Abstract
Objective: The purpose of this study was to examine the impact of prior authorization policies on the receipt of antipsychotic medication for Medicaid-enrolled children.
Methods: Using de-identified administrative Medicaid data from two large, neighboring, mid-Atlantic states from November 2007 through June 2011, we identified subjects <18 years of age using antipsychotics, from the broader group of children and adolescents receiving behavioral health services or any psychotropic medication. Prior authorization for antipsychotics was required for children in State A <6 years of age from September 2008, and for children <13 years of age from August 2009. No such prior authorizations existed in State B during that period. Filled prescriptions were identified in the data using national drug codes. Using a triple-difference strategy (using differences among the states, time periods, and differences in antidepressant prescribing rates among states over the same time periods), we examined the effect of the prior authorization policy on the rate at which antipsychotic prescriptions were filled for Medicaid-enrolled children and adolescents.
Results: The impact of prior authorization policies on antipsychotic medication use varied by age: Among 6–12 year old children, the impact of the prior authorization policy on antipsychotic medication prescribing was a modest but statistically significant decrease of 0.47% after adjusting for other factors; there was no effect of the prior authorization among children 0–5 years.
Conclusions: Prior authorization policies had a modest but statistically significant effect on antipsychotic use in 6–12 year old children, but had no impact in younger children. Future research is needed to understand the utilization and clinical effects of prior authorization and other policies and interventions designed to influence antipsychotic use in children.
Introduction
Since the United States Food and Drug Administration (FDA)'s approval of the first atypical (or second generation) antipsychotic medication (hereafter referred to as antipsychotics) approximately two decades ago, the use of such medication in children and adolescents (hereafter referred to as children) has increased dramatically (Zito et al. 2000; Pappadopulos et al. 2002; Zito et al. 2003; Pathak et al. 2004; Cooper et al. 2006; Olfson et al. 2006; Domino and Swartz 2008; Zito et al. 2008a,b). Currently, 15% of all individuals receiving antipsychotics are <18 years of age (Domino and Swartz 2008). Among United States children, antipsychotic prescriptions more than quadrupled from 1995 to 2001 (Olfson et al. 2002; Cooper et al. 2006), a trend that appears to be continuing (Olfson et al. 2012). In fact, the rate of growth of atypical antipsychotic medication use in children exceeds growth of all other psychotropic medication classes (Patel et al. 2005). The use of antipsychotics appears to be greatest in clinical populations for which there is no current FDA indication for antipsychotic use (either for children or adults) and for which evidence showing clinical benefit is modest (Wolraich 2003; Cooper et al. 2006; Olfson et al. 2006; Patel et al. 2006; Thomas et al. 2006; Findling 2008; Loy et al. 2012; Seida et al. 2012). Whereas the increase in antipsychotic use has occurred in all child populations (Patel et al. 2002; Cooper et al. 2006; Olfson et al. 2006; Thomas et al. 2006; Constantine and Tandon 2008; Domino and Swartz 2008; Olfson et al. 2010; Pathak et al. 2010), rates of antipsychotic use in Medicaid-enrolled children has undergone the greatest growth, with one study documenting a 62% increase in the use of antipsychotics among Medicaid-enrolled children between 2002 and 2007 (Matone et al. 2012). Such growth may be related to challenges Medicaid-enrolled children have in accessing specialty non-pharmacologic mental health treatment (Rubin 2013), and challenges administrators and clinicians to undertake robust efforts to ensure that the use of such medications is a clinically appropriate and efficient use of states' limited Medicaid resources.
Policy makers' concern about the increased use of antipsychotics in children, particularly among children with disorders for which there is limited evidence of efficacy, is driven in part by strong evidence of the adverse effects of antipsychotic use, and by mounting evidence that children may be at even greater risk for adverse effects from these medications than adults (Correll and Carlson 2006; McIntyre and Jerrell 2008; Correll et al. 2009; Crystal et al. 2009), including weight gain (Correll and Carlson 2006) and diabetes (Hammerman et al. 2008). States' concerns about the increased use of antipsychotics and the associated costs (Farley et al. 2008; Jerrell et al. 2012), coupled with the increased risk of adverse effects in individuals for whom there is limited support for the effectiveness of these medications, has led states to begin implementing a range of policies designed to influence the use of antipsychotics (Texas Health and Human Services Commission 2010; Vogt et al. 2011). The most common approach, prior authorization—requiring physicians and patients to obtain approval before using a medication—has now been adopted by more than a third of state Medicaid programs (Soumerai et al. 2008).
The impact of prior authorization policies on adult prescribing has been mixed. A number of studies have raised concerns about prior authorization policies in Medicaid populations, such as the greater treatment discontinuities observed among adults subject to a prior authorization policy for antipsychotic medications (Soumerai et al. 2008). Other studies, however, have not found any such negative effects of prior authorization policies reducing the use of antipsychotics (Cunningham 2005; Soumerai et al. 2008; Adams et al. 2009; Abouzaid et al. 2010; Simeone et al. 2010; Walthour et al. 2010; Vogt et al. 2011). With respect to children, little is known about the impact of prior authorization policies on antipsychotic medication use, despite increasing state attention to Medicaid-enrolled child use of antipsychotic medications, highlighted by the establishment of a Medicaid Medical Directors' Learning Network to address use of antipsychotics among children (Foti, 2010), and two subsequent, multi-state, quality collaboratives developed to support states' efforts to improve psychotropic prescribing practices.
