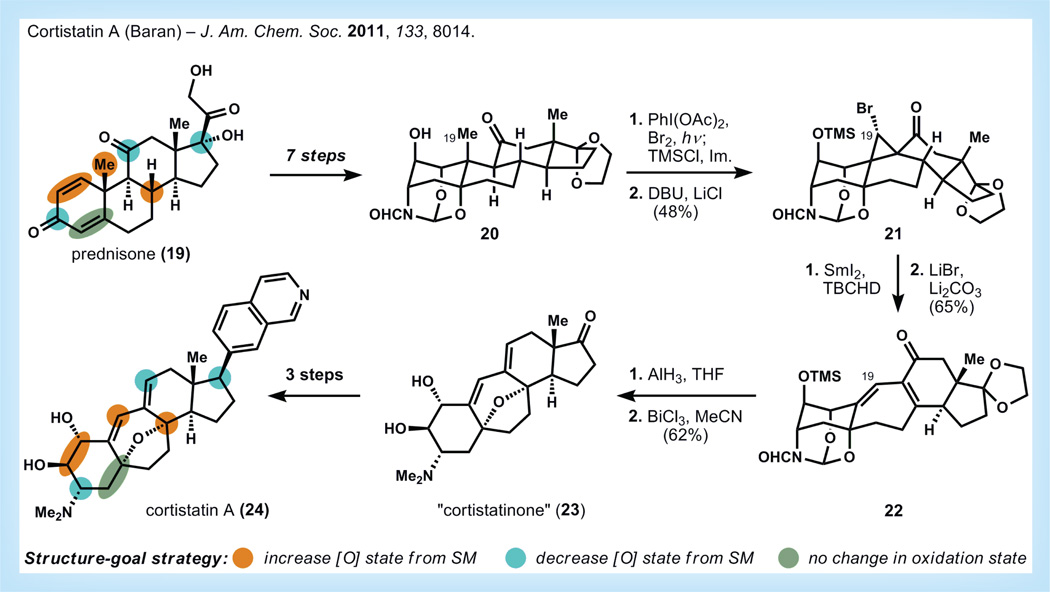
Cortistatin A
The synthesis of cortistatin A by Baran and co-workers (Figure 6) is a recent example of the power of the ‘semi-synthesis’ of steroids in a modern context [31,32]. Cortistatin A belongs to a small family of marine-derived terpenoids that exhibit potent anti-angiogenic activity and therefore may be useful for the treatment of cancer, macular degeneration and other diseases [33]. The retrosynthesis was analyzed primarily from a structure-goal perspective, with an intact steroid skeleton as the terminal goal. Secondarily, the retrosynthesis was refined by exploring the oxidation states of carbons within the cortistatins, and these carbons were mapped onto several commercially available steroids. Of the inexpensive steroids, prednisone (1.2 US$/g) exhibited the closest similarity in oxidation state to the cortistatins; the oxidation state changes separating them are highlighted in Figure 6.
Figure 6. Synthesis of cortistatin A by Baran [31,32].
DBU: 1,8-diazabicyclo[5.4.0]undec-7-ene; Im: Imidazole; SM: Starting material; TBCHD: 2,4,4,6-tetrabromo-2,5-cyclohexadienone; THF: Tetrahydrofuran; TMSCI: Trimethylsilyl chloride.
The A-ring of prednisone requires the most adjustment, so Baran and co-workers first modify the dienone into the fully stereo-defined cortistatin A-ring in five steps (two steps are also used to degrade the D-ring pregnane side chain). A novel geminaldi bromination procedure is utilized to access the correct oxidation state of the C19 methyl; the adjacent alcohol is protected in situ at low temperature as the trimethylsilyl ether to prevent backside attack of the now electrophilic carbon. Alkylation of the proximal ketone then affords as a single diastereomer the unusual bromocyclopropane 21, which is engaged in a ring expansion cascade using samarium (II) iodide and 2,4,4,6-tetrabromo-2,5-cyclohexadienone. The resultant α-bromoketone is then eliminated using lithium bromide and lithium carbonate to form dienone 22. Alane reduction followed by addition of potassium carbonate provides the amino tetraol. In the initial publication, a three-step sequence was required to install the tetrahydrofuran ring, but eventually the group found that treatment of the tetraol with bismuth trichloride could install this moiety directly. Deketalization is also accomplished in this step to give what is termed ‘cortistatinone’ 23, an intermediate from which arylated analogs can be derived. Installation of the naturally occurring isoquinoline was performed in three steps to give cortistatin A (24) in 16 steps overall. At the time of the publication of the second-generation synthesis, there were two contract companies preparing cortistatin A using the original synthetic route.
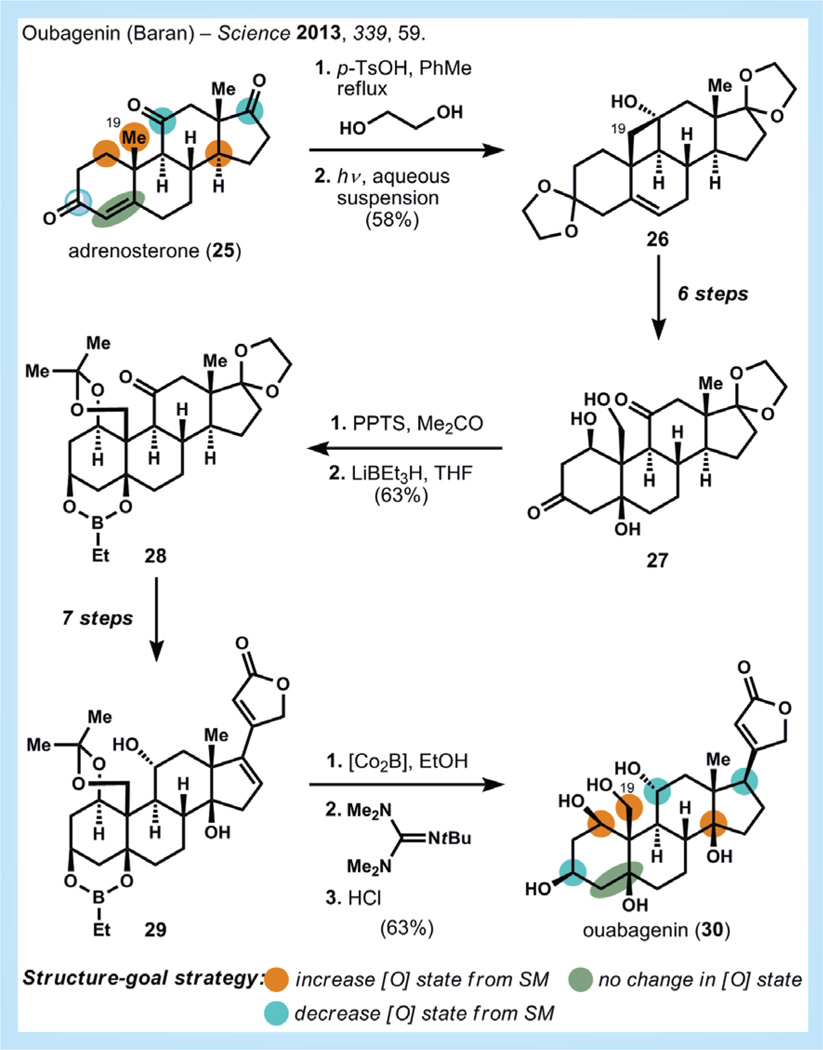
Ouabagenin
A similar semi-synthetic strategy was also pursued by Baran and co-workers in their synthesis of ouabagenin (30, Figure 7), whose glycoside, ouabain, is a cardiotonic steroid with a narrow therapeutic window that has resisted expansion despite some effort in medicinal chemistry [34–36]. Therefore, it was envisaged that semi-synthetic conversion of an inexpensive steroid might increase the opportunities for derivatization of the ouabain core and potentially lead to better tolerated treatments of, for instance, congestive heart failure.
Figure 7. Synthesis of ouabagenin by Baran [34].
PPTS: Pyridinium para-toluene sulfonate; SM: Starting material; THF: Tetrahydrofuran.
Similar to the strategy pursued in cortistatin A, a simple mapping of oxidation states between ouabagenin and commercially available steroids identified adrenosterone 25 as a viable precursor. This molecule contains the appropriate functionality for elaboration to ouabagenin – as long as the appropriate transformations can be found to efficiently modify the oxidation state of several key carbons, including the isolated C19 methyl (compare with cortistatin strategy, above).
Like cortistatin, the synthesis of oubagenin focuses first on addressing the oxidation state changes necessary to convert the A-ring of 25 and its angular methyl (C19) to the polyhydroxylated ouabagenin core. Functionalization of C19 is accomplished using a solid-state irradiation technique for a Norrish type II reaction and allows 26 to be synthesized in two steps from adrenosterone. These atypical conditions (suspension in water and irradiation in a standard photoreactor) prevent Norrish type I fragmentation of the carbon skeleton. Several more oxidation events are used to convert 26 to 27 in six steps. Formation of an acetonide followed by reduction with lithium triethylborohydride affords the stable ethyl boronic ester 28, which is useful as a protecting group and is carried through subsequent steps. Installation of the butenolide and another formal C-H oxidation event (accomplished by ketone unsaturation, isomerization and Mukaiyama hydration) occurs over seven steps. Reduction of the final double bond and placement of the final stereochemistry of the butenolide proved to be a difficult task. Most reducing conditions delivered hydrogen from the convex face and resulted in incorrect stereochemistry for the natural product. Eventually, it was found that in situ-generated cobalt boride reduces the extended conjugated system to give the non-conjugated tetrasubstituted olefin. Treatment with Barton’s base reforms the butenolide and provides a 3:1 mixture favoring the correct diastereomer. Final deprotection with acid affords ouabagenin in 20 overall steps, 0.56% yield and six net redox manipulations. To date, over 100 mg of ouabagenin has been synthesized using this route and the synthesis of analogs is now being investigated. Whether this route is able to address the narrow therapeutic index of ouabain remains to be seen, but certainly a foundation has been laid for more extensive work to address this worthy problem.
