Abstract
Background
To effectively treat differentiated thyroid cancer (DTC) with radioiodine (RAI) it is necessary to raise serum thyrotropin (TSH) levels either endogenously by thyroid hormone withdrawal (THW) or exogenously by administration of recombinant human TSH (rhTSH). The aim of our study was to compare the relative efficacy and side effect profile of rhTSH versus THW preparation for RAI therapy of metastatic DTC.
Methods
Fifty-six patients (31 women and 25 men) with RAI-avid distant metastases of DTC treated with either rhTSH-aided (n=15) or THW-aided RAI (n=41) and followed for 72±36.2 months were retrospectively analyzed. The groups were comparable in regard to mean size of target lesions (rhTSH vs. THW 6.4 vs. 4.8 cm, p=0.41), mean baseline thyroglobulin level (6995 vs. 5544 ng/mL, p=0.83), distribution of micronodular and macronodular pulmonary metastases (67% vs. 63%, p=0.54, 13% vs. 15% p=0.64, respectively), osseous (53% vs. 29%, p=0.09), brain (0% vs. 2%, p=0.73), and liver/kidney metastases (13% vs. 2%, p=0.61). Patients in the rhTSH group were older (rhTSH vs. THW mean 62 vs. 49 years, p=0.01), and received lower cumulative RAI dose (256 vs. 416 mCi, p=0.03), which was more frequently based on dosimetric calculations (80% vs. 46%, p=0.024). Responses to treatment were based on RECIST 1.1 criteria.
Results
Adjusted by age rates of complete response (CR), stable disease (SD), progressive disease (PD), and progression free survival (PFS) were not different between the groups (rhTSH vs. THW CR hazard ratio [HR] 0.97, 95% CI 0.08–11.42, p=0.982; SD HR 3.22, 95% CI 0.79–13.18, p=0.104, PD HR 0.26, 95% CI 0.52–1.26, p=0.094; PFS HR 0.41, 95% CI 0.14–1.23, p=0.112). The only independent risk factor for nonresponding to treatment and presentation with PD was age (HR 1.06, 95% CI 1.02–1.11, p=0.008). Age was also an independent factor affecting PFS (HR 1.04 for each year, 95% CI 1.02–1.07, p=0.001). Rates of leukopenia, thrombocytopenia, xerostomia, and restrictive pulmonary disease after RAI were not significantly different (rhTSH vs. THW 30% vs. 28%, p=0.61, 10% vs. 0%, p=0.37, 0% vs. 12%, p=0.20, 0% vs. 2%, p=0.73, respectively).
Conclusions
Patients with metastatic DTC prepared with rhTSH achieve comparable benefit of RAI therapy as those treated after THW.
Introduction
The routine management of patients with differentiated thyroid cancer (DTC) presenting with distant metastases (DM) consists of thyroidectomy with or without lymph node dissection, as appropriate, followed by the therapy with radioiodine (RAI) (1). American Thyroid Association (ATA) guidelines underscore the main goals of administration of RAI: (i) remnant ablation (to facilitate detection of recurrent disease and initial staging), (ii) adjuvant therapy (to decrease risk of recurrence and disease specific mortality by destroying suspected, but unproven metastatic disease), or (iii) RAI therapy (to treat known persistent disease). Administration of RAI requires thyrotropin (TSH) stimulation which may be achieved by two possible methods: (i) levothyroxine (LT4) withdrawal (thyroid hormone withdrawal [THW]) to provoke endogenous TSH elevation or (ii) exogenous stimulation by recombinant human TSH (rhTSH) (Thyrogen®). rhTSH was approved by the European Medicines Agency (EMEA) in 2005 for the ablation of remnant thyroid tissue in low-risk patients who have undergone total/near total thyroidectomy and by the United States in 2007 for RAI treatment in patients without evidence of DM (1,2). Use of rhTSH for therapy of metastatic disease is not Food and Drug Administration (FDA) and EMEA approved and the only available data bearing on efficacy in this group of patients are derived from the studies based on the Thyrogen Compassionate Use Program (TCUP). On clinical grounds, high-risk patients with metastatic disease may be older and more likely to have comorbid medical conditions. In such patients, a potential advantage of using rhTSH-aided treatment is to obviate the signs and symptoms of hypothyroidism that might be poorly tolerated by these subjects. Another theoretical advantage of the use of rhTSH in high-risk patients relates to the possibility that use of rhTSH means shorter term potential stimulation of tumor growth compared with 3–6 weeks of withdrawal preparation for adequate endogenous TSH stimulation. Another rationale for the use of rhTSH is the more rapid whole-body clearance of RAI after rhTSH in the euthyroid patient, which results in a lower total-body, bone-marrow, and gastrointestinal radiation exposure for a given administered activity (3–6).
However, there are minimal and contradictory data regarding the actual relative efficacy of rhTSH-aided versus THW-aided RAI therapy for the treatment of patients with DM of DTC, some of them suggesting similar efficacy of both methods of TSH stimulation (7–9) and others showing lower RAI uptake in metastatic lesions after preparation with rhTSH (10,11). These discrepancies formed the rationale for our study, the aim of which was to compare the relative efficacy and side effect profile of rhTSH versus THW preparation for RAI therapy of metastatic DTC.