In this article, we begin to address this gap in empirical evidence by examining whether there is an impact of prior authorization policies on physician prescribing of antipsychotic medications for Medicaid-enrolled children, and if so, the magnitude of the impact, using Medicaid data from two large, neighboring, mid-Atlantic states. Children in State A were subject to a prior authorization policy implemented in September 2008, requiring physicians prescribing any antipsychotic medication for a child <6 years of age to obtain a prior authorization to prescribe the medication. Approximately a year later in August 2009, a second prior authorization policy was implemented in State A requiring physicians prescribing any antipsychotic medication for children <13 years of age to receive a prior authorization before prescribing an antipsychotic. Both pre-authorization policies presented a relatively modest burden, requiring physicians only to submit a form indicating their specialty, providing information about the child's diagnosis/diagnoses, symptoms, and history of medication trials, and indicating why prior medication trials were ineffective, with an opportunity to appeal if the authorization was denied. No such prior authorization policies were implemented in State B. We hypothesized that use of antipsychotics among children would decrease in State A following the implementation of the prior authorization policies, compared with use in State B.
Methods
Population and variables
Using de-identified Medicaid claims data for Medicaid-enrolled children <18 years of age, in two large, neighboring, mid-Atlantic states, we identified a cohort of children who had received any behavioral health services or treatment between November 2007 and June 2011 (defined as having received specialty outpatient behavioral health services or filled a prescription for a psychotropic medication). For each month during the study period, we then used National Drug Classification (NDC) codes to identify 22,409 children from State A and 61,566 children from State B who had received an antipsychotic medication between November 2007 and June 2011. Using the same method, we identified 24,378 children from State A and 66,979 from State B who had received an antidepressant medication during the same period. Children were categorized as having received the respective medication in a given month if they had received a prescription or had any days' supply remaining from a previously filled prescription for any days in that month. Children were grouped into age cohorts corresponding to the age groups (0–5 years old and 6–12 years old) affected by the prior authorization policies, and an age group (13–17 years old) unaffected by prior authorization policies. We defined our main outcome measure, the antipsychotic prescription rate (APR), as the fraction of children in the cohort who received an antipsychotic in each month. The study was approved by the University of Pittsburgh's Institutional Review Board (IRB), the New York State Office of Mental Health's IRB, and the New York State Psychiatric Institute's IRB.
Analyses
We first conducted descriptive analyses, calculating the APR and the antidepressant prescription rate (ADR) for each age cohort for each month from November 2007 through June 2011, the mean difference between those rates within a state for each month, and the difference between those means across the states, and describing prescribing trends in both states over time.
Using both the prior authorization for children 0–5 years of age that went into effect in September 2008, and the prior authorization affecting children 6–12 years of age that went into effect in August 2009, we used a difference-in-difference-in-difference approach (i.e., a “triple difference” strategy; an extension of a traditional difference-in-difference analysis) to examine the impact of prior authorization policies on APRs among Medicaid children. A traditional difference-in-difference approach would compare the change in the monthly APR in State A (from the period before the passage of prior authorization policy for that age cohort to the period after the passage of the prior authorization policy for that age cohort), to the change in the monthly APR in state B (where no prior authorization law was in place during that period) over the same time periods. Such an approach would allow us to control for pre-implementation differences in APR between State A and B, and also allow us to control for time trends that might otherwise be confounded with the prior authorization policy. However, it would not control for changes in State A that could be affecting the use of all psychotropic medications, such as a lower Medicaid reimbursement rate for medication checks or increased community concerns about the use of psychotropic medications in children. We therefore used the APR for the same populations and over the same time periods to conduct a triple difference analysis. As antidepressants are not directly targeted by the antipsychotic prior authorization policies, this approach allowed us to control for time-varying, state-specific factors that might affect psychotropic prescribing, which are unrelated to the antipsychotic medication prior authorization policies. We conducted separate regressions for each age cohort (0–5-year-olds, and 6–12-year-olds) aggregating data and clustering standard errors at the state-month level, using month-fixed effects, and weighting the data by the sample size in each state-month cell. As a further attempt to ensure that we were assessing the effects of the prior authorization policies, we also re-ran our analyses for 13–17-year-olds, who would be unaffected by either of the prior authorization policies.
Results
Patterns of antipsychotic and antidepressant medication use prior to the prior authorization policy
Prior to the August 2009 prior authorization policy start in State A, the average monthly rate of antipsychotic use among 6–12-year-old children receiving behavioral health services or treatment was 9.8% (Table 1). In State B, for the same time period and population, the average monthly rate of antipsychotic use was 5.9%. The average monthly rate of antidepressant use among the same cohort over the same time period (4.4% and 1.8% in States A and B, respectively) was lower than the average monthly rate of antipsychotic use in both states (Table 1).
Table 1.
