Abstract
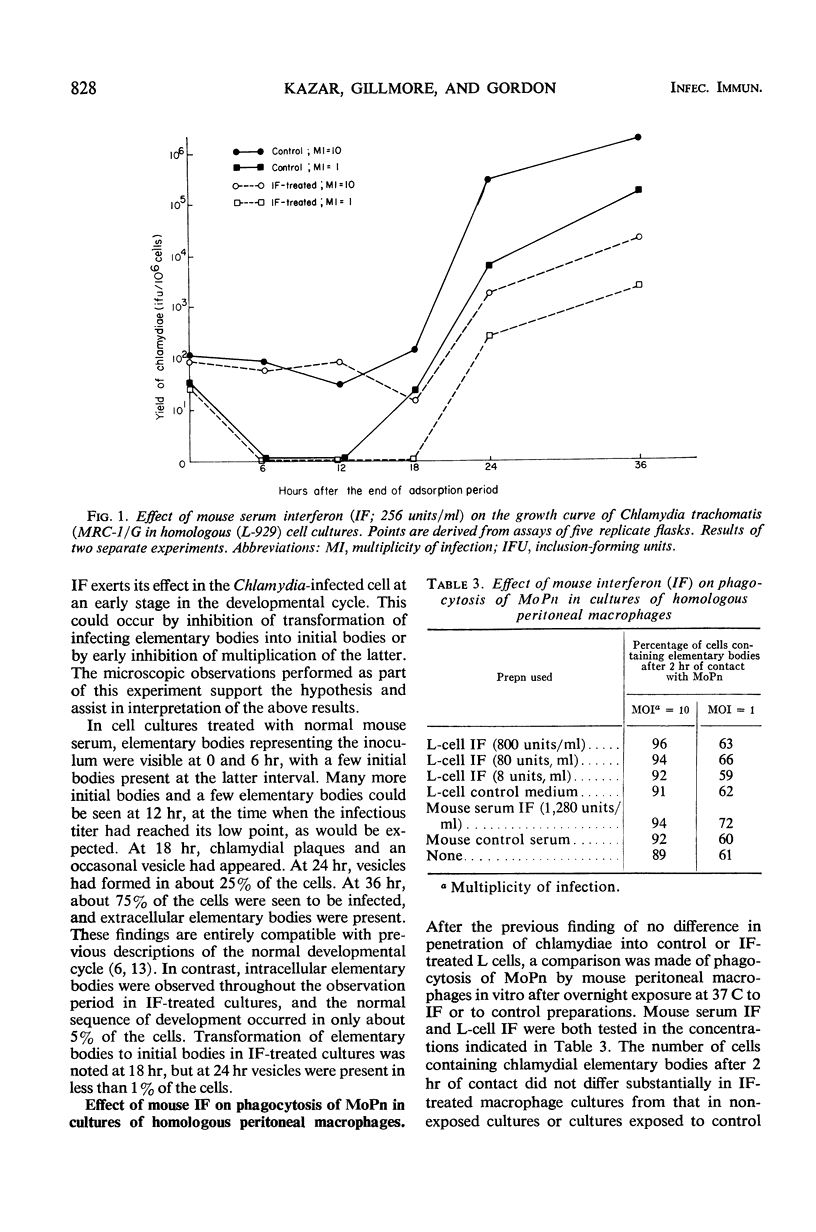
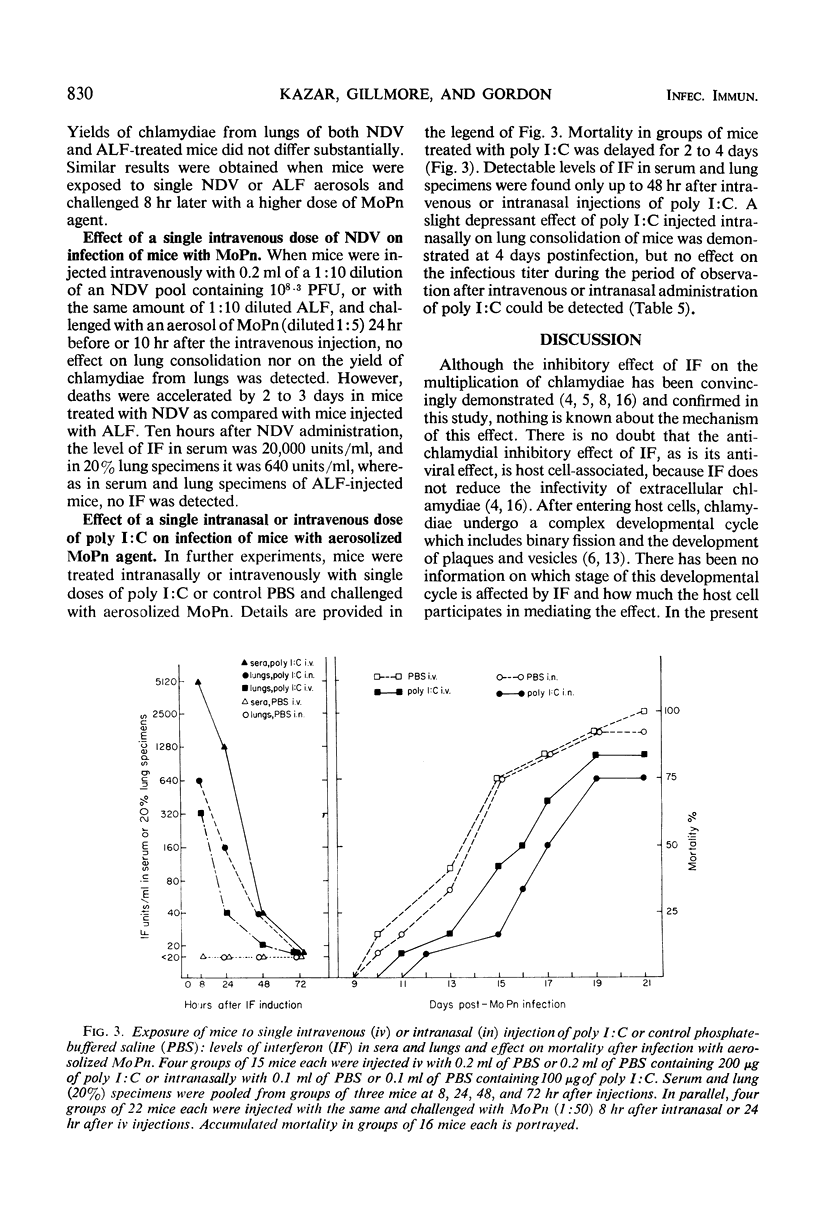
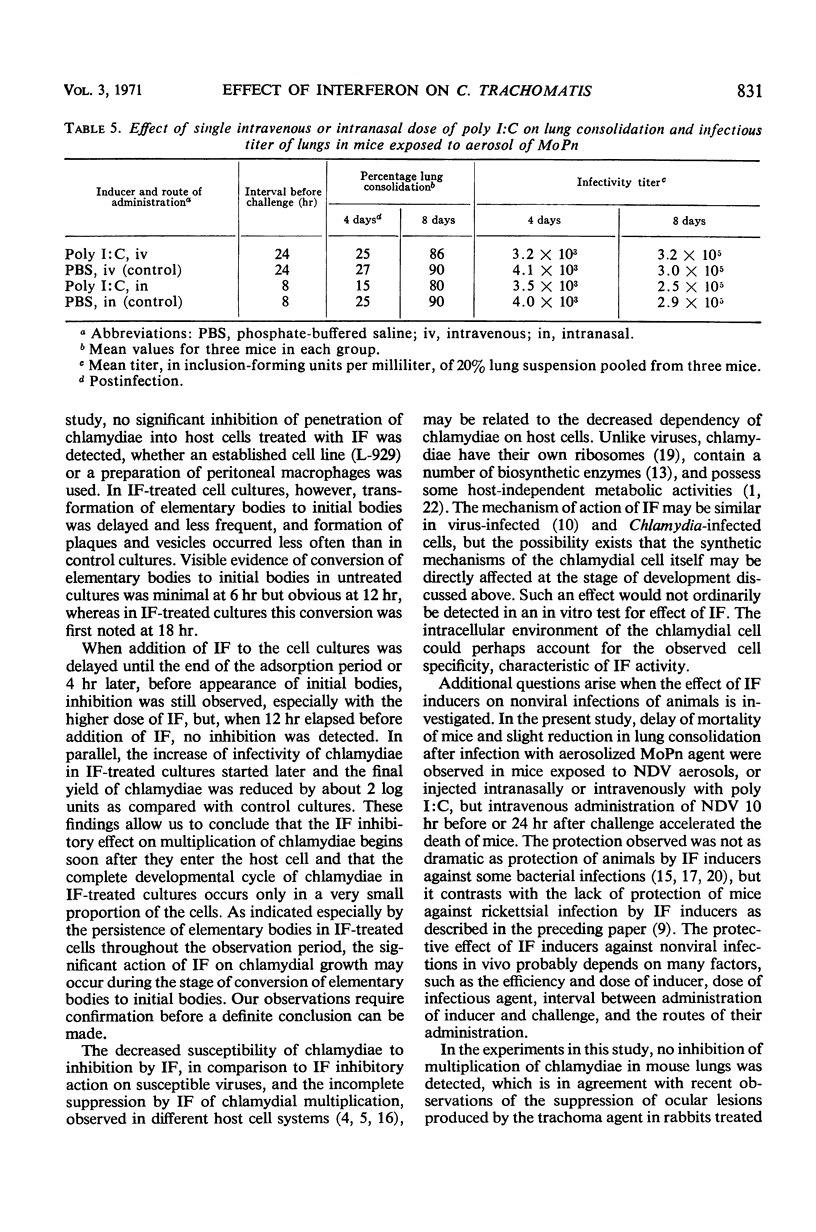
The effect of mouse interferon (IF) on the multiplication of Chlamydia trachomatis (strain MRC-1/G) in homologous (L-929) cell cultures and the effect of the IF inducers Newcastle disease virus (NDV) and polyriboinosinic acid-polyribocytidylic acid complex (poly I:C) on the experimental infection of mice with aerosolized C. trachomatis (strain MoPn) were investigated. Treatment of infected cell cultures with IF reduced the number of cells containing chlamydial inclusions and depressed the yield of chlamydiae as determined by titrations for infectivity. Growth of chlamydiae was reduced when cultures were exposed to IF 6 or 18 hr before infection, and slight reduction of the yield was also detectable in cell cultures treated with IF at early intervals (0 or 4 hr) after chlamydial infection. No effect of IF on penetration of chlamydiae into mouse cells was observed, whether phagocytic cells from peritoneal washings or L-929 cells were used, indicating that the inhibitory effect of IF occurs after chlamydiae enter the host cell. Additional evidence was obtained that a significant effect of IF occurs at an early stage in maturation of the intracellular chlamydiae. In mice exposed repeatedly to NDV aerosols and challenged with aerosolized MoPn 8 hr after the first exposure to NDV, mortality was delayed by 2 to 3 days and lung consolidation was slightly reduced at 3 days after infection. Yields of chlamydiae from lung pools of NDV-treated mice, taken at 3, 