Abstract
Background
CDH13 is a novel tumor suppressor gene often inactivated by aberrant promoter methylation in human cancers. Previous studies have shown that CDH13 methylation correlated with advanced disease and poor prognosis in non-muscle invasive bladder cancer (NMIBC). The aim of the current study was to investigate the correlations between CDH13 methylation and disease recurrence as well as progression of NMIBC.
Material/Methods
The methylation status of CDH13 in 178 NMIBC samples and 38 normal bladder epithelial tissues was examined by methylation-specific PCR (MSP), and then correlated with clinicopathological features.
Results
We found that CDH13 methylation occurs frequently in NMIBC, and significantly correlates with high grade, advanced stage, larger tumor size, and tumor recurrence and progression. Moreover, patients with methylated CDH13 exhibited significantly shorter recurrence-free survival (P<0.0001) and progression-free survival (P=0.0060) than patients with unmethylated CDH13. In addition, a multivariate Cox proportional hazard model analysis suggests that CDH13 methylation is an independent predictor for the recurrence (P=0.0043) and progression (P=0.0016) of NMIBC after initial transurethral resection.
Conclusions
Our findings demonstrate that CDH13 methylation is a frequent event in NMIBC, and is associated with unfavorable tumor features. It should be used as an independent predictor for the recurrence and progression of NMIBC, and may be useful for the design of individualized therapeutic modalities.
MeSH Keywords: Biological Markers, Cadherins, DNA Methylation, Urinary Bladder Neoplasms
Background
Bladder cancer is one of the most common malignancies in humans, with an estimated 72 570 new cases and 15 210 deaths in the United States in 2013, and is a common disease in China [1,2]. Of newly diagnosed bladder cancer cases, more then 90% are transitional cell carcinoma on histology, and over 70% of new cases are non-muscle-invasive tumors [3]. These non-muscle-invasive tumors can be treated by transurethral resection; unfortunately, most of these will relapse after initial curative treatment, and 10–20% will progress to muscle-invasive disease [4]. In addition, the outcome of bladder cancer is worse with tumor progression. Recurrence and progression are the main characteristics of bladder cancer, and it is a serious challenge to identify patients with high risk of recurrence and progression who need more aggressive treatment, and those with low risk needing less intensive surveillance after initial adequate therapy. Because bladder cancer is a heterogeneous disease, pathologically similar tumors can behave differently [5]. Thus, additional biomarkers are needed, in addition to common clinicopathologic features [6,7].
Bladder cancer arises from the accumulation of genetic and epigenetic changes that lead to the activation of proto-oncogenes, or silencing of tumor suppressor genes [8]. DNA methylation, the most common and best-characterized epigenetic change, mainly occurs in cytosine guanine dinucleotide-rich areas, known as CPG islands, in the promoter regions. Aberrant DNA methylation is a crucial mechanism of silencing tumor suppressor genes, and plays an important role in the initiation and progression of human tumors [9–11]. For these reasons, aberrant DNA methylation can be used as useful biomarkers in human tumors, especially when the methylation silences tumor suppressor genes [12]. In recent years, the association of CDH13 with human tumors has been proposed, including bladder cancer. CDH13, a novel tumor suppressor gene, belongs to the cadherin family and is frequently inactivated by aberrant promoter methylation in human tumors [12]. In previous studies, we reported that CDH13 is frequently inactivated by promoter methylation in bladder cancer, and CDH13 methylation is a potential biomarker for the malignancy of bladder cancer and independently predicting the worse outcomes of patients with bladder cancer [13–16]. Our previous findings led us to investigate the clinical significance of CDH13 methylation in NMIBC.
In the current study, the methylation status of CDH13 in primary bladder cancer tissues was evaluated by MSP. Then, we evaluated the correlation between CDH13 methylation and clinicopathologic characteristics. In addition, we also assessed the relationship between CDH13 methylation and recurrence and progression in NMIBC.