Effects of Prior Authorization (PA) Laws on Child Antipsychotic Prescription Rate (APR) and Child Antidepressant Prescription Rate (ADR) in Two States
| 0–5 year old children | 6–12 year old children | 13–17 year old childrena | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| State A (with PA policies) | Before PA policy (%) | After PA policy (%) | Decrease (increase) in rate after PA policy | Difference between antipsychotic and antidepressant change | Before PA policy (%) | After PA policy (%) | Decrease (increase) in rate after PA policy | Difference between antipsychotic and antidepressant change | Before PA policy (%) | After PA policy (%) | Decrease (Increase) in rate after PA policy | Difference between antipsychotic and antidepressant change |
| APR | 1.744 | 2.065 | (0.321) | 9.844 | 9.544 | 0.30 | 11.315 | 11.082 | 0.232 | |||
| ADR | 0.381 | 0.745 | (0.364) | 0.043 | 4.370 | 4.837 | (0.467) | 0.767 | 10.391 | 11.143 | (0.752) | 0.985 |
| State B (without PA policies) | Before PA policy (%) | After PA policy (%) | Decrease (increase) in rate after PA policy | Difference between antipsychotic and antidepressant change | Before PA policy (%) | After PA policy (%) | Decrease (increase) in rate after PA policy | Difference between antipsychotic and antidepressant change | Before PA policy (%) | After PA policy (%) | Decrease (increase) in rate after PA policy | Difference between antipsychotic and antidepressant change |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APR | 0.658 | 0.649 | 0.009 | 5.934 | 5.858 | 0.076 | 7.373 | 6.791 | 0.583 | |||
| ADR | 0.089 | 0.103 | (0.014) | 0.023 | 1.844 | 2.143 | (0.299) | 0.375 | 4.572 | 4.615 | (0.044) | 0.626 |
| Difference in Changes between State A and State B (triple difference) | Difference in Changes between State A and State B (triple difference) | Difference in Changes between State A and State B (triple difference) |
|---|---|---|
| −0.020 | −0.392* | −0.358 |
p<0.01
Reference timing is introduction of prior authorization for 6–12-year-old children. When timing of introduction for 0–5-year-old children is used as the reference, the triple difference estimate is −0.203 m.
Similarly, among 0–5-year-old children, before the start of the prior authorization policy in State A in September 2008, the average monthly rate of antipsychotic use was ∼1.7%, (Table 1), whereas for the same time period and population in State B, the average monthly rate of antipsychotic use was ∼0.66%. The average monthly rate of antidepressant use among the same cohort over the same time period was lower than the average monthly rate of antipsychotic use in both State A (0.38%) and State B (0.09%) (Table 1).
Impact of prior authorizations on antipsychotic use
After the August 2009 start of the prior authorization policy affecting 6–12-year- old children in State A, the average monthly rate of antipsychotic use decreased from 9.8% to 9.5%, a decrease of 0.30% (Table 1). In State B, for the same time period and population, there was a very slight decrease in antipsychotic medication use of 0.08%, leaving the average monthly rate of antipsychotic use essentially unchanged at 5.9%.
Among 0–5-year-old children, after the implementation of prior authorization policy in September 2008 in State A, the average monthly rate of antipsychotic use was ∼2.1% (Table 1), an increase of 0.32% from before the implementation of the policy. In State B, for the same time period and population, the average monthly rate of antipsychotic use decreased by 0.009%, to 0.65% from the 0.66% rate prior to the policy (Table 1).
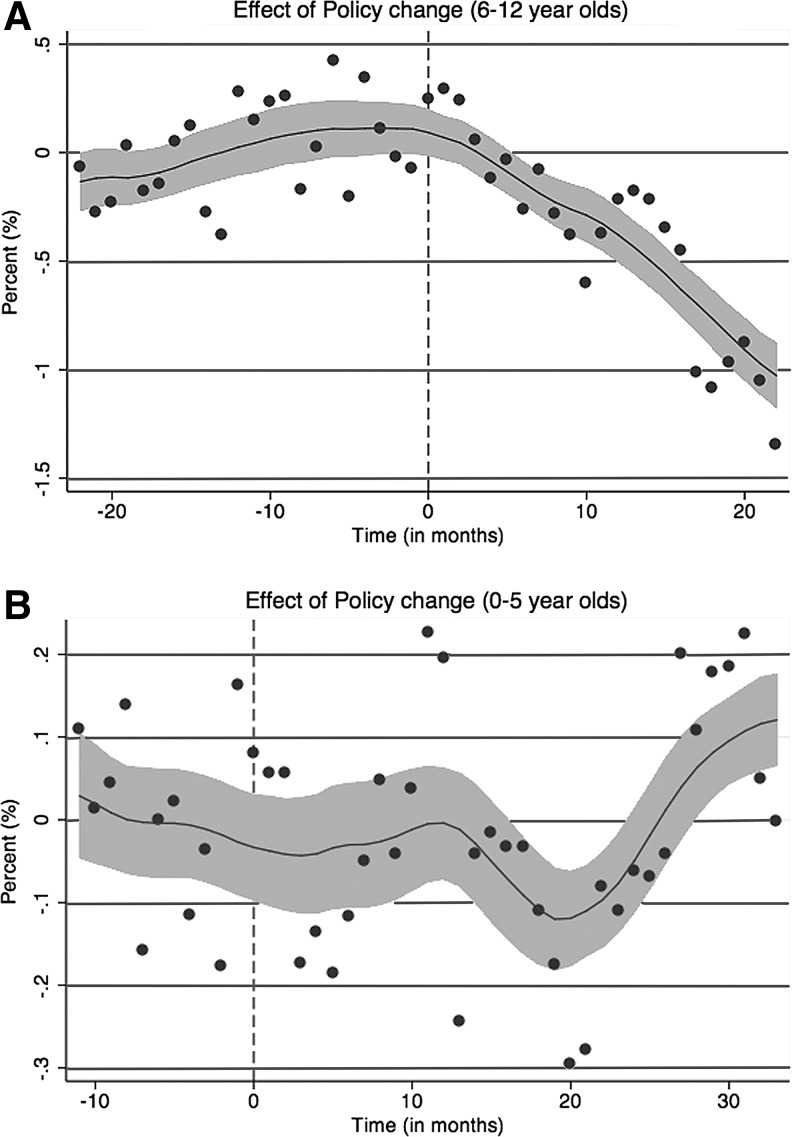
Figures 1A and B illustrate the impact of the prior authorization policies on 6–12- and 0–5-year-olds, respectively, by plotting the difference between the rate of use of antipsychotics and antidepressants in State A minus the same difference in State B for each month, using the mean difference between State A and State B in the pre-authorization period for the respective cohorts as the baseline. The vertical line indicates when the prior authorization was introduced in State A. As the smoothed regression line illustrates, there was a substantial decrease in the use of antipsychotic medications among 6–12-year-olds in State A following the prior authorization policy, but not a comparable change in 0–5-year-olds. When we conduct the triple difference analysis to examine the impact of the prior authorization policies on antipsychotic use, controlling for non-time varying state factors and state-specific trends in psychotropic use, we find that among 6–12-year-olds the prior authorization policy resulted in a 0.4% decrease from the baseline rate in the use of antipsychotic medications (p<0.01; Table 1) in the months after the prior authorization. In contrast, a comparable analysis reveals that the prior authorization policy had a negligible effect (0.02% decrease, ns) on the number of 0–5-year-old children in State A using antipsychotics (Table 1). As expected (given no change in prior authorization policies for this age group), comparable analysis of antipsychotic use among 13–17-year-old children found no significant change in the use of antipsychotic medications among children in State A after either of the prior authorizations was implemented, compared with the rate of use of antipsychotic medications in periods before the prior authorizations.