Methods
We retrospectively analyzed medical records of patients with DTC treated and/or monitored at Washington Hospital Center (WHC) and Georgetown University Hospital (GUH), Washington, DC between 1996 and 2009. Inclusion criteria were: (i) confirmed diagnosis of DTC after total or near total thyroidectomy, (ii) evidence of DM based on imaging studies (computed tomography [CT], magnetic resonance imaging [MRI], positron emission tomography [PET-CT]), (iii) RAI-avid disease documented by uptake on post-therapy RAI whole-body scans (WBS), (iv) at least one complete follow-up examination after 131I therapy including CT and other imaging techniques (MRI, PET-CT, WBS) if indicated, and serum thyroglobulin (Tg) levels. All CT scans were performed without administration of intravenous radiocontrast using a slice thickness no greater than 5 mm. The study group was divided into two subgroups: (i) rhTSH—patients exclusively treated with rhTSH-aided RAI and (ii) THW—patients exclusively treated with THW-aided RAI. Patients from rhTSH group were given 0.9 mg rhTSH intramuscularly on days 1 and 2, followed by a therapeutic dose of 131I ∼24–30 hours later. Patients returned for a post-therapy WBS approximately one week later. If the patients in the THW group were taking LT4, it was discontinued about 6 weeks prior to the treatment. Patients in the THW group were administered tri-iodothyronine for 4 weeks after total thyroidectomy and then withdrawn from it 2 weeks before RAI therapy. The post-therapy scan was performed approximately one week following the therapeutic dose of 131I. All patients in both groups adhered to a low iodine diet for at least 2 weeks prior to the treatment. The study protocol was approved by the Institutional Review Boards of WHC and GUH.
The response to therapy was defined according to the revised RECIST 1.1 criteria (12). The study included patients with target lesions and exclusively nontarget lesions. Definitions of target and nontarget lesions and definitions of response to RAI therapy (complete response [CR], partial response [PR], stable disease [SD], progressive disease [PD]) are presented in Tables 1 and 2. The best overall response was assessed based on CT scans performed after the end of 131I therapy (12). Progression free survival (PFS) was assessed from the day of the last RAI therapy until the last CT scan performed during the follow-up period. The effect of RAI therapy on the percentage change in Tg levels (ΔTg) between the (i) baseline nonstimulated Tg measured after surgery but before 131I therapy and (ii) suppressed Tg measured after the last follow-up study was assessed. During the subsequent years of follow-up, Tg measurements were performed with three immunometric assays with functional sensitivities of 0.2, 0.5, and 0.9 ng/mL and were analyzed by Quest Diagnostics (Madison, NJ), LabCorp (Burlington, NC), and the WHC Laboratory (Washington, DC). All patients were screened for anti-Tg antibody using the chemiluminescence immunoassay (ICMA) at all three laboratories. The frequency and grade of leukopenia and thrombocytopenia were documented by complete blood counts performed ∼4–6 weeks after 131I therapy (Table 3) (13). Frequency of xerostomia was assessed for the individuals for whom there was clinical record information regarding the presence/absence of symptoms of chronic dry mouth after 131I therapy. Occurrence of pulmonary fibrosis was assessed based on documentation of the presence or absence of respiratory signs or symptoms warranting further diagnostic procedures, including pulmonary function tests.
Table 1.
Baseline Documentation of Target and Nontarget Lesions
| Lesions | Definition | Baseline documentation |
|---|---|---|
| Target lesions | RAI-avid areas with the largest dimension of more than or equal to 1 cm by CT scan, and in the case of metastatic lymph nodes as more than or equal to 1.5 cm in short axis when assessed by CT scan | All target lesions up to a maximum of five lesions total and a maximum of two lesions per organ were measured and the sum of the longest diameter (or in case of lymph nodes of the short axis) was recorded. |
| Nontarget lesions | All other metastatic lesions, lesions which were subjected to additional locoregional treatment such as additional surgery or external beam radiation therapy were also considered nontarget lesions. | All nontarget lesions were recorded at baseline as present, absent, countable, uncountable, or multiple. |
Source: Eisenhauer et al. (12).
CT, computed tomography; RAI, radioiodine.
Table 2.
Evaluation of the Response to the Treatment with Radioiodine
| Response | Definition |
|---|---|
| CR | (i) Disappearance of all target and nontarget lesions; (ii) Reduction of lymph node short axis to <1 cm; (iii) Undetectable serum Tg both during suppression and after TSH stimulation. |
| PR | Decrease of 30% or more in the sum of diameters of target lesions, taking as a reference the baseline sum diameter. |
| SD | Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD or non-PD/non-CR for nontarget disease |
| PD | At least a 20% increase in the sum of diameters of target lesions taking as a reference the smallest sum on the study and an increase of at least 5 mm or appearance of one or more new lesions. |
Source: Eisenhauer et al. (12).
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; Tg, thyroglobulin.
Table 3.
World Health Organization Classification of Side Effects After the Treatment with 131I
| Leukopenia | WBC |
|---|---|
| Grade 1 | Lower normal laboratory limit |
| Grade 2 | 2000–3000/μL |
| Grade 3 | 1000–2000/μL |
| Grade 4 | Below 1000/μL |
| Grade 5 | Death |
| Thrombocytopenia | PLT |
|---|---|
| Grade 1 | 75,000–150,000/μL |
| Grade 2 | 50,000–75,000/μL |
| Grade 3 | 25,000–50,000/μL |
| Grade 4 | Below 25,000/μL |
| Grade 5 | Death |
Source: Van Nostrand and Freitas (13).
Statistical analysis
Continuous variables were compared by t-test and Mann–Whitney test. Pearson's χ2 and Fisher's exact tests were employed for comparison of categorical variables. Cox logistic regression models were performed to assess adjusted by age response to treatment and PFS.