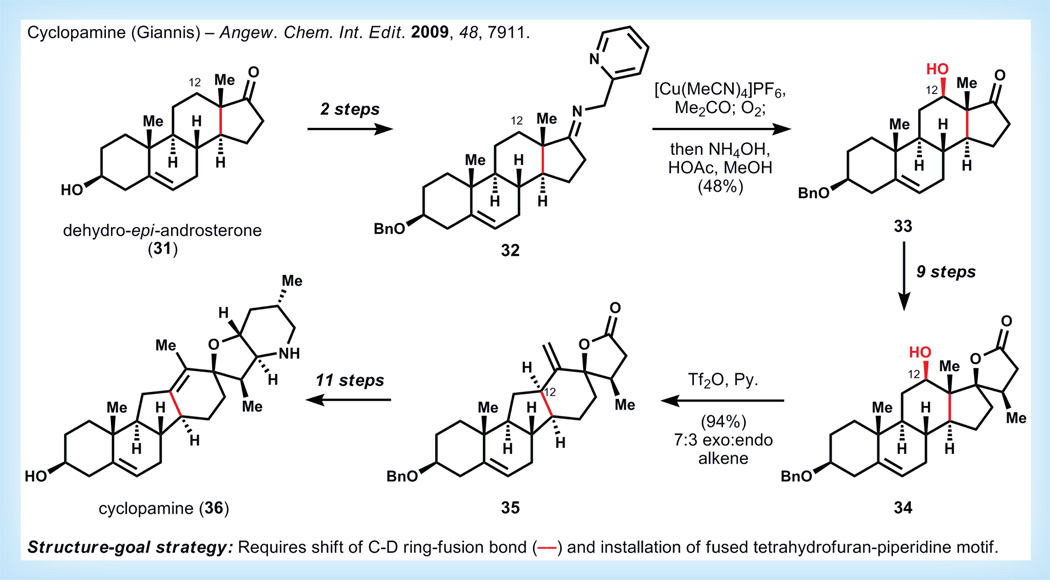
Cyclopamine
Another recent example of steroid semi-synthesis applied to a molecule of significant medical importance appeared when Giannis published his approach to the plant alkaloid cyclopamine (36, Figure 8) [37]. This remarkable compound antagonizes a G-protein-coupled receptor called Smoothened, an integral component of the Hedgehog (Hh) signaling pathway, which is overactive in basal cell carcinoma and other cancers [38– 40]. Numerous small molecule therapeutics are currently in development to target this pathway, including derivatives of cyclopamine itself, which was the first identified Hh signaling inhibitor. Unfortunately, (36) is metabolically unstable, as a consequence of the acid-labile allylic tert-alkyl ether functionality, which is readily cleaved via tetrahydrofuran ring-opening to yield a mixture of inactive compounds. Giannis anticipated that chemical synthesis of cyclopamine might allow identification of potent but metabolically stable derivatives.
Figure 8. Synthesis of cyclopamine by Giannis [37].
Since 36 is a rearranged steroidal alkaloid, an inexpensive steroid skeleton was retrosynthetically targeted as a viable starting point, in the anticipation that a method could be developed to allow migration of the C-D ring-fusion bond to provide C-nor-D-homo skeleton of cyclopamine.
Starting from a commercially available steroid, dehydro-epi-androsterone (31), Giannis and co-workers access the 2-picolylimine derivative 32 in two steps. This directing group, in concert with tetrakis(acetonitrilo)copper (I) hexafluorophosphate and molecular oxygen, effects a C-H oxidation with excellent regio- and diastereostereo-selectivity. Installation of this oxidation state at C12 is critical to enable skeletal rearrangement of the C-D bond later in the sequence. The spirolactone of cyclopamine is installed over nine steps to give 34. The alcohol of 34 is then triflated, and under these conditions a Wagner–Meerwein shift occurs to give the rearranged product, 35, in high yield and with reasonable selectivity for the exo-alkene. Although the endo-alkene is the desired isomer in the final product, it was determined that the exo-alkene could be isomerized later on in the sequence with little difficulty. Giannis and co-workers were able to elaborate 36 to cyclopamine in eleven steps (20 steps overall, 1% overall yield).
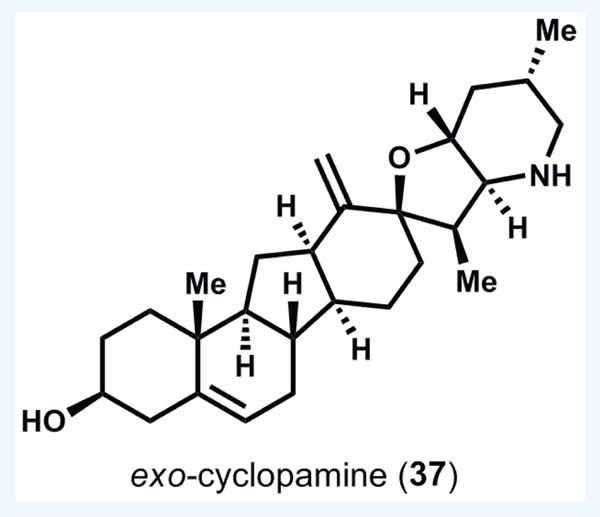
Although this synthesis was an excellent proof of concept for chemical access to cyclopamine, the real fruit of this project was reported 2 years later. By conversion of the major exo-alkene isomer 35 to exo-cyclopamine (37, see Figure 9), Giannis was able to compare the activity of this close analog with cyclopamine itself, and found that the potency of 37 against Hh-signaling is tenfold greater than that of 36 [41,42]. Even more remarkably, 37 is stable over 24 h at pH 1.5, whereas cyclopamine degrades rapidly under the same conditions. The acid stability of 37 versus 36 is easy to rationalize on the basis of either decreased kinetic basicity of 37 (assuming protonation is the rate-determining step), or the greater stability of the carbenium ion derived from ionization of the C-O bond in cyclopamine 36 (assuming a late transition state and that ionization is the rate-determining step). Most importantly, 37 represents an important lead in developing systemically viable and potent cyclopamine-derived Hh-signaling inhibitors.
Figure 9. exo-cyclopamine[41,42].
Transform-based strategies: the power of the ‘key step′
Analysis of a target structure occasionally identifies a key retrosynthetic transform (Tf. – the exact reverse of a synthetic reaction) that has the potential to dramatically simplify the synthesis of a complex scaffold. Determination of the best point within the retrosynthesis to apply this Tf. then allows a bidirectional search to connect the intermediates with the final target and with possible starting materials. In this section, we will discuss recent syntheses of bioactive terpenoids in which a key reaction enables a rapid build-up in structural complexity and leads to a highly efficient chemical synthesis.
Neothiobinupharidine
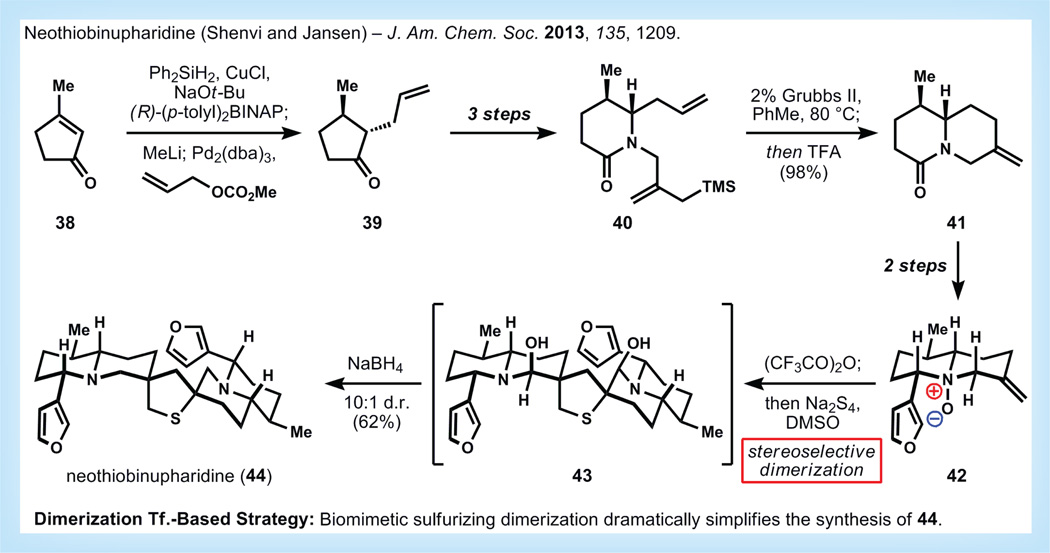
The Nuphar alkaloids are fresh water plant metabolites that exhibit promising antimetastatic activity both in vitro and in mouse studies [43,44]. Although recent biological work has shown promise for these compounds as therapeutic agents, these studies relied on impure plant extracts due to the limited availability of the pure alkaloids [45]. Current biological work has focused on determination of the discrete mechanism of action associated with the Nuphar dimers, which may involve inhibition of NFκB signaling. An insightful proposal by LaLonde suggested that these dimers might arise in nature by a sulfurizing dimerization of quinolizidine monomers [46]. Realization of such a dimerization in the laboratory would vastly simplify the synthesis of these compounds. However, while the biosynthetic proposal was a useful guide, it is important to note that this dimerization pathway was not expected to be selective for the formation of a naturally occurring stereoisomer; in fact, conformational analysis predicts the formation of the only stereoisomer never isolated. Nevertheless, the level of simplification provided by the dimerization Tf. prompted the authors of this review to explore the corresponding transformation in the laboratory [47].