FIG. 1.
(A) The difference between State A's antipsychotic prescription rate minus the antidepressant prescription rate and State B's antipsychotic prescription rate minus the antidepressant prescription rate for 6–12-year-old children in the period before and after the passage of a prior authorization policy in State A. (B) The difference between State A's antipsychotic prescription rate minus the antidepressant prescription rate and State B's antipsychotic prescription rate minus the antidepressant prescription rate for children <6 years old in the period before and after the passage of a prior authorization policy in State A.
Discussion
We found that new prior authorization policies for antipsychotic medication resulted in a modest but statistically significant decrease in their use among 6–12-year-olds, but did not have a significant effect on antipsychotic use among 0–5-year-olds. Despite the increasing interest among state Medicaid programs in interventions to influence the use of antipsychotic medication in children (Naylor et al. 2007; Hilt et al. 2009; Foti et al. 2010; Hilt 2012), we are unaware of empirical studies that have examined this issue. The findings from this analysis contribute to filling this important gap in knowledge regarding the impact of prior authorization policies on influencing the use of antipsychotic medication in children.
The magnitude of the effect of the prior authorization policy we found on the use of antipsychotic medications by 6–12-year-olds, although statistically significant, was modest, with an unadjusted decrease in the rate of antipsychotic medication use of only 0.30%. Among 0–5-year-olds there was no significant impact of the antipsychotic prior authorization policy, with the rate of antipsychotic medication use increasing after the prior authorization policy by 0.32%, only slightly less than the 0.36% increase in the use of antidepressants in 0–5-year-olds in the state in the same time frame. Although no comparable data from studies of Medicaid-enrolled children are available, effects of comparable magnitude have been observed in similar studies of adults (Simeone et al. 2010; Vogt et al. 2011). Several factors could contribute to this modest policy effect. First, as others have suggested (Law et al. 2008), prior authorization policies may have a lesser effects on antipsychotic prescribing because, compared with many other medications, children may be more likely to be using antipsychotic medications on an ongoing basis, and the impact of prior authorization policies may be greater when an individual is starting a new medication than when a prescription for a medication refill is being written. It may also be that clinicians (and/or families) may be more willing to seek prior authorization for antipsychotic medications for children because of a perceived lack of available treatment alternatives; this hypothesis is consistent with research showing challenges to families in accessing specialty child mental health treatment (Horwitz et al. 2007; Cunningham 2009) in the context of a limited child mental health specialty workforce (Manderscheid and Henderson 2002; Kim 2003; Thomas and Holzer 2006). We note, however, that in some situations, prior authorization policies have been associated with changes in the use of antipsychotic medications in youth. For example, a prior authorization policy in Illinois decreased the concurrent use of two or more antipsychotic medications among youth <18 years of age in the child welfare system (Naylor 2013), whereas a program in Washington involving elective and mandatory prior authorization telephone consultations with a child psychiatrist decreased antipsychotic use among Medicaid-enrolled children (Hilt et al. 2012). Similar to the substantial variation in state approaches to prior authorizations for the use of antidepressants in Medicaid-enrolled youth (Fischer et al. 2007), there is likely substantial variation across states in the processes and goals of antipsychotic prior authorization policies. As a result, it will be useful for future studies of prior authorization policies to examine such policies at a more granular level with respect to the level of burden, the specific target of the policy, and the context (including other concurrent interventions) in which the policy is being implemented. It will also be useful to examine how such prior authorization policies interact with patient, prescriber, practice, and payer factors.
It is also unclear why the effect of a prior authorization policy we did observe existed among 6–12-year-olds but not among children <6 years of age. Among children 6–12 years old, antipsychotics are commonly used to treat off-label disorders such as attention-deficit/hyperactivity disorder (ADHD), other disruptive behavioral disorders, and depressive disorders (Wolraich 2003; Cooper et al. 2006; Olfson et al. 2006; Patel et al. 2006; Thomas et al. 2006; Aparasu and Bhatara 2007; Findling 2008; Crystal et al. 2009; Olfson et al. 2010; Pathak et al. 2010; Constantine et al. 2011). Younger children, who are less likely to receive these diagnoses, are more likely to be prescribed antipsychotics for autism spectrum disorders and other complex clinical situations. One possibility, therefore, is that prior authorization policies may be more likely to influence prescribing practices for 6–12-year-olds because the disorders for which they are commonly prescribed in this age group, such as ADHD and other disruptive behavior disorders, often have more alternative, evidence-based psychosocial and pharmacological treatment options, which were not subject to prior authorization. In contrast, disorders such as autism spectrum disorder have relatively fewer alternative evidence-based treatments that can substitute for antipsychotics. Unfortunately, the absence of information about children's diagnoses in our data prevents us from examining this issue directly. Further research is needed to better understand the impact of prior authorization policies on the prescribing of antipsychotic medications for populations of children with different diagnoses.