Results
The study group consisted of 56 patients (31 women and 25 men) whose tumors were histologically classified as papillary thyroid cancers (PTC) in 42.9% of patients, follicular variant of PTC in 21.4% of patients, follicular thyroid cancers in 14.3 % of patients, Hurthle cell thyroid cancers in 12.5% of patients, insular in 7.1% of patients, and tall cell variant of thyroid cancer in 1.8% of patients. There was no difference in the histological breakdown between the group of patients who underwent rhTSH-aided and THW-aided RAI treatment (p=0.981). Within the study group, 70.9% of patients had exclusively nontarget lesions, 27.3% had both target and nontarget lesions and the remaining 1.8% had exclusively target lesions. The sum of the largest diameters of the target lesions in patients treated with rhTSH-aided RAI was similar to those treated with THW-aided RAI (6.4±2.3 cm vs. 4.8±4.2 cm, p=0.407, respectively). There were no differences between the study groups in terms of the distribution of patients presenting with micro- and macronodular pulmonary metastases, in bone metastases, or in atypical metastases to the brain, kidney, or liver, or in baseline nonstimulated Tg levels (Table 4). Patients prepared for the RAI therapy with rhTSH were significantly older compared with the THW group (rhTSH vs. THW 62.4±12.6 years vs. 48.8±18.2 years, p=0.010) and received lower cumulative dose of RAI (rhTSH vs. THW mean 256±136.7 vs. 414.7±257, p=0.027); prescribed activity was more commonly based on individualized dosimetric evaluation rather than on empiric dosage of RAI (dosimetry-based RAI treatment rhTSH vs. THW 80% vs. 46.3% of patients, p=0.024) (Table 5).
Table 4.
Baseline Characteristics of the Study Groups
| rhTSH (n=15) | THW (n=41) | p-Value | |
|---|---|---|---|
| Age (years) | 62.4±12.6 | 48.8±18.2 | 0.010a |
| Sex | 73.3% females | 48.8% females | 0.090 |
| 26.7% males | 51.2% males | ||
| T (cm) | 4.0±0.75 | 4.3±0.46 | 0.717 |
| % of patients with extrathyroidal extension | 35.7% | 45.5% | 0.387 |
| % of patients with pulmonary metastases countable micronodular | 13.3% | 14.6% | 0.637 |
| % of patients with pulmonary metastases countable macronodular | 13.3% | 14.6% | 0.637 |
| % of patients with micronodular multiple pulmonary metastases | 66.7% | 63.4% | 0.541 |
| % of patients with bone metastases | 53.3% | 29.3% | 0.090 |
| % of patients with a-typical metastases (brain, liver, kidney) | 13.3% | 12.2% | 0.612 |
| The sum of max diameter of target lesions (cm) | 6.4±2.3 | 4.8±4.2 | 0.407 |
| Baseline nonstimulated Tg (ng/mL) before the treatment | 6995±15,879 | 5544±22,163 | 0.372 |
Statistically significant difference.
rhTSH, recombinant human thyrotropin; THW, thyroid hormone withdrawal.
Table 5.
Therapy Applied to the Study Groups
| rhTSH-aided RAI therapy | THW-aided RAI therapy | p-Value | |
|---|---|---|---|
| Mean first dose activity (mCi) | 222.4±120.8 | 203.5±61.5 | 0.444 |
| Mean TSH at the time of the first dose application (mIU/mL) | 42.3±44.9 | 65.9±40.2 | 0.126 |
| D-Rx-aided treatment | 80% | 46.3% | 0.024a |
| Number of repeated dosages | I dose: 80% | I dose: 46.3% | 0.188 |
| II doses: 20% | II doses: 34.1% | ||
| III doses: 0% | III doses: 9.8% | ||
| IV doses: 0% | IV doses: 9.8% | ||
| Mean total cumulative dose (mCi) | 256±136.7 | 414.7±257 | 0.027a |
| Mean urine iodine (μg/L) | 117.7±54.8 | 86.7±72.2 | 0.279 |
| Additional surgical treatment of metastatic lesions | 13.3% | 31.7% | 0.150 |
| Additional radiotherapy treatment | 33.3% | 22% | 0.294 |
| Treatment with zolendronic acid | 33.3% | 14.6% | 0.121 |
| Total duration of follow-up (months) | 33.5±24.2 | 86.2±224.7 | 0.372 |
| Duration of follow-up after the last dose of 131I (months) | 25.2±24.9 | 32.5±27.3 | 0.368 |
Statistically significant difference.
RAI, radioiodine.
During the follow-up period (mean 72±36.2 months) patients from both groups received similar adjunctive treatment such as additional surgical excision of metastatic lesions (rhTSH vs. THW 13.3% of patients vs. 31.7%, p=0.150), radiotherapy for the bone lesions (rhTSH vs. THW 33.3% vs. 22%, p=0.294), treatment with zoledronic acid for the bone lesions (rhTSH vs. THW 33.3% vs. 14.6%, p=0.121) (Table 5). The rates of CR, PR, SD, and PD were not different between the groups (rhTSH vs. THW CR 7% vs. 12%, p=0.48, PR 0% vs. 0%, p=n/a, SD 73% vs. 56%, p=0.20, PD 20% vs. 32%, p=0.31). CR once obtained was present during the subsequent follow-up period, while SD was transient in 1/15 (6.7%) of patients who underwent rhTSH-aided RAI therapy and in 6/41 (14.6%) of patients who received THW-aided therapy (rhTSH vs. THW p=0.388). These patients developed PD documented by imaging studies performed on median every 6.1 months over 25.2±24.9 months in the rhTSH group and every 6.3 months over 32.5±27.3 months in the THW group. PFS was not different between the study groups (rhTSH vs. THW mean 20 vs. 24 months, p=0.55).