Chirality can be introduced in the synthesis (Figure 10), with high enantioselectivity using Buchwald’s conditions for asymmetric enone reduction of 38, followed by palladium-catalyzed allylation [48]. It should be noted that this in-house procedure for reductive allylation of cyclopentenones is the only reaction that cleanly provides 39 in reasonable yield. Lactam 40 is synthesized in three more steps, and is primed for ring-closing metathesis followed by in situ protodesilylation in the manner of Vanderwal to give the bicyclic lactam 41 [49]. Installation of the furan using a modification of Fowler’s procedure and N-oxidation provides amine-oxide 42 [50]. Treatment of 42 with trifluoroacetic anhydride effects a highly regioselective Polonovski elimination at only one of three possible positions, and subsequent addition of sodium tetrasulfide in DMSO yields dimeric intermediate 43. This intermediate proved difficult to isolate and purify, but in situ reduction with sodium borohydride provided neothiobinupharidine 44 in good yield and high stereoselectivity in preference to three other possible stereoisomers. The solvent for dimerization proved vital to good diastereo selection, which could be roughly correlated to the solvent’s dispersion force component of its Hansen parameters [51]. Subsequently, 43 has been isolated in pure form, which sets the stage for exploration of its biological mechanism of action.
Figure 10. Synthesis of neothiobinupharidine by Shenvi [47].
d.r.: Diastereomeric ratio; Tf.: Transform; TFA: Trifluoroacetic acid.
Hyperforin
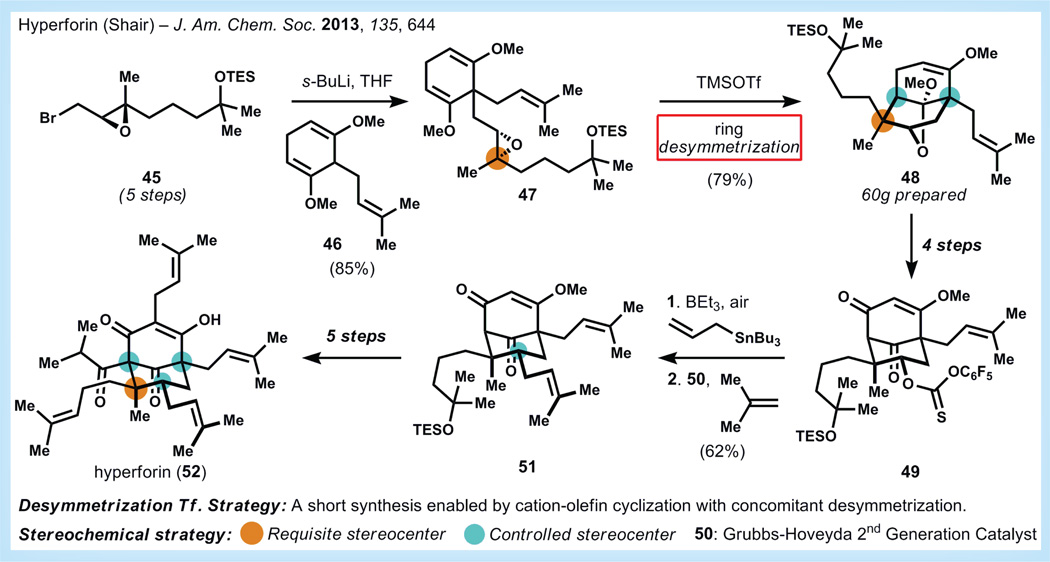
Hyperforin (52, Figure 11) is a terpenoid plant metabolite believed to be responsible for the antidepressant activity ascribed to St John’s Wort (Hypericumperforatum L.) [52] Unfortunately, its off-target effects and inherent instability limit its immediate application as a small molecule therapeutic. Therefore, Shair and co-workers targeted a modular synthetic sequence with the aim of improving the biological properties of 52 [53]. If a single, key insight can be attributed to both the success and efficiency of the synthesis, it is probably the realization of latent symmetry within phloroglucinol core, which might be accessed by relaying the stereochemistry of a chiral sidechain into a symmetrical cyclohexane (red box, Figure 11). The implementation of this approach is shown below.
Figure 11. Synthesis of hyperforin by Shair [53].
TES: Triethylsilyl; THF: Tetrahydrofuran; TMSOTf: Trimethylsilyl triflate.
Starting from the inexpensive polyprenolgeraniol, Shair and co-workers are able to synthesize epoxide 45 in five steps. The anion derived from prenylcyclohexadiene 46 displaces the alkyl bromide of 45 and sets the stage for the key desymmetrization reaction. Exposure of 47 to trimethylsilyltriflate and 2,6-lutidine cleanly inverts the epoxide stereochemistry (as observed in related terpenoid systems [54]) and yields 48 as the only isolable product. It is noteworthy that two chiral all-carbon quaternary stereocenters are formed in this step, and the scalability of this sequence is demonstrated by the preparation of 60 g of 48. Thionocarbonate 49 is then prepared in four steps, and allows for installation of the allyl group using Keck allylation followed by olefin cross-metathesis with isobutene [55,56]. Shair and co-workers then install another two prenyl units over the next five steps to afford hyperforin in a longest linear sequence of 18 steps. Over 40 mg of hyperforin had been prepared at the time of their publication.
Ingenol
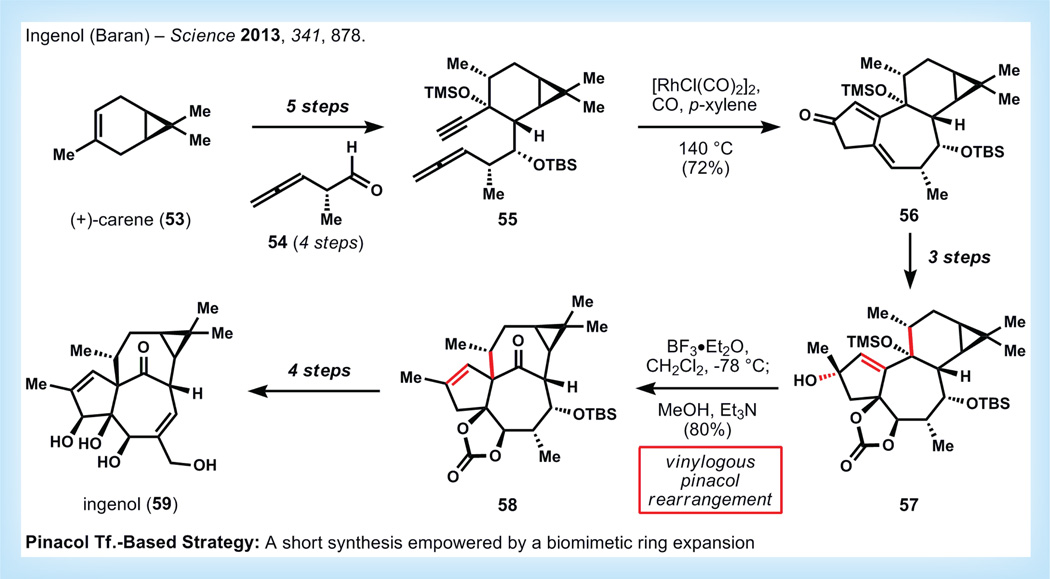
The mebutate ester of ingenol was recently approved by the US FDA for topical treatment of actinic keratosis, and clinical trials are in progress for treatment of basal cell carcinoma [57]. Ingenol itself (59, Figure 12) is a diterpenoid that was first isolated from Euphorbia ingens in 1968 [58]. The limited quantities of 59 available from plant extraction (390 mg, 59, from 2 kg dried seeds) [59] and the diverse phenotypic responses of cells to ingenol analogs indicate that the development a practical, diversifiable synthetic route to this metabolite might enable significant contributions to medicine and biology [59]. Three previous total syntheses of ingenol have been published, all requiring between 37 and 45 steps [60–63]. Although these exercises have made important contributions to understanding the reactivity of ingenanes, they are impractical for analog exploration or large-scale production. Part of the difficulty of synthesizing ingenol is developing a strategy to create its unique ‘in, out’ [4.4.1] bicycloundecane architecture. This unusual scaffold arises in nature via a 1,2-pinacol-type shift from a related (tigliane) [5.4.0] bicycloundecane skeleton [57], which was demonstrated by Cha to be easily reproduced by chemical methods [64]. Embedding this transform into a retrosynthetic analysis allows a bidirectional search for routes to synthesize the tigliane skeleton, and strategies to convert the pinacol product to ingenol. This strategy formed the basis of a remarkably concise synthesis of ingenol from the Baran laboratory.
Figure 12. Synthesis of Ingenolby Baran [57].
TBS: tert-Butyldimethylsiloxy; TMS: Trimethylsiloxy.