Similar to our findings, other studies have also found substantial variation across states in the use of psychotropic medications (Raghavan et al. 2010; Leslie et al. 2011; Merikangas et al. 2012), including variations in rates of use of antipsychotic medications (Foti et al. 2010; Merikangas et al. 2012), and have shown that a range of factors are likely to influence the rates of use of psychotropic medications, beyond the clinical characteristics of the patients. Such factors may include Medicaid eligibility requirements, shortages of child mental health specialty providers (Koplewicz 2010; Caccavale 2012; Musgrove 2012), differences in services available for vulnerable populations, pharmaceutical promotion (Berndt and Donohue 2008), or quality improvement efforts (Wisdom et al. unpublished data) such as phone consultation programs (Naylor et al. 2007; Hilt et al. 2009; Hilt 2012; Hilt et al. 2013). Further research is needed to better understand the impact of such factors on variations in the use of child antipsychotic medications across states and regions.
Limitations
As with all studies, our results must be considered within the context of its limitations. In our examination of the impact of the prior authorization policies on the use of antipsychotic medications in children, we attempted to control for other factors that may influence their use by using data from both a neighboring state and from the use of another psychotropic medication. This allowed us to control for any unobserved changes that might be influencing the prescribing of antipsychotic medications. Our triple difference strategy has strong internal validity and only requires that there be no unobserved factors that are correlated with the prior authorization policy change but not the prescribing of other psychotropic medications. Our approach also assumes that physicians are not substituting antidepressant medications for antipsychotic medications, an assumption that we believe is reasonable, as the two classes of medications are commonly used to treat different disorders. We note, however, that there was an increase in the use of antidepressants among 6–12-year-old children following the introduction of the prior authorization policy. It is possible that in cases in which physicians were uncertain whether an antipsychotic would offer particular clinical benefits compared with another psychotropic agent, they may have used an antidepressant instead of an antipsychotic medication after the prior authorization, although there was also an increase in antidepressant use among 6–12-year-olds in State B over the same time frame, which would not have been influenced by a prior authorization policy. Such a possibility reinforces the importance of examining not only the rate of use of medication after the introduction of a prior authorization policy, but also, to the extent possible, the appropriateness of observed shifts in prescribing patterns to other related agents. Unfortunately, we did not have access to diagnostic information, making us unable to determine to what extent utilization of antipsychotic medications varied by child clinical status. Similar to other studies of the impact of prior authorization policies in adults (Cunningham 2005; Soumerai et al. 2008; Adams et al. 2009; Abouzaid et al. 2010; Simeone et al. 2010; Walthour et al. 2010; Vogt et al. 2011), we do not have any way to determine the burden on physicians of the prior authorization policies. Physician burden would be important to measure in future studies, as it might explain the variability in prior authorization policy impacts observed in different studies and/or different states. Physician behavior would also be more likely to be influenced in situations in which the rates of authorizations not being approved was higher; unfortunately, we do not know how frequently sought authorizations were not being approved, nor if the rate of not being approved varied significantly for authorizations sought for 6–12-year-old children versus 0–5-year-old children. Finally, we were unable to compare rates of antipsychotic use in our population of Medicaid-enrolled children receiving any psychotropic medication or behavioral health services with studies of antipsychotic use in populations of all Medicaid-enrolled children reported by others.
Conclusions
Despite these limitations, this article contributes to our understanding of the impact of prior authorization policies on the use of antipsychotic medications among children. Our findings suggest that, even when effective, the prior authorization policy impact may be modest, and in some populations it may have little or no impact. Given the range of potential negative health effects of antipsychotics (Correll and Carlson 2006; Correll 2007; Hammerman et al. 2008; McIntyre and Jerrell 2008; Correll et al. 2009; Crystal et al. 2009), and that the cost of such medications now far exceeds that of any other drug class in Medicaid (Crystal et al. 2009), it is reasonable to anticipate that states will continue to make efforts to ensure that antipsychotic medications are appropriately used in children, and are not overused or misused. Our study highlights the limitations of prior authorization policies alone in influencing prescriber behavior, as well as the fact that such policies may not influence prescribing patterns equally for all groups of children. It also suggests the need for evaluations of more targeted and/or clinically nuanced and informed approaches, such as efforts to work with prescribers whose use of antipsychotics are outside the norm (Becker et al. 2013), or telephone consultation programs, second opinion programs, or consultation and feedback systems (Naylor et al. 2007; Hilt et al. 2009; Foti et al. 2010; Hilt 2012; Hilt et al. 2013). Given that the ultimate goal, however, is helping children and families, it is important that evaluations of such efforts move beyond examining the impact of such initiatives on the prescribing of antipsychotic medications, and examine the impact on the clinical and functional outcomes of the children receiving such medications and their families.
Clinical Significance
In recent years, there has been increasing attention and concern regarding the use of antipsychotic medication in children, especially given the increasing off-label use of such medications, their costs, and the increased risk of adverse effects in children. This study of the impact of prior authorization policies for use of antipsychotic medications in children suggests that even when significant, the impact of such policies may be quite modest, and suggests the need for more targeted and clinically nuanced and informed approaches to meaningfully influence the prescribing patterns of antipsychotics in children.