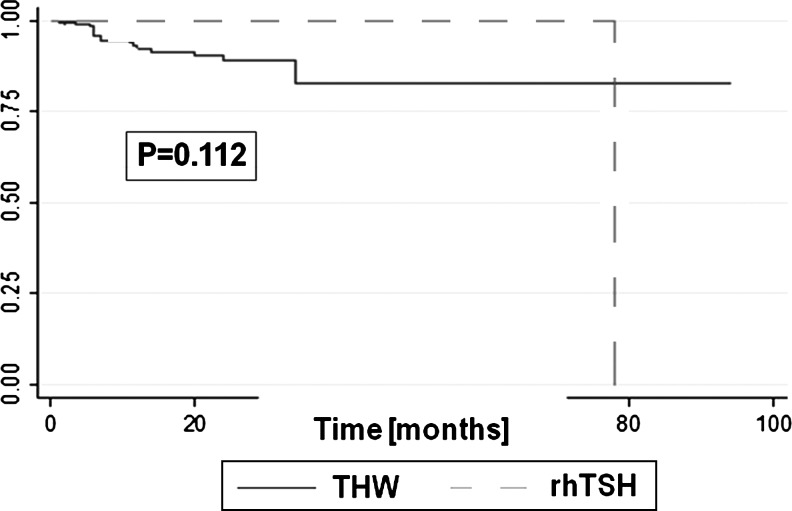
Since the study groups were imbalanced in terms of age, we provided adjustments for this risk factor potentially affecting response to the treatment. A logistic regression model, adjusting for age revealed similar treatment efficacy after preparation with rhTSH and THW (Table 6). The only independent factor increasing the risk for not responding to the treatment and presentation with PD was age (Table 6). Also PFS was not affected by the method of TSH stimulation, but by age (Table 6). PFS curves, adjusted for age, were not significantly different between the study groups (Fig. 1).
Table 6.
Response to the Radioiodine Treatment After Preparation with Recombinant Human Thyrotropin Versus Thyroid Hormone Withdrawal
| rhTSH vs. THW | Age | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p-Value | Hazard ratio | 95% CI | p-Value | |
| CR | 0.97 | 0.08–11.42 | 0.982 | 0.96 | 0.91–1.01 | 0.153 |
| PR | N/A | N/A | N/A | N/A | N/A | N/A |
| SD | 3.22 | 0.79–13.18 | 0.104 | 0.97 | 0.94–1.00 | 0.099 |
| PD | 0.26 | 0.52–1.26 | 0.094 | 1.06 | 1.02–1.11 | 0.008a |
| PFS | 0.41 | 0.14–1.23 | 0.112 | 1.04 | 1.02–1.07 | 0.001a |
Age was the only significant factor affecting treatment efficacy (older age associated with increased risk of nonresponding to treatment and presentation with PD and shorter duration of PFS).
Statistically significant difference.
N/A, not applicable; PFS, progression free survival.
FIG. 1.
No differences in adjusted by age progression free survival (PFS) curves between recombinant human thyrotropin (rhTSH) and thyroid hormone withdrawal (THW) group.
The assessment of ΔTg after therapy was not possible in 1/15 patients treated with rhTSH-aided RAI due to interfering anti-Tg antibodies and in 11/41 patients treated with THW-aided RAI. In five cases this was due to the presence of interfering anti-Tg antibodies and in six cases this was due to the lack of baseline nonstimulated Tg measurements after surgery. Tg decreased after treatment in 79% of patients treated with rhTSH-aided RAI and 70% of patients treated with THW-aided RAI (rhTSH vs. THW p=0.42).
During the follow-up period, 3/15 (20%) patients from rhTSH group and 3/41 (7.3%) from THW group died (rhTSH vs. THW p=0.188). Direct causes of death were not available for this analysis.