Starting from the inexpensive monoterpene(+)-carene (53), Baran and co-workers install the requisite functionality for a Pauson–Khand cyclization, and use Brummond’s conditions to effect the intramolecular variant of this reaction, producing dienone 56 [65]. Functional group interconversions over the next three steps provide 57, which is ready for skeletal rearrangement to install the ‘in, out’ [4.4.1] bicycloundecane framework. Initial attempts with various Lewis acid catalysts failed, but rigorous control of temperature (−78 to −40°C) during treatment with boron trifluoride etherate allows the desired vinylogous pinacol rearrangement to occur to provide ingenane 58. Over the next four steps, 58 is converted to ingenol 59, which ultimately arrives in only 14 steps from 53 and 1.4% overall yield.
FG-based strategy: the power of new methods
Retrosynthetic analysis of a complex structure will identify multiple reasonable pathways that may lead to a viable laboratory synthesis. Choice of the ‘best’ pathway can be assisted by identifying the problem to be addressed (scalability, modularity and predictability; see above) and eliminating the routes that would not fulfill the correct criteria. Among the remaining routes, some stand out as more concise and elegant than others, usually as a result of judicious application of Tf.s) that efficiently remove complexity from the target molecule [66]. Occasionally, these Tf.s are purely theoretical, and correspond to chemical reactions that, while probable, have not been previously reported. In this section, we will highlight syntheses where new reactions are invented to ‘jump the gaps’ in the retrosynthetic logic. We will also cover syntheses that take advantage of newly invented methods that dramatically simplify the construction of complex terpenes.
Amphilectene & the isocyanoterpenes
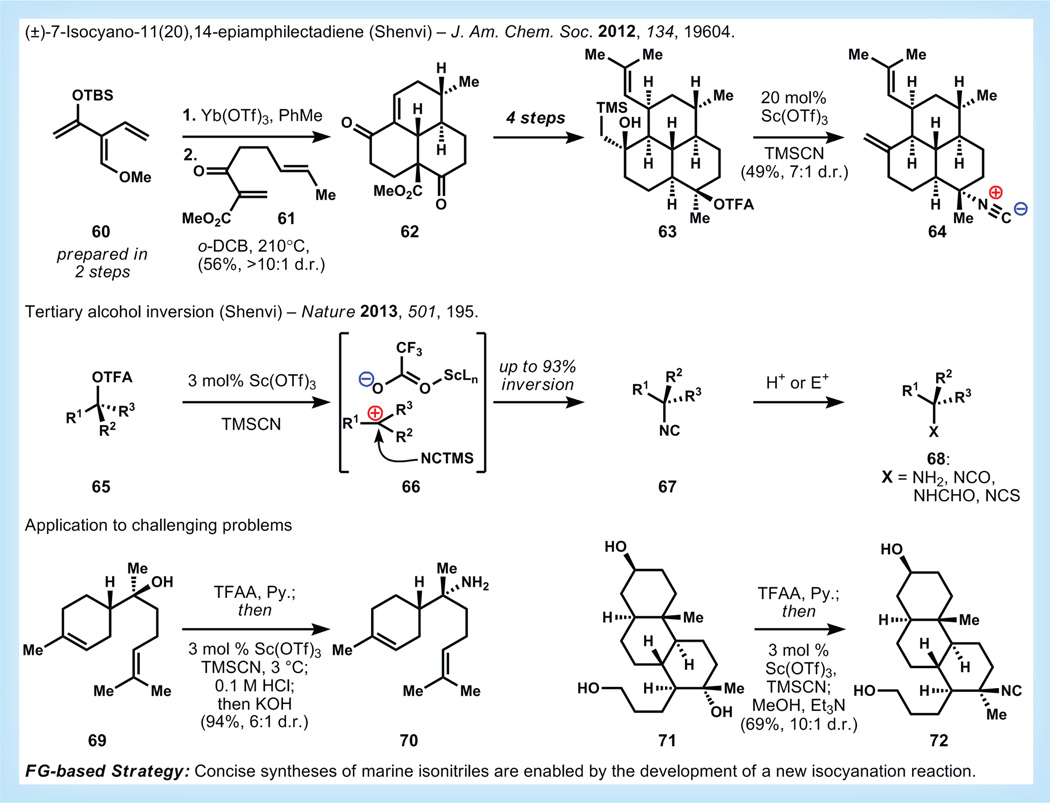
Resistance to the front-line medication artemisinin (see above) has recently been reported in Southeast Asia, and therefore the search for new antimalarials is of utmost importance [67]. Over the last 40 years, a large family of marine terpenes has accrued in the literature, and has received attention for its unusual biosynthetic incorporation of nitrogen atoms derived from cyanide [68]. These secondary metabolites have also shown potent and selective activity against Plasmodium parasites (the causative agents of malaria) and due to the presence of one or more isonitrile functions in the molecules, it has been theorized that these compounds may disrupt heme detoxification in blood stage parasites (see also ‘Artemisinin’ section above) [69]. Unfortunately, the mechanism of action of these compounds is unknown, and in vitro experiments to support heme binding do not account for the efficacy of related terpenes that contain formamide instead of isonitrile groups.
Our group undertook the synthesis of the most potent member of the amphilectene isocyano terpenes (64, Figure 13) in order to access both bulk material and eventually photoaffinity-labeled analogs to discover the antimalarial mechanism of action of this class [70,71]. There were no efficient methods reported to install the key C-N bond, which clued us in to the potential for the development of a new method. Additionally, there were no efficient approaches to the carbon skeleton of the amphilectenes and related terpenes. To solve these problems, we invented a new type of linear polyene, which we termed a ‘Danishef-skydendralene,’ that is capable of undergoing iterative cycloadditions with high levels of regio- and stereo-selectivity [72]. To address the C-N bond, we recognized stereoinversion of a tertiary alcohol to be the shortest route to install the isonitrile pharmacophore, but no method existed for such a reaction. Therefore, we designed a Lewis-acid catalyzed solvolysis reaction to invert the stereochemistry of a chiral alcohol and simultaneously install the tert-alkyl isonitrile. The combined results of these methodological advances are illustrated in the synthesis of amphilectene 64 in Figure 13.
Figure 13. Synthesis of an amphilectene by Shenvivia tertiary alcohol inversion [71,73].
d.r.: Diastereomeric ratio; e.r.: Enantiomeric ratio; o-DCB: ortho-Dichlorobenzene; TBS: tert-Butyldimethylsilyl; TFAA: Trifluoroacetic anhydride; TMSCN: Trimethylsilyl cyanide.
In the first two steps of the synthesis, our designed polyene, the parent [3]-Danishefsky-dendralene 60 reacts with double-dienophile 61 to provide the full amphilectene core 62 as the major stereoisomer. Four steps are required to elaborate this tricycle to the penultimate intermediate 63, which when subjected to our stereoinversion reaction provides the targeted molecule 64 with high diastereoselectivity. This final step likely proceeds via contact ion pair inversion (see 66), and can be used to install multiple nitrogenous functional groups since the isonitrile is easily degraded to the parent amine (see 67→68) [73]. This reaction can be extended to more challenging substrates that illustrate its potential to convert abundant terrestrial terpenes (bisabolol, 69) to scarce marine terpenes (70), or to selectively modify tertiary alcohols in the presence of secondary and primary alcohols – a selectivity which is orthogonal to standard SN2 reactions. It is anticipated that these conditions for Lewis-acid catalyzed solvolysis may lead to a small arsenal of stereoinversion reactions for tertiary alcohols.
Pumilaside aglycon
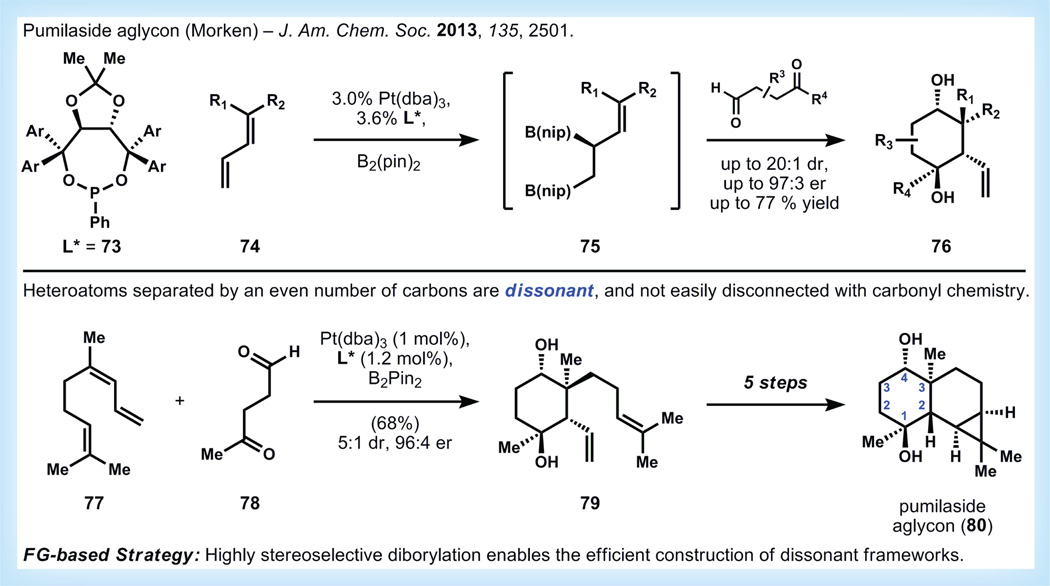
Oxygenated terpenoids are usually biosynthesized by two main reaction pathways: capture of water to terminate cationic polycyclizations, and oxidation of terpenecarbocycles by cytochrome P450 enzymes [1] In the laboratory, chemical mimicry of either of these enzyme-mediated steps is possible, but far from general. Instead, use of the innate reactivity and/or ‘charge affinity pattern’ of an oxygenated carbocycle [74] can be used to easily dissect the molecule using the chemistry of carbonyls: aldol, Michael and Mannich transforms, among others. If the functional groups do not signal these facile maneuvers (the groups are ‘dissonant,’ to use Evans’ terminology [74]), then dissection of the scaffold becomes more challenging and less uniform, which usually corresponds to more functional group interconversions and a longer step count.