Material and Methods
Patients and samples
A total of 178 consecutive patients with NMIBC who received transurethral resection were included in the current study, from September 2004 to September 2007, at the Department of Urology, Third Hospital of Hebei Medical University. The criteria for the enrollment of patients with NMIBC were histopathological diagnosis of bladder transitional cell carcinoma for the first time, no history of other malignant tumors, and not receiving any form of anti-cancer treatment prior to surgery. Patients with incomplete follow-up data were excluded to make the study more robust. All tumors were graded and staged according to the 1973 WHO grading system and the 7th edition of the Tumor Lymph Node Metastasis (TNM) classification by experienced pathologists in our department [17–19]. The NMIBC includes Ta, T1, and Tis; and muscle-invasive bladder cancer includes T2, T3, and T4. The tumor treatment and follow-up strategies were performed according to international guidelines on bladder cancer [17–20]. Patients with intermediate-risk or high-risk disease received 1 cycle of intravesical treatment using mitomycin-C, and no patients received a maintenance schedule [21,22]. All of the patients were followed up and managed in accordance with standard recommendations [18,20]. Recurrence was defined as a new tumor observed in the bladder after initial curative resection, and progression was defined as a disease with a higher TNM stage when relapsed within 5 years [21–25]. Recurrence-free survival was defined as the time form the initial surgery to the date of the first documented bladder cancer relapse, and progression-free survival was defined as the time form the initial surgery to the date of the first documented bladder cancer progression. The samples of normal bladder epithelium were obtained from 38 in-patients with bladder stones; these samples were examined pathologically to exclude the possibility of incidental tumors. All of the samples were quickly frozen in liquid nitrogen and stored at –80°C until used. This study was approved by the ethics committee of the Third Hospital of Hebei Medical University. Written informed consent was obtained from each participant.
DNA extraction, Bisulfite modification, and MSP
Genomic DNA was extracted from frozen tissues using the DNeasy Tissue Kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. The genomic DNA was treated with bisulfite using the EpiTect Bisulfite Kit (Qiagen, Valencia, CA) according to the manufacture’s protocol. The methylation status of CDH13 was examined using primers specific for unmethylated and methylated CDH13 sequences, as we previously reported [15]. The following primers were used: unmethylated: forward 5′-TTGTGGGGTTGTTTTTTGT-3′ and reverse 5′-AACTTTTCATTCATACACACA-3′; methylated: forward 5′-TCGCGGGGTTCGTTTTTCGC-3′ and reverse 5′-GACGTTTTCATTCATACACGCG-3′. The cycling program involved preliminary denaturation at 95°C for 5 min, followed by 33 cycles at 95°C for 30 s, annealing at 60°C for 1 min, and elongation at 72°C for 1 min for the unmethylated reaction; or 29 cycles at 95°C for 30 s, annealing at 70°C for 1 min, and elongation at 72°C for 1 min for the methylated reaction, followed by a final step at 72°C for 5 min, as we reported previously [14,15]. Water blanks were included with each assay as a blank control. Normal lymphocyte DNA methylated in vitro with SssI methylase (New England Biolabs, Beverly, MA, USA) was used as a methylation positive control, and normal lymphocyte DNA as an unmethylated positive control. PCR products were separated in 2% agarose gel, stained with ethidium bromide, and visualized under ultraviolet illumination. Samples were scored as methylation-positive when methylated alleles were present in the methylated DNA lane and methylation negative when bands were present only in the unmethylated DNA lane.
Statistical analysis
Fisher’s exact test was used to assess the difference in CDH13 methylation status between bladder cancer patients and controls. The chi-square test was used to assess the relationship between CDH13 methylation and clinicopathologic features. Kaplan-Meier survival analysis and log-rank test were used to assess the differences in recurrence-free, progression-free, and 5-year overall survival between patients with CDH13 methylated and unmethylated. The multivariate Cox proportional hazard model analysis was used to assess the independent prognostic effect of CDH13 methylation. A 2-sided p value <0.05 was considered statistically significant. Statistical analysis was performed using SAS version 8.0 (SAS Institute, Cary, N.C., USA).