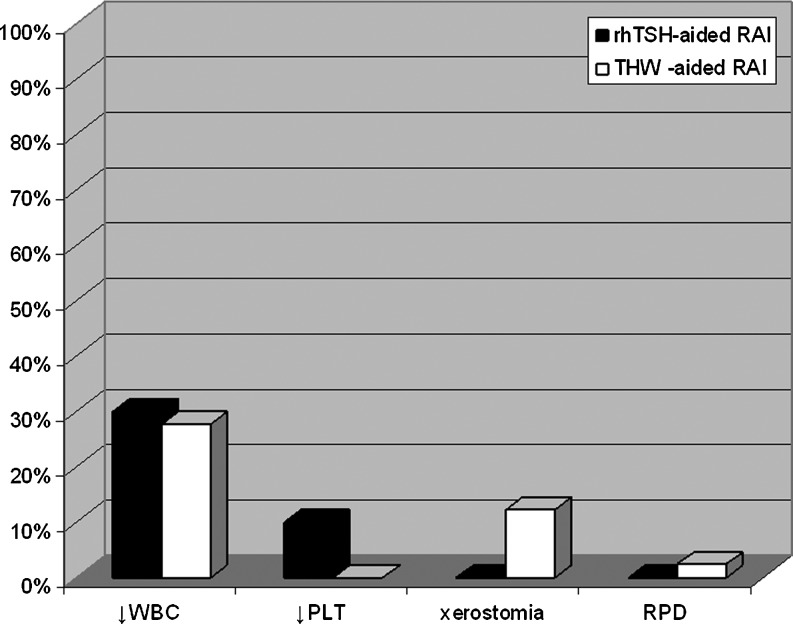
Frequency of side effects
We assessed side effects related to RAI treatment. The rates of leucopenia, thrombocytopenia, and xerostomia were not different between patients prepared for RAI therapy with rhTSH versus those prepared with THW (Fig. 2). Total leukocyte (WBC) counts before and 4–6 weeks after the therapy were available for 10/15 patients from rhTSH-aided RAI treatment group and for 18/41 patients from THW group, while platelet counts were available for 10/15 patients from rhTSH group and 13/41 from the THW group. Leukopenia was observed in 3/10 (30%) of patients prepared for RAI therapy with rhTSH (grade I according to WHO criteria in one patient and grade II in two patients) and in 5/18 (28%) of patients prepared for the RAI therapy with THW (only grade I) (rhTSH vs. THW p=0.61). Thrombocytopenia was observed in 1/10 (10%) from rhTSH group (grade II) and none of the patients from THW group (rhTSH vs. THW, p=0.37). However, transient decreases in blood counts, but not reaching the threshold value for diagnosis of leukopenia or thrombocytopenia were common. The total WBC decreased ∼4–6 weeks after the treatment by more than 10% from the baseline level in 70% of patients from rhTSH group and in 72.2% of patients treated with THW-aided RAI (rhTSH vs. THW p=0.615). Platelet counts decreased by more than 10% in 60% of patients from the rhTSH group and in 77% of patients from the THW group (rhTSH vs. THW p=0.337). Xerostomia was not observed in patients from the rhTSH group, while it was documented in 5/41 (12.2%) of patients undergoing THW (rhTSH vs. THW p=0.20). One patient from the THW group treated with a dosimetry based dose of RAI developed restrictive pulmonary disease despite the fact that the 48 hours whole-body 131I retention was lower than 80 mCi (3.0 GBq), the level considered to be consistent with little risk of pulmonary fibrosis in patients with high volume pulmonary disease (14). This was a 59-year-old man with PTC who presented with multiple micronodular pulmonary metastases and who was treated with two dosimetry-based 131I-doses up to total cumulative dose of 558.2 mCi. This was equal to 89.1% of maximum tolerable activity. The restrictive pulmonary disease with moderately reduced carbon monoxide diffusing capacity (DLCO) was documented by pulmonary function tests (PFTs) after the first dose of RAI. Restrictive pulmonary disease in this case could have been radiation induced and due to the underlying pulmonary disease. The recent PFTs of this patient revealed mild restrictive lung defect (total lung capacity 75% predicted), mild reduction in DLCO (67% predicted), and mild hypoxemia.
FIG. 2.
No differences in the frequency of adverse side effects in patient treated with rhTSH-aided radioiodine compared with THW-aided therapy.
Discussion
The results of our retrospective study suggest similar efficacy and safety profile of preparation with rhTSH and THW for RAI treatment of RAI-avid metastatic thyroid cancer. We observed similar rates of radiological (CR, SD) and biochemical responses after the RAI treatment in both groups of patients. The absence of patients obtaining PR after treatment was deemed due to the high prevalence of individuals with exclusively nontarget disease, for whom PR could not be assessed.
Because the use of rhTSH has not been approved by the U.S. FDA for the treatment of patients with metastatic disease, the only available data bearing on efficacy in this group of patients is derived from the studies based on TCUP, dedicated to the individuals with (i) coincidental hypothalamic-pituitary disorders that preclude the ability to elevate endogenous TSH after LT4 withdrawal; (ii) sufficient tumor bulk to produce levels of thyroid hormone inhibiting the proper elevation of TSH after withdrawal; and finally (iii) with comorbidities, making induction of hypothyroidism medically contraindicated (15). The latter situation is most commonly seen in older patients. In our study patients who did receive rhTSH-aided RAI treatment were older compared with the patients prepared for the RAI treatment with THW. Despite the presence of this well established risk factor for worse prognosis, they responded to the treatment similarly to patients prepared with THW.
The vast majority of published data focusing on rhTSH-aided treatment for metastatic thyroid cancer consists of case reports and case series (16–22). Luster et al. summarized the outcome of many of these cases and showed that ∼65% of patients obtained either partial remission (36%), disease stabilization (27%), or rarely, complete remission (2%) (23).
A retrospective study by Robbins et al. documented that rhTSH-aided treatment of 115 patients with locoregional and/or DM resulted in improvement of cancer-related symptoms in 24.3% of patients, stabilization in 54.1% of patients, and worsening in 21.6% of patients after a mean of one year of follow-up (4). Consistent with our data, this study documented, that serum Tg in Tg-antibody negative patients decreased or became undetectable in 72.7% of patients (4). A prospective study by Luster et al. indicated that 63% (7/11) of patients with widespread thyroid cancer had an average 30% reduction in serum Tg by 2–10 months after RAI treatment (23).
Importantly, none of above mentioned studies compared the relative efficacy of rhTSH-aided RAI treatment versus THW-aided. One of the first reports focused on this important clinical question was a retrospective study by Jarzab et al., who compared the early radiological, clinical, and biochemical outcomes after rhTSH-aided 131I treatment to the responses seen after prior THW-aided therapy in the same patients (7). They concluded that 52% of patients had identical outcomes after endogenous or exogenous TSH stimulation, and 27% actually achieved a superior response to rhTSH-aided treatment (one of the patient had a CR whereas the previous THW-aided treatment resulted in a PR), and 16% had a superior response after THW-aided treatment. Although having the patients serve as their own historical control is an attractive model, this method of comparison introduces some potential bias. Results of the second intervention may be influenced by factors related to the earlier treatment such as different administered radiation activities, number of prior RAI therapies, and the different time intervals between the courses of treatment.