Acknowledgments
The authors thank Mike Naylor and Robert Hilt for information regarding state Medicaid prior authorization programs, and Gina Boyd of the RAND Corporation for research assistance and manuscript preparation.
Disclosures
Qingxian Chen has no disclosures to report. Ka Ho Brian Chor has no disclosures to report. Molly Finnerty is currently supported by the New York State Office of Mental Health. Dr. Finnerty currently receives research support from Agency for Healthcar Research and Quality (AHRQ). Dr. Finnerty also currently serves as principal investigator on a project in which her research staff is supported by a Sunovion contract, and in the past has served as the principal investigator on a project in which her research staff was supported by Bristol Myers Squibb Foundation. Emily Leckman-Westin has no disclosures to report. Edward Okeke has no disclosures to report. Deborah Scharf has no disclosures to report. Mark Sorbero has no disclosures to report. Bradley Stein has previously received research support from Pfizer and served on an Advisory Board for Otsuka Pharmaceuticals, and is currently receiving research support from the California Mental Health Services Authority, the National Institute of Mental Health (NIMH), National Institute on Drug Abuse (NIDA), and Substance Abuse and Mental Health Services Administration (SAMHSA). Dr. Stein was previously employed by Community Care Behavioral Health Organization, a nonprofit managed behavioral health organization, when this work was done, and has previously received research support from Ortho-MacNeil Jannsen. Jennifer Wisdom has no disclosures to report.
References
- Abouzaid S, Jutkowitz E, Foley KA, Pizzi LT, Kim E, Bates J: Economic impact of prior authorization policies for atypical antipsychotics in the treatment of schizophrenia. Popul Health Manag 13:247–254, 2010 [DOI] [PubMed] [Google Scholar]
- Adams AS, Zhang F, LeCates RF, Graves AJ, Ross–Degnan D, Gilden D, McLaughlin TJ, Lu C, Trinacty CM, Soumerai SB: Prior authorization for antidepressants in Medicaid: Effects among disabled dual enrollees. Arch Intern Med 169:750, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparasu RR, Bhatara V: Patterns and determinants of antipsychotic prescribing in children and adolescents, 2003–2004. Curr Med Res Opin 23:49–56, 2007 [DOI] [PubMed] [Google Scholar]
- Becker ER, Constantine RJ, McPherson MA, Jones ME: Antipsychotic polypharmacy prescribing patterns and costs in the Florida adult and child Medicaid populations. J Health Care Finance 40:40–67, 2013 [PubMed] [Google Scholar]
- Berndt ER, Donohue JM: The economics of advertising in health care markets. In: Incentives and Choice in Health Care. Edited by Sloan F. and Kasper H. Cambridge, MA: MIT Press; 2008. pp 131–162 [Google Scholar]
- Caccavale J: Psychiatry In Crisis: Impacts on Primary Care, Patient Safety and Public Healthcare Policy. I. Available at http://www.truthindrugs.org/pdf/crisis.pdf Accessed May21, 2014
- Constantine R, Tandon R: Changing trends in pediatric antipsychotic use in Florida's Medicaid program. Psychiatr Serv 59:1162–1168, 2008 [DOI] [PubMed] [Google Scholar]
- Constantine RJ, Tandon R, McPherson M, Andel R: Early diagnoses and psychotherapeutic medication treatment experiences of a cohort of children under 6 years old who received antipsychotic treatment in Florida's Medicaid program. J Child Adolesc Psychopharmacol 21:79–84, 2011 [DOI] [PubMed] [Google Scholar]
- Cooper WO, Arbogast PG, Ding H, Hickson GB, Fuchs DC, Ray WA: Trends in prescribing of antipsychotic medications for US children. Ambul Pediatr 6:79–83, 2006 [DOI] [PubMed] [Google Scholar]
- Correll CU: Weight gain and metabolic effects of mood stabilizers and antipsychotics in pediatric bipolar disorder: A systematic review and pooled analysis of short-term trials. J Am Acad Child Adolesc Psychiatry 46:687, 2007 [DOI] [PubMed] [Google Scholar]
- Correll CU, Carlson HE: Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry 45:771–791, 2006 [DOI] [PubMed] [Google Scholar]
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK: Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302:1765–1773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal S, Olfson M, Huang C, Pincus H, Gerhard T: Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood) 28:w770–781, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham PJ: Beyond parity: Primary care physicians' perspectives on access to mental health care. Health Aff (Millwood) 28:w490–501, 2009 [DOI] [PubMed] [Google Scholar]
- Cunningham PJ: Medicaid cost containment and access to prescription drugs. Health Aff (Millwood) 24:780–789, 2005 [DOI] [PubMed] [Google Scholar]
- Domino ME, Swartz MS: Who are the new users of antipsychotic medications? Psychiatr Serv 59:507–514, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley JF, Cline RR, Schommer JC, Hadsall RS, Nyman JA: Retrospective assessment of Medicaid step-therapy prior authorization policy for atypical antipsychotic medications. Clin Ther 30:1524–1539, 2008 [DOI] [PubMed] [Google Scholar]
- Findling RL: Atypical antipsychotic treatment of disruptive behavior disorders in children and adolescents. J Clin Psychiatry 69Suppl 4:9–14, 2008 [PubMed] [Google Scholar]