6, and 9 days after challenge, were not significantly different from those of controls. Similar results were obtained when mice were challenged with MoPn 8 hr after intranasal injection with 100 μg of poly I:C or 24 hr after intravenous injection with 200 μg of poly I:C. In contrast, administration of 0.2 ml of NDV (108.3 plaque-forming units) intravenously 10 hr before or 24 hr after challenge with MoPn accelerated mortality of mice by 2 to 3 days. In all experiments, detectable levels of IF in sera or 20% lung suspensions were found only up to 48 to 72 hr after exposure of mice to IF inducers.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J. Separation of protein synthesis in meningopneumonitisgent from that in L cells by differential susceptibility to cycloheximide. J Bacteriol. 1968 Feb;95(2):327–332. doi: 10.1128/jb.95.2.327-332.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L. ISOLATION OF THE TRACHOMA AGENT IN CELL CULTURE. Proc Soc Exp Biol Med. 1965 Feb;118:354–359. doi: 10.3181/00379727-118-29841. [DOI] [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L. OCCURENCE OF GLYCOGEN IN INCLUSIONS OF THE PSITTACOSIS-LYMPHOGRANULOMA VENEREUM-TRACHOMA AGENTS. J Infect Dis. 1965 Apr;115:186–196. doi: 10.1093/infdis/115.2.186. [DOI] [PubMed] [Google Scholar]
- HIGASHI N. ELECTRON MICROSCOPIC STUDIES ON THE MODE OF REPRODUCTION OF TRACHOMA VIRUS AND PSITTACOSIS VIRUS IN CELL CULTURES. Exp Mol Pathol. 1965 Feb;76:24–39. doi: 10.1016/0014-4800(65)90021-3. [DOI] [PubMed] [Google Scholar]