An important study bearing on relative efficacy of rhTSH-aided RAI versus THW-aided RAI was provided by Tuttle et al. (8). This retrospective review described the clinical outcome of 84 thyroid cancer patients in whom RAI-avid lesions outside the thyroid bed were first identified at the time of RAI remnant ablation. THW and rhTSH-stimulated RAI ablation had similar efficacy in eliminating RAI-avid locoregional metastases (42/60, 70% of rhTSH and 10/16, 63% of THW, p=0.65) and pulmonary metastases (3/4, 75% of rhTSH and 1/4, 25% of THW, p=0.41). Finally, the latest study by Tala et al. very elegantly documented similar 5 years survival rate for 175 patients with RAI-avid DM of thyroid cancer, who were treated after the preparation with rhTSH or THW or combination of both methods (9).
There are case series of concern, however, showing decreased RAI uptake in metastatic lesions after rhTSH-aided radioactive iodine treatment compared to THW preparation (10,11). Other workers have argued that dosimetric determination of the RAI activities to be administered are necessary rather than employing empiric fixed doses in view of the very different RAI biokinetics in euthyroid individuals being prepared with rhTSH (24–26). Pötzi et al. showed a lower uptake of 123-I under rhTSH stimulation than after THW. The median half-life in tumor tissue was longer after THW (39.8 hours) then after rhTSH stimulation (21.9 hours). Further, the cumulative activity in metastatic tissue was lower after rhTSH than during hypothyroidism, with considerable variation between individual lesions (27). In our institution all patients with metastatic thyroid cancer undergo individualized dosimetric evaluations enabling the calculation of the maximum safe RAI dose not exceeding 200 rads to the bone marrow, thus diminishing the likelihood of adverse bone marrow effects (28). However, some patients in our study have received high empiric RAI activities before the referral to our institution. Therefore, 80% patients prepared for the RAI treatment with rhTSH did undergo exclusively individualized dosimetric while in the THW group only 46% of patients were solely treated with dosimetry-based approach. The remaining patients received either high empiric activities or, when treated multiple times, combination of empiric and dosimetry-based RAI dosage. Surprisingly, dosimetry-based approach to RAI treatment more commonly seen in rhTSH group resulted in lower cumulative activity of RAI compared with THW group. This observation might be due to the older age of patients from rhTSH group, which often warrants administration of lower maximum safe to the bone marrow RAI activity based on dosimetric evaluations (29,30).
In the current study we observed similar rates of RAI treatment–related side effects like leucopenia, thrombocytopenia, and xerostomia in patients treated either with rhTSH or THW-aided RAI. Unfortunately, due to the retrospective nature of our study, we were not able to assess the subjective side effects related to the method of TSH stimulation. Duntas and Biondi (31) in the review focused on side effects of both methods of TSH stimulation pointed out that the short-term hypothyroidism after LT4-withdrawal severely impairs quality of life, deranges lipid profile, and might be hazardous for patients with underlying cardiovascular diseases, especially in elderly individuals. Schroeder et al. (32) in a multicenter study including 228 patients undergoing diagnostic follow-up evaluations for thyroid cancer documented that the quality of life significantly declines after THW and can be abrogated by usage of rhTSH. Similar results were obtained in another study by Haugen et al. showing significantly better quality of life after rhTSH compared with THW in areas including performance of physical activities, physical health, pain, and emotional problems (33).
The strength of this study is the inclusion of patients with RAI-avid disease who were prepared for the treatment either exclusively with rhTSH or solely with THW, thus enabling the clear distinction between these two methods of TSH stimulation. Moreover, the comparison of the relative efficacy of rhTSH versus THW–aided RAI treatment is justified by the relatively equivalent tumor burden documented by the similar baseline dimensions of target lesions and baseline Tg values and similar distribution of patients with micro- and macropulmonary metastases, bone lesions, and atypical metastases. Noteworthy, the treatment efficacy was based on the same RECIST criteria of the response to treatment. Duration of follow-up was also relatively long (mean 6 years), however in this population of patients longer follow-up data would strengthen the results.
Our study has some limitations that include its retrospective design, and potential confounders that could affect treatment efficacy, of which age was the major factor. Unfortunately, the study sample did not allow adjustments for confounders other than age. The availability of prospective randomized studies is hampered by the relative numbers and heterogeneity of patients with metastatic thyroid cancer that are seen at any given medical center.