- Fischer MA, Servi AD, Polinski JM, Wang PS: Restrictions on antidepressant medications for children: A review of Medicaid policy. Psychiatr Serv 58:135–138, 2007 [DOI] [PubMed] [Google Scholar]
- Foti ME, Harper G, Moon R, Oestreich G, Snow R, Thompson J, Finnerty M, Freeman E, Knapp P, Muse NJ, Parks J, Crystal S, Gerhard T, Wilson N: Antipsychotic Medication Use in Medicaid Children and Adolescents: Report and Resource Guide From a 16-State Study. MMDLN/Rutgers CERTs, 2010 [Google Scholar]
- Hammerman A, Dreiher J, Klang SH, Munitz H, Cohen AD, Goldfracht M: Antipsychotics and diabetes: An age-related association. Ann Pharmacother 42:1316–1322, 2008 [DOI] [PubMed] [Google Scholar]
- Hilt RJ: Monitoring psychiatric medications in children. Pediatr Ann 41:157–163, 2012 [DOI] [PubMed] [Google Scholar]
- Hilt R, McDonell MG RC, Golombek A, Thompson J: The Partnership Access Line: Establishing an empirically based child psychiatry consultation program for Washington State. Rep Emot Behav Disord Youth 9:9–12, 2009 [Google Scholar]
- Hilt RJ, Romaire MA, McDonell MG, Sears JM, Krupski A, Thompson JN, Myers J, Trupin EW: The Partnership Access Line: Evaluating a child psychiatry consult program in Washington State. JAMA Pediatr 167:162–168, 2013 [DOI] [PubMed] [Google Scholar]
- Hilt R, Varley C, Thompson J: Antipsychotic utilization changes for Medicaid children associated with Washington state PAL and 2nd opinion telephone mental health consult services. Neuropsychiatr Enfance et Adolesc 60:S185–S186, 2012 [Google Scholar]
- Horwitz SM, Kelleher KJ, Stein RE, Storfer–Isser A, Youngstrom EA, Park ER, Heneghan AM, Jensen PS, O'Connor KG, Hoagwood KE: Barriers to the identification and management of psychosocial issues in children and maternal depression. Pediatrics 119:e208–218, 2007 [DOI] [PubMed] [Google Scholar]
- Jerrell JM, McIntyre RS, Black GB: Economic grand rounds: economic costs of failure to monitor adverse effects of second-generation antipsychotics: An underestimated factor. Psychiatr Serv 63:202–204, 2012 [DOI] [PubMed] [Google Scholar]
- Kim WJ: Child and adolescent psychiatry workforce: A critical shortage and national challenge. Acad Psychiatry 27:277–282, 2003 [DOI] [PubMed] [Google Scholar]
- Koplewicz H. Answers About Child Psychiatry.The New York Times, May26, 2010 [Google Scholar]
- Law MR, Ross–Degnan D, Soumerai SB: Effect of prior authorization of second-generation antipsychotic agents on pharmacy utilization and reimbursements. Psychiatr Serv 59:540–546, 2008 [DOI] [PubMed] [Google Scholar]
- Leslie LK, Raghavan R, Hurley M, Zhang J, Landsverk J, Aarons G: Investigating geographic variation in use of psychotropic medications among youth in child welfare. Child Abuse Negl 35:333–342, 2011 [DOI] [PubMed] [Google Scholar]
- Loy J, Merry S, Hetrick S, Stasiak K: Atypical antipsychotics for disruptive behaviour disorders in children and youths. Cochrane Database Syst Rev 9:CD008559, 2012 [DOI] [PubMed] [Google Scholar]
- Manderscheid RW, Henderson MJ: Mental health practitioners and trainees. In: Mental Health, United States, 2002. Rockville, MD: United States Department of Health and Human Services (USDHHS), Substance Abuse and Mental Health Services Administration; 2002, pp. 332–373 [Google Scholar]
- Matone M, Localio R, Huang YS, Dosreis S, Feudtner C, Rubin D: The relationship between mental health diagnosis and treatment with second-generation antipsychotics over time: A national study of U.S. Medicaid-enrolled children. Health Serv Res 47:1836–1860, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Jerrell JM: Metabolic and cardiovascular adverse events associated with antipsychotic treatment in children and adolescents. Arch Pediatr Adolesc Med 162:929–935, 2008 [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He J, Rapoport J, Vitiello B, Olfson M: Medication use in US youth with mental disorders. Arch Pediatr Adolesc Med:1–8, 2012 [DOI] [PubMed] [Google Scholar]
- Musgrove B. Use of antipsychotic drugs up sharply among poor children in Kentucky. I. Available at http://www.mcclatchydc.com/2012/10/10/171084/use–of-antipsychotic-drugs-up.html Accessed May21, 2014
- Naylor M: Monitoring the use of antipsychotic medications in foster children: The Illinois model. In: Proceedings of the 60th Annual Meeting of the American Academy of Child and Adolescent Psychiatry Orlando, FL, 2013 [Google Scholar]
- Naylor MW, Davidson CV, Ortega–Piron DJ, Bass A, Gutierrez A, Hall A: Psychotropic medication management for youth in state care: Consent, oversight, and policy considerations. Child Welfare 86:175–192, 2007 [PubMed] [Google Scholar]
- Olfson M, Blanco C, Liu SM, Wang S, Correll CU: National trends in the office-based treatment of children, adolescents, and adults with antipsychotics. Arch Gen Psychiatry 69:1247–1256, 2012 [DOI] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Liu L, Moreno C, Laje G: National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry 63:679–685, 2006 [DOI] [PubMed] [Google Scholar]