Results
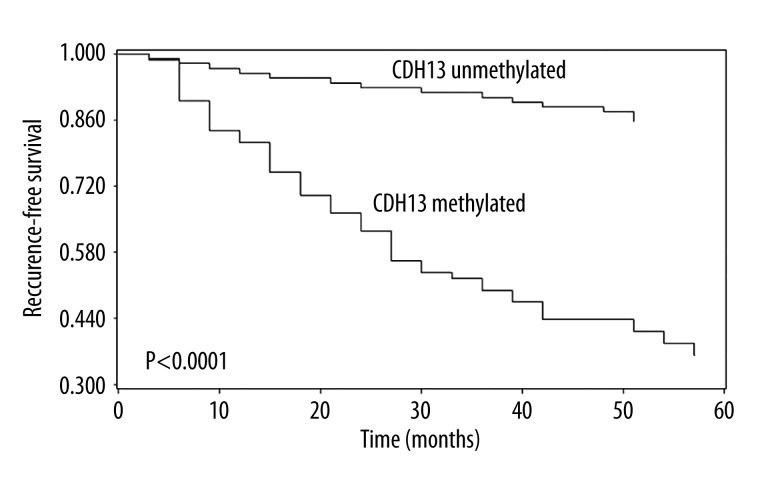
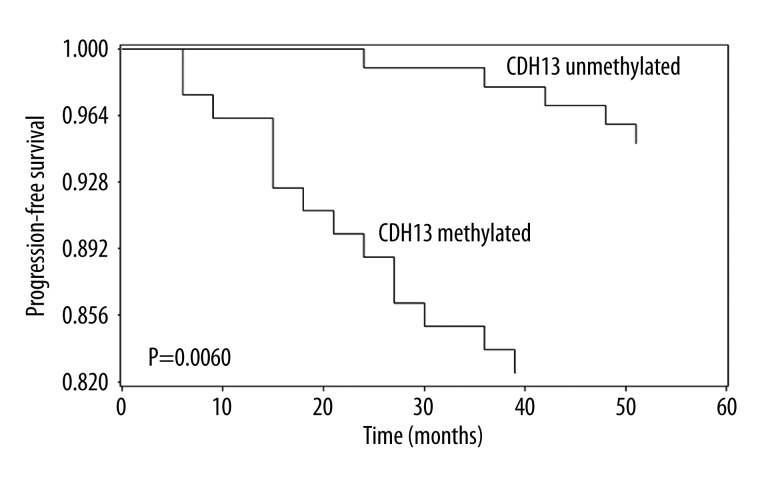
In the current study, the methylation status of CDH13 in bladder cancer tissues and normal bladder epithelial tissues was measured by MSP, CDH13 methylation was detected in 44.9% (80/178) bladder cancer patients, but no methylation was detected in controls, and the difference between these 2 groups was statistically significant (P<0.0001). Then we linked the methylation status of CDH13 to clinicopathologic characteristics of patients with bladder cancer, and found that CDH13 methylation was correlated with high grade (P=0.0186), advanced stage (P=0.0001), larger tumor size (P=0.0002), tumor recurrence (P<0.0001), and progression (P=0.0077); but no association was found between CDH13 methylation and age, sex, or tumor number (Table 1). Moreover, patients with CDH13 methylation in tumor tissues exhibited significantly shorter recurrence-free survival (log-rank test, P<0.0001, Figure 1) and progression- free survival (log-rank test, P=0.0060, Figure 2) than patients with CDH13 unmethylated. Multivariate Cox proportional hazard model analysis was performed to examine the predictive value of CDH13 for tumor recurrence and progression. Surprisingly, CDH13 methylation in bladder cancer tissues independently predicted NMIBC recurrence (P=0.0043) and progression (P=0.0016) (Tables 2 and 3).
Table 1.
Relationship between CDH13 methylation and clinicopathologic features in non muscle invasive bladder cancer (n=178).
| Features | Variables | No. | M (%) | U (%) | P |
|---|---|---|---|---|---|
| Age, years | ≤65 | 66 | 24 (36.4) | 42 (63.6) | 0.0773 |
| >65 | 112 | 56 (50.0) | 56 (50.0) | ||
| Gender | Male | 124 | 61 (49.2) | 63 (50.8) | 0.0841 |
| Female | 54 | 19 (35.2) | 35 (64.8) | ||
| Number | Single | 102 | 45 (44.1) | 57 (55.9) | 0.7974 |
| Multiple | 76 | 35 (46.1) | 41 (53.9) | ||
| Size | ≤3 cm | 99 | 32 (32.3) | 67 (67.7) | 0.0002 |
| >3 cm | 79 | 48 (60.8) | 31 (39.2) | ||
| Grade | G1 | 61 | 20 (32.8) | 41 (67.2) | 0.0186 |
| G2/G3 | 117 | 60 (51.3) | 57 (48.7) | ||
| Stage | Ta | 65 | 17 (26.2) | 48 (73.8) | 0.0001 |
| T1 | 113 | 63 (55.8) | 50 (44.2) | ||
| Recurrence | No | 115 | 31 (27.0) | 84 (73.0) | <0.0001 |
| Yes | 63 | 49 (77.8) | 14 (22.2) | ||
| Progression | No | 159 | 66 (41.5) | 93 (58.5) | 0.0077 |
| Yes | 19 | 14 (73.7) | 5 (26.3) |
Figure 1.