Clearly, the results have potential significant clinical implications, providing important evidence on the similar efficacy and safety profile of preparation with rhTSH relative to the standard THW approach for the treatment with metastatic RAI-avid thyroid cancer.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1221. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.Tuttle RM. Brokhin M. Omry G. Martorella AJ. Larson SM. Grewal RK. Fleisher M. Robbins RJ. Recombinant human TSH-assisted radioactive iodine remnant ablation achieves short-term clinical recurrence rates similar to those of traditional thyroid hormone withdrawal. J Nucl Med. 2008;49:764–770. doi: 10.2967/jnumed.107.049072. [DOI] [PubMed] [Google Scholar]
- 3.Hänscheid H. Lassmann M. Luster M. Thomas SR. Pacini F. Ceccarelli C. Ladenson PW. Wahl RL. Schlumberger M. Ricard M. Driedger A. Kloos RT. Sherman SI. Haugen BR. Carriere V. Corone C. Reiners C. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med. 2006;47:648–654. [PubMed] [Google Scholar]
- 4.Robbins RJ. Robbins AK. Clinical review 156: Recombinant human thyrotropin and thyroid cancer management. J Clin Endocrinol Metab. 2003;88:1933–1938. doi: 10.1210/jc.2002-021979. [DOI] [PubMed] [Google Scholar]
- 5.Duntas LH. Cooper DS. Review on the occasion of a decade of recombinant human TSH: prospects and novel uses. Thyroid. 2008;18:509–516. doi: 10.1089/thy.2007.0331. [DOI] [PubMed] [Google Scholar]
- 6.Remy H. Borget I. Leboulleux S. Guilabert N. Lavielle F. Garsi J. Bournaud C. Gupta S. Schlumberger M. Ricard M. 131I effective half-life and dosimetry in thyroid cancer patients. J Nucl Med. 2008;49:1445–1450. doi: 10.2967/jnumed.108.052464. [DOI] [PubMed] [Google Scholar]
- 7.Jarzab B. Handkiewicz-Junak D. Roskosz J. Puch Z. Wygoda Z. Kukulska A. Jurecka-Lubieniecka B. Hasse-Lazar K. Turska M. Zajusz A. Recombinant human TSH-aided radioiodine treatment of advanced differentiated thyroid carcinoma: a single-centre study of 54 patients. Eur J Nucl Med Mol Imaging. 2003;30:1077–1086. doi: 10.1007/s00259-003-1190-5. [DOI] [PubMed] [Google Scholar]
- 8.Tuttle RM. Lopez N. Leboeuf R. Minkowitz SM. Grewal R. Brokhin M. Omry G. Larson S. Radioactive iodine administered for thyroid remnant ablation following recombinant human thyroid stimulating hormone preparation also has an important adjuvant therapy function. Thyroid. 2010;20:257–263. doi: 10.1089/thy.2009.0401. [DOI] [PubMed] [Google Scholar]
- 9.Tala H. Robbins R. Fagin JA. Larson SM. Tuttle RM. Five-year survival is similar in thyroid cancer patients with distant metastases prepared for radioactive iodine therapy with either thyroid hormone withdrawal or recombinant human TSH. J Clin Endocrinol Metab. 2011;96:2105–2111. doi: 10.1210/jc.2011-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taïeb D. Jacob T. Zotian E. Mundler O. Lack of efficacy of recombinant human thyrotropin versus thyroid hormone withdrawal for radioiodine therapy imaging in a patient with differentiated thyroid carcinoma lung metastases. Thyroid. 2004;14:465–467. doi: 10.1089/105072504323150804. [DOI] [PubMed] [Google Scholar]
- 11.Driedger AA. Kotowycz N. Two cases of thyroid carcinoma that were not stimulated by recombinant human thyrotropin. J Clin Endocrinol Metab. 2004;89:585–590. doi: 10.1210/jc.2003-031650. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA. Therasse P. Bogaerts J. Schwartz LH. Sargent D. Ford R. Dancey J. Arbuck S. Gwyther S. Mooney M. Rubinstein L. Shankar L. Dodd L. Kaplan R. Lacombe D. Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–242. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Van Nostrand D. Freitas J. Side effects of 131-I for ablation, treatment of well differentiated thyroid carcinoma. In: Wartofsky L, editor; Van Nostrand D, editor. Thyroid Cancer: A Comprehensive Guide to Clinical Management. 2nd. Humana Press; Totowa, NJ: 2006. pp. 459–485. [Google Scholar]
- 14.Sgouros G. Song H. Ladenson PW. Wahl RL. Lung toxicity in radioiodine therapy of thyroid carcinoma: development of a dose-rate method and dosimetric implications of the 80-mCi rule. J Nucl Med. 2006;47:1977–1984. [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins RJ. Driedger A. Magner J U.S. and Canadian Thyrogen Compassionate Use Program Investigator Group. Recombinant human thyrotropin-assisted radioiodine therapy for patients with metastatic thyroid cancer who could not elevate endogenous thyrotropin or be withdrawn from thyroxine. Thyroid. 2006;16:1121–1230. doi: 10.1089/thy.2006.16.1121. [DOI] [PubMed] [Google Scholar]
- 16.Rudavsky AZ. Freeman LM. Treatment of scan-negative, thyroglobulin-positive metastatic thyroid cancer using radioiodine 131I and recombinant human thyroid stimulating hormone. J Clin Endocrinol Metab. 1997;82:11–14. doi: 10.1210/jcem.82.1.3696. [DOI] [PubMed] [Google Scholar]
- 17.Masiukiewicz US. Nakchbandi IA. Stewart AF. Inzucchi SE. Papillary thyroid carcinoma metastatic to the pituitary gland. Thyroid. 1999;9:1023–1027. doi: 10.1089/thy.1999.9.1023. [DOI] [PubMed] [Google Scholar]