- Hanna L., Merigan T. C., Jawetz E. Inhibition of TRIC agents by virus-induced interferon. Proc Soc Exp Biol Med. 1966 Jun;122(2):417–421. doi: 10.3181/00379727-122-31150. [DOI] [PubMed] [Google Scholar]
- Jenkin H. M., Lu Y. K. Induction of interferon by the Bour strain of trachoma in HeLa 229 cells. Am J Ophthalmol. 1967 May;63(5 Suppl):1110–1115. doi: 10.1016/0002-9394(67)94091-3. [DOI] [PubMed] [Google Scholar]
- Kazar J., Krautwurst P. A., Gordon F. B. Effect of Interferon and Interferon Inducers on Infections with a Nonviral Intracellular Microorganism, Rickettsia akari. Infect Immun. 1971 Jun;3(6):819–824. doi: 10.1128/iai.3.6.819-824.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus P. I., Salb J. M. Molecular basis of interferon action: inhibition of viral RNA translation. Virology. 1966 Nov;30(3):502–516. doi: 10.1016/0042-6822(66)90126-7. [DOI] [PubMed] [Google Scholar]
- Merigan T. C., Hanna L. Characteristics of interferon induced in vitro and in vivo by a TRIC agent. Proc Soc Exp Biol Med. 1966 Jun;122(2):421–424. doi: 10.3181/00379727-122-31151. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. The relation of the psittacosis group (Chlamydiae) to bacteria and viruses. Annu Rev Microbiol. 1966;20:107–130. doi: 10.1146/annurev.mi.20.100166.000543. [DOI] [PubMed] [Google Scholar]
- Oh J. O., Ostler H. B., Schachter J. Protective effect of a synthetic polynucleotide complex (poly I: C) on ocular lesions produced by trachoma agent in rabbits. Infect Immun. 1970 Jun;1(6):566–573. doi: 10.1128/iai.1.6.566-573.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindak F. F. Protection of mice against bacterial infection by interferon inducers. Infect Immun. 