Kaplan-Meier survival curves for 178 patients with bladder cancer, according to CDH13 methylation status in tumor tissues. Patients with CDH13 methylated (n=80) vs. CDH13 unmethylated (n=98), P<0.0001 log-rank test.
Figure 2.
Time to progression in non–muscle-invasive bladder cancer according to CDH13 methylation status, Kaplan-Meier survival curves, and log-rank test, P=0.0060.
Table 2.
Multivariate Cox proportional hazard analysis for prediction of recurrence in non muscle invasive bladder cancer (n=178).
| Variable | HR (95% confidence interval) | P |
|---|---|---|
| CDH13 methylation (M vs. U) | 5.147 (2.071–20.177) | 0.0043 |
| Age (>65 vs. ≤65) | 1.013 (0.725–5.051) | 0.8642 |
| Sex (male vs. female) | 1.525 (0.667–4.946) | 0.7136 |
| Number (multiple vs. single) | 1.786 (0.837–10.418) | 0.7005 |
| Size (>3 cm vs. ≤3 cm) | 3.504 (0.796–11.587) | 0.5486 |
| Grade (G2/G3 vs. G1) | 3.713 (0.917–11.028) | 0.0894 |
| Stage (T1 vs. Ta) | 4.963 (2.567–14.622) | 0.0265 |
HR – hazard ratio; M – methylated; U – unmethylated.
Table 3.
Multivariate Cox proportional hazard analysis for prediction of progression in non muscle invasive bladder cancer (n=178).
| Variable | HR (95% confidence interval) | P |
|---|---|---|
| CDH13 methylation (M vs. U) | 6.563 (2.241–21.707) | 0.0016 |
| Age (>65 vs. ≤65) | 1.133 (0.705–6.017) | 0.6412 |
| Sex (male vs. female) | 1.125 (0.568–4.136) | 0.7003 |
| Number (multiple vs. single) | 1.868 (0.737–10.418) | 0.4713 |
| Size (>3 cm vs. ≤3 cm) | 2.004 (0.754–10.417) | 0.2481 |
| Grade (G2/G3 vs. G1) | 2.881 (0.837–13.213) | 0.0796 |
| Stage (T1 vs. Ta) | 4.037 (1.968–17.542) | 0.0185 |
HR – hazard ratio; M – methylated; U – unmethylated.
Discussion
Bladder cancer is a common human malignancy and is notorious for its high frequency of recurrence and progression after initial curative treatment [3,4]. In NMIBC, tumors with similar morphology may behave differently, and it is difficult to predict which tumor will relapse and/or progress after initial therapy, which is a serious problem for clinical decision-making, and new biomarkers are needed beyond the conventional clinicopathologic parameters [3,4]. In recent years, there is increasing interest in the utilization of methylation markers, as aberrant DNA methylation is a major characteristic of bladder cancer and plays a crucial role in tumor initiation and progression [9]. To identify these methylation markers, we focused on the aberrant methylation of CDH13. CDH13, a novel tumor suppressor gene belonging to the cadherin family, plays a crucial role in processes such as cell-cell adhesion, tumor proliferation, and invasion. The human CDH13 gene is mapped to the 16q24 chromosome, which is often inactivated by aberrant promoter hypermethylation in human cancers, including bladder cancer [12]. In our previous studies, we reported that aberrant CDH13 methylation contributed to its silencing in bladder cancer and associated unfavorable tumor features, including advanced stage, higher grade, larger tumor size, and worse outcomes [13–16]. However, the sample populations in these studies included both NMIBC and muscle-invasive bladder cancer patients, and the clinical significance of CDH13 methylation in NMIBC needs to be further elucidated.