- 18.Müller V. Bohuslavizki KH. Klutmann S. Clausen M. Value of recombinant human thyrotropin in high-dose radioiodine therapy: a case report. J Nucl Med Technol. 2002;30:185–188. [PubMed] [Google Scholar]
- 19.Rotman-Pikielny P. Reynolds JC. Barker WC. Yen PM. Skarulis MC. Sarlis NJ. Recombinant human thyrotropin for the diagnosis and treatment of a highly functional metastatic struma ovarii. J Clin Endocrinol Metab. 2000;85:237–244. doi: 10.1210/jcem.85.1.6261. [DOI] [PubMed] [Google Scholar]
- 20.Risse JH. Grünwald F. Bender H. Schüller H. Van Roost D. Biersack HJ. Recombinant human thyrotropin in thyroid cancer and hypopituitarism due to sella metastasis. Thyroid. 1999;9:1253–1256. doi: 10.1089/thy.1999.9.1253. [DOI] [PubMed] [Google Scholar]
- 21.Lippi F. Capezzone M. Angelini F. Taddei D. Molinaro E. Pinchera A. Pacini F. Radioiodine treatment of metastatic differentiated thyroid cancer in patients on L-thyroxine, using recombinant human TSH. Eur J Endocrinol. 2001;144:5–11. doi: 10.1530/eje.0.1440005. [DOI] [PubMed] [Google Scholar]
- 22.Luster M. Lassmann M. Haenscheid H. Michalowski U. Incerti C. Reiners C. Use of recombinant human thyrotropin before radioiodine therapy in patients with advanced differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2000;85:3640–3646. doi: 10.1210/jcem.85.10.6903. [DOI] [PubMed] [Google Scholar]
- 23.Luster M. Lippi F. Jarzab B. Perros P. Lassmann M. Reiners C. Pacini F. rhTSH-aided radioiodine ablation and treatment of differentiated thyroid carcinoma: a comprehensive review. Endocr Relat Cancer. 2005;12:49–64. doi: 10.1677/erc.1.00830. [DOI] [PubMed] [Google Scholar]
- 24.Mazzaferri EL. Kloos RT. Using recombinant human TSH in the management of well-differentiated thyroid cancer: current strategies and future directions. Thyroid. 2000;10:767–778. doi: 10.1089/thy.2000.10.767. [DOI] [PubMed] [Google Scholar]
- 25.Luster M. Sherman SI. Skarulis MC. Reynolds JR. Lassmann M. Hänscheid H. Reiners C. Comparison of radioiodine biokinetics following the administration of recombinant human thyroid stimulating hormone and after thyroid hormone withdrawal in thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2003;30:1371–1377. doi: 10.1007/s00259-003-1230-1. [DOI] [PubMed] [Google Scholar]
- 26.Menzel C. Kranert WT. Döbert N. Diehl M. Fietz T. Hamscho N. Berner U. Grünwald F. rhTSH stimulation before radioiodine therapy in thyroid cancer reduces the effective half-life of (131)I. J Nucl Med. 2003;44:1065–1068. [PubMed] [Google Scholar]
- 27.Pötzi C. Moameni A. Karanikas G. Preitfellner J. Becherer A. Pirich C. Dudczak R. Comparison of iodine uptake in tumour and nontumour tissue under thyroid hormone deprivation and with recombinant human thyrotropin in thyroid cancer patients. Clin Endocrinol (Oxf) 2006;65:519–523. doi: 10.1111/j.1365-2265.2006.02626.x. [DOI] [PubMed] [Google Scholar]
- 28.Van Nostrand D. Atkins F. Yeganeh F. Acio E. Bursaw R. Wartofsky L. Dosimetrically determined doses of radioiodine for the treatment of metastatic thyroid carcinoma. Thyroid. 2002;12:121–134. doi: 10.1089/105072502753522356. [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni K. Van Nostrand D. Atkins F. Aiken M. Burman K. Wartofsky L. The relative frequency in which empiric dosages of radioiodine would potentially overtreat or undertreat patients who have metastatic well-differentiated thyroid cancer. Thyroid. 2006;16:1019–1023. doi: 10.1089/thy.2006.16.1019. [DOI] [PubMed] [Google Scholar]
- 30.Tuttle RM. Leboeuf R. Robbins RJ. Qualey R. Pentlow K. Larson SM. Chan CY. Empiric radioactive iodine dosing regimens frequently exceed maximum tolerated activity levels in elderly patients with thyroid cancer. J Nucl Med. 2006;47:1587–1591. [PubMed] [Google Scholar]
- 31.Duntas LH. Biondi B. Short-term hypothyroidism after levothyroxine-withdrawal in patients with differentiated thyroid cancer: clinical and quality of life consequences. Eur J Endocrinol. 2007;156:13–19. doi: 10.1530/eje.1.02310. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder PR. Haugen BR. Pacini F. Reiners C. Schlumberger M. Sherman SI. Cooper DS. Schuff KG. Braverman LE. Skarulis MC. Davies TF. Mazzaferri EL. Daniels GH. Ross DS. Luster M. Samuels MH. Weintraub BD. Ridgway EC. Ladenson PW. A comparison of short-term changes in health-related quality of life in thyroid carcinoma patients undergoing diagnostic evaluation with recombinant human thyrotropin compared with thyroid hormone withdrawal. J Clin Endocrinol Metab. 2006;91:878–884. doi: 10.1210/jc.2005-2064. [DOI] [PubMed] [Google Scholar]
- 33.Haugen BR. Pacini F. Reiners C. Schlumberger M. Ladenson PW. Sherman SI. Cooper DS. Graham KE. Braverman LE. Skarulis MC. Davies TF. DeGroot LJ. Mazzaferri EL. Daniels GH. Ross DS. Luster M. Samuels MH. Becker DV. Maxon HR., 3rd Cavalieri RR. Spencer CA. McEllin K. Weintraub BD. Ridgway EC. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab. 1999;84:3877–3885. doi: 10.1210/jcem.84.11.6094. [DOI] [PubMed] [Google Scholar]