1970 Mar;1(3):271–273. doi: 10.1128/iai.1.3.271-273.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Synthetic polyanions protect mice against intracellular bacterial infection. Nature. 1970 Apr 25;226(5243):361–363. doi: 10.1038/226361a0. [DOI] [PubMed] [Google Scholar]
- Sueltenfuss E. A., Pollard M. Cytochemical Assay of Interferon Produced by Duck Hepatitis Virus. Science. 1963 Feb 15;139(3555):595–596. doi: 10.1126/science.139.3555.595. [DOI] [PubMed] [Google Scholar]
- Tamura A. Isolation of ribosome particles from meningopneumonitis organisms. J Bacteriol. 1967 Jun;93(6):2009–2016. doi: 10.1128/jb.93.6.2009-2016.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M. J., Waitz J. A., Came P. E. Induction of resistance to bacterial infections of mice with poly I-poly C. Nature. 1970 Apr 11;226(5241):170–170. doi: 10.1038/226170a0. [DOI] [PubMed] [Google Scholar]
- Weiss E. Adenosine Triphosphate and Other Requirements for the Utilization of Glucose by Agents of the Psittacosis-Trachoma Group. J Bacteriol. 1965 Jul;90(1):243–253. doi: 10.1128/jb.90.1.243-253.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Comparative metabolism of rickettsiae and other host dependent bacteria. Zentralbl Bakteriol Orig. 1968 Apr;206(3):292–298. [PubMed] [Google Scholar]
- Youngner J. S., Stinebring W. R. Interferon appearance stimulated by endotoxin, bacteria, or viruses in mice pre-treated with Escherichia coli endotoxin or infected with Mycobacterium tuberculosis. Nature. 1965 Oct 30;208(5009):456–458. doi: 10.1038/208456a0. [DOI] [PubMed] [Google Scholar]