In the current study, we examined the methylation status of CDH13 using MSP, in tissue samples collected from NMIBC patients and controls, and then correlated with clinicopathologic features and outcomes of bladder cancer cases. MSP is a useful method for methylation detection; it is rapid, simple, sensitive, specific, cost-effective, and allows rapid examination of multiple samples, which is convenient for routine clinical practice [26,27]. CDH13 methylation was detected in 80 (44.9%) bladder cancer patients, but no methylation was found in controls, indicating that CDH13 methylation is tumor-specific, is an early event in bladder tumorigenesis, and may be involved in the recurrence and/or progression of bladder cancer. Subsequently, we grouped the patients into 2 categories according to the methylation status of CDH13, and we found that CDH13 methylation was significantly correlated with advanced stage, high grade, larger tumor size, and tumor recurrence and progression; these are all unfavorable features of bladder cancer and are commonly used to predict the outcomes of patients clinically [28,29]. To investigate the value of CDH13 methylation in predictive the recurrence and progression of NMIBC, Kaplan-Meier survival analysis and log-rank test were performed, respectively. To our surprise, patients with CDH13 methylated showed shorter recurrence-free survival and progression-free survival than patients with CDH13 unmethylated. Moreover, multivariate Cox proportional hazard model analysis suggests that CDH13 methylation independently predicts the recurrence and progression of NMIBC after initial curative treatment. Our result is similar with that of a previous report on CDH13 methylation in primary lung cancer [30]. In this regard, CDH13 methylation in tumor samples after the initial transurethral resection is a reliable predictor of tumor recurrence and progression in NMIBC. It can be used to separate patients with high risk of recurrence and progression who require more aggressive adjuvant therapy from those with low risk of recurrence and progression who need less intensive surveillance; this is very important for clinical decision-making.
To the best of our knowledge, this is the first report to investigate the correlation between CDH13 methylation and NMIBC. One of the strengths of the present study is the relatively large study population; another is that this work was performed within a homogenous study population that included only NMIBC patients. Thus, the findings of the study are relatively reliable. The major limitation of the current study is that this was a single-center study, and future studies are needed to confirm our findings before being used routinely in clinical practice.
Conclusions
In conclusion, our data demonstrate that CDH13 methylation occurs frequently in NMIBC, is associated with unfavorable tumor features, should be used as an independent predictor for the recurrence and progression of NMIBC, and may facilitate the design of personalized therapeutic modalities.
Footnotes
Conflicts of interest
None.
Source of support: This study was supported by Xuzhou Medical Talented Youth Project. No: 2014007
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Isa F, Xie LP, Hu Z, et al. Dietary consumption and diet diversity and risk of developing bladder cancer: results from the South and East China case-control study. Cancer Causes Control. 2013;24(5):885–95. doi: 10.1007/s10552-013-0165-5. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374(9685):239–49. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs BL, Lee CT, Montie JE. Bladder cancer in 2010: how far have we come? Cancer J Clin. 2010;60(4):244–72. doi: 10.3322/caac.20077. [DOI] [PubMed] [Google Scholar]
- 5.Kawamoto K, Enokida H, Gotanda T, et al. p16INK4a and p14ARF methylation as a potential biomarker for human bladder cancer. Biochem Biophys Res Commun. 2006;339(3):790–96. doi: 10.1016/j.bbrc.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Qi F, Cao Y, et al. Down-regulated microRNA-101 in bladder transitional cell carcinoma is associated with poor prognosis. Med Sci Monit. 2014;20:812–17. doi: 10.12659/MSM.890300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Zhang J, Liu Y, et al. Increased expression of DNA repair gene XPF enhances resistance to hydroxycamptothecin in bladder cancer. Med Sci Monit. 2012;18(4):BR156–62. doi: 10.12659/MSM.882618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yegin Z, Gunes S, Buyukalpelli R. Hypermethylation of TWIST1 and NID2 in tumor tissues and voided urine in urinary bladder cancer patients. DNA Cell Biol. 2013;32(7):386–92. doi: 10.1089/dna.2013.2030. [DOI] [PubMed] [Google Scholar]