With these challenges in mind, the Morken group sought to explore the application of their recently developed asymmetric diborylation technology (74→75, Figure 14) to the synthesis of complex oxygenated metabolites [75–77]. Remarkably, addition of a 1,4-dicarbonyl to the reaction mixture containing a chiral diboryl alkene results in tandem diallylation of the dicarbonyl to produce cyclic structures (76) containing four new stereocenters with high diastereo-and enantio-selectivity. These structures map well onto several known terpenoids, including pumilaside aglycon (80) [78]. The Morken group synthesizes this complex tricycle in only six steps first by diborylation of neryl-derived triene 77 followed by addition of 4-oxopentanal to provide cyclohexanediol 79 as the major stereoisomer. Elaboration of 79 to the target molecule requires only five steps of simple functional group interconversions, and more importantly, this method provides access to highly complex building blocks for terpene synthesis.
Figure 14. Synthesis of pumilaside aglycon by Morken via asymmetric diborylation [77].
d.r.: Diastereomeric ratio; e.r.: Enantiomeric ratio; FG: Functional group.
Peyssonol A
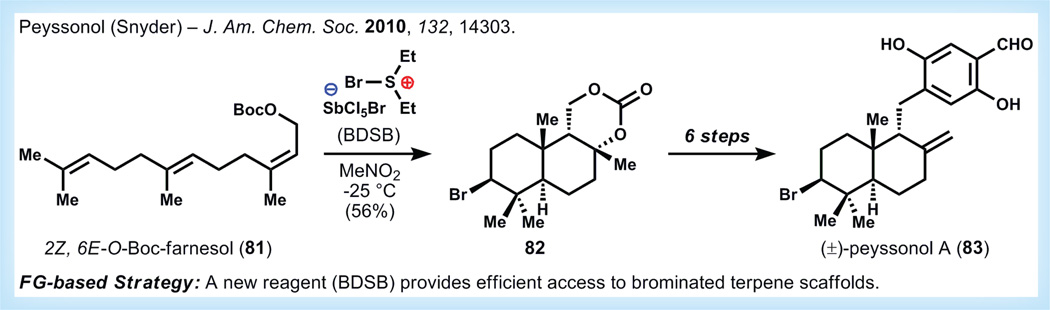
In contrast to terrestrial terpenes, marine terpenes are frequently subject to oxidative modification by halogenating enzymes that add bromine and chlorine atoms to their substrates. Given the abundance of halide anions in seawater, these halonium biosynthetic pathways are perhaps not surprising [79,80]. However, regio- and stereo-selective incorporation of halogens into terpenes scaffolds using chemical methods is by no means straightforward, and is the subject of considerable current research.
Peyssonol A (83, Figure 15) is a secondary metabolite isolated from Red Sea alga (Peyssonnelia sp.) and is likely biosynthesized via halonium-mediated polycyclization of a farnesyl chain [81]. This metabolite possesses allosteric inhibitory activity for HIV reverse transcriptase, paralleling the activity of related sesquiterpenes and avaroland avarone, which have been shown to inhibit HIV-1 transcriptase at concentrations as low as 300 nM [82]. Nevertheless, many compounds from this family suffer from high cytotoxicity. While the pharmaceutical relevance of this family of compounds remains uncertain, generation of analogs is crucial for understanding SAR and reducing the cytotoxicity of these molecules.
Figure 15. Synthesis of peyssonol A by Snyder viabromonium ion induced polycyclization [84–86].
BDSB: Bromo diethyl sulfonium pentachloro antimonite (V); FG: Functional group.
When replicating halonium cyclizations in the laboratory, a confounding feature is the ability of the counter anion to pre-emptively terminate the cyclization process [83]. Nevertheless, these polycyclization cascades can be vastly simplifying for the synthesis of polycyclic terpenoids because of the commercial availability of linear precursors derived from geraniol, nerol and farnesol. Snyder and co-workers developed bromo diethyl sulfonium bromo pentachloro antimonate (V) (BDSB) as a novel reagent for halonium ion promoted cyclizations [84,85]. This reagent benefits from its non-nucleophilic antimonate counter anion that does not interrupt the cation cyclization. Reactions are typically completed in less than 10 min and provide acceptable yields when using farnesyl-based substrates.
For the synthesis of peyssonol A, Snyder and co-workers subjected 2Z,6E-farnesyl carbonate 81 to BDSB, which causes efficient halonium-induced cyclization to provide tricycle 82. This intermediate is carried forward six steps to 83, which matches the spectroscopic data from the isolation literature and revises the original assignment of a cis-decalin topology of the natural product. Snyder and co-workers disclosed in follow-up work that they were able to use this technology to generate several simple analogs possessing antiviral activity against HIV in the low micromolar to high nanomolar range while having good therapeutic indices (>20), an excellent proof of principle for translating methodological innovation into synthetic application and on to medicinal chemistry exploration [86].
Frondosin B
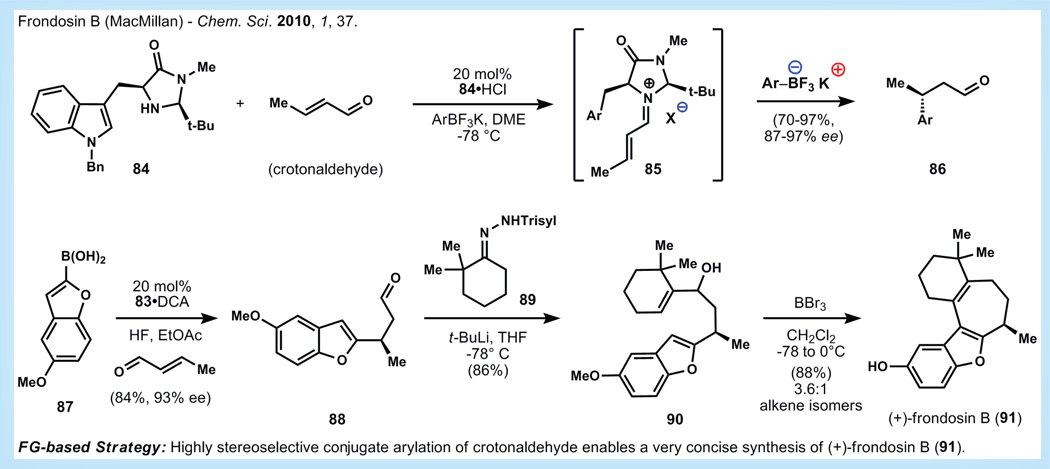
Bioassay-guided fractionation of the marine sponge Dysidea frondosa identified frondosin B (91, Figure 16) and several of its family members as potential anti-inflamatory compounds [87]. Initial work showed that frondosin B had micromolar inhibitory activity against IL-8Rα, IL-8Rβ and PKC-α. The authors suggested that the inhibitory activity of the frondosin family members might be a result of general reactivity of the compounds rather than true agonist/antagonist activity. A second report also suggested that other members of the frondosin family might have moderate, but variable, anti-HIV activity [88]. During the isolation process it was noted that several of the compounds have opposite optical rotation values and consequently they are thought to occur in nature as scalemic mixtures.
Figure 16. Synthesis of frondosin B by MacMillan via asymmetric Friedel-Crafts alkylation [94] .
ee: Eenantiomeric excess; DCA: Dichloroacetic acid; DME: 1,2-dimethoxyethane; THF: Tetrahydrofuran.
Frondosin B is characterized by a benzofuran ring system attached to a norsesquiterpenoid framework that contains a single stereogenic methyl group. Despite the seemingly simple framework, efforts to install the lone benzylic stereocenter have proved difficult, as evidenced by lengthy asymmetric syntheses. The first asymmetric synthesis of natural (+)-frondosin B was published by Danishefsky in 2001 and suffered from high step count and a low overall yield of 0.7% [89]. In 2002, Trauner published the synthesis ent-(-)-frondosin B and called into question the absolute configuration of the natural product. Trauner’s synthesis also suffered from high step count, but a more reasonable overall yield of 7.3% was obtained [90,91]. More recently, Ovaska published an eight-step racemic synthesis of frondosin B in 2007 and followed up with a ten-step asymmetric synthesis of (-)-frondosin B in 2009 [92,93] .
MacMilllan and co-workers published a synthesis of (+)-frondosin B in 2010 [94], which relies on the laboratory’s previous work in asymmetric catalysis [95]. The MacMillan laboratory previously reported the conjugate addition of aryltrifluoroborate potassium salts to chiral iminium ions generated by the condensation of aldehydes with amino acid-derived catalysts. These reactions provide chiral β-disubstituted aldehydes in good yield and excellent enantioselectivity. Application to the synthesis of 91 entailed reaction of commercially available boronic acid 87 with crotonaldehyde in the presence of catalyst 84 and hydrofluoric acid in ethyl acetate, to provide 88 in high yield and enantioselectivity. When 88 is treated with the alkenyl lithium reagent derived from the Shapiro reaction of 89 and t-BuLi, 90 is produced in good yield as an inconsequential mixture of diastereomers. A two-step process involving a molybdenum catalyst and BBr3 was initially used to synthesize (+)-frondosin B, but upon further experimentation it was discovered that treatment of 90 with BBr3 causes formation of the final seven-membered, demethylationthe phenoxy ether, and isomerization of the olefin to the desired endocyclic isomer, supplying (+)-frondosin B in only three steps (from 87) and an outstanding 50% overall yield.