- Olfson M, Crystal S, Huang C, Gerhard T: Trends in antipsychotic drug use by very young, privately insured children. J Am Acad Child Adolesc Psychiatry 49:13–23, 2010 [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Weissman MM, Jensen PS: National trends in the use of psychotropic medications by children. J Am Acad Child Adolesc Psychiatry 41:514–521, 2002 [DOI] [PubMed] [Google Scholar]
- Pappadopulos E, Jensen PS, Schur SB, MacIntyre JC, 2nd, Ketner S, Van Orden K, Sverd J, Sardana S, Woodlock D, Schweitzer R, Rube D: “Real world” atypical antipsychotic prescribing practices in public child and adolescent inpatient settings. Schizophr Bull 28:111–121, 2002 [DOI] [PubMed] [Google Scholar]
- Patel NC, Crismon ML, Hoagwood K, Johnsrud MT, Rascati KL, Wilson JP: Physician specialty associated with antipsychotic prescribing for youths in the Texas Medicaid program. Med Care 44:87–90, 2006 [DOI] [PubMed] [Google Scholar]
- Patel NC, Crismon ML, Hoagwood K, Johnsrud MT, Rascati KL, Wilson JP, Jensen PS: Trends in the use of typical and atypical antipsychotics in children and adolescents. J Am Acad Child Adolesc Psychiatry 44:548–556, 2005 [DOI] [PubMed] [Google Scholar]
- Patel NC, Sanchez RJ, Johnsrud MT, Crismon ML: Trends in antipsychotic use in a Texas medicaid population of children and adolescents: 1996 to 2000. J Child Adolesc Psychopharmacol 12:221–229, 2002 [DOI] [PubMed] [Google Scholar]
- Pathak P, West D, Martin BC, Helm ME, Henderson C: Evidence-based use of second-generation antipsychotics in a state Medicaid pediatric population, 2001–2005. Psychiatr Serv 61:123–129, 2010 [DOI] [PubMed] [Google Scholar]
- Pathak S, Arszman SP, Danielyan A, Johns ES, Smirnov A, Kowatch RA: Psychotropic utilization and psychiatric presentation of hospitalized very young children. J Child Adolesc Psychopharmacol 14:433–442, 2004 [DOI] [PubMed] [Google Scholar]
- Raghavan R, Lama G, Kohl P, Hamilton B: Interstate variations in psychotropic medication use among a national sample of children in the child welfare system. Child Maltreat 15:121–131, 2010 [DOI] [PubMed] [Google Scholar]
- Rubin D: Conflicting data on psychotropic use by children: Two pieces to the same puzzle. JAMA Pediatr 126, 189–190, 2013 [DOI] [PubMed] [Google Scholar]
- Seida J, Schouten J, Mousavi S, Hamm M, Beaith A, Vandermeer B, Dryden D, Boylan K, Newton A: First-and second-generation antipsychotics for children and young adults. Comparative Effectiveness Review No. 39. Rockville, MD: Agency for Healthcare Research and Quality; 2012 [PubMed] [Google Scholar]
- Simeone JC, Marcoux RM, Quilliam BJ: Cost and utilization of behavioral health medications associated with rescission of an exemption for prior authorization for severe and persistent mental illness in the Vermont Medicaid program. J Manag Care Pharm 16:317–328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumerai SB, Zhang F, Ross–Degnan D, Ball DE, LeCates RF, Law MR, Hughes TE, Chapman D, Adams AS: Use of atypical antipsychotic drugs for schizophrenia in Maine Medicaid following a policy change. Health Aff (Millwood) 27:w185–195, 2008 [DOI] [PubMed] [Google Scholar]
- Texas Health and Human Services Commission: Safety and Appropriateness of Antipsychotic Medications for Medicaid Children Under Age 16. Report to the Texas Legislature as Required by H.B. 2163, 81st Legislature, Regular Session, 2009, 2010
- Thomas CP, Conrad P, Casler R, Goodman E: Trends in the use of psychotropic medications among adolescents, 1994 to 2001. Psychiatr Serv 57:63–69, 2006 [DOI] [PubMed] [Google Scholar]
- Thomas CR, Holzer CE, 3rd: The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry 45:1023–1031, 2006 [DOI] [PubMed] [Google Scholar]
- Vogt WB, Joyce G, Xia J, Dirani R, Wan G, Goldman DP: Medicaid cost control measures aimed at second-generation antipsychotics led to less use of all antipsychotics. Health Aff (Millwood) 30:2346–2354, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walthour A, Seymour L, Tackett R, Perri M: Assessment of changes in utilization of health–care services after implementation of a prior authorization policy for atypical antipsychotic agents. Ann Pharmacother 44:809–818, 2010 [DOI] [PubMed] [Google Scholar]
- Wolraich ML: Annotation: The use of psychotropic medications in children: An American view. J Child Psychol Psychiatry 44:159–168, 2003 [DOI] [PubMed] [Google Scholar]
- Zito J, Safer D, de Jong-van den Berg LT, Janhsen K, Fegert JM, Gardner JF, Glaeske G, Valluri SC.: A three-country comparison of psychotropic medication prevalence in youth. Child Adolesc Psychiatry Ment Health 2:26–33, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F: Trends in the prescribing of psychotropic medications to preschoolers. JAMA 283:1025–1030, 2000 [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, DosReis S, Gardner JF, Magder L, Soeken K, Boles M, Lynch F, Riddle MA: Psychotropic practice patterns for youth: A 10-year perspective. Arch Pediatr Adolesc Med 157:17–25, 2003 [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, Sai D, Gardner JF, Thomas D, Coombes P, Dubowski M, Mendez–Lewis M: Psychotropic medication patterns among youth in foster care. Pediatrics 121:e157–163, 2008b [DOI] [PubMed] [Google Scholar]