- 9.Kandimalla R, van Tilborg AA, Zwarthoff EC. DNA methylation-based biomarkers in bladder cancer. Nat Rev Urol. 2013;10(6):327–35. doi: 10.1038/nrurol.2013.89. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Xie PG, Lin YL, et al. Aberrant Methylation of PCDH10 Predicts Worse Biochemical Recurrence-Free Survival in Patients with Prostate Cancer After Radical Prostatectomy. Med Sci Monit. 2014;20:1363–68. doi: 10.12659/MSM.891241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin YL, Xie PG, Wang L, et al. Aberrant methylation of protocadherin 17 and its clinical significance in patients with prostate cancer after radical prostatectomy. Med Sci Monit. 2014;20:1376–82. doi: 10.12659/MSM.891247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreeva AV, Kutuzov MA. Cadherin 13 in cancer. Genes Chromosomes Cancer. 2010;49(9):775–90. doi: 10.1002/gcc.20787. [DOI] [PubMed] [Google Scholar]
- 13.Lin YL, He ZK, Li ZG, et al. Downregulation of CDH13 expression promotes invasiveness of bladder transitional cell carcinoma. Urol Int. 2013;90(2):225–32. doi: 10.1159/000345054. [DOI] [PubMed] [Google Scholar]
- 14.Lin YL, Sun G, Liu XQ, et al. Clinical significance of CDH13 promoter methylation in serum samples from patients with bladder transitional cell carcinoma. J Int Med Res. 2011;39(1):179–86. doi: 10.1177/147323001103900119. [DOI] [PubMed] [Google Scholar]
- 15.Lin YL, Liu XQ, Li WP, et al. Promoter methylation of H-cadherin is a potential biomarker in patients with bladder transitional cell carcinoma. Int Urol Nephrol. 2012;44(1):111–17. doi: 10.1007/s11255-011-9961-6. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y, Sun G, Liu X, et al. Clinical significance of T-cadherin tissue expression in patients with bladder transitional cell carcinoma. Urol Int. 2011;86(3):340–45. doi: 10.1159/000322962. [DOI] [PubMed] [Google Scholar]
- 17.Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002;87(7):13–15. [PubMed] [Google Scholar]
- 18.Oosterlinck W, Lobel B, Jakse G, et al. Guidelines on bladder cancer. Eur Urol. 2002;41(2):105–12. doi: 10.1016/s0302-2838(01)00026-4. [DOI] [PubMed] [Google Scholar]
- 19.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–74. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 20.Babjuk M, Oosterlinck W, Sylvester R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59(6):997–1008. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Jeong P, Min BD, Ha YS, et al. RUNX3 methylation in normal surrounding urothelium of patients with non-muscle-invasive bladder cancer: potential role in the prediction of tumor progression. Eur J Surg Oncol. 2012;38(11):1095–100. doi: 10.1016/j.ejso.2012.07.116. [DOI] [PubMed] [Google Scholar]
- 22.Kim YJ, Yoon HY, Kim JS, et al. HOXA9, ISL1 and ALDH1A3 methylation patterns as prognostic markers for nonmuscle invasive bladder cancer: array-based DNA methylation and expression profiling. Int J Cancer. 2013;133(5):1135–42. doi: 10.1002/ijc.28121. [DOI] [PubMed] [Google Scholar]
- 23.Kim WT, Yun SJ, Park C, et al. Identification of C16or f74 as a marker of progression in primary non-muscle invasive bladder cancer. LoS One. 2010;5(12):e15260. doi: 10.1371/journal.pone.0015260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Wang J, Xiao W, et al. Epigenetic inactivation of KLF4 is associated with urothelial cancer progression and early recurrence. J Urol. 2014;191(2):493–501. doi: 10.1016/j.juro.2013.08.087. [DOI] [PubMed] [Google Scholar]
- 25.Tada Y, Wada M, Taguchi K, et al. The association of death-associated protein kinase hypermethylation with early recurrence in superficial bladder cancers. Cancer Res. 2002;62(14):4048–53. [PubMed] [Google Scholar]
- 26.Herman JG, Graff JR, Myöhänen S, et al. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93(18):9821–26. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinoshita K, Minagawa M, Takatani T, et al. Establishment of diagnosis by bisulfite-treated methylation-specific PCR method and analysis of clinical characteristics of pseudohypoparathyroidism type 1b. Endocr J. 2011;58(10):879–87. doi: 10.1507/endocrj.k10e-364. [DOI] [PubMed] [Google Scholar]
- 28.Guo G, Xu Y, Gong M, et al. USP28 is a potential prognostic marker for bladder cancer. Tumour Biol. 2014;35(5):4017–22. doi: 10.1007/s13277-013-1525-1. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Carbayo M. Hypermethylation in bladder cancer: biological pathways and translational applications. Tumour Biol. 2012;33(2):347–61. doi: 10.1007/s13277-011-0310-2. [DOI] [PubMed] [Google Scholar]
- 30.Kim JS, Han J, Shim YM, et al. Aberrant methylation of H-cadherin (CDH13) promoter is associated with tumor progression in primary nonsmall cell lung carcinoma. Cancer. 2005;104(9):1825–33. doi: 10.1002/cncr.21409. [DOI] [PubMed] [Google Scholar]