Conclusion
Terpenoids provide an excellent foundation for the development of new small molecule therapeutics. The increasing focus in academia on generating concise and scalable routes to these molecules should allow a much broader range of terpene scaffolds to be considered viable foundations for medicinal chemistry campaigns. Similarly, the economical production of complex terpenes on multikilogram scale through chemical synthesis is increasingly possible as a less-expensive alternative to isolation from living organisms (this has been true of the monoterpene menthol for several years). Key to the development of a chemical solution to a given terpenoid structure is identification of the most important problem to be addressed by synthesis – diverse analog production, large-scale production, increased chemical/metabolic stability and generation of mechanistic probes, among others. In support of any of these goals, the development of a concise synthetic route that minimizes the number of steps is an additional, crucial agenda. The strategies listed above to minimize synthetic operations should provide a general approach to reduce the costs of terpene synthesis at multiple levels. It is anticipated that the brevity, practicality and favorable economies of modern chemical synthesis will reinvigorate the use of terpenes in the drug discovery process. Investment in the right scaffolds, synthesis strategies and methodological advances are likely to yield a high return for any drug discovery team.
Future perspective
Chemical synthesis – a central, foundational industry in society – is not anticipated to recede in the next decade. Instead, molecules of increasing complexity, including terpenes, will be increasingly accessible through synthesis and ever more applied to fields that interface with chemistry, especially medicine. A focus on simplicity of synthesis through the invention of operationally straightforward, predictable and air/water/cell-tolerant reactions will predominate. Most importantly, application of both new reactions and new molecules to modulate biological function will increase in power, breadth and predictability. The artificial, conceptual barrier separating ‘natural product-like’ from ‘drug-like’ molecules is likely to fall, as drugs become more complex, and intersperse more stereocenters, rotatable bonds and common metabolite motifs such as polyketide chains. Similarly, research in chemical synthesis will become equally liberated to address problems at the interface of other diverse fields, rather than remaining insular and self-serving. The prospects for discovery in organic chemistry are therefore both exciting and limitless.
Executive summary.
Background
Terpenes are successful therapeutics, yet challenging starting points rational medicinal chemistry using chemical synthesis.
Identifying the problem
A chemical synthesis must be tailored to the problem it is trying to solve, and therefore identification of the actual problem is crucial. The problem often dictates choice of higher-level strategy employed.
Stereochemistry-based strategies: the power of substrate relay
Stereochemical relay from the substrate can be an effective tool to rapidly increase complexity, as long as the appropriate choreography of bond disconnections can be determined.
Structure-goal strategies: the power of ‘semi-synthesis’
‘Total’ synthesis does not totally solve a problem any more than ‘semi-’ synthesis partially solves it. Rather, the unique structural, biological, physical, ecological and economical problems associated with a given molecule dictate the tools that may best be applied.
Transofrm-based strategies: the power of the ‘key step’
Analysis of a target structure occasionally identifies a key retrosynthetic transform that has the potential to radically simplify the synthesis of a complex scaffold.
FG-based strategy: the power of new methods
Retrosyntheses occasionally identify excellent transforms that are purely theoretical, and therefore new reactions must be invented to ‘jump the gaps’ in the retrosynthetic logic.
Conclusion
Application of both new reactions and new molecules to modulate biological function will increase in power, breadth, and predictability.
Acknowledgments
Financial support for this work was provided by the NIH (GM104180). The authors are grateful to the Scripps Research Institute, Eli Lilly, Boehringer Ingelheim, Amgen, the Alfred P Sloan Foundation and the Baxter Foundation for additional financial support.
Key terms
- Terpenes
Organic molecules composed of repeating units of isoprene, occasionally rearranged, that are biosynthesized from polyisoprenyldiphosphates.
- Semi-synthesis
Possibly outdated term that involves the use of a ‘complex’ starting material (in terms of skeletal arrangement, number of atoms and number of stereocenters, among others) to synthesize a structurally related complex molecule.
- Total synthesis
Possibly outdated term that refers to the synthesis of a complex molecule from ‘simple’ chemicals (chemical feedstock).
- Chemical synthesis
Use of one or more chemical reactions to make a molecule.
- Higher-level strategy
Overarching strategy apparent from the application of a series of transforms used to synthesize a discrete chemical compound.
- Formal synthesis
Chemical synthesis of an intermediate that was previously synthesized and elaborated to a target molecule.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:.
• of interest.
- 1.Dewick PM. Medicinal Natural Products: A Biosynthetic Approach. West Sussex, UK: Wiley; 2009. [Google Scholar]
- 2.Kotz J. Phenotypic screening, take two. SciBX. 2012;5(15):1–3. [Google Scholar]
- 3. Gaich T, Baran PS. Aiming for the ideal synthesis. J. Org. Chem. 2010;75(14):4657–4673. doi: 10.1021/jo1006812.. • Provides good thoughts on ‘ideal’ synthesis concepts.
- 4. Hudlicky T. Design constraints in practical syntheses of complex molecules: current status, case studies with carbohydrates and alkaloids, and future perspectives. Chem. Rev. 1996;96(1):3–30. doi: 10.1021/cr950012g.. • Provides good thoughts on ‘ideal’ synthesis concepts.
- 5. Wender PA, Verma VA, Paxton TJ, Pillow TH. Function-oriented synthesis, step economy, and drug design. Acc. Chem. Res. 2007;41(1):40–49. doi: 10.1021/ar700155p.. • Provides good thoughts on ‘ideal’ synthesis concepts.
- 6.Mathison IW, Solomons WE, Tidwell RR. Structural features and pharmacologic activity. In: Foye WO, editor. Principles of Medicinal Chemistry. PA, USA: Williams and Wilkins; 1995. [Google Scholar]
- 7.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review. MD, USA: National Cancer Institute; 2013. pp. 1975–2010. [Google Scholar]
- 8.Ratnayake R, Covell D, Ransom TT, Gustafson KR, Beutler JA. Englerin A, a selective inhibitor of renal cancer cell growth, from. Phyllanthus engleri. Org. Lett. 2009;11(1):57–60. doi: 10.1021/ol802339w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sourbier C, Scroggins BT, Ratnayake R, et al. Englerin A stimulates PKCθ to inhibit insulin signaling and to simultaneously activate HSF1: pharmacologically induced synthetic lethality. Cancer Cell. 2013;23(2):228–237. doi: 10.1016/j.ccr.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willot M, Radtke L, Konning D, et al. Total synthesis and absolute configuration of the guaiane sesquiterpene englerin A. Angew. Chem. Int. Ed. 2009;48(48):9105–9108. doi: 10.1002/anie.200905032. [DOI] [PubMed] [Google Scholar]
- 11.Radtke L, Willot M, Sun HY, et al. Total synthesis and biological evaluation of (−)-englerin A and B. Synthesis of analogues with improved activity profile. Angew. Chem. Int. Ed. 2011;50(17):3998–4002. doi: 10.1002/anie.201007790. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez-Nunez E, Claverie CK, Nieto-Oberhuber C, Echavarren AM. Prinscyclizations in Au-catalyzed reactions of enzymes. Angew. Chem. Int. Ed. 2006;45(33):5452–5455. doi: 10.1002/anie.200601575. [DOI] [PubMed] [Google Scholar]
- 13.Molawi K, Delpont N, Echavarren AM. Enantioselective synthesis of (−)-englerins A and B. Angew. Chem. Int. Ed. 2010;49(20):3517–3519. doi: 10.1002/anie.201000890. [DOI] [PubMed] [Google Scholar]
- 14.Zhou QH, Chen XF, Ma DW. Asymmetric, protecting-group-free total synthesis of (−)-englerin A. Angew. Chem. Int. Ed. 2010;49(20):3513–3516. doi: 10.1002/anie.201000888. [DOI] [PubMed] [Google Scholar]
- 15.Li ZW, Nakashige M, Chain WJ. A brief synthesis of (−)-englerin A. J. Am. Chem. Soc. 2011;133(17):6553–6556. doi: 10.1021/ja201921j. [DOI] [PubMed] [Google Scholar]
- 16.Miller LH, Su X. Artemisinin: discovery from the Chinese herbal garden. Cell. 2011;146(6):855–858. doi: 10.1016/j.cell.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paddon CJ, Westfall PJ, Pitera DJ, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496(7446):528–532. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- 18.Levesque F, Seeberger PH. Continuous-flow synthesis of the anti-malaria drug artemisinin. Angew. Chem. Int. Ed. 2012;51(7):1706–1709. doi: 10.1002/anie.201107446. [DOI] [PubMed] [Google Scholar]
- 19.Ro DK, Paradise EM, Ouellet M, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440(7086):940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- 20.Turconi J, Griolet F, Guevel R, et al. Semisynthetic artemisinin, the chemical path to industrial production. Org. Process Res. Dev. 2014;18(3):417–422. [Google Scholar]
- 21.Zhu CY, Cook SP. A concise synthesis of (+)-artemisinin. J. Am. Chem. Soc. 2012;134(33):13577–13579. doi: 10.1021/ja3061479. [DOI] [PubMed] [Google Scholar]
- 22.Jarugumilli GK, Zhu C, Cook SP. Re-evaluating the nucleophilicity of zinc enolates in alkylation reactions. Eur. J. Org. Chem. 2012;2012(9):1712–1715. [Google Scholar]
- 23.Jarugumilli GK, Cook SP. A simple, nontoxic iron system for the allylation of zinc enolates. Org. Lett. 2011;13(8):1904–1907. doi: 10.1021/ol200059u. [DOI] [PubMed] [Google Scholar]
- 24.Sy LK, Brown GD. The mechanism of the spontaneous autoxidation of dihydroartemisinic acid. Tetrahedron. 2002;58(5):897–908. [Google Scholar]
- 25.Posner GH, Parker MH, Northrop J, et al. Orally active, hydrolytically stable, semisynthetic, antimalarial trioxanes in the artemisinin family. J. Med. Chem. 1998;42(2):300–304. doi: 10.1021/jm980529v. [DOI] [PubMed] [Google Scholar]
- 26.Posner GH, Cumming JN, Woo S-H, Ploypradith P, Xie S, Shapiro TA. Orally active antimalarial 3-substituted trioxanes: new synthetic methodology and biological evaluation. J. Med. Chem. 1998;41(6):940–951. doi: 10.1021/jm970686e. [DOI] [PubMed] [Google Scholar]
- 27.Vennerstrom JL, Arbe-Barnes S, Brun R, et al. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature. 2004;430(7002):900–904. doi: 10.1038/nature02779. [DOI] [PubMed] [Google Scholar]
- 28. Nicolaou KC, Vourloumis D, Winssinger N, Baran PS. The art and science of total synthesis at the dawn of the twenty-first century. Angew. Chem. Int. Ed. 2000;39(1):44–122.. • Provides historical perspectives on chemical synthesis and its value to society.
- 29. Nicolaou KC, Sorensen EJ. Classics in total synthesis : targets, strategies, methods. Weinheim, NY, USA: Wiley-VCH; 1996. . • Provides historical perspectives on chemical synthesis and its value to society.
- 30.American Chemical Society International Historic Chemical Landmarks. The “Marker Degradation” and Creation of the Mexican Steroid Hormone Industry. :1938–1945. www.acs.org/content/acs/en/education/whatischemistry/landmarks/progesteronesynthesis.html.
- 31.Shenvi RA, Guerrero CA, Shi J, Li CC, Baran PS. Synthesis of (+)-cortistatin. A. J. Am. Chem. Soc. 2008;130(23):7241–7243. doi: 10.1021/ja8023466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi J, Manolikakes G, Yeh CH, et al. Scalable synthesis of cortistatin A and related structures. J. Am. Chem. Soc. 2011;133(20):8014–8027. doi: 10.1021/ja202103e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoki S, Watanabe Y, Sanagawa M, Setiawan A, Kotoku N, Kobayashi M. Cortistatins A, B, C, and D, anti-angiogenic steroidal alkaloids, from the marine sponge Corticium simplex. J. Am. Chem. Soc. 2006;128(10):3148–3149. doi: 10.1021/ja057404h. [DOI] [PubMed] [Google Scholar]
- 34.Renata H, Zhou Q, Baran PS. Strategic redox relay enables a scalable synthesis of ouabagenin. A bioactive cardenolide. Science. 2013;339(6115):59–63. doi: 10.1126/science.1230631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erhardt PW. In search of the digitalis replacement. J. Med. Chem. 1987;30(2):231–237. doi: 10.1021/jm00385a001. [DOI] [PubMed] [Google Scholar]
- 36.Kamano Y, Kotake A, Hashima H, et al. Structure–cytotoxic activity relationship for the toad poison bufadienolides. Bioorg. Med. Chem. 1998;6(7):1103–1115. doi: 10.1016/s0968-0896(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 37.Giannis A, Heretsch P, Sarli V, Stossel A. Synthesis of cyclopamine using a biomimetic and diastereoselective approach. Angew. Chem. Int. Ed. 2009;48(42):7911–7914. doi: 10.1002/anie.200902520. [DOI] [PubMed] [Google Scholar]
- 38.Taipale J, Chen JK, Cooper MK, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406(6799):1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 39.Taipale J, Beachy PA. The Hedgehog and Wnt signaling pathways in cancer. Nature. 2001;411(6835):349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 40.Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304(5678):1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- 41.Heretsch P, Buettner A, Tzagkaroulaki L, Zahn S, Kirchner B, Giannis A. Exo-cyclopamine-a stable and potent inhibitor of hedgehog-signaling. Chem. Commun. 2011;47(26):7362–7364. doi: 10.1039/c1cc11782c. [DOI] [PubMed] [Google Scholar]
- 42.Moschner J, Chentsova A, Eilert N, Rovardi I, Heretsch P, Giannis A. Cyclopamine analogs bearing exocyclic methylenes are highly potent and acid-stable inhibitors of hedgehog signaling. Beilstein J. Org. Chem. 2013;9:2328–2335. doi: 10.3762/bjoc.9.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuda H, Morikawa T, Oda M, Asao Y, Yoshikawa M. Potent anti-metastatic activity of dimeric sesquiterpene thioalkaloids from the rhizome of nupharpumilum. Bioorg. Med. Chem. Lett. 2003;13(24):4445–4449. doi: 10.1016/j.bmcl.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 44.Matsuda H, Yoshida K, Miyagawa K, Nemoto Y, Asao Y, Yoshikawa M. Nuphar alkaloids with immediately apoptosis-inducing activity from nupharpumilum and their structural requirements for the activity. Bioorg. Med. Chem. Lett. 2006;16(6):1567–1573. doi: 10.1016/j.bmcl.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 45.Ozer J, Eisner N, Ostrozhenkova E, et al. Nuphar lutea thioalkaloids inhibit the nuclear factor kappa B pathway, potentiate apoptosis and are synergistic with cisplatin and etoposide. Cancer Biol. Ther. 2009;8(19):1860–1868. doi: 10.4161/cbt.8.19.9567. [DOI] [PubMed] [Google Scholar]
- 46.Wong CF, Lalonde RT. 6-hydroxyneothiobinupharidine and 6’-hydroxyneothiobinupharidine - monohemiaminals from nuphar luteum and their possible role in thiaspirane nuphar alkaloid biogenesis. Experientia. 1975;31(1):15–16. doi: 10.1007/BF01924651. [DOI] [PubMed] [Google Scholar]
- 47.Jansen DJ, Shenvi RA. Synthesis of (+)-neothiobinupharidine. J. Am. Chem. Soc. 2013;135(4):1209–1212. doi: 10.1021/ja310778t. [DOI] [PubMed] [Google Scholar]
- 48.Yun J, Buchwald SL. One-pot synthesis of enantiomerically enriched 2,3-disubstituted cyclopentanones via copper-catalyzed 1,4-reduction and alkylation. Org. Lett. 2001;3(8):1129–1131. doi: 10.1021/ol015577f. [DOI] [PubMed] [Google Scholar]
- 49.Dowling MS, Vanderwal CD. Ring-closing metathesis of allylsilanes as a flexible strategy toward cyclic terpenes. short syntheses of teucladiol, isotencladiol, poitediol, and dactylol and an attempted synthesis of caryophyllene. J. Org. Chem. 2010;75(20):6908–6922. doi: 10.1021/jo101439h. [DOI] [PubMed] [Google Scholar]
- 50.Hwang YC, Fowler FW. Diels-alder reaction of 1-azadienes. a total synthesis of deoxynupharidine. J. Org. Chem. 1985;50(15):2719–2726. [Google Scholar]
- 51.Hansen C. Hansen Solubility Parameters: A User’s Handbook. Second Edition. FL, USA: CRC Press; 2007. [Google Scholar]
- 52.Chatterjee SS, Bhattacharya SK, Wonnemann M, Singer A, Muller WE. Hyperforin as a possible antidepressant component of hypericum extracts. Life Sci. 1998;63(6):499–510. doi: 10.1016/s0024-3205(98)00299-9. [DOI] [PubMed] [Google Scholar]
- 53.Sparling BA, Moebius DC, Shair MD. Enantioselective total synthesis of hyperforin. J. Am. Chem. Soc. 2013;135(2):644–647. doi: 10.1021/ja312150d. [DOI] [PubMed] [Google Scholar]
- 54.Corey EJ, Staas DD. Demonstration of a common concerted mechanistic pathway for the acid-catalyzed cyclization of 5,6-unsaturated oxiranes in chemical and enzymatic systems. J. Am. Chem. Soc. 1998;120(14):3526–3527. [Google Scholar]
- 55.Keck GE, Enholm EJ, Yates JB, Wiley MR. One electron C-C bond forming reactions via allylstannanes - scope and limitations. Tetrahedron. 1985;41(19):4079–4094. [Google Scholar]
- 56.Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. Efficient and recyclable monomeric and dendritic Ru-based metathesis catalysts. J. Am. Chem. Soc. 2000;122(34):8168–8179. [Google Scholar]
- 57.Jorgensen L, Mckerrall SJ, Kuttruff CA, Ungeheuer F, Felding J, Baran PS. 14-step synthesis of (+)-ingenol from (+)-3-carene. Science. 2013;341(6148):878–882. doi: 10.1126/science.1241606. [DOI] [PubMed] [Google Scholar]
- 58.Hecker E. Cocarcinogenic prinicples from seed oil of croton tiglium and from other euphorbiaceae. Cancer Res. 1968;28(11):2338. [PubMed] [Google Scholar]
- 59.Appendino G, Tron GC, Cravotto G, Palmisano G, Jakupovic J. An expeditious procedure for the isolation of ingenol from the seeds of Euphorbia lathyris. J. Nat. Prod. 1999;62(1):76–79. doi: 10.1021/np980218n. [DOI] [PubMed] [Google Scholar]
- 60.Winkler JD, Rouse MB, Greaney MF, Harrison SJ, Jeon YT. The first total synthesis of (+/-)-ingenol. J. Am. Chem. Soc. 2002;124(33):9726–9728. doi: 10.1021/ja026600a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanino K, Onuki K, Asano K, et al. Total synthesis of ingenol. J. Am. Chem. Soc. 2003;125(6):1498–1500. doi: 10.1021/ja029226n. [DOI] [PubMed] [Google Scholar]
- 62.Nickel A, Maruyama T, Tang H, et al. Total synthesis of ingenol. J. Am. Chem. Soc. 2004;126(50):16300–16301. doi: 10.1021/ja044123l. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe K, Suzuki Y, Aoki K, Sakakura A, Suenaga K, Kigoshi H. Formal synthesis of optically active ingenol via ring-closing olefin metathesis. J. Org. Chem. 2004;69(23):7802–7808. doi: 10.1021/jo048833l. [DOI] [PubMed] [Google Scholar]
- 64.Epstein OL, Cha JK. Rapid access to the ‘in,out’-tetracyclic core of ingenol. Angew. Chem. Int. Ed. 2005;44(1):121–123. doi: 10.1002/anie.200461807. [DOI] [PubMed] [Google Scholar]
- 65.Brummond KM, Chen HF, Fisher KD, et al. An allenic Pauson-Khand-type reaction: a reversal in pi-bond selectivity and the formation of seven-membered rings. Org. Lett. 2002;4(11):1931–1934. doi: 10.1021/ol025955w. [DOI] [PubMed] [Google Scholar]
- 66. Corey EJ. The Logic of Chemical Synthesis. NY, USA: John Wiley & Sons; 1995. Cheng X-M.. • Provides organic chemists with the foundational logic for retrosynthetic analysis. More concepts than those provided in this review can be found here. See chapters 1–6 for discussion of transforms and other terminology.
- 67.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2009;361(5):455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wratten SJ, Faulkner DJ, Hirotsu K, Clardy J. Diterpenoid isocyanides from the marine sponge hymeniacidonamphilecta. Tetrahedron Lett. 1978;19(45):4345–4348. [Google Scholar]
- 69.Meunier B, Robert A. Heme as trigger and target for trioxane-containing antimalarial drugs. Acc. Chem. Res. 2010;43(11):1444–1451. doi: 10.1021/ar100070k. [DOI] [PubMed] [Google Scholar]
- 70.Wright AD, Mccluskey A, Robertson MJ, Macgregor KA, Gordon CP, Guenther J. Anti-malarial, anti-algal, anti-tubercular, anti-bacterial, anti-photosynthetic, and anti-fouling activity of diterpene and diterpeneisonitriles from the tropical marine sponge Cymbastelahooperi. Org. Biomol. Chem. 2011;9(2):400–407. doi: 10.1039/c0ob00326c. [DOI] [PubMed] [Google Scholar]
- 71.Pronin SV, Shenvi RA. Synthesis of a potent antimalarial amphilectene. J. Am. Chem. Soc. 2012;134(48):19604–19606. doi: 10.1021/ja310129b. [DOI] [PubMed] [Google Scholar]
- 72.Hopf H, Sherburn MS. Dendralenes branch out: cross-conjugated oligoenes allow the rapid generation of molecular complexity. Angew. Chem. Int. Ed. 2012;51(10):2298–2338. doi: 10.1002/anie.201102987. [DOI] [PubMed] [Google Scholar]
- 73.Pronin SV, Reiher CA, Shenvi RA. Stereoinversion of tertiary alcohols to tertiary-alkyl isonitriles and amines. Nature. 2013;501(7466):195–199. doi: 10.1038/nature12472. [DOI] [PubMed] [Google Scholar]
- 74.Evans DA. Manuscript 242A: An Organizational Format for the Classification of Functional Groups. Application to the Construction of Difunctional Relationships. MA, USA: Harvard University; 1971. [Google Scholar]
- 75.Kliman LT, Mlynarski SN, Ferris GE, Morken JP. Catalytic enantioselective 1,2-diboration of 1,3-dienes: versatile reagents for stereoselective allylation. Angew. Chem. Int. Ed. 2012;51(2):521–524. doi: 10.1002/anie.201105716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kliman LT, Mlynarski SN, Morken JP. Pt-catalyzed enantioselective diboration of terminal alkenes with B2(pin)2. J. Am. Chem. Soc. 2009;131(37):13210–13211. doi: 10.1021/ja9047762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferris GE, Hong K, Roundtree IA, Morken JP. A catalytic enantioselective tandem allylation strategy for rapid terpene construction: application to the synthesis of pumilaside A glycon. J. Am. Chem. Soc. 2013;135(7):2501–2504. doi: 10.1021/ja400506j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kitajima J, Kimizuka K, Tanaka Y. Three new sesquiterpenoid glucosides of Ficuspumila fruit. Chem. Pharm. Bull. 2000;48(1):77–80. doi: 10.1248/cpb.48.77. [DOI] [PubMed] [Google Scholar]
- 79.Gribble GW. Naturally occurring organohalogen compounds. Acc. Chem. Res. 1998;31(3):141–152. [Google Scholar]
- 80.Neumann CS, Fujimori DG, Walsh CT. Halogenation strategies in natural product biosynthesis. Chem. Biol. 2008;15(2):99–109. doi: 10.1016/j.chembiol.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 81.Talpir R, Rudi A, Kashman Y, Loya Y, Hizi A. 3 new sesquiterpene hydroquinones from marine origin. Tetrahedron. 1994;50(14):4179–4184. [Google Scholar]
- 82.Sarin PS, Sun D, Thornton A, Muller WEG. Inhibition of replication of the etiologic agent of acquired-immune-deficiency-syndrome (human T-lymphotropic retrovirus lymphadenopathy-associated virus) by avarol and avarone. J. Natl Cancer Inst. 1987;78(4):663–666. [PubMed] [Google Scholar]
- 83.Pronin SV, Shenvi RA. Synthesis of highly strained terpenes by non-stop tail-to-head polycyclization. Nature Chem. 2012;4(11):915–920. doi: 10.1038/nchem.1458. [DOI] [PubMed] [Google Scholar]
- 84.Snyder SA, Treitler DS. Et2SBr-SbCl5Br: an effective reagent for direct bromonium-induced polyene cyclizations. Angew. Chem. Int. Ed. 2009;48(42):7899–7903. doi: 10.1002/anie.200903834. [DOI] [PubMed] [Google Scholar]
- 85.Snyder SA, Treitler DS, Brucks AP. Simple reagents for direct halonium-induced polyene cyclizations. J. Am. Chem. Soc. 2010;132(40):14303–14314. doi: 10.1021/ja106813s. [DOI] [PubMed] [Google Scholar]
- 86.Treitler DS, Li Z, Krystal M, Meanwell NA, Snyder SA. Evaluation of HIV-1 inhibition by stereoisomers and analogues of the sesquiterpenoid hydroquinone peyssonol A. Bioorg. Med. Chem. Lett. 2013;23(7):2192–2196. doi: 10.1016/j.bmcl.2013.01.098. [DOI] [PubMed] [Google Scholar]
- 87.Patil AD, Freyer AJ, Killmer L, et al. Frondosins, five new sesquiterpene hydroquinone derivatives with novel skeletons from the sponge Dysidea frondosa: inhibitors of interleukin-8 receptors. Tetrahedron. 1997;53(14):5047–5060. [Google Scholar]
- 88.Hallock YF, Cardellina JH, Boyd MR. (−)-Frondosins A and D, HIV-inhibitory sesquiterpene hydroquinone derivatives from Euryspongia sp. Natural Product Lett. 1998;11(2):153–160. [Google Scholar]
- 89.Inoue M, Frontier AJ, Danishefsky SJ. The total synthesis of frondosin B. Angew. Chem. 2000;112(4):777–780. [PubMed] [Google Scholar]
- 90.Hughes CC, Trauner D. Concise total synthesis of (−)-frondosin B using a novel palladium-catalyzed cyclization. Angew. Chem. Int. Ed. 2002;41(9):1569–1572. doi: 10.1002/1521-3773(20020503)41:9<1569::aid-anie1569>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 91.Hughes CC, Trauner D. Palladium-catalyzed couplings to nucleophilicheteroarenes: the total synthesis of (−)-frondosin B. Tetrahedron. 2004;60(43):9675–9686. [Google Scholar]
- 92.Li X, Ovaska TV. Total synthesis of (+/−)-frondosin B. Org. Lett. 2007;9(19):3837–3840. doi: 10.1021/ol701633z. [DOI] [PubMed] [Google Scholar]
- 93.Ovaska TV, Sullivan JA, Ovaska SI, Winegrad JB, Fair JD. Asymmetric synthesis of seven-membered carbocyclic rings via a sequential oxyanionic 5-exo-dig cyclization/claisen rearrangement process. total synthesis of (−)-frondosin B. Org. Lett. 2009;11(12):2715–2718. doi: 10.1021/ol900967j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reiter M, Torssell S, Lee S, MacMillan DWC. The organocatalytic three-step total synthesis of (+)- frondosin B. Chem. Sci. 2010;1(1):37–42. doi: 10.1039/C0SC00204F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paras NA, MacMillan DWC. New strategies in organic catalysis: the first enantioselective organocatalytic Friedel-Crafts alkylation. J. Am. Chem. Soc. 2001;123(18):4370–4371. doi: 10.1021/ja015717g. [DOI] [PubMed] [Google Scholar]